Abstract
Episodic memory impairment is a frequently reported symptom in schizophrenia. It has been shown to be associated with reduced neural activity of the hippocampus and prefrontal cortex. Given the high heritability of schizophrenia the question arises if alterations in brain activity are modulated by susceptibility genes and might be detectable in healthy risk allele carriers. The present study investigated the effect of the single nucleotide polymorphism (SNP) rs1018381 (P1578) of the dystrobrevin‐binding protein 1 (DTNBP1) on brain activity in 84 healthy subjects assessed by functional magnetic resonance imaging (fMRI) while they performed an episodic memory task comprising encoding and retrieval of faces. During encoding, the group of risk allele carriers (n = 29) showed enhanced neural activity in the left middle frontal gyrus (BA 11) and bilaterally in the cuneus (BA 17, 7) when compared with the nonrisk carrier group (n = 55). During retrieval, the risk group (compared to the non risk group) showed increased right hemispheric neural activity comprising the medial frontal gyrus (BA 9), inferior frontal gyrus (BA 9), and inferior parietal lobule (BA 40). Since there were no behavioral performance differences, increased neural activity of the risk group might be interpreted as a correlate of higher effort or differing cognitive strategies in order to compensate for a genetically determined slight cognitive deficit. Interestingly, the laterality of increased prefrontal activity is in accordance with the well known hemispheric encoding/retrieval asymmetry (HERA) model of episodic memory. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: fMRI, genetic imaging, HERA, prefrontal cortex, schizophrenia
INTRODUCTION
Memory impairments belong to the predominant and well‐documented cognitive deficits in patients with schizophrenia. Several domains of memory have been shown to be typically affected in schizophrenia patients, including encoding and retrieval of episodic events (for a review see Aleman et al. [1999]. In an attenuated degree, deficits in episodic memory can be found as well in nonpsychotic relatives of schizophrenia patients. Accordingly, episodic memory impairment might index genetic liability and be regarded as a candidate endophenotype for schizophrenia [Gallinat et al.,2008; Sitskoorn et al.,2004; Snitz et al.,2006]. In this context it is of high interest to investigate the neural correlates of episodic memory in schizophrenia patients and groups at increased genetic risk compared to healthy subjects, to elucidate the mediating factor in the causal chain from genes to the manifestation of a cognitive deficit.
In healthy subjects, episodic memory encoding and retrieval has been consistently associated with neural activity of prefrontal and medial‐temporal regions. Encoding has often been shown to be lateralized to the left prefrontal cortex, while prefrontal activations during retrieval show a clear tendency for right lateralization [Cabeza and Nyberg,2000]. This lateralization has been described by Tulving et al. [1994 a,b] as the hemispheric encoding/retrieval asymmetry (HERA) model of prefrontal activation.
Compared with healthy subjects, episodic memory impairment in schizophrenia has been shown to be consistently associated with a robust pattern of reduced neural activity in the prefrontal cortex and in the medial temporal lobe/hippocampus [Achim and Lepage,2005; Weiss and Heckers,2001]. A meta‐analysis of numerous studies showed that within the frontal lobe, the left inferior prefrontal cortex was the most consistent area of reduced neural activity in schizophrenia patients during both encoding and retrieval. These differential activations were interpreted as their inability to use efficient strategies for encoding and retrieval. Furthermore, reduced activity was found in the right middle and medial frontal gyrus during encoding, and in the left middle and medial frontal gyrus during retrieval [Achim and Lepage,2005].
Besides the group of healthy subjects and schizophrenia patients, there is a group of individuals with a genetic risk for schizophrenia who might suffer from slight cognitive deficits. These can be relatives of schizophrenia patients and/or carriers of risk alleles in susceptibility genes which are known to enhance the risk for schizophrenia. Several fMRI studies have investigated the neural correlates of various cognitive domains in relatives of schizophrenia patients (for a review see [Macdonald et al., in press]) but only three of those investigated episodic memory. In a study by Whyte et al. [2006], a high‐risk group showed increased neural activity in the right inferior frontal gyrus during encoding and in the right cerebellum during retrieval of verbal material when compared with a control group. Similarly, in a study by Bonner‐Jackson et al. [2007] inferior frontal regions were activated greater in the risk group, however, in this study bilaterally. Finally, Thermenos et al. [2007] found bilaterally enhanced neural activity of the anterior parahippocampus in the risk group. In summary, performing episodic memory tasks was accompanied by hypoactivations in schizophrenia patients but hyperactivations in relatives of patients in several areas when compared with healthy subjects.
The mentioned studies have shown that genetic liability in general affects neural correlates of cognitive functions. However, schizophrenia has a polygenetic pattern of heredity and genetic effects on brain functions have scarcely been demonstrated on a single gene level by functional imaging studies [Kircher et al.,2009; Krug et al.,2008]. In the last years several susceptibility genes for schizophrenia have been detected including dysbindin 1 (DTNBP1), neuregulin 1 (NRG1), catechol‐O‐methyltransferase (COMT), disrupted‐in‐schizophrenia 1 (DISC1) regulator of G‐protein signalling 4 (RGS4), G72, proline dehydrogenase (PRODH), and d‐amino acid oxidase (DAAO) [Harrison and Weinberger,2005; O'Tuathaigh et al.,2007; Owen et al.,2004b]. Among these, DTNBP1 has been regarded as one of the best‐supported susceptibility genes [Owen et al.,2004a; Williams et al.,2005]. It has been shown to have an effect on schizotypy and attention capacity [Stefanis et al.,2007], executive function [Luciano et al.,2009], intelligence [Burdick et al.,2006, 2007; Luciano et al.,2009; Zinkstok et al.,2007], and memory [Donohoe et al.,2007; Luciano et al.,2009] in both healthy subjects and patients with schizophrenia. The effect of DTNBP1 on cognitive functions has been supposed to be mediated by the glutamate neurotransmitter system, acting via the prefrontal cortex [Fallgatter et al.,2006]. Several single‐nucleotide polymorphisms of DTNBP1 have been detected and discussed to be risk factors for schizophrenia [Straub et al.,2002]. The most significant association with schizophrenia has been shown for the SNP rs1018381 (P1578) [Funke et al.,2004] and this was the only SNP that showed a significant effect on general cognitive ability in a study by Burdick et al. [2006].
The aim of the present study was to investigate the effects of SNP rs1018381 of the DTNBP1 gene on neural correlates of episodic memory in healthy subjects. Based on the above mentioned studies, we hypothesized that the risk variant (A allele) leads to an enhancement of neural activity, mainly in the prefrontal cortex, during encoding and retrieval of an episodic memory task.
MATERIALS AND METHODS
Subjects
Eighty‐four right‐handed participants (as tested with the Edinburgh Laterality Scale [Oldfield,1971]) were recruited from the RWTH Aachen university. Inclusion criteria were age (18–55 years) and no psychiatric disorder according to ICD‐10. The study protocol was approved by the local ethics committee and written informed consent was obtained from each subject.
Genetic Analysis
DNA was isolated from peripheral lymphocytes by a simple salting out procedure. The SNP rs1018381 [Straub et al.,2002] was genotyped using Applied Biosystems 7900HT Fast Real‐Time PCR System and TaqMan‐probes designed by Applied Biosystems (Foster City, CA). The following primers and VIC/FAM‐probe sequences for rs101838 detection were used: Forward‐5′‐GAGTTACAAGTAAATGAAACGTCATGCA‐3′; Reverse‐5′‐GCTGAGATCTGCCGGTGATTC‐3′; 5′‐VIC‐ACAGCGTGCGGAAC‐3′; 5′‐FAM‐AACAGCATGCGGAAC. Note, that the common C allele reported in previous studies is equivalent to our G allele and analogous the risk T allele [Burdick et al.,2006; Funke et al.,2004; Straub et al.,2002] is equivalent to our A allele. Actual and expected frequencies of the SNP rs1018381 did not show a statistical significant difference, assessed by Hardy‐Weinberg equilibrium (HWE) using Haldane's exact test [Elston and Forthofer,1977].
fMRI Task
Task and stimuli
The fMRI task was designed with “Presentation” software (Neurobehavioral Systems Inc., San Francisco, CA) and comprised an encoding and a retrieval episode in a block design as described in a previous experiment by Leube et al. [2001]. During encoding, subjects were required to memorize consecutively presented standardized pictures of male or female faces that were presented on a black background for 4,000 ms in a pseudorandomized order, followed by a blank screen of 1,000 ms. In order to ensure their attention, subjects were instructed to press a button (LUMItouch™ Lightwave Technologies, Richmond, B.C., Canada) with their left and right index finger to indicate if the presented face was male or female. In a second condition, subjects had to press the button whenever the symbol “#” was presented (low‐level baseline). Face encoding and baseline were presented in 10 alternating blocks of 30 seconds (five blocks of each condition), requiring six responses in each block, resulting in 30 faces to be encoded and 30 button presses as baseline. The second part of the fMRI task consisted in the retrieval of the memorized faces. For this purpose, two pictures of faces were presented simultaneously side by side, each trial comprising a previously presented face and a new face, randomly positioned at the left or right side. Subjects were requested to select the previously presented face and forced to make a choice by pressing the corresponding button with the left or right index finger. Background image, low level baseline, timing, and block design were exactly the same as in the encoding episode of the experiment.
Data acquisition
FMRI was performed on a 3T Trio MR scanner (Siemens, Erlangen, Germany) in the Institute of Neuroscience and Biophysics—Medicine, Research Centre Jülich, using a T 2*‐weighted echo planar imaging (EPI) sequence (time repetition = 2,250 ms, time echo = 30 ms, flip angle = 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior‐posterior commissural line (AC‐PC). A total of 137 functional images were acquired, each consisting of 36 slices (3 mm thickness, 20 × 20 cm field of view, 64 × 64 image matrix). The initial three images were excluded from further analysis in order to remove the influence of T1 stabilization effects.
fMRI data analysis
Analysis of fMRI data was done by SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Functional images were realigned, normalized (to a voxel size of 2 × 2 × 2 mm), smoothed (6 mm isotropic Gaussian filter) and high‐pass filtered (cut‐off period 120 s). At the first level, the BOLD responses were modeled by a boxcar function convolved with the canonical hemodynamic response function. For each subject, neural activity related to episodic encoding and retrieval was contrasted with activity at the low‐level baseline. At the second level, the individual β‐contrasts of the first level analyses were used to calculate two sample t‐tests to investigate for genotype effects (G/A vs. G/G). In order to correct for multiple comparisons within a search volume we applied a cluster extent threshold determined by Monte Carlo simulations [Slotnick et al.,2003]. For spatial properties as present in this study, 10.000 simulations resulted in an extent threshold of 26 or 33 resampled voxels for a threshold at the voxel level of P = 0.001 or P = 0.005, respectively. This procedure prevented a false positive rate above 5% due to multiple testing. Brain activations were displayed on the anatomical SPM template.
RESULTS
Subjects
Genetic analysis of SNP rs1018381 revealed 29 subjects with the risk allele variant A/G and 55 subjects with the non risk variant G/G. Risk group and non risk group did not differ concerning sex ratio, age, education and estimated IQ (see Table I)
Table I.
Sociodemographic variables of the sample
| Sample (n = 84) | Risk group (n = 29) | Nonrisk group (n = 55) | Statistics | P |
|---|---|---|---|---|
| Sex ratio (men/women) | 22/7 | 35/20 | χ2 = 1.3 | NS |
| Age (yr) | 22.6 (2.3) | 23.7 (3.2) | F = 2.68 | NS |
| Education (yr) | 15.4 (2.2) | 15.9 (2.7) | F = 0.84 | NS |
| Estimated IQ | 113.8 (11.3) | 111.5 (11.9) | F = 0.74 | NS |
Means, standard deviations (SD) in parentheses.
IQ was estimated using the MWT‐B [Lehrl,2005].
NS = nonsignificant (P > 0.05).
Behavioral Data
The mean of the correctly recognized faces was 23.4 (SD 2.8) in the A/G risk genotype group and 24.4 (SD 3.1) in the G/G genotype group. Differences were statistically not significant (F = 1.93, P = 0.17).
fMRI Data
Group differences were calculated separately for encoding and retrieval. During encoding, the risk group showed increased neural activity of the left middle frontal gyrus and bilaterally of the cuneus when compared to the non risk group (see Table II and Fig. 1). During retrieval, the risk group showed increased right hemisphere neural activity of the medial and inferior frontal gyrus and of the inferior parietal lobule (see Table III and Fig. 1). The reverse contrasts (nonrisk > risk group) did not show any significant differences.
Table II.
Neural activity during encoding
| Risk group > nonrisk group | |||||||
|---|---|---|---|---|---|---|---|
| C | Region | BA | Side | RS | z‐value | ||
| x | y | z | |||||
| −26 | 37 | −4 | Middle frontal gyrus | 11 | L | 58 | 3.30 |
| −8 | −95 | 7 | Cuneus | 17 | L | 49 | 3.28 |
| 24 | −83 | 10 | Cuneus | 17 | R | 66 | 3.19 |
| 2 | −72 | 33 | Cuneus | 7 | R | 36 | 2.98 |
TC, Talairach coordinates; BA, Brodmann area; RS, region size (number of voxels), threshold >33 determined by Monte Carlo simulations, P < 0.005 uncorrected).
Figure 1.
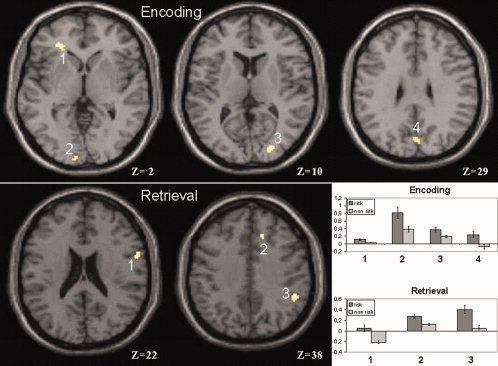
Increased neural activity during encoding and retrieval: risk group > nonrisk group. Mean effects (β) and standard errors of each region as bar charts.
Table III.
Neural activity during retrieval
| Risk group > nonrisk group | |||||||
|---|---|---|---|---|---|---|---|
| TC | Region | BA | Side | RS | z‐value | ||
| x | y | z | |||||
| 14 | 29 | 32 | Medial frontal gyrus | 9 | R | 35 | 4.08 |
| 59 | 5 | 22 | Inferior frontal gyrus | 9 | R | 62 | 3.75 |
| 50 | −43 | 39 | Inferior parietal lobule | 40 | R | 101 | 3.92 |
TC, Talairach coordinates; BA, Brodmann area; RS, region size (number of voxels), threshold >26 determined by Monte Carlo simulations, P < 0.001 uncorrected).
DISCUSSION
In the present study, the effect of the single nucleotide polymorphism (SNP) rs1018381 of the dystrobrevin‐binding protein 1 (DTNBP1) on episodic memory performance and its neural correlates was investigated in healthy subjects. Retrieval performance did not differ significantly between the A/G risk allele group and the G/G nonrisk group. On the functional imaging level, the risk group showed enhanced neural activity both during encoding and retrieval. In general, this result is in accordance with previous episodic memory studies that showed increased neural activity in relatives of schizophrenia patients when compared with control subjects with no family history of schizophrenia [Bonner‐Jackson et al.,2007; Thermenos et al.,2007; Whyte et al.,2006]. These studies were based on the assumption that relatives of patients should have a general genetic liability and therefore might show different patterns of neural activity when performing cognitive tasks [for a review see Macdonald et al., in press].
In our study now we disclosed the impact of dysbindin 1 on neural correlates of encoding and retrieval of nonverbal material. Increased neural activity was found in the prefrontal lobe both during encoding and retrieval. This is in accordance with functional imaging and neurocognition studies that have consistently shown abnormalities of prefrontal information processing in patients with schizophrenia and in unaffected individuals who are genetically at risk for schizophrenia [Weinberger et al.,2001]. It has been supposed that risk alleles may directly affect the development, maturation, and adult function of the dorsolateral prefrontal cortex [Weinberger et al.,2001]. Likewise, Donohoe et al. [2007] suggested that the increased risk for schizophrenia associated with dysbindin may be partly mediated by its influence on prefrontal function. Numakawa et al. [2004] showed that dysbindin is involved in the presynaptic protein expression and release of glutamate, a key neurotransmitter related to cognitive dysfunction in schizophrenia. Patients with schizophrenia showed reduced dysbindin mRNA levels especially in the dorsolateral prefrontal cortex [Weickert et al.,2004]. In summary, the effect of DTNBP1 on cognitive functions has been supposed to be mediated by the glutamate neurotransmitter system, acting via the prefrontal cortex [Fallgatter et al.,2006].
Since episodic memory is known to rely on neural networks including the prefrontal cortex [Blumenfeld and Ranganath,2007; Cabeza and Nyberg,2000; Fernandez and Tendolkar,2001], a dysfunction of this region caused by DTNBP1 is likely to interfere with episodic memory. Increased prefrontal neural activity in the risk group of our study can be interpreted as a neural correlate for encoding and retrieval strategies in order to maintain or improve task performance and compensate for a genetically determined deficit. Such an involvement of prefrontal activity in association with the attempt to improve episodic memory performance has been demonstrated in previous studies in both healthy subjects and schizophrenia patients. Miotto et al. [2006] showed the engagement of bilateral prefrontal regions when healthy subjects applied effortful encoding strategies. Bonner‐Jackson et al. [2005] demonstrated that during deep encoding schizophrenia patients activated several prefrontal regions that were not activated during shallow encoding. In a recent study, incidental versus intentional encoding was compared in a group of schizophrenia patients [Bonner‐Jackson et al.,2008]. Better retrieval performance after incidental encoding was correlated with increased neural activity in an extended neural network including several prefrontal regions. However, in general, schizophrenia patients show hypoactivity of the prefrontal cortex [Achim and Lepage,2005]. Thus it can be assumed that subjects at genetic risk for schizophrenia are still able to compensate for their deficit, reflected by increased neural activity of the prefrontal cortex [Bonner‐Jackson et al.,2007a; Whyte et al.,2006] while the onset of the disorder goes along with a collapse of these compensating mechanisms reflected in a decrease of prefrontal neural activity [Achim and Lepage,2005].
The lateralization of the increased prefrontal activity was in accordance with the hemispheric encoding/retrieval asymmetry (HERA) model of episodic memory [Tulving et al., 1994a,b]. This model asserts that encoding and retrieval of episodic information are specifically associated with enhanced neural activity of left and right prefrontal cortical regions, respectively.
Apart from frontal regions, increased neural activity in the risk group was found in the cuneus (bilaterally) during encoding. Neural activity in this region has previously shown to be common during nonverbal retrieval [Cabeza and Nyberg,2000]. In our study it might indicate the effort to improve encoding performance by imagery operations. Risk allele carriers might (unconsciously) try to compensate their disadvantage in “spontaneous” encoding by means of intensified visual associations in order to link new information to previously encoded information, later on resulting in a better retrieval performance.
Finally, increased neural activity in the risk group was found at the right inferior parietal lobe during retrieval. This region has been previously described to be involved in the recall of episodic information [Cabeza et al.,1997; Tulving et al., 1994 a,b] though more for the retrieval of information about spatial location than for object identity [Moscovitch et al.,1995]. However, the literature concerning genetic liability to schizophrenia is widely divergent and in general the right parietal cortex (along with the right ventral prefrontal cortex) has been found to be the most consistent region of increased neural activity regardless of the cognitive domain [Macdonald et al., in press].
Since the effects of single SNPs on neural activity and cognitive abilities in general are rather small, further studies would be desirable to investigate the additive and interactive effects of several SNPs or risk genes. A direct comparison of a young and unimpaired risk group (as we investigated) with an older risk group that is more likely to show memory deficits [Luciano et al.,2009] and with schizophrenia patients would also be a promising approach.
In summary, our results demonstrate that the risk allele variant A/G of the SNP rs1018381 leads to enhanced neural activity in networks associated with encoding and retrieval of episodic information. This can be interpreted as a neural correlate of higher effort or differing cognitive strategies in order to compensate for a genetically determined episodic memory alteration.
REFERENCES
- Achim AM, Lepage M ( 2005): Episodic memory‐related activation in schizophrenia: Meta‐analysis. Br J Psychiatry 187: 500–509. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS ( 1999): Memory impairment in schizophrenia: A meta‐analysis. Am J Psychiatry 156: 1358–1366. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C ( 2007): Prefrontal cortex and long‐term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13: 280–291. [DOI] [PubMed] [Google Scholar]
- Bonner‐Jackson A, Csernansky JG, Barch DM ( 2007): Levels‐of‐processing effects in first‐degree relatives of individuals with schizophrenia. Biol Psychiatry 61: 1141–1147. [DOI] [PubMed] [Google Scholar]
- Bonner‐Jackson A, Haut K, Csernansky JG, Barch DM ( 2005): The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry 58: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner‐Jackson A, Yodkovik N, Csernansky JG, Barch DM ( 2008): Episodic memory in schizophrenia: The influence of strategy use on behavior and brain activation. Psychiatry Res 164: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK ( 2007): DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res 89: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, Kucherlapati R, Malhotra AK ( 2006): Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet 15: 1563–1568. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Kapur S, Craik FIM, McIntosh AR, Houle S, Tulving E ( 1997): Functional neuroanatomy of recall and recognition: A PET study of episodic memory. J Cogn Neurosci 9: 254–265. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and FMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, Garavan H, Robertson IH, Gill M, Corvin A ( 2007): Variance in neurocognitive performance is associated with dysbindin‐1 in schizophrenia: A preliminary study. Neuropsychologia 45: 454–458. [DOI] [PubMed] [Google Scholar]
- Elston RC, Forthofer R ( 1977): Testing for Hardy‐Weinberg equilibrium in small samples. Biometrics 33: 536–542. [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, Deckert J ( 2006): DTNBP1 (Dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology 31: 2002–2010. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I ( 2001): Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: Human declarative memory formation at the system level. Brain Res Bull 55: 1–9. [DOI] [PubMed] [Google Scholar]
- Funke B, Finn CT, Plocik AM, Lake S, DeRosse P, Kane JM, Kucherlapati R, Malhotra AK ( 2004): Association of the DTNBP1 locus with schizophrenia in a U.S. population Am J Hum Genet 75: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Bauer M, Heinz A ( 2008): Genes and neuroimaging: Advances in psychiatric research. Neurodegener Dis 5: 277–285. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR ( 2005): Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry 10: 40–68. [DOI] [PubMed] [Google Scholar]
- Kircher T, Thienel R, Wagner M, Reske M, Habel U, Kellermann T, Frommann I, Schwab S, Wolwer W, von Wilmsdorf M, Braus DF, Schmitt A, Rapp A, Stocker T, Shah NJ, Henn FA, Sauer H, Gaebel W, Maier W, Schneider F ( 2009): Neuregulin 1 ICE‐single nucleotide polymorphism in first episode schizophrenia correlates with cerebral activation in fronto‐temporal areas. Eur Arch Psychiatry Clin Neurosci 259: 72–79. [DOI] [PubMed] [Google Scholar]
- Krug A, Markov V, Eggermann T, Krach S, Zerres K, Stocker T, Shah NJ, Schneider F, Nothen MM, Treutlein J, Rietschel M, Kircher T ( 2008): Genetic variation in the schizophrenia‐risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. Neuroimage 42: 1569–1576. [DOI] [PubMed] [Google Scholar]
- Lehrl S ( 2005). Der Mehrfachwahl‐Wortschatz‐Intelligenztest. Göttingen: Hogrefe. [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT ( 2001): Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport 12: 2773–2777. [DOI] [PubMed] [Google Scholar]
- Luciano M, Miyajima F, Lind PA, Bates TC, Horan M, Harris SE, Wright MJ, Ollier WE, Hayward C, Pendleton N, Gow AJ, Visscher PM, Starr JM, Deary IJ, Martin NG, Payton A ( 2009): Variation in the dysbindin gene and normal cognitive function in three independent population samples. Genes Brain Behav 8: 218–227. [DOI] [PubMed] [Google Scholar]
- Macdonald AW III, Thermenos HW, Barch DM, Seidman LJ: Imaging genetic liability to schizophrenia: Systematic review of FMRI studies of patients' nonpsychotic relatives. Schizophr Bull (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MG, Iaki S, Amaro E Jr ( 2006): Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Hum Brain Mapp 27: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch C, Kapur S, Kohler S, Houle S ( 1995): Distinct neural correlates of visual long‐term memory for spatial location and object identity: A positron emission tomography study in humans. Proc Natl Acad Sci USA 92: 3721–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R ( 2004): Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet 13: 2699–2708. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL ( 2007): Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev 31: 60–78. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O'Donovan MC ( 2004a): Dysbindin‐1 and schizophrenia: From genetics to neuropathology. J Clin Invest 113: 1255–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O'Donovan MC ( 2004b): The molecular genetics of schizophrenia: New findings promise new insights. Mol Psychiatry 9: 14–27. [DOI] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS ( 2004): Cognitive deficits in relatives of patients with schizophrenia: A meta‐analysis. Schizophr Res 71: 285–295. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J Jr. ( 2003): Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17: 75–82. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW III, Carter CS ( 2006): Cognitive deficits in unaffected first‐degree relatives of schizophrenia patients: A meta‐analytic review of putative endophenotypes. Schizophr Bull 32: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis NC, Trikalinos TA, Avramopoulos D, Smyrnis N, Evdokimidis I, Ntzani EE, Ioannidis JP, Stefanis CN ( 2007): Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol Psychiatry 62: 784–792. [DOI] [PubMed] [Google Scholar]
- Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris‐Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS ( 2002): Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 71: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Seidman LJ, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT ( 2007): Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry 61: 564–574. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S ( 1994a): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S ( 1994b): Neuroanatomical correlates of retrieval in episodic memory: Auditory sentence recognition. Proc Natl Acad Sci USA 91: 2012–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE ( 2004): Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 61: 544–555. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE ( 2001): Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50: 825–844. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S ( 2001): Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 42: 239–250. [DOI] [PubMed] [Google Scholar]
- Whyte MC, Whalley HC, Simonotto E, Flett S, Shillcock R, Marshall I, Goddard NH, Johnstone EC, Lawrie SM ( 2006): Event‐related FMRI of word classification and successful word recognition in subjects at genetically enhanced risk of schizophrenia. Psychol Med 36: 1427–1439. [DOI] [PubMed] [Google Scholar]
- Williams NM, O'Donovan MC, Owen MJ ( 2005): Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull 31: 800–805. [DOI] [PubMed] [Google Scholar]
- Zinkstok JR, de Wilde O, van Amelsvoort TA, Tanck MW, Baas F, Linszen DH ( 2007): Association between the DTNBP1 gene and intelligence: A case‐control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]


