Abstract
Extrastriate, parietal, and frontal brain regions are differentially involved in distinct kinds of body movements and motor cognition. Using functional magnetic resonance imaging, we investigated the neural mechanisms underlying the observation and mental imagery of meaningful face and limb movements with or without objects. The supplementary motor area was differentially recruited by the mental imagery of movements while there were differential responses of the extrastriate body area (EBA) during the observation conditions. Contrary to most previous reports, the EBA responded to face movements, albeit to a lesser degree than to limb movements. The medial wall of the intraparietal sulcus and adjacent intraparietal cortex was selectively recruited by the processing of meaningful upper limb movements, irrespective of whether these were object‐related or not. Besides reach and grasp movements, the intraparietal sulcus may thus be involved in limb gesture processing, that is, in an important aspect of human social communication. We conclude that subregions of a frontal–parietal network differentially interact during the cognitive processing of body movements according to the specific motor‐related task at hand and the particular movement features involved. Hum Brain Mapp, 2009. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, extrastriate body area, intraparietal cortex, motor cognition, inhibitory control, mirror neurons
INTRODUCTION
The primary motor (M1) and premotor cortices, the supplementary motor area (SMA), lateral inferior prefrontal, superior temporal, extrastriate, and parietal areas are all known components of a neural network supporting the cognitive processing of movements [see, e.g., Decety et al., 1994; Jeannerod, 1994, 2001]. The specific contribution of these areas is likely to depend upon task demands and body parts involved, and other features of movement processing.
Clinical studies demonstrated a dissociation of buccofacial and limb apraxia in patients with differential location of frontal and parietal brain lesions [e.g., Raade et al., 1991; Watson et al., 1986] suggesting that—besides the somatotopic representation of distinct body parts in primary motor and somatosensory cortices e.g. Fink et al., 1997; Buccino et al., 2001—the neural processing of face and limb movements is supported at least in part by differential brain areas. Moreover, functional neuroimaging studies have shown that regions of the extrastriate cortex respond differentially to body movements depending on the body part involved. A region located in the posterior fusiform gyrus, the “fusiform face area” (FFA), has selectively been implicated in face perception [Grill‐Spector et al., 2004; Kanwisher et al., 1997] while the extrastriate body area (EBA), situated in the more posterior portion of the temporooccipital junction, has predominantly been associated with the observation of limb movements [see, e.g., Downing et al., 2001; Urgesi et al., 2004], however, also showed EBA activation in response to faces).
Motor imagery [i.e., a mental representation of movements which does not imply muscular activity for its implementation; Denis, 1985; Porro et al., 1996] may also trigger the EBA. For example, Solodkin et al. [2004] showed evidence for EBA activity during both the execution and (visual and kinesthetic) mental imagery of finger movements. Peigneux et al. [2000] implicated the EBA in the performance of naming and orientation tasks on static pictures of meaningful and meaningless gestures. The activated region extended into area MT/V5, which is known to be involved in the analysis of perceived [Tootell et al., 1995] and mentally imagined motion [Goebel et al., 1998]. The findings of Peigneux et al. [2000] suggest that the EBA and MT/V5 interact during the cognitive processing of motor acts and, importantly, do not only respond to real paths of movements, but are also involved in the extraction of movements from static pictures. It is worth noting that motor imagery is likely to play a key role when someone mentally derives the appropriate movement dynamics from the static display of a gesture.
Some areas of the extrastriate cortex interact with parietal regions in visuomotor cognition [see, e.g., Grefkes et al., 2004; Newman et al., 2005], in particular, during target‐directed actions. For example, an fMRI study by Grefkes et al. [2004] showed that the medial wall (putative human area MIP) of the intraparietal sulcus (IPS) is activated in concert with MT/V5 by visuomotor transformation processes related to goal‐directed arm and hand movements. These findings in humans are in good accordance with monkey data, suggesting that area MIP acts as a key component in the planning and execution of goal‐directed reaches [Colby et al., 1988], which require the visuomotor transformation of target coordinates into planned and ongoing reaching movements [Cohen and Andersen, 2002; Eskandar and Assad, 2002]. It is not clear, however, whether MIP engagement in goal‐directed reaches requires the involvement of a real object or not.
Frontal areas implicated in motor processing comprise M1, the premotor cortex, the SMA, and inferior prefrontal regions (e.g., Broca's area). It is well known that M1 contains a somatotopic motor representation of body parts [Förster, 1931; Penfield and Jasper, 1954]. M1 somatotopic activation has particularly been corroborated by neuromagnetic studies of movement observation [e.g., Hari et al., 1998; Jarvelainen et al., 2004: for a review, see Grezes and Decety, 2001] and neuroimaging studies of motor imagery [Ehrsson et al., 2003; Stippich et al., 2002]. The macaque inferior frontal gyrus contains “mirror neurons” that respond during both the execution and observation of body movements [for a review, see Rizzolatti et al., 2001]. Together with premotor and inferior parietal areas, the inferior frontal cortex (including Broca's area) is assumed to constitute the human equivalent of the macaque “mirror neuron system” [Buccino et al., 2004; Jeannerod, 2001]. The SMA and the premotor cortex have specifically been related to the mental imagery of body movements [Dechent et al. 2004; Porro et al., 2000; Solodkin et al., 2004; Stephan et al., 1995] and other forms of higher‐order motor cognition, such as the timing and preparation of movements [Macar et al., 2004; Michelon et al., 2006]. It is important to note that the mental imagery of movements also requires the inhibition of motor execution and, therefore, engages prefrontal areas involved in executive control [e.g., Lotze et al., 1999; Nakata et al., 2005; Watanabe et al., 2002].
The present study aims at characterizing the common and differential brain mechanisms which underlie the observation and motor imagery of meaningful buccofacial and unimanual upper limb movements with or without object using event‐related fMRI. A 2 × 2 × 2 experimental design with the factors task (observation/motor imagery), body part (upper limb/face), and object relatedness (with/without object) was applied to disentangle the brain mechanisms supporting the perception‐ and imagery‐related cognitive processing of the differential types of human body movements of interest. We expected that the factor body part would differentially modulate movement representations in extrastriate regions (differential EBA engagement in limb movements, differential FFA involvement in face movement) and that object‐related limb movements would specifically trigger the human equivalent of the macaque area MIP of the IPS. We also assumed that the SMA and regions of the lateral inferior prefrontal cortex would be modulated by the distinct cognitive demands associated with the observation and mental imagery tasks. In particular, we hypothesized differential SMA and prefrontal involvement in motor inhibition during motor imagery; stronger activation of mirror neurons in the inferior frontal gyrus during the observation relative to the motor imagery task.
MATERIALS AND METHODS
Subjects
Fourteen male, right‐handed subjects (mean age + SD = 25.18 + 4.25 years) with no history of psychiatric or neurological disorder were enrolled in the experiment. Handedness was tested using the Edinburgh Inventory [Oldfield, 1971]. Kinesthetic and visual motor imagery abilities were assessed by the revised Movement Imagery Questionnaire [MIQ‐R; Hall and Martin, 1997]. The study was accomplished in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all subjects prior to participation, and the study was approved by the local ethics committee. MR images of two subjects were excluded from data analysis because of a subarachnoidal cyst in one case and technical problems in the other.
Experimental Design
A 2 × 2 × 2 factorial event‐related fMRI experiment was conducted contrasting the cognitive processes involved in the observation or mental imagery (factor 1 = task) of meaningful buccofacial and unimanual upper limb movements (factor 2 = body part) with or without object (factor 3 = object relatedness). Examples of movements included in each of the resulting four stimulus categories are illustrated in Figure 1. The Appendix provides a verbal description of all movements used for the fMRI experiment. All movements were meaningful with respect to human everyday experience. However, movements were heterogeneous concerning their abstractness and concrete practical meaning. In particular, movements with and without objects “naturally” differ along these dimensions. This aspect might represent a potential limitation of our study.
Figure 1.

Examples of the meaningful body movements shown in video clips during the fMRI experiment. Half of the movements of each stimulus category—buccofacial movements either with or without object (a) and upper limb movements either with or without object (b)—were performed by a male, and the other half by a female.
Video clips were used for the presentation of stimulus movements. Actors performed all upper limb movements unimanually using their left hand. Participants were instructed to imagine themselves performing each movement with the right hand, since we expected that the “mirror” observation of left‐hand movements would facilitate the mental imagery of one's own performance of each movement using the right hand [Aziz‐Zadeh et al., 2006a]. Given the current knowledge on the lateralization of motor imagery and other motor‐related brain activation [e.g., Aziz‐Zadeh et al., 2006a, b; Ehrsson et al., 2003], we assumed that the brain circuits involved in our experimental tasks would be activated bilaterally, but with left‐hemispheric predominance. Altogether, 80 different videos were used (20 for each of the four distinct movement types, as defined by the factors body part and object relatedness). Each video was shown only once throughout the whole experiment. The video clips of stimulus movements had different durations of 4–10 s according to the distinct natural duration of movements. Typically, the stimulus movement was performed only once in each video clip. However, in some stimuli, there was an inherent repetition of movement components which was necessary to warrant the natural unfolding of the movement sequence (e.g., to wave, to tap one's own chest, to chew). In the analysis, the different lengths of the stimulus movement clips were accounted for by modeling the video events as mini blocks with respective differential durations (see later).
Half of the movements included in each movement category were performed by a male, and the other half by a female. The factor task (observation/motor imagery) was implemented by the inclusion of distinct event types into each trial which consisted of five events (see Fig. 2): (1) cue (black square, 3 s), (2) movement observation (video clip, 4–10 s), (3) pause (fixation cross, 2–8 s), (4) motor imagery of the movement observed in the preceding video clip (black frame on white background, 7 s), (5) baseline condition (fixation cross, 6–12 s), in that fixed order. A blink of the fixation cross (event 3) indicated that the motor imagery task (event 4) would begin within the next second. Event durations were jittered to exclude correlation of event regressors in order to reliably separate brain activation related to the events of interest. To control for the maintenance of the subjects' alertness throughout the fMRI session, a checkerboard was presented for 500 ms in 50% of the baseline events, balanced across movement types and runs. Participants were instructed to press a button with their right index finger as fast as possible whenever it appeared. To avoid confounds which could have resulted from checkerboard presentations and the associated motor responses, these events were explicitly modeled by including extra regressors into the design matrix for fMRI data analysis. These regressors accounted for blood oxygen level‐dependent (BOLD) signal changes that were associated with checkerboard detections and motor responses.
Figure 2.
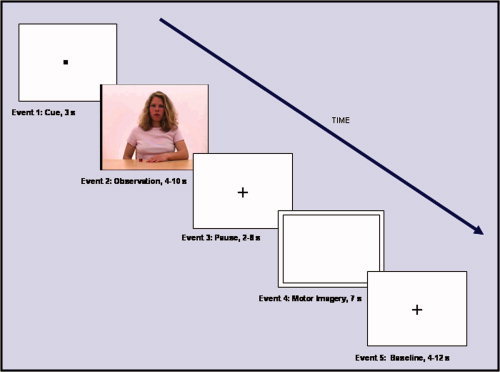
Order and duration of events included in each experimental trial. The cue event at the beginning was always followed by an observation (video clip), a pause (fixation cross), a motor imagery (motor imagery of the movement seen in the preceding video clip), and a “baseline” event (fixation cross). Event duration was jittered to prevent correlation of event regressors. A reaction time task was included in the experiment to control for the subjects' alertness during the experiment (button press response upon detection of a checkerboard presented for 500 ms in 50% of the baseline events).
Presumably, motor imagery of one's own movements is based on a combination of both kinesthetic and visual imagery albeit with a lower degree of recruitment of the visual aspect [e.g., De Felippo et al., 1995; Imbiriba et al., 2006; Livesey, 2002; Sacco et al., 2006; Sirigu and Duhamel, 2001] Accordingly, the imagery task of the present experiment was assumed to require mainly kinesthetic imagery of one's own limbs and face, but would also include some visual imagery. To control for these aspects and for general motor imagery abilities, subjects completed the MIQ‐R [Hall and Martin, 1997] prior to the fMRI investigation. The questionnaire requires (1) real performance of four distinct body movements, (2) kinesthetic, and (3) visual imagery of the same movements. Imagery performance was rated on a seven‐point scale, ranging from 1 = very hard to visually/kinesthetically imagine to 7 = very easy to visually/kinesthetically imagine). Subjects who achieved values less than 4 (4 = neutral, i.e. neither easy nor hard to visually/kinesthetically imagine) on any rating were excluded from the study. Participants were familiarized with the experimental setup and the tasks prior to the fMRI measurement. They were instructed not to concentrate on the checkerboard since the reaction time task was subordinate to the action observation and motor imagery tasks. Before entering the MR scanner, 30 min of training of the experimental tasks were accomplished by each subject. None of the stimuli used for the training session was employed during the fMRI experiment to avoid repetition effects.
Stimuli were presented visually by projecting them onto a mirror placed on a standard head coil. Five experimental runs were performed, each consisting of 16 trials (four trials of each movement type; order of trial types was counterbalanced across runs and subjects). One hundred seventy‐three volumes images were acquired per run. Four images at the beginning of each run were discarded, to allow the MR signal to reach steady state. Thus, 845 volumes per subject for the whole fMRI measurement were entered into the data analysis.
Immediately after the fMRI experiment, participants accomplished a debriefing questionnaire that required them to rate their task performance on a five‐point scale (ranging from 5 = very good or always (depending on the specific item) to 1 = not at all) separately for each of the 80 movements seen in the video clips, while each item was shown again on a computer screen. Subjects indicated how difficult it was (1) to recognize each movement shown in the video clips, (2) to separate the different tasks required by the events of each trial from each other, (3) to kinesthetically, and (4) visually imagine self‐performance of the movement.
MR Hardware and Technical Parameters
Scanning was performed on a 1.5‐T whole‐body MR system (Siemens Sonata, Erlangen, Germany) with echo‐planar imaging (EPI) capability. A standard radiofrequency head coil was used for transmitting and receiving of the MR signal. Prior to the fMRI measurement, high‐resolution anatomical images were acquired using a T1‐weighted high‐resolution 3D magnetization‐prepared, rapid acquisition gradient‐echo pulse sequence. Functional images were acquired in axial plane with a gradient‐echo EPI pulse sequence using BOLD contrast. Sequence parameters were as follows: TE = 66 ms, TR = 3 s, flip angle = 90°, slice thickness = 4 mm, interslice‐gap = 0.4 mm, FOV = 200 mm, in‐plane resolution = 3.125 mm × 3.125 mm, matrix = 64 × 64, and 29 transversal slices. The 29 slices covered a subject's brain from the cerebellar vermis up to the vertex and were oriented along the anterior–posterior commissure line using a midsagittal scout image.
Image Processing
For image processing and all statistical calculations, MATLAB 6.5 (The Mathworks, Natick, MA) and SPM2 (Statistical Parametric Mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk) were used. Slice‐timing and correction for head movements between scans and runs were performed on the 169 volume images of each time series. Images were transformed into standard stereotactic space of a representative brain from the Montreal Neurological Institute (MNI) series [Evans et al. 1994], using linear proportions and a nonlinear sampling algorithm. Normalized data were smoothed with a Gaussian kernel of 8 mm (full width half maximum) for the group analysis.
Statistical Analysis
For each single subject, the onset and the duration of each stimulus was modeled in a general linear model, according to the distinct stimulus types. Events were considered as mini blocks. Fourteen regressors were included into the analysis, each of which referred to one of the event types of interest. These 14 regressors were modeled separately for each of the five runs. Regressors were convolved with the canonical hemodynamic response function. Specific effects were assessed by the application of appropriate linear contrasts to the parameter estimates of the events and the baseline resulting in t‐statistics for each voxel. For the group analysis, corresponding contrast images created for each subject were entered into an analysis of variance (ANOVA), constituting a random‐effect model [Friston et al., 1995]. The statistical height threshold was set to P < 0.05, family wise error (FWE) corrected for multiple comparisons. No extent threshold was applied. The exact anatomical location of activations was assessed by reference to the structural MR images of the volunteers and the anatomical brain atlas of Duvernoy [1999]. Moreover, an anatomical toolbox [Eickhoff et al., 2005] was used, which allows for the integration of probabilistic cytoarchitectonic maps of the brain and functional neuroimaging data.
RESULTS
Behavioral Data
Behavioral data were analyzed using repeated measures ANOVAs. Parametric statistical tests were applied, since a Kolmogorov–Smirnov test revealed a normal distribution of data.
Reaction times and error rates in the subordinate alertness task during the fMRI experiment
The subjects' reaction times in the subordinate alertness task (button press response upon detection of a checkerboard during the baseline event) did not differ significantly between the trial types (two‐factorial ANOVA; P < 0.05, corrected for multiple comparisons; F(3,8) = 0.405; P = 0.754), suggesting that the overall level of attention was the same during task performance on all movement categories. There was a linear decrease in the reaction times across runs approaching significance, that is, the subjects' responses became faster in the course of the fMRI measurement (one‐factorial ANOVA; P < 0.05, corrected for multiple comparisons; F(4,7) = 3.168; P = 0.087).
Prescanning assessment of visual and kinesthetic body movement imagery abilities
The MIQ‐R [Hall and Martin, 1997] data did not reveal statistically significant differences between the subjects' ability of visual and kinesthetic imagery of body movements, neither on the MIQ‐R single item level [one‐factorial ANOVAs; P < 0.05, corrected for multiple comparisons; leg movement: F(1,13) = 0.881; P = 0.365; jump movement: F(1,13) = 1.825; P = 0.200; arm movement: F(1,13) = 0.582; P = 0.459; toe movement: F(1,13) = 0.807; P = 0.385] nor for the combined items (F(1,13) = 0.108; P = 0.748). Mean ratings of the difficulty of kinesthetic and visual imagery were >5 (somewhat easy to feel/visualize the movement) for each single movement and the combined movements (range: 5.00–6.21).
Postscanning debriefing procedures
The subjects' self‐ratings of performance on limb compared to face movements were significantly higher (one‐factorial ANOVAs; P < 0.05, corrected for multiple comparisons) for the items recognition (F(1,11) = 29.479; P = 0.000), separation between tasks (F(1,11) = 13.836; P= 0.004), combined kinesthetic and visual imagery (F(1,9) = 11.919; P = 0.007), and visual imagery only (F(1,11) = 11.560; P = 0.008). A trend of better task performance on limb relative to buccofacial movements was also observed for the kinesthetic imagery domain (F(1,11) = 6.662; P = 0.030). There were no statistically significant differences between the subjects' ratings of movements with and without object. The corresponding data are given in Table I.
Table I.
Postscanning rating of task performance during the fMRI experiment
| Main effect of body part | Main effect of object use | |||||
|---|---|---|---|---|---|---|
| Mean: limb ± SD | Mean: bucco ± SD | P value | Mean: without ± SD | Mean: with ± SD | P value | |
| Recognition | 4.58 ± 0.82 | 3.80 ± 0.62 | 0.000* | 4.09 ± 0.49 | 4,30 ± 0.46 | 0.074 |
| Separation between tasks | 4.07 ± 0.24 | 3.69 ± 0.35 | 0.004* | 3.80 ± 0.36 | 3.96 ± 0.38 | 0.030 |
| Imagery: kinesthetic/visual | 4.32 ± 0.32 | 3.85 ± 0.47 | 0.007* | 4.05 ± 0.35 | 4.11 ± 0.39 | 0.955 |
| Imagery: kinesthetic | 4.36 ± 0.34 | 3.86 ± 0.51 | 0.030 | 4.07 ± 0.36 | 4.12 ± 0.35 | 0.798 |
| Imagery: visual | 4.17 ± 0.36 | 3.84 ± 0.48 | 0.008* | 3.91 ± 0.38 | 4.11 ± 0.46 | 0.113 |
Significant at P < 0.05, corrected for multiple comparisons, corrected α = 0.01.
The subjects' (n = 14) mean ratings (±SD) of their performance during the fMRI measurement. Subjects rated their performance on a rating scale ranging from 1 to 5: 1 = not at all, 2 = not good, 3 = medium, 4 = good, 5 = very good. Ratings of performance on limb (relative to face) movements were significantly higher for the items recognition, separation between tasks, combined kinesthetic and visual imagery, and visual imagery only. There were no statistically significant differences between the subjects' ratings of movements with and without object. See Results for statistical parameters.
Neuroimaging Data
Conjunction analysis
We used a strict conjunction analysis across all experimental conditions to asses common significant increases in neural activity (P < 0.05, FWE corrected) related to both the observation and motor imagery of each movement type (relative to baseline). A bilateral network of extrastriate, parietal, and frontal brain areas was activated, including the inferior parietal cortex and the supramarginal gyri, the lingual and superior occipital gyri, the cuneus, EBA, SMA, as well as the precentral and inferior frontal gyri. Most of the activations showed a left hemispheric predominance. The MNI coordinates of the local maxima of activation are given in Table II. Areas with significant BOLD signal change, as revealed by the conjunction analysis, are displayed in Figure 3. The activation peak is located in left precentral gyrus in an area corresponding to the superior region of the ventral premotor cortex (PMv).
Table II.
Conjunction analysis of all experimental conditions
| Region | Side | x | y | z | T value |
|---|---|---|---|---|---|
| Conjunction analysis: Brain areas involved in all experimental conditions of interest (observation and motor imagery of all movement types) | |||||
| Precentral gyrus (PMv) | L | −36 | −4 | 48 | 10.77 |
| R | 44 | 0 | 48 | 8.21 | |
| Lingual gyrus | R | 16 | −76 | −6 | 7.21 |
| L | −18 | −76 | −8 | 6.38 | |
| EBA (extending into MT/V5) | L | −52 | −58 | 2 | 7.14 |
| Superior occipital gyrus | L | −12 | −98 | 12 | 5.74 |
| Cuneus/superior occipital gyrus | R | 20 | −98 | 16 | 6.38 |
| Inferior frontal gyrus | L | −54 | 14 | 30 | 6.10 |
| SMA | L | −6 | 2 | 58 | 5.99 |
| Supramarginal gyrus | L | −56 | −34 | 28 | 5.45 |
| Inferior parietal lobule | L | −34 | −42 | 44 | 5.34 |
Brain regions in which common relative significant BOLD signal increases were observed during both the observation and motor imagery of all movement types. For each region of activation, the coordinates in standard stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest T value.
x, distance (mm) to right (+) or left (−) of the midsagittal plane; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; z, distance above (+) or below (−) the intercommissural anterior–posterior commissure (AC‐PC) plane.
The height threshold for statistical significance of activations was set to P < 0.05 (corresponding to T = 5.11), FWE corrected for multiple comparisons across the whole brain volume. No extent threshold was applied.
Figure 3.
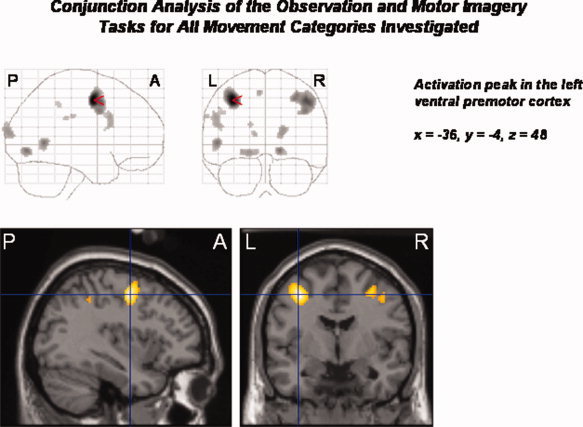
Conjunction analysis of all experimental conditions of interest (observation and motor imagery of buccofacial movements without object, buccofacial movements with object, upper limb movements without object, upper limb movements with object; relative to the baseline condition). There is increased neural activity in a bilateral network of frontal, parietal, occipital, and extrastriate brain areas, including the precentral and inferior frontal gyri, the SMA, the inferior parietal lobule, lingual, supramarginal, and superior occipital gyri, the cuneus, and the EBA. Activations predominated in the left hemisphere. The activation peak was located in the left superior PMv. Statistical threshold: P < 0.05, FWE corrected across the whole brain volume (T = 5.11); k = 0 voxel. P, posterior; A, anterior; L, left; R, right.
The factor body part
Main effect.
Analysis of the main effect of body part (i.e., irrespective of the factors object relatedness and task) showed that the processing of limb (relative to face) movements differentially activated area MIP (extending into the adjacent intraparietal cortex; IPC), the EBA, calcarine sulcus, and lingual gyrus (extending into the fusiform gyrus in the left hemisphere) bilaterally, the left postcentral gyrus/inferior parietal lobule, superior/middle frontal gyrus, superior occipital regions, and the right precuneus (P < 0.05, FWE corrected). The reverse contrast (face relative to limb movements) revealed differential neural responses in the postcentral and inferior frontal gyri bilaterally, as well as in the right supramarginal and precentral gyri (P < 0.05, FWE corrected). Figure 4a,b show SPMs of the main effects of upper limb and face movements. Figure 4c anatomically illustrates MIP/IPC regions that were differentially activated during the processing of upper limb (relative to face) movements. Table IIIa,b displays the respective MNI coordinates of the local maxima of activated areas.
Figure 4.
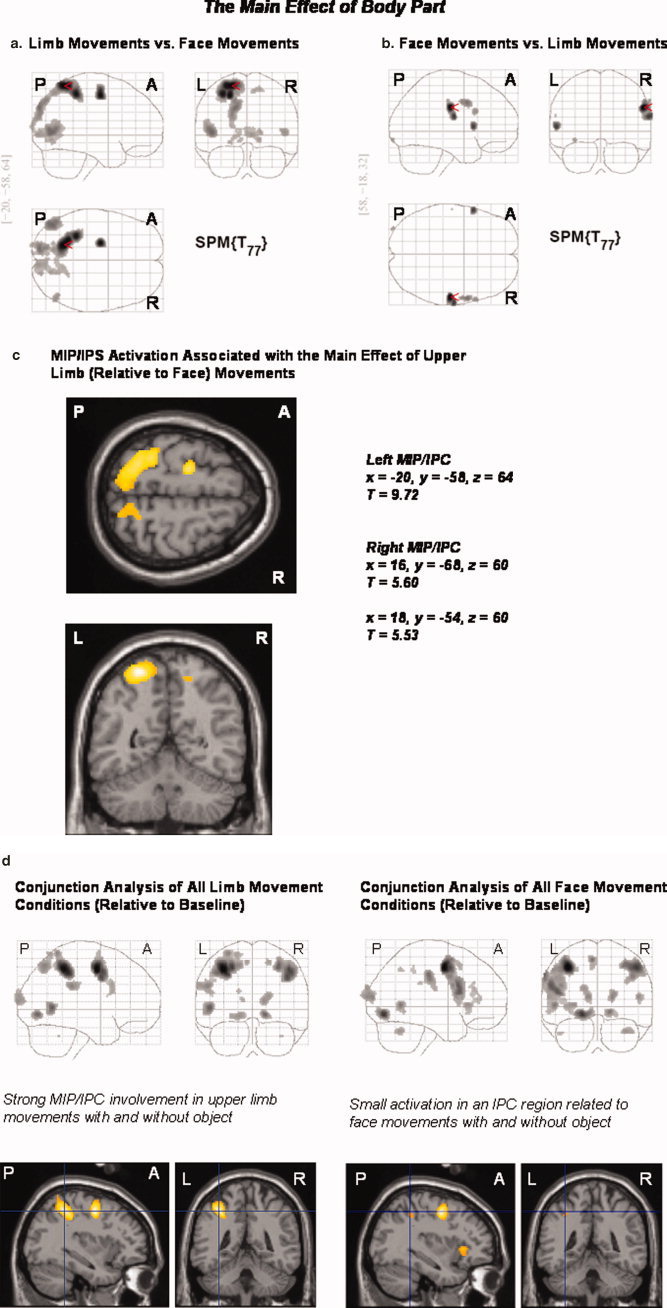
(a–d) Main effect of the factor “body part” and separate conjunction analyses for face and upper limb movements (each with and without object): The processing of limb (relative to face) movements bilaterally activated area MIP and the adjacent IPC, the EBA, calcarine sulci, and lingual gyri (extending into the fusiform gyrus in the left hemisphere), the left postcentral gyrus/inferior parietal lobule, superior/middle frontal, and superior occipital gyri, as well as the right precuneus. The reverse contrast (the processing of face relative to limb movements) revealed differential neural responses in the postcentral and inferior frontal gyri bilaterally, as well as the right supramarginal and precentral gyri (a–c). A conjunction analysis of the limb conditions (relative to baseline) showed that both limb movements with and without object reference activated the left MIP and adjacent IPC. A respective conjunction analysis of the face conditions revealed only small bilateral IPC activations in distinct anatomical regions (d). Statistical threshold: P < 0.05, FWE corrected across the whole brain volume (T = 5.11); k = 0 voxel. P, posterior; A, anterior; L, left; R, right.
Table III.
The main effect of body part
| Region | Side | x | y | z | T value |
|---|---|---|---|---|---|
| a. Observation and motor imagery of limb relative to face movements (irrespective of object use) | |||||
| Area MIP (extending into IPC) | L | −20 | −58 | 64 | 9.72 |
| R | 16 | −68 | 60 | 5.60 | |
| Inferior parietal lobule/ postcentral gyrus | L | −32 | −42 | 54 | 8.99 |
| Superior/middle frontal gyrus | L | −22 | −10 | 52 | 9.27 |
| EBA (extending into MT/V5) | L | −46 | −74 | 4 | 6.94 |
| R | 50 | −68 | 4 | 6.11 | |
| Calcarine sulcus | R | 6 | −92 | 0 | 6.01 |
| L | −16 | −78 | 12 | 5.24 | |
| Superior occipital gyrus | L | −16 | −90 | 36 | 6.76 |
| Lingual gyrus | R | 16 | −74 | −6 | 5.87 |
| Lingual/fusiform gyrus | L | −22 | −72 | −8 | 5.58 |
| Precuneus | R | 10 | −60 | 58 | 5.60 |
| b. Observation and motor imagery of face relative to limb movements (irrespective of object use) | |||||
| Postcentral gyrus | R | 58 | −18 | 32 | 7.64 |
| L | −60 | −16 | 22 | 5.17 | |
| Supramarginal gyrus | R | 66 | −22 | 42 | 5.44 |
| Inferior occipital gyrus | L | −34 | −96 | −12 | 5.52 |
| Inferior frontal gyrus (operculum) | L | −58 | 12 | 6 | 6.63 |
| R | 62 | 16 | 26 | 5.68 | |
| Precentral gyrus | R | 58 | 4 | 38 | 5.99 |
The main effect of “body part”: Brain regions showing differential significant BOLD signal increases (a) during the processing of limb relative to face movements and (b) face relative to limb movements. For each region of activation, the coordinates in MNI standard stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest T value.
See legend of Table II for the use of statistical thresholds and display of coordinates.
Conjunction analyses of either limb or face movements (each with and without object).
A conjunction analysis of limb conditions (relative to the baseline condition; conjunction analysis) showed that both limb movements with and without object reference strongly activated left area MIP and the adjacent left IPC. The respective conjunction analysis of face conditions revealed only small bilateral activations in distinct IPC regions (see Fig. 4d).
The factor object use
The processing of movements which did not involve an object (relative to object‐related movements) differentially activated the left middle frontal gyrus and inferior parietal cortex (P < 0.05, FWE corrected). The reverse contrast (object‐related movements relative to movements without object) revealed a differential involvement of the EBA bilaterally, the left LOC, supramarginal gyrus, and inferior parietal cortex. Figure 5a,b displays the brain regions which were sensitive to the factor object use. Table IVa,b provides the respective MNI coordinates of the differential activation peaks associated with the processing of movements with or without object, respectively.
Figure 5.
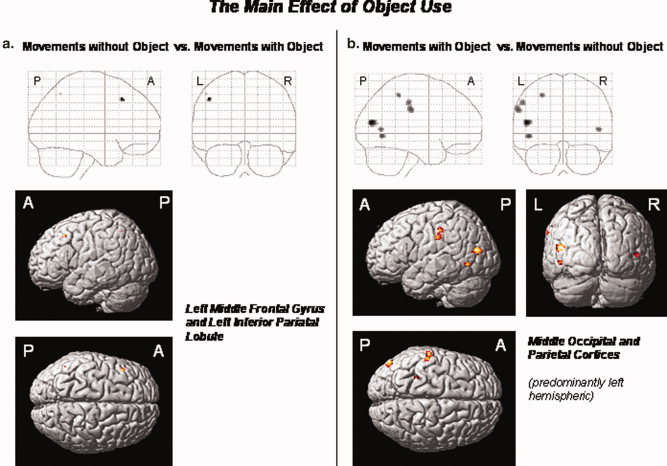
(a,b) The main effect of the factor “object use”: The processing of movements without object (relative to object‐related movements) differentially activated the left middle frontal gyrus and inferior parietal lobule (a). The reverse contrast (object‐related versus nonobject‐related movements) revealed differential neural responses in the EBA and adjacent area MT/V5 bilaterally, the left middle occipital and supramarginal gyri, and the left inferior parietal lobule (b). Statistical threshold: P < 0.05, FWE corrected across the whole brain volume (T = 5.11); k = 0 voxel. P, posterior; A, anterior; L, left; R, right.
Table IV.
The main effect of object use
| Region | Side | x | y | z | T value |
|---|---|---|---|---|---|
| a. Observation and mental imagery of movements without object relative to movements with object (irrespective of the body part involved) | |||||
| Middle frontal gyrus | L | −46 | 22 | 46 | 5.89 |
| Inferior parietal lobule | L | −50 | −58 | 52 | 5.13 |
| b. Observation and mental imagery of movements with object relative to movements without object (irrespective of the body part involved) | |||||
| Middle occipital gyrus | L | −50 | −78 | 16 | 6.24 |
| EBA (extending into MT/V5) | L | −46 | −64 | −4 | 5.73 |
| R | 48 | −66 | 6 | 5.63 | |
| Supramarginal gyrus | L | −60 | −24 | 32 | 5.70 |
| Inferior parietal lobule/supramarginal gyrus | L | −54 | −28 | 42 | 5.62 |
| Inferior parietal lobule/postcentral gyrus | L | −28 | −42 | 52 | 5.61 |
The main effect of “object use”: Brain regions showing differential significant BOLD signal increases (a) during the processing of movements without object relative to movements with object and (b) movements with object relative to movements without object. For each region of activation, the coordinates in MNI standard stereotactic space are given referring to the maximally activated focus within an area of activation, as indicated by the highest T value.
See legend of Table II for statistical thresholds and the display of coordinates.
The factor task
The observation (relative to the mental imagery) of all movement types investigated differentially activated the inferior occipital gyrus bilaterally, the right EBA, inferior frontal gyrus, IPC, thalamus, and medial cerebellum. The reverse contrast (motor imagery relative to the observation of all movement types) revealed differential activations of the SMA and middle frontal gyrus bilaterally, the right precuneus and insula (extending into the temporal pole), the left rolandic operculum, and posterior cingulate cortex (extending into the hippocampal region) (P < 0.05, FWE corrected; see Fig. 6a,b and Table Va,b).
Figure 6.
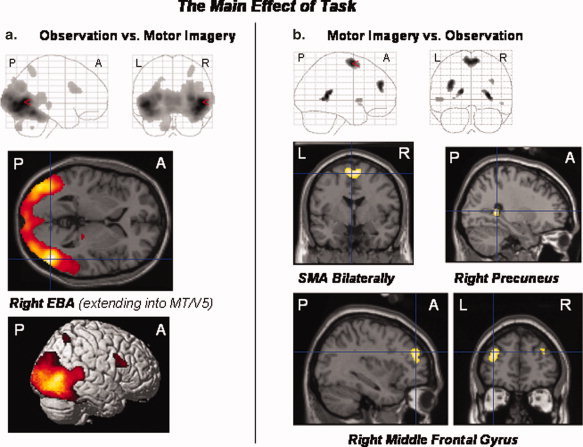
(a,b) The main effect of the factor “task”: The observation (relative to the mental imagery) of all movement types differentially activated the right EBA, the inferior occipital gyrus bilaterally, the right inferior frontal gyrus, intraparietal sulcus and adjacent IPC, thalamus, and cerebellum (a). The reverse contrast (motor imagery relative to the observation of all movement types) revealed differential activations of the right precuneus, SMA and middle frontal gyri bilaterally, the left rolandic operculum and posterior cingulate cortex (extending into the hippocampal region), as well as the right insula (extending into the temporal pole) (b). Statistical threshold: P < 0.05, FWE corrected across the whole brain volume (T = 5.11); k = 0 voxel. P, posterior; A, anterior; L, left; R, right.
Table V.
The main effect of task
| Region | Side | x | y | z | T value |
|---|---|---|---|---|---|
| a. Observation versus mental imagery of all movement categories | |||||
| EBA (extending into MT/V5) | R | 50 | −70 | 2 | 22.32 |
| Inferior occipital gyrus | R | 44 | −78 | −8 | 20.57 |
| Inferior/middle occipital gyrus | L | −44 | −84 | −2 | 19.67 |
| Inferior frontal gyrus (pars triangularis) | R | 40 | 16 | 22 | 7.26 |
| Thalamus | R | 22 | −26 | −2 | 6.29 |
| Intraparietal sulcus (extending into IPC) | R | 30 | −58 | 54 | 6.16 |
| Cerebellum (vermis) | R | 0 | −56 | −42 | 5.93 |
| b. Mental imagery versus observation of all movement categories | |||||
| Precuneus | R | 28 | −44 | 8 | 7.25 |
| SMA | R | 6 | −2 | 64 | 6.89 |
| L | −2 | −2 | 62 | 6.85 | |
| Posterior cingulate gyrus | L | −16 | −40 | 12 | 6.77 |
| Middle frontal gyrus | L | −34 | 46 | 28 | 6.75 |
| R | 34 | 48 | 30 | 5.69 | |
| Rolandic operculum | L | −50 | 8 | 0 | 5.72 |
| Insula/temporal pole | R | 50 | 12 | −6 | 5.26 |
The main effect of “task”: Brain regions showing differential significant BOLD signal increases (a) during observation relative to motor imagery of all movement types and (b) during motor imagery relative to observation of all movement types. For each region of activation, the coordinates in MNI standard stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest T value.
See legend of Table II for statistical thresholds and the display of coordinates.
Interactions
There were no significant interactions between the three experimental factors body part, object use, and task (P < 0.05, FWE corrected).
DISCUSSION
Behavioral Data
The postscanning debriefing data indicate that the factor body part affected the subjects' ratings of task difficulty, while the factor object use did not. Task performance during the fMRI measurement was rated significantly better for limb than for face movements. This effect may at least in part depend on the demands of the imagery task which required the subjects to mentally imagine themselves performing each movement. It is reasonable to assume that the motor imagery of self‐executed movements can be accomplished more easily for limb than for face movements, since face movements are fine grained and subtle (compared to limb movements which extend into peripersonal space). Moreover, the assumed contribution of both kinesthetic and visual imagery to a mental representation of one's own body movements might also implicate that motor imagery is easier for limb than face movements: typically, limb movements executed by oneself can be seen by the performer while movements of one's own face are usually inaccessible to the performer's vision. Our MIQ‐R data do not clarify this issue, since the questionnaire involves limb movements only.
The finding that the subjects' reaction times in the subordinate alertness task became slightly faster during the course of the fMRI indicates that the participants' overall alertness did not decline in the course of the MR measurement.
Neuroimaging Data
Our data provide evidence that both common and differential brain mechanisms are involved in the observation and mental imagery of face and limb movements with or without objects. The region of common maximum activation revealed by the conjunction analysis was located in the left PMv. Further maxima of activation common to all conditions were found in the lingual and superior occipital gyri bilaterally, in the left anterior EBA, inferior frontal and supramarginal gyri, inferior parietal lobule, and SMA. As expected, main areas were involved bilaterally, but activations predominated in the left hemisphere. Extrastriate regions, the SMA, a region of the middle frontal gyrus, area MIP, and adjacent IPC regions, as well as the precuneus, were differentially modulated by the experimental factors. Our present data may help specifying the roles of these areas in the observation and motor imagery of differential types of human body movements. In particular, our data demonstrate that human area MIP is involved in the cognitive processing of meaningful limb movements irrespective of whether they are object‐related or not.
The Frontal Cortex
The left PMv was involved in the observation and motor imagery of all movement types investigated (and to a lesser degree also the right hemisphere homologue). It is in good accordance with this finding that a coupling between the representations of observed and imagined movements has been ascribed to this premotor area, referred to as a matching process between stored and observed movements [Jeannerod, 2001]. In animal experiments, this kind of perception–action matching was modeled in terms of a “vocabulary of motor acts” [Rizzolatti et al., 1988], which is stored in the PMv and may be assessed internally (e.g., by movement planning and motor imagery) or by appropriate external cues (e.g., observation of movements). In the present study, it is likely that a matching between stored and observed movements occurred during the observation and motor imagery of each movement category, thus explaining the common PMv activation. Besides PMv, an adjacent region of the left inferior frontal gyrus corresponding to Broca's area (BA 44/45) was activated in the conjunction analysis. In both monkeys and humans, this region is supposed to contain mirror neurons [e.g., Gallese et al., 1996]. However, it has also been implicated in motor inhibition [Casey et al., 1997; Garavan et al., 1999; Konishi et al., 1999] and other types of executive functions [e.g., working memory; D'Esposito et al., 1998]. Our data suggest that neurons in the PMv and the adjacent inferior frontal cortex closely interact in higher‐order movement processing, most likely constituting modules of a neural circuit which integrates the matching, mirror, and control mechanisms involved in motor cognition.
The middle frontal gyrus and the SMA were activated bilaterally during the motor imagery (relative to the observation) of all movement types. Both regions are known to be involved in motor control [Matsui et al., 2002; Mostofsky et al., 2003]. The region of the middle frontal gyrus which showed differential activation related to motor imagery in the present study has been implicated in the inhibition of imitative response tendencies [Brass et al., 2001]. Viewing a movement may yield a “contagion‐like” tendency in the observer to execute this movement [Brass et al., 2001; Fadiga et al., 1995; Schürmann et al., 2005]. Therefore, imitation appears to be a prepotent response tendency. In the imagery event, our subjects were required to imagine themselves performing each movement seen in the preceding video clip, but without actually moving. It is thus conceivable that demands on the inhibition of movement execution were even stronger during the imagery than in the observation task, as reflected by increased neural activity in the middle frontal gyrus. Such an assumption is also supported by previous functional neuroimaging studies on executive control, which implicated this brain region in the inhibition of motor reactions in response to respective cueing during a go/no‐go task [Casey et al., 1997; De Zubicaray et al., 2000; Kawashima et al., 1996]. Moreover, the middle frontal gyrus has been implicated in other executive processes, such as the internal generation of a motor program [e.g., Basho et al., 2007] and working memory [e.g., Leung et al., 2002]. These data could also explain our finding of motor imagery‐related activation of the middle frontal gyrus: both the generation of a motor program and working memory are most likely more strongly involved in motor imagery than in movement observation.
The SMA and the pre‐SMA are supposed to play a general role in motor control. The pre‐SMA has been implicated in the inhibition of unwanted movements during a go/no‐go task [Mostofsky et al., 2003] as well as antisaccades and antipointing [Connolly et al., 2000]. In our study, the area of peak activation in the superior frontal gyrus related to movement imagery was located more posterior in the SMA proper. The activated region, however, extended into the pre‐SMA, suggesting that SMA and pre‐SMA neurons operate in concert to inhibit motor execution during the mental imagery of one's own body movements.
Note, the SMA has also been implicated in self‐control and self‐inhibition. In a study on the correlation between gray matter volume of specific brain structures and scores on the self‐control subscales of the Minnesota Multiphasic Personality Inventory, low self‐control scores were negatively correlated with SMA and precuneus gray matter density [Matsui et al., 2002]. These regions are known to govern voluntary movement and motor imagery [e.g., Ogiso et al., 2000; Roland et al., 1980a, b]. In the present study, the areas of maximum activation during the motor imagery of one's own body movements (relative to the observation task) were located in the right precuneus and the SMA bilaterally, thus supporting the hypothesis that these regions may act as modulators in self‐related movement control, in particular, in the inhibition of unwanted movements. It is in good line with these findings that functional neuroimaging studies on the neural substrates of disturbed cognitive functions in schizophrenia (a disorder which is typically associated with impaired self‐control of movements) reported SMA and precuneus pathology in schizophrenic patients [Curtis et al., 1998; McGuire et al., 1996].
Overall, the frontal activations observed in the present study suggest that the PMv and the inferior frontal gyrus (including Broca's area) are generally involved in the observation and motor imagery of body movements. Recruitment of the middle frontal gyrus and the SMA was specifically modulated by the factor task: both regions were differentially involved in movement control during the mental imagery of movements, in particular, in the motor inhibition of imitative response tendencies.
The Extrastriate Cortex
In our experiment, the left EBA and the adjacent area MT/V5 were activated during perception‐ and imagery‐related processing of all movement categories. These regions have been implicated in tasks involving motion perception [Peigneux et al., 2000; Tootell et al., 1995; Watson et al., 1993]. Area MT/V5 has particularly been associated with perceived [Tootell et al., 1995] and mentally imagined motion analysis [Goebel et al., 1998]. Astafiew et al. [2004] demonstrated that both the EBA and MT/V5 contribute to the integration of visuomotor attention and sensorimotor signals in the neural processing of one's own body movements. The task used in that study may have also involved motor imagery: subjects pointed with their hand or foot to visual targets, with their limbs not being visible to them. It is likely that the participants had a mental image of their own moving body part when pointing at the target. One cannot be sure, however, whether the EBA was activated by motor imagery or rather by attention to a specific body part and/or motor selection in response to each cue. An fMRI study by Solodkin et al. [2004] showed more direct evidence for EBA activity during the execution as well as visual and kinesthetic mental imagery of finger movements. Furthermore, the EBA was reported to act as a higher‐order object recognition area specialized for the recognition of human body movements [e.g., Tootell et al., 2003]. Our data agree with all these previous findings in that they show both EBA and MT/V5 engagement in the higher‐order visuomotor processing of movements of one's body. Note, however, we also show evidence that these brain areas responded stronger during the observation than the motor imagery task (see Fig. 6).
Unlike Urgesi et al. [2004] who suggested that the EBA and MT/V5 do not respond to face movements, we demonstrate activation of these areas during the processing of both face and upper limb movements (see conjunction analysis; Fig. 3). This result is in accordance with the finding of Downing et al. [2001], who showed EBA responses to static displays of human bodies and body parts including whole faces and parts of faces, and also with a more recent study of this research group demonstrating general EBA responses to the category “bodies” [Downing et al., 2006; see also Peelen and Downing [2005] who reported a high degree of within‐subject reproducibility of category‐specific activations of the visual cortex]. EBA activity associated with the visual processing of whole faces was low in the experiment of Downing et al. [2001]. This was also the case in the present study: the analysis of the main effect of body part (see Fig. 4a–c) demonstrates that the cognitive processing of limb (relative to face) movements activated the EBA and MT/V5 bilaterally while the reverse contrast (face relative to limb movements) did not show extrastriate activations. This finding may indicate that these regions respond to a higher degree to limb than face movements. It is worth noting, however, that the spatial extension of motion was stronger for limb than face movements in the present experiment. These differences in the perceptual domain might also have contributed to the observed differential extrastriate activations related to limb movements.
The Parietal Cortex
Distinct areas of the parietal cortex differentially responded during the observation and motor imagery of the movement categories of interest, supporting the view of a functional segregation of parietal regions involved in motor cognition [e.g., Assmus et al., 2003, 2007; Hanakawa et al., 2005]. The main effect of body part revealed differential activations in the postcentral gyrus corresponding to somatotopic face and arm/hand representations. Limb movements activated the left‐hemispheric somatosensory upper limb region (near the junction to the inferior parietal lobule) only. This finding agrees with previous patient data, suggesting a predominant role of the left somatosensory cortex in the sensorimotor integration of complex hand/finger movements [Okuda et al., 1995]. Postcentral activations related to face movements were bilateral. Importantly, these activations extended into area 5 that has been implicated in the mental imagery of body movements [e.g., Hanakawa et al., 2005]. Area 5 putatively corresponds to the macaque parietal area FE. It connects with M1 and may act as a module of higher‐order somatosensory integration [Rizzolatti et al., 1983]. This view of the functional role of area 5 is consistent with the activation we observed in this region during higher‐order cognitive processing of fine grained and complex face movements in the present study. Currently available data do not clarify the issue whether subregions of human area 5 might be specifically involved in the decoding of face movements. To our knowledge, the functional role of this region has only been investigated with regard to its involvement in limb, but not with face movements yet.
The main effect of body part also revealed significant differential activations in the right supramarginal gyrus related to the observation and motor imagery of face (relative to upper limb) movements. This brain structure has been associated with the cognitive processing of faces previously. It is in good accordance with our data that Sugiura et al. [2000] implicated the right supramarginal gyrus in the representation of one's own face, and Campanella et al. [2001] related this region to the association between faces and names. The human supramarginal gyrus corresponds to the monkey parietal area PF [Grezes and Decety, 2001]. Area PF builds up a circuit with the ventral premotor region F5 that contains mirror neurons [Rizzolatti et al., 1998]. The supramarginal gyrus could thus well be involved in the control of mirror neuron mechanisms. Our observation of differential activation in a right supramarginal region related to the processing of face (relative to upper limb) movements may indicate that the cognitive processing of face movements requires specific and/or more intense control of mirror neuron components. This could be the case since face movements are out of the performer's vision, and thus make higher demands on the inhibitory control of “contagion”‐related perceptual decoding processes and the associated tendency to imitate. This interpretation agrees with our behavioral data, indicating that task performance was more difficult for face (relative to limb) movements.
Area MIP and the adjacent IPC as well as the lingual gyri in both hemispheres, the left inferior parietal lobule and cuneus were differentially activated during the processing of limb relative to face movements. The IPS is known to be composed of multiple subdivisions in nonhuman primates, with each portion constituting a neural circuit with subregions of the frontal premotor areas [Rizzolatti and Luppino, 2001; Rizzolatti et al., 1998]. Neuroimaging studies in humans suggest a comparable functional and anatomical organization of the human IPS [e.g., Bremmer et al., 2001; Grefkes et al., 2002]. In particular, human MIP has been implicated in visuomotor transformation during arm and hand movements directed to a target [Chaminade and Decety, 2002; Grefkes et al., 2004]. This view is in good accordance with previous electrophysiological studies in macaques, suggesting that the IPS contains specialized neuronal modules which are integrated into the parietooccipital and parietofrontal circuits mediating goal‐ or object‐directed body movements. Area MIP is supposed to constitute one of these modules. Overall, our finding of MIP and adjacent IPC activation during the higher‐order cognitive processing of upper limb movements is in accordance with these previous reports. The conjunction analysis of upper limb movements with and without object (relative to baseline; see Fig. 4d) revealed an activation peak in the left IPS/IPC. This finding indicates that MIP/IPC recruitment by goal‐directed movements does not necessarily require the involvement of a reachable real object (e.g., nail, table). MIP and the surrounding IPC region also responded to upper limb movements which were goal‐directed in the sense of social semantics, that is, meaningful in the context of social communication (e.g., shaking one's fist at someone; see Appendix). Thompson et al. [2004] showed evidence for a specific left IPS/IPC involvement in the processing of socially meaningful number information conveyed by finger movements. These data are in accordance with our results in that they implicate the left IPS/IPC region (where the present IPC activation peak was located) in the decoding of human arm and finger movements, providing socially relevant semantic information, that is, in upper limb gesture processing. The maximum of left MIP/IPC activation associated with the processing of limb movements in the present study was located superior to the IPC coordinates reported by Thompson et al. [2004]. However, IPC activations related to the cognitive processing of limb movements were not restricted to MIP, but rather extended into more inferior and anterior IPS and adjacent IPC regions (see Fig. 4a–d), suggesting that distinct functional modules of the IPS/IPC operated in concert during the processing of upper limb movement processing. Note, however, IPS/IPC activations related to limb movements either directed to a real target or aiming at a more abstract social purpose were highly similar (see Fig. 4a–d). The finding of small bilateral activations of the IPC in the conjunction analysis of face movements with and without object (relative to baseline; see Fig. 4d) might reflect IPC involvement in attention to features of faces [Ishai et al., 2002; Leibenluft et al., 2004] and socially relevant environmental signals [Salzmann, 1995]. It is conceivable that functions of the IPC might not be restricted to the processing of limb features and movements. Rather, the IPC could play a more general role in gesture‐ and mimic‐based (i.e., nonverbal) attention and social communication. However, this hypothesis needs to be further investigated.
The precuneus was differentially engaged in the motor imagery relative to the observation of all movement types. This finding agrees with previous studies implicating the precuneus in the motor imagery of various types of body movements, including locomotor [Malouin et al., 2003] and finger tapping tasks [Hanakawa et al., 2003]. Fletcher et al. [1995] referred to the precuneus as “the mind's eye,” since in their experiment the structure was differentially triggered by the visual imagery during episodic memory recall. This interpretation is in good accordance with reports on precuneus and posterior cingulate cortex involvement in episodic autobiographical memory retrieval, which is typically associated with vivid mental imagery [e.g., Gardini et al., 2006; Piefke et al., 2003]. Moreover, the precuneus has been involved in self‐processing, in particular, in first‐person perspective taking and the experience of self‐agency [for a review, see Cavanna and Trimble, 2006]. This suggestion is in line with both the aforementioned autobiographical memory (a per se self‐referential type of memory) data and our current finding of differential precuneus activity related to the motor imagery (compared to the observation) of movements. The latter provides evidence that the subjects actually imagined themselves performing each movement instead of, for example, the persons they viewed in the video clips.
CONCLUSIONS
Our data suggest a fine‐grained functional segregation of frontal, extrastriate, and parietal brain areas involved in the perception‐ and mental imagery‐based processing of distinct types of human body movements. While the PMv, EBA, and area MT/V5 appear to play a general key role in the visually based cognitive processing of human body movements, engagement of the SMA and precuneus (mental imagery of body movements and self‐control during imagery), regions of the middle frontal gyrus (motor inhibition during motor imagery), area MIP and the adjacent IPC (cognitive processing of goal‐directed upper limb movements irrespective of whether an object is involved; social semantics of upper limb and perhaps also face gestures), area 5 of the somatosensory cortex (somatosensory integration required by the cognitive processing of subtle face mimics), and the supramarginal gyrus (control of mirror neuron mechanisms) are modulated by the cognitive tasks and the movement types involved in each case. The data specify the common neural substrates of motor cognition and the functional segregation of frontal, extrastriate, and parietal regions involved in the observation and motor imagery of face and upper limb movements with or without object. In particular, our findings provide new suggestions regarding the interaction between motor and “cognitive” areas of the frontal cortex during motor imagery, the role of somatosensory integration in the processing of face movements, and IPS/IPC involvement in social communication.
Acknowledgements
We thank the Institute of Medicine, Cognitive Neurology research staff for their expert collaboration.
Table AI.
Face and upper limb movements presented during the fMRI experiment
| Face movements | |||
|---|---|---|---|
| Buccofacial movements without object | Buccofacial movements with object | ||
| German | English translation | German | English translation |
| schnalzen | to click one's tongue | Kirschkern ausspucken | to spit out the stone of a cherry |
| beißen | to bite | Lutscher lutschen | to lick a lolly |
| lächeln | to smile | aus einem Strohhalm trinken | to drink through a straw |
| pusten | to blow | Kaugummi kauen | to chew a chewing gum |
| blinzeln | to blink (with the eyes) | Kopfball schießen | to head a ball |
| kauen | to chew | Kaugummiblase machen | to blow (chewing gum) bubbles |
| gähnen | to yawn | Trillerpfeife pfeifen | to blow a whistle |
| Stirn runzeln | to frown | Feder wegpusten | to blow away a feather |
| mit den Lippen flattern | to quiver one's lips | Zigarre im Mundwinkel halten | to hold a cigar in the corner of one's mouth |
| Zunge in der Wangentasche haben | to have the tongue between one's cheeks and teeth | Papier von Tisch ansaugen | to suck paper up from a table |
| Nase rümpfen | to turn one's nose up at sth. | Nutella von der Oberlippe ablecken | to lick Nutella off one's upper lip |
| Zähne klappern | to chatter one's teeth | Schokostreusel auflecken (von einem Teller) | to lick off chocolate chips (from a plate) |
| Mund spitzen | to purse one's lips | Spatel auf der Zunge balancieren | to balance a spatula on your tongue |
| Zähne fletschen | to bare one's teeth | Bleistift auf der Oberlippe balancieren | to balance a pencil on your upper lip |
| Lippen lecken | to lick one's lips | an einem Blumenstrauß riechen | to smell a bunch of flowers |
| Zunge herausstrecken | to stick one's tongue out | Monokel halten | to hold up monocles |
| Augen rollen* | to roll one's eyes* | Rose zwischen den Zähnen halten | to hold a rose between one's teeth |
| Unterlippe vorschieben | to stick one's bottom lip out | Buch auf dem Kopf balancieren | to balance a book on one's head |
| Wangen aufblasen* | to blow one's cheeks* | Pokal küssen | to kiss a trophy |
| niesen | to sneeze | aus einer Tasse schlürfen | to slurp from a cup |
| Upper limb movements | |||
| Upper limb movements without object | Upper limb movements with object | ||
| German | English translation | German | English translation |
| mit den Fingern schnipsen | to snap one'sfingers | Tablet tbalancieren | to balance a tray |
| mit drei Fingern schwören | to swear with three fingers | hämmern | to hammer |
| mit dem Finger (auf etwas)zeigen | to point with a finger (at sth.) | Haare kämmen | to comb one's hair |
| Daumen hochhalten | to hold one's thumbs up | Tennisball werfen | to throw a tennisball |
| Mittelfinger zeigen | to show someone the finger | in einem Topf herumrühren | to stir the contents of a pan |
| Victory‐Zeichen machen | to make the Victory‐sign | dirigieren | to conduct (an orchestra) |
| Vogel zeigen* | to tap one's forehead* | Schraube eindrehen | to tighten a screw |
| eine Faust machen | to make a fist | Karten umdrehen | to turn cards over |
| sich auf die Schulter klopfen | to tap one's own shoulder | Klingel drücken (Rezeptionstheke) | to press a bell (at a reception desk) |
| Stopp signalisieren (mit einer Hand) | to indicate Stop (with one hand) | Feuerzeug anzünden | to light a lighter |
| mit dem Bizeps protzen | to flex one's arm muscles (biceps) | Nummer in das Handy eintippen | to dial a number on a mobile phone |
| sich auf die Brust schlagen | to tap one's own chest | würfeln (mit einer Hand) | to throw a dice (with one hand) |
| sich an die Stirn fassen* | to touch one's forehead* | mit einer Spraydose sprühen | to spray with an aerosol can |
| salutieren (wie ein Soldat) | to salute (like a soldier) | mit einer Glocke klingeln | to ring a bell |
| winken (mit einer Hand) | to wave (with one hand) | Suppe löffeln | to spoon a soup |
| Achseln zucken | to shrug | mit einem Pinsel malen | to paint with a brush |
| sich am Kopf kratzen | to scratch one's head | Glas einschenken | to fill a glass |
| aufzeigen (z.B. im Schulunterricht) | to put one's hand up (e.g., in a school lesson) | Buch durchblättern | to leaf through a book |
| Abwinken (mit einer Hand) | to dismiss (with one hand) | stempeln | to stamp |
| sich die Haare zurückstreichen | to run hands through one's own hair | mit Stäbchen essen | to eat with sticks |
Note that not all social gestures are universal across nationalities and cultures. For example, in Germany, tapping one's own forehead when talking with someone (German: “Vogel zeigen”) indicates one's assumption that the other person has bats in the belfry. This gesture does not exist in UK. In Italy, the gesture is performed using a slightly different movement. In the appendix, nonuniversal gestures are marked with a *.
REFERENCES
- Assmus A,Marshall JC,Ritzl A,Noth J,Zilles K,Fink GR ( 2003): Left inferior parietal cortex integrates time and space during collision judgements. Neuroimage 20 ( Suppl 1): S82–S88. [DOI] [PubMed] [Google Scholar]
- Assmus A,Giessing C,Weiss PH,Fink GR ( 2007): Functional interactions during the retrieval of conceptual action knowledge: An fMRI study. J Cogn Neurosci 19: 1004–1012. [DOI] [PubMed] [Google Scholar]
- Astafiew SV,Stanley CM,Shulman GL,Corbetta M ( 2004): Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci 7: 422–423. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L,Iacoboni M,Zaidel E ( 2006a): Hemispheric sensitivity to body stimuli in simplke reaction time. Exp Brain Res 170: 116–121. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L,Koski L,Zaidel E,Mazziotta J,Iacoboni M ( 2006b): Lateralization of the human mirror neuron system. J Neurosci 26: 2964–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basho S,Palmer ED,Rubio MA,Wulfeck B,Müller RA ( 2007): Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and over speech. Neuropsychologia 45: 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M,Zysset S,von Cramon DY ( 2001): The inhibition of imitative response tendencies. Neuroimage 14: 1416–1423. [DOI] [PubMed] [Google Scholar]
- Bremmer F,Schlack A,Shah NJ,Kubischik M,Hoffmann KP,Zilles K,Fink GR ( 2001): Polymodal motion processing in posterior parietal and premotor cortex: A human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29: 287–296. [DOI] [PubMed] [Google Scholar]
- Buccino G,Binkofski F,Fink GR,Fadiga L,Fogassi L,Gallese V,Seitz RJ,Zilles K,Rizzolatti G,Freund HJ ( 2001): Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Buccino G,Vogt S,Ritzl A,Fink GR,Zilles K,Freund HJ,Rizzolatti G ( 2004): Neural circuits underlying imitation learning of hand actions: An event‐related fMRI study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Campanella S,Joassin F,Rossion B,De Volder A,Bruyer R,Crommelinck M ( 2001): Association of the distinct visual representations of faces and names: A PET activation study. Neuroimage 14: 873–882. [DOI] [PubMed] [Google Scholar]
- Casey BJ,Trainor RJ,Orendi JL,Schubert AB,Nystrom LE,Giedd JN,Castellanos FX,Haxby JV,Noll DC,Cohen JD,Forman SD,Dahl RE,Rapoport JL ( 1997): A developmental fMRI study of prefrontal activation during performance of a go/no‐go task. J Cogn Neurosci 9: 835–847. [DOI] [PubMed] [Google Scholar]
- Cavanna AE,Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Chaminade T,Decety J ( 2002): Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport 14: 1975–1978. [DOI] [PubMed] [Google Scholar]
- Cohen YE,Andersen RA ( 2002): A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci 3: 553–562. [DOI] [PubMed] [Google Scholar]
- Colby CL,Gattass R,Olson CR,Gross CG ( 1988): Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: A dual tracer study. J Comp Neurol 269: 392–413. [DOI] [PubMed] [Google Scholar]
- Connolly JD,Goodale MA,Desouza JF,Menon RS,Vilis T ( 2000): A comparison of frontoparietal fMRI activation during anti‐saccades and anti‐pointing. J Neurophysiol 84: 1645–1655. [DOI] [PubMed] [Google Scholar]
- Curtis VA,Bullmore ET,Brammer MJ,Wright IC,Williams SC,Morris RG,Sharma TS,Murray RM,McGuire PK ( 1998): Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 155: 1056–1063. [DOI] [PubMed] [Google Scholar]
- Decety J,Perani D,Jeannerod M,Bettinardi V,Tadary B,Woods R,Mazziotta JC,Fazio F ( 1994): Mapping motor representations with positron emission tomography. Nature 371: 600–602. [DOI] [PubMed] [Google Scholar]
- Dechent P,Merboldt KD,Frahm J ( 2004): Is the human primary motor cortex involved in motor imagery? Brain Res Cogn Brain Res 20: 533. [DOI] [PubMed] [Google Scholar]
- D'Esposito MD,Aguirre GK,Zarahn E,Ballard D,Shin RK,Lease J ( 1998): Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res 7: 1–13. [DOI] [PubMed] [Google Scholar]
- De Felippo CL,Sims DG,Gottermeier L ( 1995): Linking visual and kinaesthetic imagery in lipreading instruction. J Speech Hear Res 38: 244–256. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI,Andrew C,Zelaya FO,Williams SCR,Dumanoir C ( 2000): Motor response suppression and the prepotent tendency to respond: A parametric fMRI study. Neuropsychologia 38: 1280–1291. [DOI] [PubMed] [Google Scholar]
- Denis M ( 1985): Visual imagery and the use of mental practice in the development of motor skills. Can J Appl Sport Sci 10 ( Suppl): S4–S16. [PubMed] [Google Scholar]
- Downing PE,Jiang Y,Shuman M,Kanwisher N ( 2001): A cortical area selective visual processing of the human body. Science 293: 2470–2473. [DOI] [PubMed] [Google Scholar]
- Downing PE,Chan AWY,Peelen MV,Dodds CM,Kanwisher N ( 2006): Domain specifity in visual cortex. Cereb Cortex 16: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Duvernoy H ( 1999): The Human Brain: Surface, Three‐Dimensional Sectional Anatomy, and MRI. New York: Springer; 491 pp. [Google Scholar]
- Ehrsson HH,Geyer S,Naito E ( 2003): Imagery of voluntary movements fingers, toes, and tongue activates corresponding body‐part‐specific motor representations. J Neurophysiol 90: 3304–3316. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB,Stephan KE,Mohlberg H,Grefkes C,Fink GR,Amunts K,Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eskandar EN,Assad JA ( 2002): Distinct nature of directional signals among parietal cortical areas during visual guidance. J Neurophysiol 88: 1777–1790. [DOI] [PubMed] [Google Scholar]
- Evans AC,Kamber M,Collins DL,MacDonald D ( 1994): An MRI‐based probabilistic atlas of neuroanatomy In: Shorvon S, Fish D,Andermann F, Bydder GM, Stefan H, editors. Magnetic Resonance Scanning and Epilepsy. New York: Plenum; pp 263–274. [Google Scholar]
- Fadiga L,Fogassi L,Pavesi G,Rizzolatti G ( 1995): Motor facilitation during action observation: A magnetic stimulation study. J Neurophysiol 73: 2608–2611. [DOI] [PubMed] [Google Scholar]
- Fink GR,Frackowiak RSJ,Pietrzyk U,Passingham RE ( 1997): Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Frith CD,Baker SC,Shallice T,Frackowiak RSJ,Dolan RJ ( 1995): The mind's eye—Precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Förster O ( 1931): The cerebral cortex in man. Lancet 221: 309–312. [Google Scholar]
- Friston KJ,Holmes A,Worsley KJ,Poline JB,Frith CD,Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gallese V,Fadiga L,Fogassi L,Rizzolatti G ( 1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Garavan H,Ross TJ,Stein EA ( 1999): Right hemispheric dominance of inhibitory control: An event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini S,Cornoldi C,De Beni R,Venneri A ( 2006): Left mediotemporal structures mediate the retrieval of episodic autobiographical mental images. Neuroimage 30: 645–655. [DOI] [PubMed] [Google Scholar]
- Goebel R,Khorram‐Sefat D,Muckli L,Hacker H,Singer W ( 1998): The constructive nature of vision: Direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci 10: 1563–1573. [DOI] [PubMed] [Google Scholar]
- Grefkes C,Weiss PH,Zilles K,Fink GR ( 2002): Crossmodal processing of object features in human anterior intraparietal cortex: A fMRI study implies equivalencies between humans and monkeys. Neuron 35: 173–184. [DOI] [PubMed] [Google Scholar]
- Grefkes C,Ritzl A,Zilles K,Fink GR ( 2004): Human medial intraparietal cortex subserves visuomotor coordinate transformation. Neuroimage 23: 1494–1506. [DOI] [PubMed] [Google Scholar]
- Grezes J,Decety J ( 2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K,Knouf N,Kanwisher N ( 2004): The fusiform face area subserves face perception, not generic within‐category identification. Nat Neurosci 7: 555–562. [DOI] [PubMed] [Google Scholar]
- Hall CR,Martin KA ( 1997): Measuring movement imagery abilities: A revision of the movement imagery questionnaire. J Ment Imag 21: 143–154. [Google Scholar]
- Hanakawa T,Immisch I,Keiichiro T,Dimyan MA,van Geldern P,Hallett M ( 2003): Funtional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89: 989–1002. [DOI] [PubMed] [Google Scholar]
- Hanakawa T,Parikh S,Bruno MK,Hallett M ( 2005): Finger and face representations in ipsilateral precentral motor areas in humans. J Neurophysiol 93: 2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R,Forss N,Avikainen S,Kirveskari E,Salenius S,Rizzolatti G ( 1998): Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci USA 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbiriba LA,Rodrigues EC,Magalhães J,Vargas CD ( 2006): Motor imagery in blind subjects: The influence of the previous visual experience. Neurosci Lett 400: 181–185. [DOI] [PubMed] [Google Scholar]
- Ishai A,Haxby JV,Ungerleider LG ( 2002): Visual imagery of famous faces: Effects of memory and attention revealed by fMRI. Neuroimage 17: 1729–1741. [DOI] [PubMed] [Google Scholar]
- Jarvelainen J,Schurmann M,Hari R ( 2004): Activation of the human primary motor cortex during observation of tool use. Neuroimage 23: 187–192. [DOI] [PubMed] [Google Scholar]
- Jeannerod M ( 1994): The representing brain: Neural correlates of motor intention and imagery. Behav Brain Sci 17: 187–245. [Google Scholar]
- Jeannerod M ( 2001): Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 14 ( Suppl): S103–S109. [DOI] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: A module in the human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R,Satoh K,Itoh H,Ono S,Furumoto S,Gotoh R,Koyama M,Yoshioka S,Takahashi TG,Takahashi K,Yanagisawa T,Fukuda H ( 1996): Functional anatomy of go/no‐go discrimination and response selection: A PET study in man. Brain Res 728: 78–89. [PubMed] [Google Scholar]
- Konishi S,Nakajima K,Uchida I,Kikyo H,Kameyama M,Miyashita Y ( 1999): Common inhibitory mechanism in human inferior prefrontal cortex revealed by event‐related functional MRI. Brain 122: 981–991. [DOI] [PubMed] [Google Scholar]
- Leibenluft E,Gobbini MI,Harrison T,Haxby JV ( 2004): Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry 56: 225–232. [DOI] [PubMed] [Google Scholar]
- Leung HC,Gore JC,Goldman‐Rakic PS ( 2002): Sustained mnemonic response in the human middle frontal gyrus during on‐line storage of spatial memoranda. J Cogn Neurosci 14: 659–671. [DOI] [PubMed] [Google Scholar]
- Livesey DJ ( 2002): Age differences in the relationship between visual movement imagery and performance on kinesthetic acuity tests. Dev Psychol 38: 279–287. [PubMed] [Google Scholar]
- Lotze M,Montoya P,Erb M,Hulsmann E,Flor H,Klose U,Birbaumer N,Grodd W ( 1999): Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J Cogn Neurosci 11: 491–501. [DOI] [PubMed] [Google Scholar]
- Macar F,Anton JL,Bonnet M,Vidal F ( 2004): Timing functions of the supplementary motor area: An event‐related fMRI study. Brain Res Cogn Brain Res 21: 206–215. [DOI] [PubMed] [Google Scholar]
- Malouin F,Richards CL,Jackson PL,Dumas F,Dovon J ( 2003): Brain activation during motor imagery of locomotor‐related tasks: A PET study. Hum Brain Mapp 19: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M,Yoneyama E,Sumiyoshi T,Noguchi K,Nohara S,Suzuki M,Kawasaki Y,Seto H,Kurachi M ( 2002): Lack of self‐control as assessed by a personality inventory is related to reduced volume of supplementary motor area. Psychiatry Res Neuroimaging 116: 53–61. [DOI] [PubMed] [Google Scholar]
- McGuire PK,Silbersweig DA,Murray RM,David AS,Frackowiak RS,Frith CD ( 1996): Functional anatomy of inner speech and auditory verbal imagery. Psychol Med 26: 29–38. [DOI] [PubMed] [Google Scholar]
- Michelon P,Vettel JM,Zacks JM ( 2006): Lateral somatotopic organization during imagined and prepared movements. J Neurophysiol 95: 811–822. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH,Schafer JGB,Abrams MT,Goldberg MC,Flower AA,Boyce A,Courtney SM,Calhoun VD,Kraut MA,Denckla MB,Pekar JJ ( 2003): FMRI evidence that the neural basis of response inhibition is task‐dependent. Cogn Brain Res 17: 419–430. [DOI] [PubMed] [Google Scholar]
- Nakata H,Inui K,Wasaka T,Akatsuka K,Kakigi R ( 2005): Somato‐motor inhibitory processing in humans: A study with MEG and ERP. Eur J Neurosci 22: 1784–1792. [DOI] [PubMed] [Google Scholar]
- Newman SD,Klatzky RL,Lederman SJ,Just MA ( 2005): Imagining material versus geometric properties of objects: An fMRI study. Brain Res Cogn Brain Res 23: 235–246. [DOI] [PubMed] [Google Scholar]
- Ogiso T,Kobayashi K,Sugishita M ( 2000): The precuneus in motor imagery: A magnetoencephalographic study. Neuroreport 11: 1345–1349. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Okuda B,Tanaka H,Tomino Y,Kawabata K,Tachibana H,Sugita M ( 1995): The role of the left somatosensory cortex in human hand movement. Exp Brain Res 106: 493–498. [DOI] [PubMed] [Google Scholar]
- Peelen MV,Downing PE ( 2005): Within‐subject reproducibility of category‐specific visual activation with functional MRI. Hum Brain Mapp 25: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P,Salmon E,van der Linden M,Garraux G,Aerts J,Delfiore G,Degueldre C,Luxen A,Orban G,Franck G ( 2000): The role of lateral occipitotemporal junction and area MT/V5 in the visual analysis of upper‐limb postures. Neuroimage 11: 644–655. [DOI] [PubMed] [Google Scholar]
- Penfield W,Jasper H ( 1954): Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little, Brown, & Co; 896 pp. [Google Scholar]
- Piefke M,Weiss PH,Zilles K,Markowitsch HJ,Fink GR ( 2003): Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126: 650–668. [DOI] [PubMed] [Google Scholar]
- Porro CA,Francescato MP,Cettolo V,Diamond ME,Baraldi P,Zuiani C,Bazzocchi M,di Prampero PE ( 1996): Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance study. J Neurosci 16: 7688–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA,Cettelo V,Francescano MP,Baraldi P ( 2000): Ipsilateral involvement of primari motor cortex during motor imagery. Eur J Neurosi 12: 3059–3063. [DOI] [PubMed] [Google Scholar]
- Raade AS,Rothi LJ,Heilman KM ( 1991): The relationship between buccofacial and limb apraxia. Brain Cogn 16: 13–46. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Luppino G ( 2001): The cortical motor system. Neuron 31: 889–901. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Matelli M,Pavesi G ( 1983): Deficits in attention and movement of following the removal of postarcuate (area 6) and prearcuate (area 5) cortex in macaque monkeys. Brain 106: 655–673. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Camarda R,Fogasssi L,Gentilucci M,Lupppino G,Metalli M ( 1988): Functional organisation of area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71: 491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Luppino G,Matelli M ( 1998): The organization of the cortical motor system electroencephalograph. Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fogassi L,Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Roland PE,Larsen B,Lassen NA,Skinhoj B ( 1980a): Supplementary motor area and other cortical areas in organization of voluntary movement in man. J Neurophysiol 43: 118–136. [DOI] [PubMed] [Google Scholar]
- Roland PE,Skinhoj E,Lassen NA.,Larsen B ( 1980b): Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol 43: 137–150. [DOI] [PubMed] [Google Scholar]
- Sacco K,Cauda F,Cerliani L,Mate D,Duca S,Geminiani GC ( 2006): Motor imagery of walking following training in locomotor attention. The effect of “the tango lesson”. Neuroimage 32: 1441–1449. [DOI] [PubMed] [Google Scholar]
- Salzmann E ( 1995): Attention and memory trials during neuronal recording from the primate pulvinar and posterior parietal cortex (area PG). Behav Brain Res 6: 241–253. [DOI] [PubMed] [Google Scholar]
- Schürmann M,Hesse MD,Stephan KE,Saarela M,Zilles K,Hari R,Fink GR ( 2005): Yearning to yawn: The neural basis of contagious yawning. Neuroimage 24: 1260–1264. [DOI] [PubMed] [Google Scholar]
- Sirigu A,Duhamel JR ( 2001): Motor and visual imagery as complementary but neurally dissociable mental processes. J Cogn Neurosci 13: 910–919. [DOI] [PubMed] [Google Scholar]
- Solodkin A,Hlustik P,Chen EE,Small SE ( 2004): Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex 14: 1246–1255. [DOI] [PubMed] [Google Scholar]
- Stephan KM,Fink GR,Passingham RE,Silbersweig D,Ceballos‐Baumann AO,Frith CD,Frackowiak RSJ ( 1995): Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386. [DOI] [PubMed] [Google Scholar]
- Stippich C,Ochmann H,Sartor K ( 2002): Somatotopic mapping of the human primary sensorimotor cortex during motor imagery and motor execution by functional magnetic resonance imaging. Neurosci Lett 331: 50–54. [DOI] [PubMed] [Google Scholar]
- Sugiura M,Kawashima R,Nakamura K,Okada K,Kato T,Nakamura A,Hatano K,Itoh K,Kojima S,Fukuda H ( 2000): Passive and active recognition of one's own face. Neuroimage 11: 36–48. [DOI] [PubMed] [Google Scholar]
- Thompson JC,Abbott DF,Wheaton KJ,Syngeniotis A,Puce A ( 2004): Digit representation is more than just hand waving. Brain Res Cogn Brain Res 21: 412–417. [DOI] [PubMed] [Google Scholar]
- Tootell RB,Reppas JB,Dale AM,Look RB,Sereno MI,Malach R.,Brady TJ,Rosen BR ( 1995): Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature 375: 139–141. [DOI] [PubMed] [Google Scholar]
- Tootell RB,Tsao D,Vanduffel W ( 2003): Neuroimaging weighs in: Humans meet macaques in “primate” visual cortex. J Neurosci 23: 3981–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C,Berlucci G,Aglioti SM ( 2004): Magnetic stimulation of the extrastriate body area impairs visual processing of nonfacial body parts. Curr Biol 14: 2130–2134. [DOI] [PubMed] [Google Scholar]
- Watanabe J,Sugiura M,Sato K,Sato Y,Maeda Y,Matsue Y,Fukuda H,Kawashima R ( 2002): The human prefrontal and parietal association cortices are involved in NO‐GO performances: An event‐related fMRI study. Neuroimage 17: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Watson RT,Fleet WS,Gonzalez‐Rothi L,Heilman KM ( 1986): Apraxia and the supplementary motor area. Arch Neurol 43: 787–792. [DOI] [PubMed] [Google Scholar]
- Watson JD,Myers R,Frackowiak RS,Hajnal JV,Woods RP,Mazziotta JP,Shipp S,Zeki S ( 1993): Evidence from a combined study using positron emossion tomography and functional magnetic resonance imaging. Cereb Cortex 3: 79–94. [DOI] [PubMed] [Google Scholar]


