Abstract
The experience of being liked is a key social event and fundamental to motivating human behavior, though little is known about its neural underpinnings. In this study, we examined the experience of being liked in a group of 15‐ to 24‐year‐old: a cohort for whom forming friendships has a great degree of salience, and for whom the explicit representation of relationships is familiar from their frequent use of social networking technologies. Study participants (n = 19) were led to believe that other participants had formed an opinion on their likability based on their appearance in a photograph, and during fMRI scanning viewed the photographs of people who had purportedly responded favorably to them (alongside photographs of control participants). Results indicated that being liked activated primary reward‐ and self‐related regions, including the nucleus accumbens, midbrain (in an area corresponding to the ventral tegmentum), ventromedial prefrontal cortex, posterior cingulate cortex (including retrosplenial cortex), amygdala, and insula/opercular cortex. Participants showed greater activation of ventromedial prefrontal cortex and amygdala in response to being liked by people that they regarded highly compared to those they regarded less so. Finally, being liked by the opposite compared to the same gender activated the right caudal orbitofrontal cortex and right anterior insula: areas important for the representation of primary somatic rewards. This study demonstrates that neural response to being liked has features that are consistent with response to other rewarding events, but it has additional features that reflect its intrinsically interpersonal character. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: social cognition, faces, self, gender, reward, fMRI
INTRODUCTION
Social events such as falling in love and making new friends provide our lives with richness and meaning. They are not only pleasurable, but tap into a more fundamental sense of who we are. The experience of being liked is intrinsically self‐affirming; and even more so if the person who likes us is held in high regard [Kaplan,1986]. It is an experience that becomes especially important during adolescence and young adulthood; an important developmental period during which a young person forms a stronger sense of his or her identity, and during which social tasks such as forming friendships and finding intimacy become so preoccupying [Davey et al.,2008]. The importance of these types of social events for young people has possibly become even more pronounced in contemporary life, with social networking sites such as Facebook and MySpace making the ventures very public affairs.
Brain responses to socially rewarding stimuli––including beautiful faces [Aharon et al.,2001; Ishai,2007; O'Doherty et al.,2003; Winston et al.,2007], participants' own babies [Bartels and Zeki,2004; Strathearn et al.,2008], and their lovers [Bartels and Zeki,2000; Aron et al.,2005]––show substantial overlap with responses to nonsocial rewards [Izuma et al.,2008; for further exploration of the similarity]. There is also overlap between regions that respond to reward outcome and those that respond to self‐relatedness. Midline cortical regions such as the ventromedial prefrontal cortex (vmPFC), posterior cingulate cortex (PCC), and precuneus are responsive to reward outcome [Berridge and Robinson,2003; Ernst et al.,2004; Knutson et al.,2001; Ramnani et al.,2004], and also to stimuli that are perceived as relevant to the self irrespective of valence [de Greck et al.,2008; Izuma et al.,2008; Moran et al.,2006; Phan et al.,2004].
The human character of the stimuli that entail social rewards differentiate them in a number of important respects from other stimuli [Beauchamp et al.,2002; Belin et al.,2000; Binder et al.,2000; Kanwisher et al.,1997; McCarthy et al.,1997]. Gender, for instance, is an important feature of human stimuli. Any relationship a person has must be with someone of the same or opposite gender; an aspect of relationships that evolutionary processes are likely to have embedded in the way they are processed by the brain [Geary,2006]. This facet of social relationships has been little investigated in the neuroimaging literature, though studies have shown that response to attractive faces of the opposite compared to the same gender results in orbitofrontal cortex (OFC) activation [Aharon et al.,2001; Ishai,2007; O'Doherty et al.,2003]. In addition, Ishai's study, and another by Kranz and Ishai [2006], showed that homosexual participants demonstrated greater OFC activation to faces of the same gender.
In this study, we present the findings of a novel functional MRI experiment that allowed us to examine the neural correlates of a salient social event for adolescents and young adults: the experience of being liked. We aimed to test the following hypotheses:
-
1
that being liked would activate reward‐related regions, including the ventral tegmental area (VTA), nucleus accumbens (NAcc), and OFC;
-
2
that being liked would activate regions associated with the self and social processes, including vmPFC, PCC, insula, and amygdala;
-
3
that the value of positive feedback would be encoded in mid‐line cortical regions and the amygdala;
-
4
that being liked by the opposite gender would activate the OFC.
METHODS
Participants
Twenty adolescents and young adults (from 15 to 24 years of age) were recruited via advertisements placed in a local daily newspaper in Melbourne, Australia. Participants had no history of mental illness, determined by the first and fourth authors using the Structured Clinical Interview for DSM‐IV Axis I Disorders [First et al.,1997], and all had normal or corrected‐to‐normal vision. All participants identified themselves as heterosexual. Two of the participants reported that they were regular (at least weekly) tobacco smokers, and the median frequency of alcohol consumption among the participants was two to three times per month. The participants provided their informed consent (or assent, if under 18 years of age, with parental consent) to participate in the study, which was approved by the ethics committees of Melbourne Health and The Royal Children's Hospital, Melbourne. Imaging data from one participant was excluded due to excessive head movement during scanning (z‐axis translation >2 mm), resulting in a final group of 12 females and 7 males with a mean age of 19 years (SD 2.9).
Experimental Design
During an initial assessment approximately 1 week prior to fMRI scanning, participants were told that they were to be part of a study that was investigating how people used first impressions to decide whether or not they liked someone. Participants had their photograph taken and understood that this would be presented to other study participants who would assess how much they thought they would like the participant based on their appearance in the photograph. The overt study design was, however, a ruse: the participants' photographs were deleted soon after they were taken, and the photographs that they viewed, which they understood were of other study participants, were in fact from a preexisting database [Martinez and Benavente,1998; http://www.ece.osu.edu/~aleix/ARdatabase.html]. The participants viewed the photographs of the 40 people they understood had been enrolled in the study to date: 20 females and 20 males, all with neutral facial expressions, and selected from the larger face database on the basis that they appeared to be of a similar age to the participants. They were asked to rate on a scale from 1 to 9 their answer to the question: “How much do you think you would like this person if you were to meet them?”
On the day of fMRI scanning, immediately prior to the scan, participants were again asked to rate the same set of photographs to refamiliarize them with the faces they would see during scanning. Once in the scanner participants viewed the “responses” from the people that had rated them. The responses had been pseudorandomly determined, ensuring balance between gender and between faces that the participants had rated highly and lowly. The high‐rated faces were determined for each participant as the four faces from each gender that they had rated the highest, and the low‐rated faces as the four from each gender that they had rated the lowest. These photographs made up the people who had apparently responded positively (henceforth referred to as “positive‐feedback” faces), and were presented during the scanning session on a green background. To provide a control condition, participants were told that not all people could be contacted for a response. These faces were selected from those rated in the mid‐range for each participant (and are referred to here as “control‐feedback” faces): they were presented on a white background. People who had apparently made unfavorable responses were not shown at all, the investigation of social rejection not being an aim of the current study.
Each of the 32 photographs included in the final fMRI paradigm (16 positive‐feedback faces [8 high‐rated and 8 low‐rated] and 16 control‐feedback faces) were presented three times over six blocks. The face blocks consisted of 16 photographs over 96 s separated by fixation blocks of 21.6 s, and each block was preceded by an instruction (for a total length of 12 min 17 s). Each photograph was displayed for 3 s and was interspersed with null events that had the effect of jittering the interstimulus interval by between 1 and 7 s (Fig. 1). Participants were not required to make a response: they were instructed to pay attention to the faces, and after the scanning session completed a recognition test in which they were shown the 32 faces they had seen in the scanner alongside 32 faces they had never seen (but from the same database). They were also asked to rate, on a 9‐point scale, how good they felt to discover that each of the people in the photographs liked them. The participants were then debriefed and the deceptive nature of the task was discussed with them.
Figure 1.
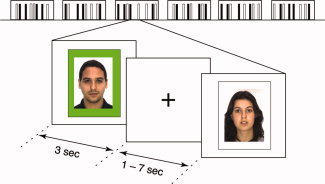
Experimental task design. The facial stimuli were presented in blocks of 16 faces over 96 s (only a portion of the stimuli are illustrated above), separated by fixation periods of 21.4 s. Positive‐feedback faces were presented on a green background, and control‐feedback faces on a white background. The faces were presented for 3 s, and the inclusion of null events had the effect of jittering the interstimulus interval by between 1 and 7 s. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Image Acquisition
A 3T Siemens Magnetom Trio magnetic resonance scanner (Erlangen, Germany) was used to acquire whole‐brain functional T2*‐weighted echo‐planar images. Functional sequences consisted of gradient‐recalled acquisition in the steady state (time of repetition [TR], 2,400 ms; time of echo [TE], 40 ms; pulse angle, 90°) within a field of view (FOV) of 210 mm, with a 64 × 64‐pixel matrix, and with a slice thickness of 3 mm (no inter‐slice gap). Thirty‐six interleaved slices, parallel to the anterior‐posterior commissure line, were acquired to cover the whole‐brain for all functional sequences. The first four (additional) images were discarded to allow the magnetization to reach steady‐state. In addition, high‐resolution T1‐weighted anatomical images were also acquired (with TR, 1,900 ms; TE, 2.1 ms; flip‐angle, 9°; voxel‐size, 1 mm × 0.5 mm × 0.5 mm; FOV, 256 mm). During scanning, participants were provided with earphones to reduce scanner noise, and foam‐rubber inserts were used to aid head stability. Stimuli were presented using Presentation software (Neurobehavioral Systems, USA) and were projected onto a half‐transparent viewing screen using an LCD projector (Epson EMP‐1810, Japan). They were viewed by the participants by way of a mirror mounted on the head‐coil.
Image Preprocessing and Analysis
Image analysis was carried out using tools from the FMRIB software library (http://www.fmrib.ox.ac.uk/fsl/). The images were realigned to compensate for small head movements, spatially smoothed using a 6 mm full‐width‐half‐maximum Gaussian kernel, and temporally filtered using a nonlinear high‐pass filter with a 128 s cut‐off. The positive‐feedback and control‐feedback face events were each modeled after convolution with canonical hemodynamic response functions, and parameter estimates calculated using a general linear model with local autocorrelation correction [Woolrich et al.,2001]. Temporal derivatives were included as covariates to improve statistical sensitivity. The individual statistical maps were registered with the participants' high‐resolution structural images, and then normalized to standard (Montreal Neurological Institute; MNI) space using nonlinear transformations. For additional analyses, the positive‐feedback faces were subdivided into high‐rated and low‐rated faces (for each participant, according to their ratings), and into same‐gender and opposite‐gender faces, and a similar univariate model was applied.
Mixed‐effects analysis was performed at a second level, and group statistical maps created using one‐sample t‐tests at each voxel for each contrast of interest [Woolrich et al.,2004]. Voxels were identified that showed greater activation to the positive‐feedback compared to control‐feedback faces by performing whole‐brain analysis with a statistical threshold of P < 0.001 (uncorrected). Whole‐brain analysis was also used to identify regions that were activated in response to the positive‐feedback and to the control‐feedback faces (for each separately, compared to baseline fixation), controlled for multiple comparisons using a false discovery rate correction (P FDR < 0.05).
Functional regions‐of‐interest (ROIs) were selected from a contrast comparing all faces to baseline (thresholded using a false discovery rate correction, P FDR < 0.05); and these were used to test for differences in activation to the high‐rated versus low‐rated faces. Finally, an exploratory whole‐brain analysis was conducted to identify the effects of gender: specifically, a two‐factor ANOVA (feedback × face gender) was used to compare the participant's responses to being liked by the opposite gender compared to the same gender (P < 0.01, uncorrected).
RESULTS
Behavioral Results
Participants' mean response to the question, “How much do you think you would like this person if you met them?” was 5.4 (SD = 1.07) on a 9‐point scale, and ratings were consistent between those at the first session and those immediately prior to the scan at the second session (mean ICC = 0.68, SD = 0.19). A two‐factor ANOVA (participant gender × face gender) was conducted to compare the effects of gender on face rating (Fig. 2). Female faces were rated significantly higher than male faces overall (F 1,17 = 16.64, P = 0.001). There was no interaction effect (F 1,17 = 1.39, P = 0.26), or main effect of participant gender (F 1,17 = 1.75, P = 0.20).
Figure 2.
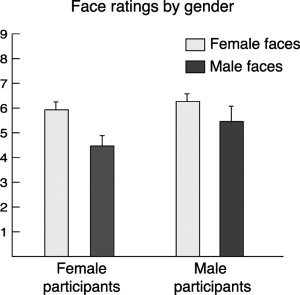
Behavioral analysis of the face ratings. Both male and female participants rated the female faces more highly than the male faces, giving rise to a significant main effect of face gender. There was no main effect of participant gender, nor interaction effect.
In post‐scan testing, participants reliably discriminated the faces they had seen during the scanning session from distractor faces (mean d′ = 3.96, SD = 1.64). The faces had been separated into high‐ and low‐rated faces for each participant according to their ratings at the first session. Postscan testing confirmed that participants found those faces they had rated highly in the prescan session as significantly more rewarding, when viewed in the scanner, than those they had rated lowly (6.9 [SD = 1.1] vs. 5.9 [SD = 0.8] on a 9‐point scale to the question, “How good did finding out that they like you make you feel?”; t 18 = 4.85, P = 0.0001). A two‐factor ANOVA (participant gender × face gender) was conducted to compare the effects of gender on the postscan face ratings. Again, female faces were rated significantly higher than male faces overall (F 1,17 = 7.11, P = 0.02); and there was neither interaction effect (F 1,17 = 0.17, P = 0.68), nor main effect of participant gender (F 1,17 = 0.79, P = 0.39). During debriefing all participants expressed surprise at the study's deception, and believed that the people they had viewed in the scanner had assessed their photographs.
Imaging Results
In a direct comparison of responses to the positive‐feedback and control‐feedback faces, greater activation was observed for the positive‐feedback faces in primary reward‐related regions (right NAcc and ventral midbrain); midline regions (vmPFC, mid‐cingulate cortex, dorsal PCC, ventral PCC extending to precuneus, and retrosplenial PCC); bilateral amygdalae; and a cluster extending from the right anterior insula to frontal opercular cortex. Activations to the positive‐feedback > control‐feedback contrast are listed in Table I and presented in Figure 3. There were no activations demonstrated for the control‐feedback > positive‐feedback contrast. In a secondary analysis, age was added as a covariate, which resulted in equivalent results to that describe earlier. Age was therefore not included as a study covariate for this and subsequent analyses.
Table I.
Significant activations for the positive‐feedback versus control‐feedback contrast
| Brain region | Brodmann area(s) | Number of voxels in cluster | Z‐score at peak voxel | MNI coordinates of peak voxel (x, y, z) | ||
|---|---|---|---|---|---|---|
| Brain regions demonstrating significant activation in response to being liked | ||||||
| Midline cortical activations | ||||||
| Ventromedial prefrontal | 10, 32 | 519 | 4.6 | 0 | 60 | −4 |
| 10 | 173 | 4.4 | −4 | 68 | 10 | |
| Pregenual anterior cingulate | 24 | 12 | 3.4 | 8 | 36 | 4 |
| Mid cingulate | 23 | 238 | 5.2 | 2 | −10 | 22 |
| Dorsal posterior cingulate | 23 | 34 | 3.5 | 0 | −32 | 28 |
| Retrosplenial posterior cingulate | 26, 29 | 11 | 3.3 | −6 | −46 | 14 |
| Precuneus/ventral posterior cingulate | 31 | 60 | 3.9 | 2 | −66 | 20 |
| Left hemisphere cortical activations | ||||||
| Superior frontal | 8 | 92 | 3.8 | −24 | 42 | 40 |
| 8 | 15 | 3.3 | −24 | 28 | 48 | |
| 9 | 14 | 3.4 | −22 | 44 | 26 | |
| Inferior temporal | 21 | 11 | 3.9 | −62 | −10 | −22 |
| Lateral parietal | 39 | 11 | 3.5 | −42 | −60 | 44 |
| Fusiform | 18, 19 | 235 | 4.6 | −22 | −78 | −20 |
| Occipital | 17 | 348 | 5.6 | −18 | −100 | 0 |
| Right hemisphere cortical activations | ||||||
| Superior frontal | 9 | 12 | 3.5 | 22 | 60 | 26 |
| Anterior insula/operculum | 13 | 157 | 4.2 | 38 | 14 | −18 |
| Inferior temporal | 20, 21 | 46 | 3.8 | 64 | −12 | −24 |
| Occipital/fusiform | 17, 18, 19 | 602 | 5.0 | 16 | −96 | −2 |
| Subcortical and cerebellar activations | ||||||
| R NAcc | 8 | 3.4 | 4 | 6 | −4 | |
| L amygdala | 25 | 3.8 | −24 | −10 | −24 | |
| R amygdala | 13 | 3.3 | 30 | −8 | −22 | |
| Ventral tegmental area | 4 | 3.3 | 0 | −20 | −18 | |
| Cerebellum | 10 | 3.4 | 26 | −48 | −24 | |
Voxels were thresholded at P < 0.001 (uncorrected), and formed clusters of at least 8 contiguous voxels.
An exception was made for the ventral tegmental area, whose anatomical volume is less than the volume represented by eight voxels [Mai et al.,2007].
Figure 3.
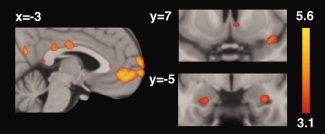
Whole‐brain analysis of the contrast comparing positive‐feedback to control‐feedback conditions. Regions demonstrating greater activation to positive‐feedback faces compared to control‐feedback faces included vmPFC, mid‐cingulate, PCC, precuneus, ventral midbrain, right NAcc, right insula/operculum, and left and right amygdalae. For this figure, and the figures that follow, activations are displayed on a high‐resolution (0.5 mm isotropic) version of the MNI152 standard brain. Corresponding color bars indicate the Z‐score ranges of the displayed activation maps. Images are displayed in neurological convention (left = left).
Activations produced by the positive‐feedback and control‐feedback faces alone, compared to baseline, indicated that each set of stimuli evoked distributed and overlapping brain regions, including the dorsomedial PFC, left anterior insula, and caudal OFC, subcortical areas encompassing the amygdala, hippocampus, and parahippocampal cortex bilaterally, and large areas of the occipital cortex, including bilateral fusiform gyri (Fig. 4). Positive‐feedback faces produced additional activations in midline regions (vmPFC, PCC, and precuneus), right anterior insula extending to caudal OFC, bilateral NAcc, and ventral midbrain.
Figure 4.
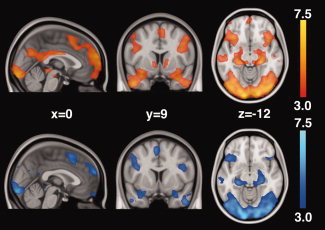
Whole‐brain analysis of activations to positive‐feedback faces (top, in orange) and control‐feedback faces (bottom, in blue). Both the positive‐feedback and control‐feedback faces activated brain regions including the dorsomedial PFC, left anterior insula and caudal OFC, subcortical areas encompassing the amygdala, hippocampus and parahippocampal gyrus, and large areas of the occipital cortex. Positive‐feedback faces produced additional activation of midline regions (vmPFC, PCC, and precuneus), right anterior insula extending to caudal OFC, bilateral NAcc and ventral midbrain.
ROI analysis was performed using selected activation clusters from a contrast comparing all faces to baseline. ROIs were identified in the vmPFC, PCC, and left and right amygdalae (the latter were extracted from a much larger cluster by identifying voxels with a 50% or greater probability of belonging to the amygdala according to the Harvard‐Oxford probabilistic atlas in FSL; Flitney et al.,2007]. These analyses demonstrated significant differences in activation to high‐ versus low‐rated faces in two regions: vmPFC (t 18 = 2.19, P = 0.04) and left amygdala (t 18 = 2.41, P = 0.03; Fig. 5).
Figure 5.
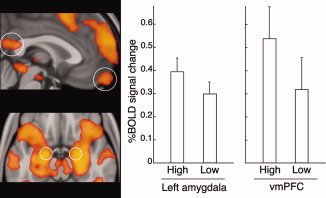
ROI analyses of the differential effects of being liked by people who individual participants had rated highly compared to those they had rated lowly. Functional ROIs (circled) were selected from a contrast comparing all faces to baseline. (Note that the circles showing the location of the amygdala ROIs are approximate; refer to the text for details of their selection). Significant differences were demonstrated in the left amygdala (P = 0.03) and vmPFC (P = 0.04).
Gender‐related effects were assessed by comparing activations to the opposite versus same gender. This contrast identified a significant interaction effect between social feedback and face gender that was represented by activation clusters in the right caudal OFC (cluster size, 32 voxels; peak voxel coordinate [x y z], 34, 20, −8; peak Z‐score, 2.84) and right anterior insular (cluster size, 31 voxels; peak voxel coordinate, 34, 30, −14; peak Z‐score, 2.95; Fig. 6). Post hoc ROI analysis confirmed significant interaction effects between feedback and face gender in the right OFC (F 1,18 = 5.54, P = 0.03) and right anterior insula (F 1,18 = 6.02, P = 0.02). As expected, there were main effects of social feedback in each region (in right OFC: F 1,18 = 5.62, P = 0.03; and in right insula: F 1,18 = 11.05, P = 0.004), and nonsignificant main effects of face gender (in right OFC: F 1,18 = 3.60, P = 0.07; and in right insula: F 1,18 = 4.19, P = 0.06). The participant's own gender did not interact with feedback and face gender in either region (interaction effect for feedback × face gender × participant gender in right OFC: F 1,17 = 0.14, P = 0.72; and in right insula: F 1,17 = 0.08, P = 0.78), suggesting that the participant's own gender had no significant influence on the effect.
Figure 6.
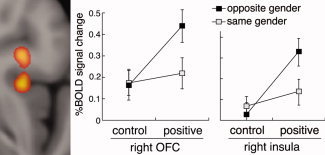
Whole‐brain analysis of the gender manifestations of social feedback. The analysis identified voxels in the right caudal OFC and right anterior insula that were activated by the interaction of feedback and gender (they showed significantly greater activation to positive feedback from the opposite gender compared to the same gender). The mean BOLD signal changes were calculated for each region to illustrate the interactions.
DISCUSSION
Being liked is a highly desired, salient social experience. This study confirms that the experience is associated with a pattern of neural activation that is consistent with response to rewarding stimuli, but it has additional features that reflect its intrinsically interpersonal character. Being liked is confirmed to activate primary reward‐related regions associated with the dopaminergic system. The ventral tegmental area and nucleus accumbens have been argued to play more important roles in the anticipation of rewards rather than response to their outcome [Berridge and Robinson,2003], though the regions are responsive to outcomes that are unexpected or novel [O'Doherty,2004]. A number of imaging studies have confirmed specific responses to reward outcome in both regions [Adcock et al.,2006; Elliott et al.,2000; Ernst et al.,2005; Rogers et al.,2004]. In this study, activation of VTA and NAcc is consistent with the role of the dopaminergic system in indexing the novelty and unexpectedness of rewarding outcomes, which were engendered by an event‐related design that jittered and intermingled the appearance of the positive‐feedback faces.
The experience of being liked produced activation of midline regions, including vmPFC, mid‐cingulate cortex, PCC, and precuneus. These regions not only respond to reward outcome, but also to the degree of self‐relatedness of the stimuli [Northoff et al.,2006]. The midline regions are key regions of the default network; a network that is highly active during passive conditions and self‐referential tasks, and that is deactivated by externally‐directed, cognitively demanding tasks [Greicius et al.,2003; Gusnard et al.,2001; Harrison et al.,2008; Raichle et al.,2001]. Midline regions play an important role in the evaluation of social stimuli, which are inherently complex. In this study, their value was determined by the participant's appraisal of a person's appearance, and their extrapolation from this to an assessment of their likeability (which our behavioral results suggested were consistent with positive hedonic feelings during the task). Being liked by someone who is held in high regard is likely to drive internal and reflective processes that relate to the self, which is consistent with involvement of a key region of the default network: the vmPFC. The region has an important role in self‐reflection, and vmPFC activity has been demonstrated to correlate with the degree of self‐relatedness of the stimuli being considered [D'Argembeau et al.,2005].
Both reward outcome and the value of reward were indexed by amydgala activation. The amygdala is well known to have a role in fear perception and fear‐conditioning, though is less well recognized for its role in the processing of reward [Baxter and Murray,2002; Gottfried et al.,2003]. In addition, the amygdala is responsive to social stimuli, and especially emotional faces [Bartels and Zeki,2000; Fitzgerald et al.,2005; Killgore and Yurgelun‐Todd,2001; Winston et al.,2007]. Each of the nuclei in the basolateral complex––the lateral, basolateral, basomedial and basoventral nuclei––contain neurons that project directly to the nucleus accumbens, and the complex is reciprocally connected to the orbital and medial prefrontal cortices [Amunts et al.,2005; Baxter and Murray,2002], suggesting that the amygdala is well placed to encode the value of social reward.
Being liked activated a region extending from the right anterior insula to the frontal operculum. The right anterior insula has an important role in the representation of internal states. It sits atop a hierarchy for somatic representation, producing an integrated image of the felt state of the body [Craig,2002, 2003]. A number of studies have shown it to be activated by rewarding experiences; particularly of a social nature [Bartels and Zeki,2000; Bartels and Zeki,2004; Strathearn et al.,2008; Winston et al.,2007].
The influence of gender‐to‐gender relationships produced a novel finding: that being liked by the opposite gender activates the right caudal OFC and right anterior insula. The OFC has a role in the representation of rewards, and the association of these rewards, by learning, with secondary rewards [Rolls,2000]. There is within the OFC an anterior‐posterior trend for the types of rewards represented, with simple primary rewards such as taste and olfaction represented caudally, and more complex rewards, such as money, represented rostrally [Kringelbach and Rolls,2004]. The right anterior insula and caudal OFC, which consist of agranular cortex that is continuous between them [Ongur et al.,2003], act in concert to represent primary, somatic rewards. The primary nature of rewards that have been previously shown to be represented by the regions acting together––such as taste and smell [de Araujo et al.,2003] and thermal sensation [Craig et al.,2000]––suggests something of equivalent primacy is represented in the regions' responsiveness to the opposite gender. The significance of gender to sexual reproduction, and the somatic nature of sexual feeling, suggests that activity in the right caudal OFC and right anterior insula may be indexing sexual possibility.
There are caveats to the study. First, facial stimuli are inherently activating, necessitating the use of an appropriate facial contrast. As a comparison for the study, we used the faces of people who we were supposedly unable to contact. While the faces controlled for lower‐level common effects (they were demonstrated to activate face‐responsive regions, in common with the positive‐feedback faces), they may also have produced their own affective experience: perhaps one of irritation or frustration. Second, the positive‐feedback condition differed from the control‐feedback condition in ways other than affective valence. While our focus in the study has been on the positive nature of the feedback, the conditions also differ on whether they included feedback at all (one way of interpreting the control‐feedback condition is that it contained no feedback). Thus the activations that we have demonstrated in response to positive feedback may simply reflect that the faces provided feedback: the valence of the feedback may not have been important. While the results provide evidence of response to the positive character of the feedback (reward‐related regions were activated) the inclusion of another feedback condition––for instance, negative feedback––may have helped to clarify the issue. Third, we were unable to dissociate the interaction of feedback and face rating. While we demonstrated that some regions were more responsive to positive feedback from faces that participants had rated highly compared to those they had rated lowly, we were unable to demonstrate a similar effect for the control‐feedback faces as a consequence of the undifferentiated way in which they had been selected.
Notwithstanding the above, we have been able to demonstrate the effects of a simple, direct, and highly salient social event: the experience of being liked. Being liked activates primary reward‐related regions, and regions that are important for an ongoing sense of self. In addition, if the experience originates from a person of the opposite gender, being liked activates regions consistent with the stirring of a primary bodily feeling. Study participants were adolescents and young adults, from an age group for whom social events have a great deal of salience. We have previously argued [Davey et al.,2008] that it is the postpubertal development of the ability to represent complex social rewards that brings with it a vulnerability to depression when the attainment of the rewards is frustrated. The current findings suggest further lines of investigation. The paradigm allows for an exploration of developmental changes, from childhood to adolescence, in the way that the brain processes social rewards. It may also prove useful in demonstrating how the neural processing of social rewards is affected by disorders such as depression, which has social disengagement as one of its characteristic and core symptoms.
Acknowledgements
Dr. Davey is supported by a National Health and Medical Research Council of Australia (NHMRC) Postgraduate Medical Scholarship (I.D. 400524), Dr. Allen is supported by a grant from the Colonial Foundation, Dr. Harrison is supported by an NHMRC Training Award (I.D. 400420) and Dr. Yücel is supported by an NHMRC Clinical Career Development Award (I.D. 509345). We thank Dr. Alex Fornito for his helpful comments on an earlier version of the study paradigm.
REFERENCES
- Adcock RA, Thangavel A, Whitfield‐Gabrieli S, Knutson B, Gabrieli JD ( 2006): Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron 50: 507–517. [DOI] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC ( 2001): Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32: 537–551. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K ( 2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol 210: 343–352. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL ( 2005): Reward, motivation, and emotion systems associated with early‐stage intense romantic love. J Neurophysiol 94: 327–337. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S ( 2000): The neural basis of romantic love. Neuroreport 11: 3829–3834. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S ( 2004): The neural correlates of maternal and romantic love. Neuroimage 21: 1155–1166. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA ( 2002): The amygdala and reward. Nat Rev Neurosci 3: 563–573. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A ( 2002): Parallel visual motion processing streams for manipulable objects and human movements. Neuron 34: 149–159. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B ( 2000): Voice‐selective areas in human auditory cortex. Nature 403: 309–312. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE ( 2003): Parsing reward. Trends Neurosci 26: 507–513. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM ( 2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E ( 2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25: 616–624. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB ( 2008): The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev 32: 1–19. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N ( 2003): Taste‐olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18: 2059–2068. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B, Northoff G ( 2008): Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. Neuroimage 39: 2066–2075. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ ( 2000): Dissociable neural responses in human reward systems. J Neurosci 20: 6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS ( 2005): Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 25: 1279–1291. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS ( 2004): Choice selection and reward anticipation: An fMRI study. Neuropsychologia 42: 1585–1597. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 1997. Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I). Washington: American Psychiatric Publishing. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL ( 2005): Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage 30: 1441–1448. [DOI] [PubMed] [Google Scholar]
- Flitney D, Webster M, Patenaude B, Seidman L, Goldstein J, Tordesillas Gutiérrez D, Eickhoff S, Amunts K, Zilles K, Lancaster J, Haselgrove C, Kennedy D, Jenkinson M, Smith S ( 2007): Anatomical brain atlases and their application in the FSLView visualisation tool. In Thirteenth Annual Meeting of the Organization for Human Brain Mapping, Chicago.
- Geary DC ( 2006): An evolutionary perspective on sexual and intimate relationships In: McAnulty RD, Burnette MM, editors. Sex and Sexuality. New York: Praeger; pp 67–86. [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ ( 2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Ortiz H, Soriano‐Mas C, Yucel M, Pantelis C, Cardoner N ( 2008): Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA 105: 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A ( 2007): Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol 63: 181–185. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N ( 2008): Processing of social and monetary rewards in the human striatum. Neuron 58: 284–294. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HB. 1986. Social Psychology of Self‐Referent Behavior. New York: Plenum. [Google Scholar]
- Killgore WD, Yurgelun‐Todd DA ( 2001): Sex differences in amygdala activation during the perception of facial affect. Neuroreport 12: 2543–2547. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D ( 2001): Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12: 3683–3687. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A ( 2006): Face perception is modulated by sexual preference. Curr Biol 16: 63–68. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET ( 2004): The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. 2007. Atlas of the Human Brain. New York: Academic Press. [Google Scholar]
- Martinez AM, Benavente R ( 1998): The AR face database. CVC Technical Report #24.
- McCarthy G, Puce A, Gore JC, Allison T ( 1997): Face‐specific processing in the human fusiform gyrus. J Cogn Neurosci 9: 605–610. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM ( 2006): Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 18: 1586–1594. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J ( 2006): Self‐referential processing in our brain: A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP ( 2004): Reward representations and reward‐related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol 14: 769–776. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ ( 2003): Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41: 147–155. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL ( 2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passingham RE ( 2004): Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage 23: 777–786. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM ( 2004): Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision‐making cognition. Biol Psychiatry 55: 594–602. [DOI] [PubMed] [Google Scholar]
- Rolls ET ( 2000): The orbitofrontal cortex and reward. Cereb Cortex 10: 284–294. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR ( 2008): What's in a smile? Maternal brain responses to infant facial cues. Pediatrics 122: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ ( 2007): Brain systems for assessing facial attractiveness. Neuropsychologia 45: 195–206. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM ( 2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM ( 2004): Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21: 1732–1747. [DOI] [PubMed] [Google Scholar]


