Abstract
We evaluated the functional magnetic resonance imaging (fMRI) correlates of simple movement performance in patients with pediatric multiple sclerosis (MS) and their relation with the extent of T2 lesion volume (LV), to improve our understanding of the mechanisms leading to their short/medium term favorable clinical course. We obtained fMRI during repetitive flexion‐extension of the last four fingers of the right hand and brain dual‐echo scans from 17 right‐handed patients with pediatric relapsing‐remitting MS and 9 sex‐ and age‐matched right‐handed healthy controls. T2 LV was measured using a local thresholding segmentation technique. fMRI activations and functional connectivity analysis were performed using SPM2. Compared to controls, pediatric MS patients had an increased recruitment of the left (L) primary sensorimotor cortex (SMC). They also showed reduced functional connectivity between the L primary SMC and the L thalamus (P = 0.03), the L insula and the L secondary sensorimotor cortex (SII) (P = 0.02), the supplementary motor area and the L SII (P = 0.02), the L thalamus and the L insula (P = 0.01) and the L thalamus and the L SII (P = 0.003). In patients with pediatric MS, the activity of the L primary SMC was significantly correlated with brain T2 LV (r = 0.78). No correlation was found between coefficients of abnormal connectivity and structural MRI measures. The maintenance of a selective and strictly lateralized pattern of movement‐associated brain activations and a modulation of its functional connections suggest a preserved functional reserve in patients with pediatric MS, which, in turn, might contribute to explain their favorable clinical evolution at short/medium term. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: pediatric multiple sclerosis, functional magnetic resonance imaging, adaptation, reserve
INTRODUCTION
A clinical onset before the age of 16 years occurs in about 3–12% of patients with multiple sclerosis (MS) [Renoux et al., 2007; Simone et al., 2002]. Natural history studies have suggested that patients with pediatric MS may have, at least at short/medium term, a more favorable clinical course than those with the more common adult form of the disease [Banwell et al., 2007a; Confavreux et al., 2003; Renoux et al., 2007; Simone et al., 2002]. This notion has been strengthened by the results of a recent epidemiological survey of 394 pediatric MS patients and 1,775 adult‐onset MS patients, which showed that the former took ∼10 years longer to reach progression of disease and accumulation of irreversible disability [Renoux et al., 2007].
In the past few years, significant effort has been spent to define the factors associated with the favorable clinical evolution of patients with pediatric MS. Although the application of conventional magnetic resonance imaging (MRI) (i.e., identification of dual‐echo lesions, gadolinium enhancing lesions, and T1‐hypointense lesions, and assessment of brain atrophy) provided inconclusive results [Balassy et al., 2001; Banwell et al., 2007b; Ebner et al., 1990; Sindern et al., 1992], two preliminary studies [Mezzapesa et al., 2004; Tortorella et al., 2006] using magnetization transfer and diffusion tensor MRI have shown that in these patients the overall amount of central nervous system (CNS) damage is relatively mild. The relative preservation of brain structural integrity is also supported by the demonstration of a selective atrophy of the thalamus in these patients, with sparing of the cortical gray matter (GM) and of the remaining deep GM nuclei [Mesaros et al., 2008].
Another factor that might play a role in limiting the clinical consequences of disease‐related pathology in patients with pediatric MS is likely to be the effectiveness of brain reorganization following tissue damage. The application of functional magnetic resonance imaging (fMRI) in adult patients with MS has indeed suggested that brain functional reorganization might have such a role [Rocca and Filippi, 2007]. To the best of our knowledge, this aspect of the disease has never been investigated in pediatric MS patients.
Against this background, the present study assessed the movement‐associated pattern of cortical activations in patients with pediatric MS with the aim to improve our understanding of the mechanisms leading to their short/medium term favorable clinical course. To provide some clues about the nature of the detected changes, if present, we also planned to investigate their correlation with the extent of T2 lesion volume (LV).
PATIENTS AND METHODS
Patients
We studied 17 right‐handed patients with pediatric relapsing‐remitting (RR) MS [Krupp et al., 2007] (11 girls and 6 boys; mean age = 14.6 years, range = 11–16 years; mean disease duration = 2.7 years, range = 0.5–11 years; median Expanded Disability Status Scale (EDSS) [Kurtzke, 1983] = 1.0, range = 0.0–3.0, mean number of previous relapses = 3.6, range = 2–8) and 9 sex‐ and age‐matched right‐handed healthy volunteers (6 girls and 3 boys; mean age = 15.6 years, range, 10–16 years). Three pediatric MS patients had had previous relapses involving the right upper limb, but had a complete clinical recovery. Handedness was established according to the 10‐item version of the Edinburgh Handedness Inventory (EHI) scale [Oldfield, 1971]. The mean laterality quotient at the EHI was 0.97 (range = 0.90–1.00) in healthy volunteers and 0.98 (range = 0.90–1.00) in pediatric MS patients. At the time MRI was performed, all patients had been relapse‐ and steroid‐free for at least 6 months. Twelve patients were taking disease‐modifying treatments (interferon β‐1a = 11 patients and mitoxantrone = 1 patient). None of the patients was taking any other concomitant therapy with the potential to interfere with CNS function, such as antidepressant, psychoactive and antifatigue (e.g., amantadine) drugs. Local Ethical Committee approval and written informed consent from each subject were obtained prior to study initiation.
Functional Assessment
Right upper limb motor functional assessment was performed for all individuals on the same day MRI was acquired, using the nine‐hole peg test (9‐HPT) and the maximum finger tapping frequency. The maximum finger‐tapping rate was observed for two 30‐s trial periods outside the magnet and the mean frequency to the nearest 0.5 Hz entered the analysis. No difference was found in the performance of these tests between MS patients and healthy controls (time to complete the 9‐HPT: mean = 20.0, SD = 1.2 s for controls; mean = 21.8, SD = 1.9 s for patients; finger‐tapping rate: mean = 3.5, SD = 0.7 Hz for controls; mean = 3.6, SD = 0.8 Hz for patients).
Experimental Design
Using a block design (ABAB), where six periods of activation were alternated with six periods of rest (each period of rest and activity consisting of five measurements), the subjects were scanned while performing a simple motor task consisting of repetitive flexion‐extension of the last four fingers of the dominant right hand moving together. A standardized frame was used to restrict the amplitude of the extension to ∼3 cm from base. The movements were paced by a metronome at a 1‐Hz frequency. Patients were trained before performing the study. The subjects were instructed to keep their eyes closed during fMRI acquisition and were monitored visually during scanning to ensure accurate task performance and to check for additional movements (e.g., mirror movements).
fMRI Acquisition
Brain MRI scans were obtained using a 1.5 Tesla machine (Vision, Siemens, Erlangen, Germany). Sagittal T1‐weighted images were acquired to define the anterior–posterior commissural (AC‐PC) plane. Functional MR images were acquired using a T2*‐weighted echo‐planar imaging sequence (TE = 66 ms, flip angle = 90°, matrix size = 128 × 128, field of view = 256 × 256 mm, TR = 5.5 s). Twenty‐four axial slices, parallel to the AC‐PC plane, with a thickness of 5 mm, covering the whole brain were acquired during each measurement. Shimming was performed for the entire brain using an auto‐shim routine, which yielded satisfactory magnetic field homogeneity.
Structural MRI Acquisition
Using the same magnet, the following sequences of the brain were also acquired: (a) dual‐echo turbo spin echo sequence (TSE) (TR = 3,300 ms, first echo TE = 16 ms, second echo TE = 98 ms, echo train length = 5), and (b) sagittal three‐dimensional (3D) T1‐weighted magnetization prepared rapid acquisition gradient echo (MP‐RAGE) (TR/TE = 11.4/4.4, flip angle = 15°, FOV = 250 × 250 mm2, matrix size = 256 × 256, slab thickness 160 mm, voxel size = 1 × 1 × 1 mm3). For the TSE scans, 24 contiguous interleaved axial slices were acquired with 5‐mm slice thickness, 256 × 256 matrix, and 250 × 250 mm2 FOV. The slices had the same positioning of fMRI data set.
FMRI Analysis
All image postprocessing was performed on an independent computer workstation (Sun Sparcstation, Sun Microsystems, Mountain View, CA). FMRI data were analyzed using the statistical parametric mapping (SPM2) software [Friston et al., 1995]. Before statistical analysis, all images were realigned to the first one to correct for subject motion, spatially normalized into the standard space of SPM, and smoothed with a 10‐mm, 3D‐Gaussian filter. Subjects were included in the subsequent statistical analysis if they had a maximum translation/rotation lower than 3.0 mm in the x,y,z planes.
Changes in blood oxygenation level dependent (BOLD) contrast associated with the performance of the motor task were assessed on a pixel‐by‐pixel basis, using the general linear model and the theory of Gaussian fields [Friston et al., 1995]. Specific effects were tested by applying appropriate linear contrasts. Significant hemodynamic changes were assessed using t statistical parametric maps (SPMt).
Analysis of Functional Connectivity
The interactions between different regions involved in the motor task were calculated using a dynamic causal model (DCM) approach [Friston et al., 2003]. Definition of brain regions included in the DCM relied on data from published fMRI studies of the motor system [Rocca and Filippi, 2007; Ward, 2006] and the results of the within‐ and between‐group analysis of this study. Time series, which were adjusted for the effect of interest, were extracted from a spherical volume (5‐mm radius) centered at the most significant voxel within an a priori defined cluster in the SPMf maps (i.e., SPM maps thresholded using an F‐contrast) in each subject. Volumes of interest were extracted from the clusters with the highest peak of activations in the primary sensorimotor cortex (SMC), bilaterally, the secondary sensorimotor cortex (SII), bilaterally, the supplementary motor area (SMA), the left thalamus, the left insula, and the right cerebellum. These regions were entered into subject‐specific DCMs. For each subject, the DCM was used to investigate the intrinsic connectivity pattern between all regions of interest previously defined. To this end, a DCM was constructed, where all regions were assumed to be connected bidirectionally with each other. We determined in healthy volunteers and MS patients, separately, which of the regions defined by the fMRI analysis was the most likely input region. To test which model fitted best the observed findings, eight different fully connected models were built, each having one of the eight regions included in the DCM model as the input region. Model evidence was computed based on Bayesian and Akaikes information criterion (BIC and AIC, respectively), as described by Penny et al. [2004]. For each subject and each model, we estimated the corresponding Bayesian factors, and then model evidence for each group, separately, was calculated by multiplying the Bayesian factors obtained for each pairs of models and for each subject. This analysis revealed the superiority of the model having the left SMC as the input region in both healthy volunteers and MS patients (Table I). Therefore, this model was applied for DCM analysis in each group and drove the contrasts between patients and controls. The intrinsic connectivity strength coefficients (A) were estimated using a Bayesian approach [Friston et al., 2003].
Table I.
Bayesian factors from the comparison of the different dynamic causal models (DCM) tested in healthy controls and pediatric MS patients
| Healthy subjects | Pediatric MS patients | |
|---|---|---|
| L SMC model vs. R cerebellum model vs. vice versa | 1.02 × 1029 vs. 2.54 × 10−13 | 9.24 × 108 vs. 1.93 × 104 |
| L SMC model vs. L insula model vs. vice versa | 3.73 × 1090 vs. 1.16 × 10−24 | 1.00 × 1033 vs. 3.60 × 10−22 |
| L SMC model vs. R SII model vs. vice versa | 3.77 × 1038 vs. 2.08 × 10−26 | 6.50 × 1029 vs. 1.50 × 10−18 |
| L SMC model vs. L SII model vs. vice versa | 6.22 × 1036 vs. 4.57 × 10−24 | 4.10 × 1027 vs. 3.10 × 10−20 |
| L SMC model vs. SMA model vs. vice versa | 1.07 × 1030 vs. 3.41 × 10−24 | 8.41 × 1036 vs. 1.4 × 10−25 |
| L SMC model vs. R SMC model vs. vice versa | 1.08 × 1057 vs. 5.61 × 10−28 | 1.20 × 1035 vs. 3.11 × 10−13 |
| L SMC model vs. L thalamus model vs. vice versa | 8.95 × 10103 vs. 1.55 × 10−24 | 9.06 × 1085 vs. 5.03 × 10−14 |
L, left; R, right; SMC, primary sensorimotor cortex; SMA, supplementary motor area; SII, secondary sensorimotor cortex.
Structural MRI Postprocessing
Total T2 LV were measured using a local thresholding segmentation technique (Jim 4.0, Xinapse System, Leicester, UK). The LV of the corpus callosum (CC) and the left and right corticospinal tracts (CST) were also calculated using a white‐matter atlas [Mori, 2005] available within the FSL library and containing labels of 50 structures. First, a lesion mask from the manually segmented lesions visible on the dual‐echo images was produced. Then, the affine transformation between the T2‐weighted images and the atlas space was calculated and applied to lesion masks. Finally, the LV within the fiber bundles of interest was calculated.
Normalized brain volumes (NBV) were measured on the 3D T1‐weighted images, using the cross‐sectional version of the Structural Imaging Evaluation of Normalized Atrophy (SIENA) software [Smith et al., 2001].
Statistical Analysis
The intragroup activations and comparisons between groups were investigated using SPM2 and a random‐effect analysis [Friston et al., 1999]. An analysis of covariance (ANCOVA) was used to compare movement‐associated activations between pediatric MS patients and controls, including age as a nuisance covariate in the analysis. We report activations below a threshold of P < 0.05 corrected for multiple comparisons (familywise error). To assess the correlation of BOLD changes with clinical variables and quantities derived from structural MRI, these metrics were entered into the SPM design matrix, using basic models and linear regression analysis [Friston et al., 1999].
A two‐tailed Student's t‐test for not‐paired data was used to compare structural MRI derived metrics and DCM metrics between patients and controls. Univariate correlations between DCM measures and structural MRI data were assessed using the Spearman Rank correlation coefficient (SPSS version 13.0).
RESULTS
Structural MRI
All controls had normal brain MRI scans. In pediatric MS patients, the mean brain T2 LV was 9.4 ± 13.3 ml, the mean CC T2 LV was 0.71 ± 0.82 ml, the mean left CST LV was 0.67 ± 1.5 ml, and the mean right CST LV was 0.30 ± 0.4 ml. NBV did not differ between MS patients and controls (NBV = 1,771, SD = 57 ml in healthy controls vs. 1,686, SD = 96 ml in MS patients, P = n.s.).
Functional MRI: Between‐Group Analysis
All subjects performed the tasks correctly and no additional movements were observed during fMRI acquisition. In Figure 1, the brain areas with significant activations detected while performing the task in healthy subjects and pediatric MS patients are shown (within‐group one‐sample t test). Compared to healthy volunteers, pediatric MS patients had a more significant activation of the contralateral (L) SMC (SPM space coordinates: −32, −28, 56) (see Fig. 1). No area was significantly more active in controls than in pediatric MS patients.
Figure 1.
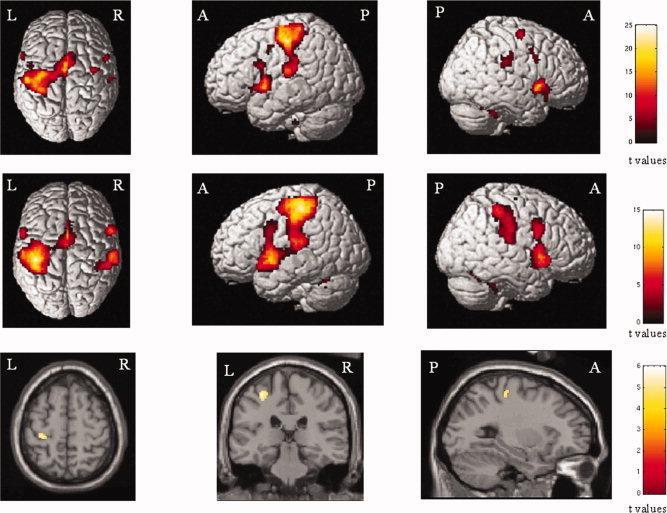
Cortical activations on a rendered brain from right‐handed healthy subjects (top row) and patients with pediatric MS (middle row) during the performance of a simple motor task with their clinically unimpaired and fully normal functioning right hands (within‐group analysis, one‐sample t tests, P < 0.05 corrected for multiple comparisons). In the bottom row, the between‐groups differences are shown: the left primary sensorimotor cortex was more significantly activated in pediatric MS patients than controls. Images are in neurological convention. See text for further details.
Functional Connectivity Analysis
In Table II and Figure 2, the results of the comparison of path coefficient strengths between healthy controls and pediatric MS patients are shown. Only connections significantly different between controls and MS patients are reported. Compared to controls, pediatric MS patients had reduced functional connectivity between the L primary SMC and the L thalamus (P = 0.03), the L insula and the L SII (P = 0.02), the SMA and the L SII (P = 0.02), the L thalamus and the L insula (P = 0.01), and the L thalamus and the L SII (P = 0.003) (see Fig. 2).
Table II.
Significant paths coefficients (mean values) between brain regions for controls and pediatric MS patients
| Dependent regions | Path regions | Controls | Pediatric MS patients |
|---|---|---|---|
| L primary SMC | L thalamus | 0.10 | 0.04 |
| SMA | L SII | 0.07 | 0.03 |
| L thalamus | L SII | 0.02 | 0.004 |
| L insula | 0.03 | 0.009 | |
| L insula | L SII | 0.06 | 0.03 |
Only connections significantly different between controls and MS patients have been reported. L, left; R, right; SMC, sensorimotor cortex; SMA, supplementary motor area; SII, secondary sensorimotor cortex; MS, multiple sclerosis.
Figure 2.
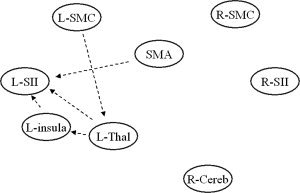
Dynamic causal model showing the results of the between‐group analysis of connectivity. Reduced strength of connection in pediatric MS patients vs. controls are reported as dotted black lines. See text for further details.
Correlations Between Functional MRI and Structural MRI Measures
No correlation was found between fMRI metrics and clinical variables.
In patients with pediatric MS, a significant correlation was found between the activity of the L primary SMC and brain T2 LV (r = 0.78, P < 0.001). No correlation was found between coefficients of abnormal connectivity and T2 LV.
DISCUSSION
In this study, we applied fMRI to interrogate the sensorimotor network of patients with pediatric MS in order to gain additional insights into the mechanisms responsible for their slower accrual of disability when compared to patients with adult‐onset MS [Banwell et al., 2007a; Confavreux et al., 2003; Renoux et al., 2007; Simone et al., 2002].
The analysis of movement‐associated brain patterns of activations showed that, compared to controls, pediatric MS patients had exclusively an increased activation of the primary SMC of the left hemisphere, which was moderately related to the extent of T2 visible lesions. This correlation suggests a possible adaptive role of the observed functional change in limiting the clinical consequences secondary to the accumulation of tissue damage. The notion that an increased activation of the contralateral primary SMC might play a critical role, at least in some phases of the disease, in counteracting MS‐related structural damage agrees with findings from fMRI studies in adult MS, which described an increased recruitment of this area in patients with clinically isolated syndromes (CIS) suggestive of MS [Filippi et al., 2004; Rocca et al., 2003a], and in those with RRMS without overt clinical disability [Rocca et al., 2002]. It is worth noting that in these studies [Filippi et al., 2004; Rocca et al., 2002, 2003a], increased activation of the contralateral primary SMC was, however, not observed in isolation, but it occurred concomitantly to the recruitment of additional areas of the “classical” sensorimotor network, mainly located in the dominant (left) cerebral hemisphere. Conversely, in more disabled patients with RRMS and in those with SPMS, a more bilateral pattern of movement‐associated activations has been described [Reddy et al., 2002; Rocca and Filippi, 2007; Rocca et al., 2003b]. Combined with the results of a cross‐sectional study, which compared the brain patterns of activations between adult MS patients at different stages of the disease [Rocca et al., 2005a], these findings suggest that there might be a hierarchy in the adaptive response of the cerebral cortex to the underlying structural damage of MS, characterized, at the beginning of the disease, by an increased activation of areas selectively devoted to the performance of a given task (the contralateral primary SMC in case of motor performance), and later on, by a bilateral activation of the same areas, as well as by the progressive recruitment of additional, more distant areas [Rocca et al., 2005a]. In this context, our findings would suggest a relative preservation of the adaptive properties of the cerebral cortex in pediatric MS patients, which might be among the factors responsible for their favorable clinical evolution at short/medium term. The notion that the maintenance of a “selective” and strictly lateralized pattern of movement‐associated brain activations is associated with a better clinical prognosis is also supported by findings in CIS patients not experiencing an evolution to definite MS after 1 year [Rocca et al., 2005b], who showed more significant activations of several areas part of the “classical” motor network in comparison to those who went on to develop MS. Conversely, in evolving CIS patients [Rocca et al., 2005b], as well as in patients at high risk of developing Alzheimer's disease [Bookheimer et al., 2000], a widespread pattern of cortical recruitment has been related to an unfavorable clinical evolution. The concept that over‐recruitment of a given network might represent a negative prognostic factor for subsequent clinical evolution is also supported by studies on stroke patients [Calautti and Baron, 2003] and MS patients with previous motor relapses [Mezzapesa et al., 2008], in whom a persistent over‐activation of the undamaged cerebral hemisphere has been associated with an absent or only poor clinical recovery.
The results of the activation analysis are strengthened by those obtained from the assessment of functional connectivity. Studies of sensorimotor system functional connectivity in MS patients are still scanty. A study in clinically unimpaired adult patients with RRMS has demonstrated an increased connectivity between the L SMA and the L primary SMC and between the R SMC and the R cerebellum [Rocca et al., 2007]. Another preliminary study of patients with early RRMS has shown an abnormal correlation between signal intensity changes in the cerebellum and the premotor and motor cortices [Saini et al., 2004]. In contrast to what has been observed in adult patients [Rocca et al., 2007; Saini et al., 2004], this study shows that pediatric MS patients have a reduced functional connectivity between several movement‐associated areas of the left cerebral hemisphere (including the SMA, the thalamus, and the insula) and the left SII, as well as between the left primary SMC and the left thalamus, and between this latter structure and the left insula. Since these patients did not differ from healthy controls in terms of age, the observed fMRI changes cannot be attributed to a different maturation of the sensorimotor network in the two study groups. Conversely, such changes are likely to reflect mechanisms of brain reorganization occurring in pediatric MS patients. Recent studies in healthy individuals have suggested that there might be an enhancement of shorter connections in local networks and a reduction of longer connections with aging [Rowe et al., 2006], indicating a potential adaptive role of increased functional short‐range connectivity with CNS damage. In this perspective, the finding of a sort of “downregulation” of these connections in pediatric MS patients might be considered as a functional reservoir, which might play a role later on in the course of the disease, when structural damage begins to accumulate at a faster pace. This, however, albeit intriguing, remains a speculation since we cannot exclude that our findings might reflect a local dysregulation of motor system circuits, due to the presence of lesions located in critical regions of the movement‐associated brain network. In this study, we quantified the extent of T2‐visible lesions in two of the major white matter (WM) fiber bundles related to the performance of the investigated task, that is, the CST and the CC, and we found no relation between these measures and those of abnormal functional connectivity. Combined with the finding of a correlation between the activity of the left primary SMC and the extent of brain T2 visible lesions, these results would suggest that the overall accumulation of macroscopic lesion burden, rather than involvement of selected WM regions, might have a role in modulating the observed fMRI changes. However, we cannot rule out that damage of other motor‐related WM fiber bundles as well as the presence of microscopic damage in the CST and CC, which were not assessed in this study, might contribute to explain our findings. Indeed, a recent voxel‐based morphometry study [Mesaros et al., 2008] described a selective damage of the thalamus in pediatric MS patients, and the majority of the abnormal connections we found may have the thalamus as a relay station. Other factors that have been shown to influence brain recruitment in adult MS patients, including the extent of normal‐appearing white and gray matter damage as well as the severity of spinal cord involvement, are likely to have had only a minimal influence on our findings, since they have been demonstrated to be modest, if not absent, in pediatric MS patients [Mezzapesa et al., 2004; Tortorella et al., 2006].
In conclusion, this study suggests that the maintenance of a selective and strictly lateralized pattern of movement‐associated brain activations may yet be an additional factor associated to the short/medium term favorable evolution of pediatric MS patients. The role of the modulation of functional connections of this network in these patients deserves further investigations, which should consider studying younger patients to evaluate age‐related changes of the characteristics of these connections, as well as adult patients with a disease onset in childhood to assess whether they might represent a functional reservoir during disease course. Our findings also call for longitudinal multiparametric studies to confirm that a different functional reorganization of the neuronal wiring of the pediatric MS brain is clinically relevant.
REFERENCES
- Balassy C,Bernert G,Wöber‐Bingöl C,Csapó B,Kornek B,Széles J,Fleischmann D,Prayer D ( 2001): Long‐term MRI observations of childhood‐onset relapsing‐remitting multiple sclerosis. Neuropediatrics 32: 28–37. [DOI] [PubMed] [Google Scholar]
- Banwell B,Ghezzi A Bar‐Or A, Mikaeloff Y,Tardieux M ( 2007a): Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol 6: 887–902 (Review). [DOI] [PubMed] [Google Scholar]
- Banwell B,Shroff M,Ness JM,Jeffery D,Schwid S,Weinstock‐Guttman B,International Pediatric MS Study Group ( 2007b): MRI features of pediatric multiple sclerosis. Neurology 68: S46–S53. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY,Strojwas MH,Cohen MS,Saunders AM,Pericak‐Vance MA,Mazziotta JC,Small GW ( 2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C,Baron JC ( 2003): Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke 34: 1553–1566. [DOI] [PubMed] [Google Scholar]
- Confavreux C,Vukusic S,Adeleine P ( 2003): Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 126: 770–782. [DOI] [PubMed] [Google Scholar]
- Ebner F,Millner MM,Justich E ( 1990): Multiple sclerosis in children: Value of serial MR studies to monitor patients. AJNR Am J Neuroradiol 11: 1023–1027. [PMC free article] [PubMed] [Google Scholar]
- Filippi M,Rocca MA,Mezzapesa DM,Ghezzi A,Falini A,Martinelli V,Scotti G,Comi G ( 2004): Simple and complex movement‐associated functional MRI changes in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Hum Brain Mapp 21: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Poline JB,Grasby PJ,Williams SC,Frackowiak RS,Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Price CJ,Büchel C,Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Harrison L,Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Krupp LB,Banwell B,Tenembaum S,International Pediatric MS Study Group ( 2007): Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 68: S7–S12 (Review). [DOI] [PubMed] [Google Scholar]
- Kurtzke JF ( 1983): Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33: 1442–1452. [DOI] [PubMed] [Google Scholar]
- Mesaros S,Rocca MA,Absinta M,Grezzi A,Milani N,Moiola L,Veggiotti P,Comi G,Filippi M ( 2008): Evidence of thalamic gray matter loss in pediatric multiple sclerosis. Neurology 70: 1107–1112. [DOI] [PubMed] [Google Scholar]
- Mezzapesa DM,Rocca MA,Falini A,Rodegher ME,Ghezzi A,Comi G,Filippi M ( 2004): A preliminary diffusion tensor and magnetization transfer magnetic resonance imaging study of early‐onset multiple sclerosis. Arch Neurol 61: 366–368. [DOI] [PubMed] [Google Scholar]
- Mezzapesa DM,Rocca MA,Rodegher M,Comi G,Filippi M ( 2008): Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp 29: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S ( 2005): MRI Atlas of Human White Matter. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Penny WD,Stephan KE,Mechelli A,Friston KJ ( 2004): Comparing dynamic causal models. Neuroimage 22: 1157–1172. [DOI] [PubMed] [Google Scholar]
- Reddy H,Narayanan S,Woolrich M,Mitsumori T,Lapierre Y,Arnold DL,Matthews PM ( 2002): Functional brain reorganization for hand movement in patients with multiple sclerosis: Defining distinct effects of injury and disability. Brain 125: 2646–2657. [DOI] [PubMed] [Google Scholar]
- Renoux C,Vukusic S,Mikaeloff Y,Edan G,Clanet M,Dubois B,Debouverie M,Brochet B,Lebrun‐Frenay C,Pelletier J,Moreau T,Lubetzki C,Vermersch P,Roullet E,Magy L,Tardieu M,Suissa S,Confavreux C,Adult Neurology Departments KIDMUS Study Group ( 2007): Natural history of multiple sclerosis with childhood onset. N Engl J Med 356: 2603–2613. [DOI] [PubMed] [Google Scholar]
- Rocca MA,Filippi M ( 2007): Functional MRI in multiple sclerosis. J Neuroimaging 17: 36S–41S (Review). [DOI] [PubMed] [Google Scholar]
- Rocca MA,Falini A,Colombo B,Scotti G,Comi G,Filippi M ( 2002): Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann Neurol 51: 330–339. [DOI] [PubMed] [Google Scholar]
- Rocca MA,Mezzapesa DM,Falini A,Ghezzi A,Martinelli V,Scotti G,Comi G,Filippi M ( 2003a): Evidence for axonal pathology and adaptive cortical reorganization in patients at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 18: 847–855. [DOI] [PubMed] [Google Scholar]
- Rocca MA,Gavazzi C,Mezzapesa DM,Falini A,Colombo B,Mascalchi M,Scotti G,Comi G,Filippi M ( 2003b): A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage 19: 1770–1777. [DOI] [PubMed] [Google Scholar]
- Rocca MA,Colombo B,Falini A,Ghezzi A,Martinelli V,Scotti G,Comi G,Filippi M ( 2005a): Cortical adaptation in patients with MS: A cross‐sectional functional MRI study of disease phenotypes. Lancet Neurol 4: 618–626. [DOI] [PubMed] [Google Scholar]
- Rocca MA,Mezzapesa DM,Ghezzi A,Falini A,Martinelli V,Scotti G,Comi G,Filippi M ( 2005b): A widespread pattern of cortical activations in patients at presentation with clinically isolated symptoms is associated with evolution to definite multiple sclerosis. AJNR Am J Neuroradiol 26: 1136–1139. [PMC free article] [PubMed] [Google Scholar]
- Rocca MA,Pagani E,Absinta M,Valsasina P,Falini A,Scotti G,Comi G,Filippi M ( 2007): Altered functional and structural connectivities in patients with MS: A 3‐T study. Neurology 69: 2136–2145. [DOI] [PubMed] [Google Scholar]
- Rowe JB,Siebner H,Filipovic SR,Cordivari C,Gerschlager W,Rothwell J,Frackowiak R ( 2006): Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage 32: 747–760. [DOI] [PubMed] [Google Scholar]
- Saini S,De Stefano N,Smith S,Guidi L,Amato MP,Federico A,Matthews MP ( 2004): Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 75: 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone IL,Carrara D,Tortorella C,Liguori M,Lepore V,Pellegrini F,Bellacosa A,Ceccarelli A,Pavone I,Livrea P ( 2002): Course and prognosis in early‐onset MS: Comparison with adult‐onset forms. Neurology 59: 1922–1928. [DOI] [PubMed] [Google Scholar]
- Sindern E,Haas J,Stark E,Wurster U ( 1992): Early onset MS under the age of 16: Clinical and paraclinical features. Acta Neurol Scand 86: 280–284. [DOI] [PubMed] [Google Scholar]
- Smith SM,De Stefano N,Jenkinson M,Matthews PM ( 2001): Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25: 466–475. [DOI] [PubMed] [Google Scholar]
- Tortorella P,Rocca MA,Mezzapesa DM,Ghezzi A,Lamantia L,Comi G,Filippi M ( 2006): MRI quantification of gray and white matter damage in patients with early‐onset multiple sclerosis. J Neurol 253: 903–907. [DOI] [PubMed] [Google Scholar]
- Ward NS ( 2006): The neural substrates of motor recovery after focal damage to the central nervous system. Arch Phys Med Rehabil 87: S30–S35. [DOI] [PubMed] [Google Scholar]


