Abstract
The language profile of patients suffering from Alzheimer's disease (AD) is characterized not only by lexicosemantic impairments but also by phonological deficits, as shown by an increasing number of neuropsychological studies. This study explored the functional neural correlates underlying phonological and lexicosemantic processing in AD. Using H215O PET functional brain imaging, a group of mild to moderate AD patients and a group of age‐matched controls were asked to repeat four types of verbal stimuli: words, wordlike nonwords (WL+), non‐wordlike nonwords (WL−) and simple vowels. The comparison between the different conditions allowed us to determine brain activation preferentially associated with lexicosemantic or phonological levels of language representations. When repeating words, AD patients showed decreased activity in the left temporo‐parietal and inferior frontal regions relative to controls, consistent with distorted lexicosemantic representations. Brain activity was abnormally increased in the right superior temporal area during word repetition, a region more commonly associated with perceptual‐phonological processing. During repetition of WL+ and WL− nonwords, AD patients showed decreased activity in the middle part of the superior temporal gyrus, presumably associated with sublexical phonological information; at the same time, AD patients showed larger activation than controls in the inferior temporal gyrus, typically associated with lexicosemantic levels of representation. Overall, the results suggest that AD patients use altered pathways to process phonological and lexicosemantic information, possibly related to a progressive loss of specialization of phonological and lexicosemantic neural networks. Hum Brain Mapp 2009. © 2007 Wiley‐Liss, Inc.
Keywords: Alzheimer's disease, positron emission tomography, language, semantic, phonology
INTRODUCTION
Alzheimer's disease (AD) is a common degenerative disorder characterized by a progressive decline of multiple cognitive functions. Language is among the functions impaired in the most early stages of AD. Many studies have found AD patients to have difficulties with tasks involving lexicosemantic knowledge such as picture naming, semantic categorization, or semantic priming, and performance on these tasks typically decline with progress of disease [Garrard et al., 2001; Giffard et al., 2002]. Although phonological processing was initially thought to be preserved in AD, mainly on the basis of spontaneous speech reports [Nicholas et al., 1985], more detailed assessments have reported impaired phonological abilities in even mild AD patients. For instance, consistent difficulties have been described in phoneme discrimination, letter fluency, or single nonword repetition tasks [Croot et al., 2000; Glosser et al., 1997, 1998].
While a number of neuroimaging studies have explored phonological and lexicosemantic processing in healthy young populations in considerable detail [Vigneau et al., 2006], relatively few have investigated the neural substrates of language processing in AD patients. With respect to lexicosemantic deficits in AD, neuroimaging studies using positron emission tomography (PET) or structural magnetic resonance imaging (MRI) techniques have investigated correlations between behavioral performance on semantic tasks and brain metabolism or structure in AD patients. These studies have shown that the extent of the semantic impairment correlates with reduced resting state brain metabolism or cortical atrophy located in the left lateral temporal, inferior parietal, or medial occipital areas [Desgranges et al., 1998; Grossman et al., 2003; Hirono et al., 2001; Zahn et al., 2004]. Furthermore, an fMRI study investigated semantic processing in AD patients by measuring hemodynamic brain responses during a pleasantness judgment task for printed words [Grossman et al., 2003]. The results confirmed that AD patients showed significantly less recruitment of the left temporoparietal, lateral frontal, and occipital areas during the task, while at the same time there was greater recruitment of the left inferior temporal cortex, relative to healthy elderly subjects. Few data are available regarding the neural bases of phonological processing in AD. Harasty et al. [2001] documented two cases of autopsy‐confirmed severe AD patients, one with prominent phonological disturbances and the other with more typical semantic deficits. Their results showed that the patient with a selective phonological deficit had a severe neuronal loss in the vicinity of Broca's area (area 45) and the anterior and posterior insula, as well as a lesion in the superior temporal gyrus. The authors interpreted these data as suggesting that the neuronal atrophy of the insula, together with the loss of receptive phonological processing capacities in the superior temporal gyrus, may have crucially impaired the linking, coordinating, and controlling aspects of phonological production.
Two limitations, however, make the interpretation of these findings uncertain. First, many of the studies mentioned earlier used correlation analyses between language performance measured outside the scanner and resting brain metabolism. It is possible that elderly controls and AD patients are differentially engaged in semantic processing during the resting state, and thus the correlations observed could reflect this differential engagement in semantic processing during resting state rather than genuine hypometabolism of the semantic processing areas [Binder et al., 1999]. Therefore, activation studies, in which the brain activity of participants is measured while directly performing a precise cognitive task represent a more direct approach to investigate the specific brain regions used by AD patients when they are actively processing phonological or lexicosemantic material. A further concern is the fact that the language tasks used in the aforementioned studies involved metalinguistic decision processes (e.g., pleasantness judgments). The hypometabolic areas highlighted in these studies could be related, at least partially, to the decline in metalinguistic rather than basic linguistic cognitive performance. Consequently, it is important to specifically investigate the neural substrates of phonological and lexicosemantic representations in AD patients by eliminating, as far as possible, the intervention of metalinguistic processes, such as decision, evaluation, or monitoring processes.
In activation studies, phonological and lexicosemantic representations can be accessed by contrasting the pattern of brain activity when processing word and nonword stimuli in very basic tasks, such as passive listening or immediate repetition. Immediate repetition paradigms have in fact been extensively used in psycholinguistic research to explore the structure of phonological and lexical representations. These studies have typically shown faster and more accurate performance for the repetition of words than for nonwords [the “lexicality effect”; Hulme et al., 1991; Vitevitch and Luce, 1998; Vitevitch et al., 1999]. This advantage has been attributed to the recruitment of lexicosemantic knowledge during word but not nonword repetition. Most interestingly, even repetition performance for nonword lists is influenced by the activation of sublexical language knowledge; this knowledge concerns the statistical properties of possible phoneme co‐occurrences in a given language. Indeed, repetition of lists of nonwords containing phoneme co‐occurrences that are frequent in native language phonology (highly wordlike stimuli) is faster and more accurate than repetition of nonwords composed of infrequent phoneme combinations (less wordlike stimuli) [see Gathercole et al., 1999; Majerus et al., 2004; Vitevitch and Luce, 1998 for a description of this “phonotactic frequency effect”]. At a behavioral level, these lexicality and phonotactic frequency effects have been reported to be relatively preserved in AD [Peters et al., 2007]. However, this does not necessarily imply that the neural networks that subserve the processing of words and nonwords do not differ between AD patients and healthy elderly subjects.
In the present PET study, we explored this issue by administering single word and nonword repetition conditions to AD patients and age‐matched control participants; the nonwords were of two types: highly wordlike or less wordlike. Brain activation, measured during repetition for the three stimulus conditions, was compared to explore the neural substrates related to lexicosemantic and phonological levels of representation. In addition, random effect statistical analyses were used in order to ensure intersubject consistency of results. On the basis of studies in healthy young participants, we know that lexicosemantic levels of language processing (e.g., when comparing word to nonword processing) involve the activation of mainly left‐sided regions in the middle and inferior temporal cortices, the inferior parietal (angular) cortex, temporoparietal junction, and the inferior frontal and dorsal prefrontal regions [Binder et al., 1996, 1997; Demonet et al., 1992, 1994; Howard et al., 1992; Majerus et al., 2002; Perani et al., 1996; Price et al., 1996; Scott et al., 2000]. By contrast, brain regions underlying (sublexical) phonological levels of language processing are primarily located in the left (or bilateral) superior temporal area, and more specifically in the superior temporal sulcus and the posterior superior temporal gyrus; these regions are activated when participants listen to both meaningful and meaningless speech (e.g., nonwords), but not when listening to acoustically matched nonverbal sounds [Binder, 2000; Binder and Price, 2001; Binder et al., 2000; Burton et al., 2000, 2005; Demonet et al., 1992, 1994; Hickok and Poeppel, 2000; Jacquemot et al., 2003; Jancke et al., 2002; Majerus et al., 2002; Mazoyer et al., 1993; Mummery et al., 1999; Perani et al., 1996; Poldrack et al., 2001; Specht and Reul, 2003]. In our study, on the basis of available neuroimaging studies that have studied language processing in AD, we expected decreased activation in left temporoparietal areas, as well as in the left lateral frontal cortex when AD patients had to repeat words and activate lexicosemantic levels of language representation [Desgranges et al., 1998; Grossman et al., 2003, 2004; Zahn et al., 2004]. With respect to phonological processing, we expected decreased activity (compared to elderly controls) in the posterior superior temporal gyrus and Broca's area when AD patients had to repeat nonwords, in line with previous structural neuroanatomical findings [Harasty et al., 2001].
MATERIALS AND METHODS
Subjects
A group of 10 patients suffering from AD (8 women and 2 men; aged 63–82 years) and a group of 12 healthy elderly controls matched for age (10 women and 2 men; aged 62–85 years) and for educational level (number of years of education; AD group's mean: 10.8; control group mean: 11.33; t(1, 20) = 0.38, P > 0.05) gave their written informed consent to take part in this study, which was approved by the Ethics Committee of the Faculty of Medicine of the University of Liège and conforms with “The Code of Ethics of the World Medical Association” (Declaration of Helsinki). All subjects were native French‐speakers and had normal auditory acuity as measured by tonal audiometry testing. The diagnosis of AD was established by an experienced neurologist (E.S.) based on a semistructured interview with the patient and a relative, Mini‐Mental State Examination (AD group mean: 18.1), and neurological and neuropsychological examinations, according to National Institute of Neurological and Communicative Disorders and Stroke/AD and Related Disorders Association criteria [McKhann et al., 1984]. Structural imaging showed mild cerebral atrophy only, and a few patients had a mild degree of leukoaraiosis. The elderly volunteers had no history of alcohol abuse, psychotropic drug use, or psychiatric disorders. They were recruited on a voluntary basis and received a small hourly fee for compensation. The Dementia Rating Scale [Mattis, 1973] was administered to all subjects. Each control subject performed above the cutoff score of 130 at the Mattis scale (AD group mean: 117.7 (100–129); control group: 142.2 (134–144)). Lastly, the mean duration of disease in AD group was 3.5 years.
Cognitive Tasks
In this study, four different conditions were administrated to the subjects: word condition [W], highly wordlike nonword condition [WL+], less wordlike nonword [WL−], and a reference (baseline) condition [vow]. In the reference condition, in order to minimize the amount of verbal content, subjects were simply asked to repeat pairs of identical vowels. Four vowels were used: /a/‐/a/, /i/‐/i/, /o/‐/o/, and /u/‐/u/. For the other three conditions, three lists of disyllabic stimuli were created: 50 words, 50 highly wordlike nonwords, and 50 less wordlike nonwords. Each stimulus had the same consonant (C) –vowel (V) structure: CVCCVC. The WL− nonwords were constructed using CV and VC diphones that are quite rare in French, and had no lexical neighbors1 (e.g., in the nonword
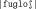 the diphones
the diphones 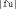 ,
, 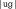 ,
, 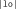 , and
, and 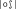 are not very frequent in French, and this nonword has no lexical neighbor in French, see Table I and Appendix A). On the other hand, the WL+ nonwords contained CV and VC diphones that are quite frequent and, whereas the mean number of lexical neighbors was also very low in WL+ nonword condition, and this was significantly greater than in the WL− nonwords condition (e.g., in the nonword
are not very frequent in French, and this nonword has no lexical neighbor in French, see Table I and Appendix A). On the other hand, the WL+ nonwords contained CV and VC diphones that are quite frequent and, whereas the mean number of lexical neighbors was also very low in WL+ nonword condition, and this was significantly greater than in the WL− nonwords condition (e.g., in the nonword 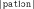 the diphones
the diphones 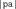 ,
, 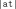 ,
, 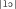 , and are very frequent in French, and this nonword has two lexical neighbors: “patronne” and “pateline”). The diphone frequencies were taken from a phonetic database of French created by Tubach and Boë [1990]. This database was developed on the basis of a phonetic transcription of formal and informal conversations between French‐speaking subjects. There was a significant difference in summed diphone frequency counts for the syllables of the wordlike+ versus wordlike‐nonwords (4444.7 vs. 297.1), F(1, 147) = 91.4, P < 0.0001, but no difference in summed diphone frequency counts for the syllables of wordlike+ nonwords versus words (4444.7 vs. 4530.7), F(1, 147) = 0.46, P > 0.05. The stimuli were recorded by a trained native French speaker. All stimuli were spoken in isolation. The stimuli were digitized at a sampling rate of 22,050 Hz. All stimuli were edited into individual files and stored on a computer disk. There were no significant differences in mean stimulus duration for the different stimulus lists: words vs. WL+ nonwords (1,291 vs. 1,294 ms), F(1, 147) < 1, P = 0.84, and WL+ vs. WL− nonwords (1,294 vs. 1,290 ms), F(1, 147) < 1, P = 0.80. Stimulus presentation was controlled via E‐Prime beta software (Psychology Software Tools, 2000) installed on a PC computer.
, and are very frequent in French, and this nonword has two lexical neighbors: “patronne” and “pateline”). The diphone frequencies were taken from a phonetic database of French created by Tubach and Boë [1990]. This database was developed on the basis of a phonetic transcription of formal and informal conversations between French‐speaking subjects. There was a significant difference in summed diphone frequency counts for the syllables of the wordlike+ versus wordlike‐nonwords (4444.7 vs. 297.1), F(1, 147) = 91.4, P < 0.0001, but no difference in summed diphone frequency counts for the syllables of wordlike+ nonwords versus words (4444.7 vs. 4530.7), F(1, 147) = 0.46, P > 0.05. The stimuli were recorded by a trained native French speaker. All stimuli were spoken in isolation. The stimuli were digitized at a sampling rate of 22,050 Hz. All stimuli were edited into individual files and stored on a computer disk. There were no significant differences in mean stimulus duration for the different stimulus lists: words vs. WL+ nonwords (1,291 vs. 1,294 ms), F(1, 147) < 1, P = 0.84, and WL+ vs. WL− nonwords (1,294 vs. 1,290 ms), F(1, 147) < 1, P = 0.80. Stimulus presentation was controlled via E‐Prime beta software (Psychology Software Tools, 2000) installed on a PC computer.
Table I.
Mean summed diphone frequency counts, mean number of lexical neighbors, and stimulus duration for the word and nonword lists used
| Summed diphone frequencya | Mean number of lexical neighborsb | Mean spoken duration (ms) | |
|---|---|---|---|
| Words | 4530.7 | 1.21 | 1290.6 |
| Wordlike + nonwords | 4444.7 | 0.16 | 1294.7 |
| Wordlike − nonwords | 297.1 | 0.00 | 1291.9 |
Diphone frequency counts are derived from a database of French phonology by Tubach and Boe [1990].
Mean number of lexical neighborhood are derived from “Lexique 2,” a french lexical database by New et al. [in press].
The stimuli were presented through headphones at a comfortable amplitude output level (∼80 dB). Moreover, practice trials for each stimulus condition were administered before the start of the experimental session in order to familiarize the participants with the task requirements. During the experiment, each subject was given two blocks of 25 words, two blocks of 25 WL+ nonwords, two blocks of 25 WL− nonwords, and two blocks of a vowel repetition condition. Before each block, subjects were told which type of stimulus they would hear and were instructed to repeat the stimuli as quickly and accurately as possible. The subjects were explicitly informed about the nature of the stimuli they would hear (and two examples were given to them) in order to avoid lexical search processes when presenting nonwords at a time when they might have been expecting to hear words. In each block, the stimuli were presented at a rate of one item every 4 s. The order of presentation of the blocks and of the stimuli within each block was pseudorandomized. Behavioral data were collected by recording the subjects' responses via a digital‐to‐analogue tape recorder connected to a microphone; response latencies (time between the onset of the stimulus and the onset of the subjects' responses) were recorded via a second microphone, installed 10 cm from the participants head, and connected to E‐Prime software. The percentages of correct responses and the mean reaction times were calculated for each block and then averaged for each list condition. Lastly, we also computed the proportion of four different types of errors: phonological errors (responses that share 50% or more of their phonemes with the corresponding target item), neologisms (responses sharing less than 50% of phonemes with the target item), lexicalizations (the target nonword being replaced by a real word), and omissions (absence of oral response).
PET Scanning
PET data were acquired on a Siemens CTI 951 R 16/31 scanner in 3D mode. The subject's head was stabilized by a thermoplastic facemask secured to the head holder (TruScan Imaging, Annapolis, MD), and a venous catheter was secured in a left antebrachial vein. First, a 20‐min. transmission scan was acquired for attenuation correction using three rotating sources of 68Ge. Then, regional cerebral blood flow, taken as a marker of local neuronal activity [Jueptner and Weiller, 1995], was estimated during eight emission scans. Subjects were scanned with eyes closed and room light dimmed. Each scan consisted of two frames: a 30‐s background frame and a 90‐s acquisition frame. The slow intravenous radiolabeled water (HO) infusion began 10 s before starting the second frame. Six mCi (222 MBq) in 5 ml saline was injected over a period of 20 s for each scan. The infusion was totally automated in order not to disturb the subject during the scanning period. Data were reconstructed using a Hanning filter (cutoff frequency: 0.5 cycles/pixel) and corrected for attenuation and background activity. Each experimental task (block) corresponded to one acquisition and started 10 s before the second frame.
Data Analysis
Data were transformed and analyzed using SPM2 software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) implemented in MATLAB (Mathworks, Sherborn, MA). First, spatial transformations were performed to accommodate within‐subject head movement in scan replications and intersubject differences in gyral and functional anatomy. For each subject, all scans were realigned together, and then normalized to a standard PET template [Friston et al., 1995]. Spatial registration and normalization of images conform to the space defined by the ICBM‐NIHP‐20 project, and approximate that of the space described in Talairach and Tournoux's stereotaxic atlas [Talairach and Tournoux, 1988]. Finally, PET images were smoothed using a Gaussian kernel of 16‐mm full width at half maximum.
Statistical analyses on imaging data were conducted using the random effect model (Holmes and Friston, 1998). The random effect model is a two‐step procedure, based on the approach using mean summary statistics on repeated measures [Frison and Pocock, 1992] applied to accommodate both interindividual and intraindividual variability in PET data. It requires all members of the population to show this effect, such that its expectation is greater than under the null hypothesis. At the first step, PET data were analyzed separately at a within‐subject level using standard subtraction analyses. Because a separable model is required to allow subsequent modeling of the between‐subject variance, the adjustment for one subject was made independent from other subjects by using an ANCOVA adjustment of the global activity. In addition, the mean accuracy score for each stimulus condition was entered as a covariate in the model in order to control for possible differences in response accuracy between word and nonword conditions, and between AD and control groups [Glosser et al., 1997].
At this step, the comparison of brain activity between the different stimulus conditions allowed us to isolate brain regions preferentially associated with lexicosemantic or phonological levels of representation in AD. For lexicosemantic knowledge, we performed two contrasts comparing brain activation between the word and nonword repetition conditions in each elderly control and each AD participant separately: word versus WL+ nonword ([W vs. WL+]), word versus WL− nonword ([W vs. WL−]). Then, three other contrasts were performed to highlight the cerebral areas associated to phonological knowledge in each participant: WL+ nonword versus vowel repetition ([WL+ vs. VOW]), WL− nonword versus vowel repetition ([WL− vs. VOW]), and WL+ nonword versus WL− nonword ([WL+ vs. WL−]). The resulting estimates (contrast images) fitted the within‐subject component of the variance and could then be used for a subsequent second‐level analysis, in which the between‐subject variance was taken into account.
At the second level, between‐subject analyses were performed with identical contrasts across stimuli to highlight the brain areas in which activity specifically underlay the processing of each stimulus type within each group: control group: ([ControlW>WL+], [ControlW>WL−], [ControlWL+>VOW], [ControlWL−>VOW], and [ControlWL+>WL−]); and AD group: ([ADW>WL+], [ADW>WL−], [ADWL+>VOW], [ADWL−>VOW], and [ADWL+>WL−]). Finally, we conducted interaction analyses in order to determine which brain regions were differentially activated in the AD and control groups, as a function of stimulus condition ([(ADW>WL+) vs. (ControlW>WL+)], [(ADW>WL−) vs. (ControlW>WL−)], [(ADWL+>VOW) vs. (ControlWL+>VOW)], [(ADWL−>VOW) vs. (ControlWL−>VOW)], and [(ADWL+>WL−) vs. (ControlWL+>WL−)]). For all analyses, the resulting set of voxel values for each contrast constituted a map of the t statistic, SPM(T), thresholded at P ≤ 0.001 (uncorrected). In addition, the mean difference of accuracy scores between list conditions was entered as a covariate in the corresponding contrast in order to control for possible differences in response accuracy between AD and control groups. An inclusive masking procedure was also applied for all interaction analyses. The masking procedure ensured that the resulting map of voxels was restricted to the areas activated for the two separate contrasts entering the interaction term; the inclusive mask was thresholded at P < 0.05. Then, small volume correction (SVC) was applied (using P ≤ 0.05, corrected) on published coordinates for a priori brain regions considered to be involved in either phonological or lexicosemantic processing. For lexicosemantic knowledge, a 10‐mm radius spherical volume was used centered on [(−55, 14, −21); Majerus et al., 2002] for the anterior superior temporal sulcus, [(−54, −48, −6), Davis, 2004] for the posterior middle temporal gyrus, [(−61, −22, −17), Majerus et al., 2002] for the inferior temporal gyrus, [(−47, −83, 25), Binder et al., 2005] for the inferior parietal lobule (angular gyrus), and [(−45, 35, −4), Binder et al., 1996] for the inferior frontal gyrus [Binder, 2000; Binder et al., 1996, 1999, 2005; Demonet et al., 1992, 1994; Howard et al., 1992; Majerus et al., 2002; Perani et al., 1996; Petersen et al., 1988; Price et al., 1996; Scott et al., 2000]. Similarly, a priori regions of interest for phonological levels of representation were based on previous studies of sublexical phonological processing of speech: SVC was centered on [(−60, −4, −10) and (66, −12, 0), Scott et al., 2000] for the bilateral superior temporal sulci and [(−53, −43, 6) and (56 −30 4), Binder et al., 2000] for the bilateral posterior superior temporal gyri [Binder, 2000; Binder and Price, 2001; Binder et al., 2000; Burton et al., 2000, 2005; Demonet et al., 1992, 1994; Hickok and Poeppel, 2000; Jacquemot et al., 2003; Jancke et al., 2002; Majerus et al., 2002; Mazoyer et al., 1993; Mummery et al., 1999; Perani et al., 1996; Poldrack et al., 2001; Specht and Reul, 2003].
RESULTS
Behavioral Performance
We performed a mixed ANOVA with group condition (Controls vs. AD) as between‐subject factor and list condition (W vs. WL+ vs. WL−) as within‐subject factor. First, mean proportion of correct responses was used as a dependent variable. Mean scores are summarized in Table I. The results revealed significant main effects for the group (F(20,1) = 32.2, P < 0.0001; Controls > AD) and list conditions (F(40,2) = 222.9, P < 0.0001; W > WL+ > WL−). In addition, a significant interaction was found (F(40, 2) = 12, P < 0.0001). Tukey's post hoc comparisons showed that performance in the word condition was similar between control and AD groups (P > 0.05), but the control group had higher scores than the AD group for WL+ (P < 0.01) and WL− nonword lists (P < 0.05). As mentioned in the Methods section, these group and condition‐related differences in performance were accounted for in the brain imaging analyses by introducing the behavioral scores as covariates. A second mixed ANOVA was conducted with reaction times as dependent variable. The results revealed a main effect of list condition (F(40, 2) = 56.84; P < 0.05; Words < WL+ = WL−), whereas the main effect of group and the interaction between group and list conditions were not significant (F(1, 20) = 0.001; P> 0.05 and F(2, 40) = 0.789; P > 0.05, respectively), suggesting that reaction times varied across list conditions but similarly in AD and control groups. Lastly, we also performed separate mixed ANOVAs with the proportion of error types as dependent variable (phonological errors, omissions, and neologisms); given the very low proportion of lexicalization errors, these were not included in this analysis. For phonological errors, the results revealed a main effect of group (F(20, 1) = 5.27; P < 0.05; Controls > AD) and a main effect of list conditions (F(40, 2) = 3.89; P < 0.05; WL+ > Words = WL–), but no significant interaction (F(40, 2) = 0.01; P > 0.05). There was also a significant main effect of group (AD > Controls) and a main effect list condition (WL− > Words = WL+) for the proportion of neologisms, but no interaction between group and list conditions. Concerning the proportion of omissions, the analyses revealed a main effect of list conditions (Words > WL+ = WL−) but no effect of group and no significant interaction. Generally, these results indicate that the same profile of errors was observed in both groups of subjects (a predominant proportion of phonological errors over other types of errors), although the responses in AD group were slightly more distorted than in the control group (as evidenced by a slightly greater proportion of neologisms; Table II).
Table II.
Task performance
| AD | Controls | |
|---|---|---|
| Words | ||
| Correct responses | 0.85 (0.06) | 0.96 (0.03) |
| Incorrect responses | ||
| Phonological errors | 0.86 (0.17) | 0.95 (0.06) |
| Omissions | 0.11 (0.13) | 0.05 (0.12) |
| Neologisms | 0.04 (0.08) | 0 (0) |
| Lexicalizations | 0 (0) | 0 (0) |
| Wordlike+ nonwords | ||
| Correct responses | 0.49 (0.12)a | 0.80 (0.11) |
| Incorrect responses | ||
| Phonological errors | 0.95 (0.05) | 0.99 (0.03) |
| Omissions | 0.03 (0.06) | 0.01 (0.03) |
| Neologisms | 0.02 (0.03) | 0 (0) |
| Lexicalizations | 0 (0) | 0 (0) |
| Wordlike− nonwords | ||
| Correct responses | 0.36 (0.14)a | 0.59 (0.13) |
| Incorrect responses | ||
| Phonological errors | 0.85 (0.11) | 0.95 (0.05) |
| Omissions | 0.03 (0.05) | 0.01 (0.02) |
| Neologisms | 0.12 (0.10) | 0.04 (0.05) |
| Lexicalizations | 0 (0) | 0 (0) |
Mean percentage of correct responses (standard deviation) in the repetition tasks as a function of list condition in a group of 12 healthy elderly participants (Controls) and 10 patients with Alzheimer's disease (AD).
Significant group difference (P < 0.05) after Tukey's post hoc multiple comparisons.
Imaging Results
Differential effects
Lexicosemantic contrasts.
For lexicosemantic knowledge, we performed two contrasts comparing brain activation between the word and nonword repetition conditions, separately for the elderly control group and the AD group ([W > WL+], [W > WL−]). In the W > WL+ contrast, the control group showed increased activation in the left angular gyrus. Moreover, contrasting words and WL− nonwords in the control group [W > WL−] revealed increased brain activity in two distinct areas of the left middle temporal gyrus (posterior and anterior parts), in the left inferior temporal gyrus and in the left inferior prefrontal cortex. In the AD group, comparison of brain activation for words and WL+ nonwords did not elicit any significant activation at the statistical thresholds chosen here. However, the right inferior temporal gyrus was significantly more activated in AD patients when comparing the word and WL− nonword conditions (Table III).
Table III.
Differential effects: Lexicosemantic contrasts
| Brain areas | Hemisphere | Cluster | Brodmann area | Z‐value | Stereotaxic coordinates | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Words > wordlike + nonwords | |||||||
| Controls | |||||||
| Angular | L | 38 | 39 | 4.05 | −48 | −80 | 32 |
| AD | |||||||
| No significant activation | |||||||
| Words > wordlike− nonwords | |||||||
| Controls | |||||||
| Inferior prefrontal | L | 26 | 47 | 3.96 | −44 | 38 | −16 |
| Posterior middle temporal | L | 63 | 21 | 3.81 | −42 | −32 | −4 |
| Middle temporal | L | 31 | 21 | 3.61 | −50 | −10 | −18 |
| Inferior temporal | L | 15 | 20 | 3.39 | −64 | −6 | −30 |
| AD | |||||||
| Inferior temporal | R | 202 | 20 | 4.42 | 54 | −14 | −20 |
| Inferior temporal | R | 59 | 20 | 4.36 | 46 | −34 | −20 |
Locus and extent of peak activations after subtraction analysis related to lexicosemantic knowledge: Word Condition minus Nonwords Conditions (wordlike+ nonword and wordlike− nonword repetition) in a group of 12 healthy elderly participants (Controls) and 10 patients with Alzheimer's disease (AD).
Phonological contrasts.
In both groups, comparison of the two nonword conditions relative to the vowel condition [WL+ > Vow, WL− > Vow] revealed increased activity in the anterior and middle portions of the superior temporal gyrus bilaterally and the left temporoparietal junction (see Table III). In the control group, additional activation foci were found in the left inferior prefrontal gyrus, whereas in the AD group, WL+ nonwords [WL+ > Vow] yielded additional activation in the anterior portion of the right middle temporal gyrus. In the control group, the posterior part of the left superior temporal gyrus and the left inferior temporal gyrus were more activated when comparing WL+ and WL− nonwords. In the AD group, the same contrast did not reveal any significant activation at the statistical thresholds chosen (Table IV).
Table IV.
Differential effects: Phonological contrasts
| Brain areas | Hemisphere | Cluster | Brodmann area | Z‐value | Stereotaxic coordinates | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Word‐like+ nonwords > vowels repetition | |||||||
| Controls | |||||||
| Inferior prefrontal | L | 958 | 47 | 3.55 | −44 | 26 | 0 |
| Temporoparietal junction | L | 958 | 22 | 3.78 | −62 | −14 | 10 |
| Superior temporal | L | 958 | 22 | 4.62 | −66 | −10 | 0 |
| Superior temporal | R | 547 | 22 | 4.28 | 65 | −10 | 2 |
| AD | |||||||
| Superior temporal | L | 47 | 22 | 3.46 | −62 | −2 | −2 |
| Superior temporal | R | 77 | 22 | 3.81 | 54 | −12 | 8 |
| Middle temporal | R | 114 | 21 | 3.83 | 58 | 2 | −24 |
| Word‐like− nonwords > vowels repetition | |||||||
| Controls | |||||||
| Inferior prefrontal | L | 58 | 47 | 3.50 | −52 | 18 | −4 |
| Temporoparietal junction | L | 32 | 42/40 | 3.61 | −34 | −28 | 22 |
| Superior temporal | L | 114 | 21/22 | 3.71 | −70 | −14 | −6 |
| Superior temporal | R | 14 | 22 | 3.37 | 62 | −18 | 6 |
| AD | |||||||
| Inferior precentral | L | 204 | 6 | 4.19 | −54 | 8 | 20 |
| Central | L | 183 | 4 | 4.60 | −50 | −8 | 42 |
| Temporoparietal junction | L | 463 | 22 | 4.25 | −44 | −38 | 16 |
| Middle temporal | L | 200 | 21 | 4.53 | −70 | −14 | −12 |
| Superior temporal | R | 172 | 38 | 4.29 | 52 | 6 | −6 |
| Wordlike + nonwords | |||||||
| Controls | |||||||
| Superior temporal | L | 91 | 22 | 4.24 | −50 | −54 | 16 |
| Inferior temporal | L | 86 | 20 | 4.12 | −58 | −18 | −26 |
| AD | |||||||
| No significant activation | |||||||
Locus and extent of peak activations after subtraction analysis related to phonological knowledge: nonword conditions (Wordlike+ and Wordlike− nonwords) minus vowels repetition condition, and Wordlike+ nonword condition minus Wordlike− nonword condition in a group of 12 healthy elderly participants (Controls) and 10 patients with Alzheimer's disease (AD).
Interaction analyses
Lexicosemantic contrasts.
Peak activations of interaction analyses are summarized in Table V. Relative to the control group, the AD group showed decreased activation in a number of language areas including the left temporoparietal junction, the left superior and middle temporal gyri, and the left inferior frontal gyrus [(AD < Control) for (W > WL−)]. AD patients also showed additional decrease of activation in the right inferior precentral gyrus compared to controls [(AD < Control) for (W > Vow)]. Conversely, AD patients activated more the right superior temporal gyrus than controls when the word condition was compared to the WL+ nonword condition [(AD > Control) for (W > WL+); Table V, Fig. 1].
Table V.
Interaction effects: Lexicosemantic representations
| Brain areas | Hemisphere | Cluster | Brodmann area | Z‐value | Stereotaxic coordinates | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Words vs. wordlike+ nonwords | |||||||
| AD < Controls | |||||||
| No significant activation | |||||||
| AD > Controls | |||||||
| Superior temporal | R | 51 | 22 | 3.99 | 60 | 4 | −4 |
| Words vs. wordlike− nonwords | |||||||
| AD < Controls | |||||||
| Inferior frontal | L | 56 | 47 | 3.61 | −42 | 38 | 0 |
| Temporoparietal junction | L | 491 | 22/39 | 4.63 | −42 | −34 | 10 |
| Posterior superior temporal | L | “ | 22 | 3.98 | −42 | −36 | 8 |
| Posterior middle temporal | L | “ | 21 | 3.76 | −42 | −32 | −4 |
| AD > Controls | |||||||
| No significant activation | |||||||
Locus and extent of peak activations after interaction analysis: Contrasts of brain activation related to lexicosemantic material (W > WL+ Nonwords; W > WL− Nonwords) in 10 patients with Alzheimer's disease (AD) compared with 12 healthy elderly controls (Controls).
Figure 1.
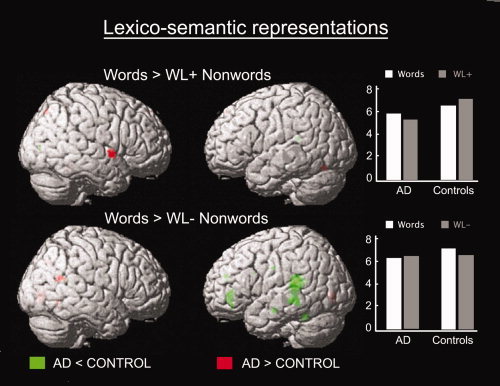
Patterns of activation illustrating interaction effects between group (healthy elderly subjects (Control) vs. Alzheimer's disease patients (AD)) and list conditions associated with processing lexicosemantic material (words > WL+ nonwords, words > WL− nonwords). The resulting set of voxels for each contrast was thresholded at P ≤ 0.001 (uncorrected). Then small volume correction was applied on published coordinates for a priori regions considered to be involved in lexicosemantic processing in healthy young subjects. The interactions illustrating reduced activation in AD patients relative to elderly controls are shown in green, while the interactions illustrating greater activation in AD patients relative to elderly controls are shown in red. On the right side of the figure, mean values of the parameter estimates in the right superior temporal gyrus [60, 4, −4] and the left temporo‐parietal junction [−42, −34, 10] (from up to bottom) are plotted.
Phonological contrasts.
Three interaction analyses were performed to investigate phonological levels of representation [WL+ > Vow; WL− > Vow; WL+ > WL−]. The results indicated decreased brain activity in the AD group relative to the control group in the left superior and middle temporal gyri when comparing Wordlike+ and wordlike‐ nonwords [(AD < Control) for (WL+ > WL−)]. When comparing the WL+ and Vow conditions, the results also showed less activity in the right inferior precentral gyrus in the AD group compared to the control group. At the same time, when AD patients processed WL− nonwords, the task elicited stronger brain activation in the left inferior temporal gyrus than in the control group [(AD > Control) for (WL− > Vow); Table VI, Fig. 2].
Table VI.
Interaction effects: Phonological representations
| Brain areas | Hemisphere | Cluster | Brodmann area | Z‐value | Stereotaxic coordinates | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Wordlike+ nonwords > vowels | |||||||
| AD < Controls | |||||||
| Orbitofrontal gyrus | L | 41 | 11 | 3.61 | −32 | 44 | −22 |
| Inferior precentral gyrus | R | 56 | 43 | 4.14 | 72 | −14 | 20 |
| AD > Controls | |||||||
| No significant activation | |||||||
| Wordlike− nonwords > vowels | |||||||
| AD < Controls | |||||||
| No significant activation | |||||||
| AD > Controls | |||||||
| Inferior temporal | L | 144 | 20 | 3.77 | −62 | −12 | −28 |
| Wordlike+ vs. wordlike− nonwords | |||||||
| AD < Controls | |||||||
| Superior temporal | L | 136 | 22 | 4.03 | −48 | −12 | −4 |
| Middle temporal | L | “ | 21 | 4.12 | −50 | −14 | −12 |
| AD > Controls | |||||||
| No significant activation | |||||||
Locus and extent of peak activations after interaction analysis: Contrasts in brain activation related to phonological material (WL+ Nonwords > Vowels repetition; WL− Nonwords > Vowels repetition; WL+ Nonwords > WL− Nonwords) in 10 patients with Alzheimer's disease (AD) compared with 12 elderly healthy controls (Controls).
Figure 2.
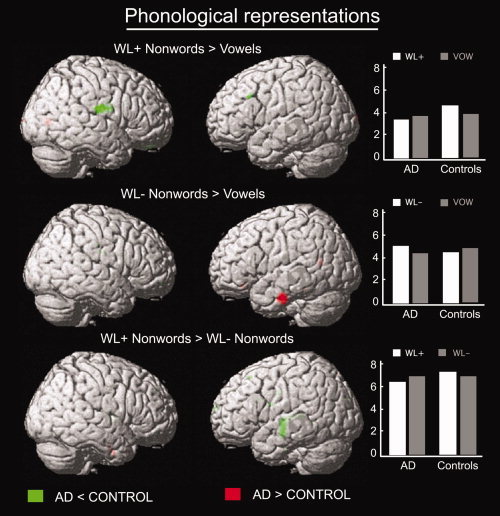
Patterns of activation illustrating interaction effects between group (healthy elderly subjects (Control) vs. Alzheimer's disease patients (AD)) and list conditions associated with processing phonological material (WL+ nonwords > vowels repetition, WL− nonwords > vowels repetition, WL+ nonwords > WL− nonwords). The resulting set of voxels for each contrast was thresholded at P ≤ 0.001 (uncorrected). Then small volume correction was applied on published coordinates for a priori regions considered to be involved in phonological processing in healthy young subjects. The interactions illustrating reduced activation in AD patients relative to elderly controls are shown in green, while the interactions illustrating greater activation in AD patients relative to elderly controls are shown in red. On the right side of the figure, mean values of the parameter estimates in the right precentral gyrus [72, −14, 20] and the left inferior [−62, −12, −28] and superior [−48, −12, −4] temporal gyri (from up to bottom) are plotted.
DISCUSSION
In this study, we investigated the neural substrates of lexicosemantic and phonological processing in AD patients and matched elderly control subjects. We observed differential activation in the language processing areas, mostly in the left hemisphere, as a function of stimulus condition and group, involving, for the AD group relative to the control group, a relative decrease of cerebral activity in the superior temporal lobe during phonological processing, and a relative decrease of activity in more posterior temporal lobe and prefrontal regions during lexicosemantic processing.
Cortical Activation for Lexicosemantic Representations in AD
We observed that elderly controls recruited several areas of the left hemisphere when processing lexicosemantic information: the angular gyrus, the anterior portion of the inferior temporal gyrus, the posterior middle temporal gyrus, and the inferior prefrontal gyrus. These activation patterns are consistent with a number of previous investigations in healthy young subjects comparing words to nonwords or to nonverbal stimuli (e.g., tones or letter strings) [Davis et al., 2004; Fiebach et al., 2002; Price et al., 2003]. Moreover, these areas are also activated during judgment tasks that specifically target semantic processing for verbal or nonverbal stimuli [Binder et al., 1997; Demonet et al., 1994; Pugh et al., 1996; Simos et al., 2002]. In summary, increased cerebral activity in the angular gyrus, inferior temporal gyrus, posterior middle temporal gyrus, and inferior prefrontal gyrus is consistent with the activation of semantic representations, regardless of stimulus modality.
With respect to AD patients, a somewhat different activation profile was observed for word processing. Interaction analyses revealed that a network including the left temporoparietal and inferior frontal regions was significantly less recruited in AD patients than in the control group, when comparing word to WL− nonword conditions. This result is in keeping with previous neuroimaging findings showing a correlation between semantic deficits in AD patients and impairment of left temporoparietal areas, as measured by structural MRI or resting brain metabolism [Desgranges et al., 1998; Grossman et al., 1997, 1998]. As suggested by Grossmann et al. [2003], impaired activity in the left temporoparietal and frontal regions in AD patients could reflect impaired integration of lexicosemantic information, regardless of the domain of knowledge or the nature of the task. In addition, when comparing word to WL+ nonword conditions, interaction analyses revealed enhanced activity in the middle part of the right superior temporal gyrus, in AD patients relative to controls. In healthy young adults, there is accumulating evidence that the right superior temporal gyrus is involved in perceptual–phonological processing, and more specifically that this region responds to increasing spectral complexity of human vocal sounds [Demonet et al., 1992; Johnsrude et al., 2000; Lattner etal., 2005; Okada and Hickock, 2006; Scott et al., 2000; Zatorre and Belin, 2001]. The AD patients' enhanced activity in the right superior temporal gyrus during word repetition could thus be related to abnormal recruitment of phonological information, maybe in an effort to counter less efficient processing of the left temporoparietal and frontal lexicosemantic networks. An alternative explanation that also has to be considered is that the increased activation of the right superior temporal gyrus could reflect the relatively greater difficulty of the word repetition condition for AD patients as compared to elderly controls. Indeed, some studies have shown that increasing task difficulty leads to recruitment of language homologue areas in the right hemisphere [Just et al., 1996; St George et al., 1999; see also Dräger et al., 2004 who did not confirmed these results]. However, task accuracy was similar during word repetition for AD and control participants, a finding that is not consistent with an interpretation in terms of group‐related differences in task difficulty; rather, abnormal activation in the right superior temporal gyrus, in conjunction with preserved levels of performance for repeating words, may be interpreted as reflecting the recruitment of alternative, compensatory lexicosemantic networks during word repetition.
Cortical Activation for Phonological Representations in AD
With respect to phonological processing, the elderly control participants recruited inferior parietal, superior temporal, and inferior prefrontal areas during nonword repetition, as compared to vowel repetition. Previous studies have consistently shown these regions to be involved in phonological processing (Scott et al., 2000; Sekiguchi et al., 2001). More interestingly, we also investigate the wordlikeness effect by comparing wordlike and less wordlike nonwords. We observed a significant increase in activation in the left inferior and posterior superior temporal gyri for WL+ nonwords versus WL− nonwords in elderly control participants. Activation in the left posterior superior temporal area has been observed in many studies exploring phonological processing and access to sublexical phonological representations [e.g., Binder et al., 1999; Majerus et al., 2005; Uppenkamp et al., 2006]. Hence, this activation in elderly controls for WL+ nonwords could be related to the more frequent sublexical sound combinations the WL+ nonwords are made of. However, although all nonwords were not obviously related to existing words (the number of lexical neighbors was very low), we cannot rule out that this activation also reflects lexical processes, given that the WL+ nonwords contained diphones more likely to occur in familiar words than the WL− nonwords. Increased activation in the left inferior temporal, often reported to be involved in lexicosemantic processing, is also in line with this possibility [e.g., Damasio et al., 1996; Vandenberghe et al., 1996]. On the other hand, we must note that actual lexicalization errors were quite rare, suggesting that all participants processed the nonwords as nonwords and not as similarly sounding words. Of course, since very few studies have investigated the neural substrates of the phonotactic frequency effect [for an exception, see Majerus et al., 2002 in which the same verbal material as in the present study was used], and none of them has explored this effect in normal aging, it is also possible that the activation observed in inferior and superior temporal areas is characteristic of nonword processing in elderly individuals. Indeed, when comparing Wordlike+ and Wordlike− nonwords conditions in a group of young participants, Majerus et al. [2002] did not found increased activity in these areas. However, future neuroimaging studies encompassing a direct comparison of the phonotactic frequency effect in young and elderly subjects are necessary to answer to this question.
When turning to the activation profile for phonological processing in AD patients, notable differences between the patient and the control group were observed. AD patients showed less activation in the middle portion of the left superior and middle temporal gyri when processing nonwords (WL+ nonwords > WL− nonwords). This decreased activity in the left superior temporal gyrus is consistent with previous studies reporting postmortem measures of cerebral atrophy in that area for AD patients with phonological disturbances [Harasty et al., 1999, 2001]. In normal young subjects, these brain areas have been related to the processing of sound structures that characterize intelligible speech sounds such as syllables, nonwords, or words [Belin et al., 2002; Binder et al., 2000; Burton et al., 2005; Poeppel et al., 2004; Scott et al., 2000]. It follows that impaired processing for WL+ nonwords in AD patients might be attributed to difficulties in accessing sublexical phonological knowledge processed in the left superior/middle temporal gyrus. In addition, interaction analysis also revealed that AD patients showed decreased activation in the right inferior precentral gyrus compared with control subjects when processing WL+ nonwords (WL+ nonwords > Vowels). The precentral gyrus is known to be involved in articulatory programming in normal young population, and many patients with brain lesions in the precentral areas are characterized by markedly impaired articulation abilities [Dronkers, 1996; Fox et al., 2000; Tanji et al., 2001]. Thus, it seems likely that decreased activation in the right precentral gyrus in AD patients could be related to reduced articulatory programming of the sequences of phonemes that form WL+ nonwords. A finding which might appear to be less consistent with previous studies concerns the absence of decreased activity in Broca's area and associated insular cortex, areas associated with basic phonological and articulatory output processing [Harasty et al., 2001]. However, we should mention that, by comparing repetition of WL+ nonwords with the repetition of WL− nonwords or vowel pairs, areas associated with basic articulatory output processing are supposedly recruited equally by all these conditions, and hence any fundamental difference in activation in these areas between AD patients and controls will be difficult to observe, given our subtractive statistical methods.
A further important difference in brain activity between AD patients and control participants is the observation of stronger activation in the left lateral inferior temporal gyrus in AD patients when processing WL− nonwords relative to single vowels. Interestingly, activation in the left inferior temporal gyrus has often been associated with lexicosemantic retrieval [Thioux et al., 2005]. Severe lexical retrieval deficits, as seen in some cases of AD and Pick's disease, have been associated with lesions confined to the left anterior temporal lobe and the inferior temporal cortex [Damasio et al., 1991; Graff‐Radford et al., 1990; Semenza and Zettin, 1989]. Furthermore, as we already noted, the control subjects activated this same region when they repeated words or WL+ nonwords but not WL− nonwords. In AD patients, despite the increased activity in the left inferior temporal gyrus, their accuracy in WL− nonword repetition was significantly reduced, suggesting that this enhanced activation did not compensate their difficulty in accessing phonological information.
Brain degeneration, such as in AD, may result in a spectrum of different changes in terms of regions or degree of activation in functional neuroimaging studies. These changes may include (1) a loss of activated regions, (2) diminished activation as compared to healthy subjects, (3) enhanced activation of regions in order to compensate for a brain deterioration (with relatively preserved accuracy), and (4) the emergence of inappropriate activated regions, normally not involved in the task, and that does not help to maintain normal levels of behavioral performance [see Cabeza, 2002 for a description of the “dedifferentiation” mechanism]. In the present study, we observed several of those changes in AD patients. On one hand, the neural substrates of lexicosemantic representation in AD patients was characterized by a reduced degree of activation in the temporoparietal junction (“type 2” change), an area that is importantly involved in semantic processing, and at the same time, by a possible compensatory activation in the right superior temporal gyrus (“type 3” change), suggesting that additional processing of the perceptual–phonological attributes of words was taking place in AD patients. On the other hand, the pattern of activation observed in AD patients for phonological representation (decreased performance associated with the recruitment of untypical cerebral areas for the given performance) could be related to the hypothesis of dedifferentiation (“type 4” change), introduced in the context of studies exploring age‐related reorganization of brain functions [Cabeza, 2002; Li and Lindenberger, 1999]. According to this hypothesis, difficulty in accessing specialized neural mechanisms is reflected by the recruitment of inappropriate regions and by a decrement in performance. In AD patients, the problems accessing phonological knowledge could therefore be related to the increase in activity in the left inferior temporal gyrus, which is associated with lexicosemantic knowledge, leading to inefficient processing of nonwords, especially for nonwords that differ maximally from familiar lexical word forms.
Methodological Limitations
Given the highly reduced levels of scanner noise (relative to fMRI), PET scanning can be considered an attractive technology especially for conducting language studies requiring the presentation of auditory verbal information and the collection of verbal responses. At the same time, this technique also entails a number of methodological limitations. In the present study, each scan was acquired for 90 s during which the subjects repeated 25 different items of the same list condition. Unfortunately, by using this type of block design, correct and incorrect responses are confounded during that period, and it is therefore impossible to separate them. One could argue that the incorrect responses added noise to the imaging data, or might even have biased contrast estimation. We should note here that, at the statistical level, we tried to control for differences in accuracy as much as is possible using PET data. We in fact included accuracy scores for each stimulus condition as a covariate in the model, hence controlling for any possible linear relationship between accuracy and brain metabolism. Second, the error analysis showed that most incorrect responses were phonological errors close to the target stimuli (although slightly more distorted in the AD group) and omissions were very rare in each group of participants. This supports the view that subjects were fully engaged in the task, and that task‐irrelevant activation was relatively limited in this study. Finally, producing erroneous responses in repetition task is inherent to the nature of speech repetition tasks and the psycholinguistic processes addressed in producing correct or phonologically related incorrect responses are the same: perceptual–phonological analysis and interfacing with speech motor processes and networks are recruited in all cases, even if the result of these processes might sometimes be faulty. This would be different if the responses are fundamentally different from the target stimulus (i.e., a word produced for a nonword or a nonword produced for a word) or if there are no responses at all. However, as presented in our error analyses, this was not the case and the majority of errors in both participant groups were phonologically related errors close to the target. As a consequence, although in general, it is probably preferable to separate correct and incorrect responses in the imaging analyses (if possible), we believe that the present results reflect task‐related brain activation, independently of response accuracy.
CONCLUSIONS
In summary, although our AD patients showed preserved levels of performance when processing words, they showed markedly different patterns of brain activation relative to controls. On the one hand, decreased activity was observed in the left temporoparietal and inferior frontal regions. This is consistent with the existence of distorted lexicosemantic representations in AD and supports previous studies that have related lesions or hypometabolism of the left temporoparietal regions with lexicosemantic deficits in AD patients. On the other hand, the AD patients' brain activity was abnormally high in the right superior temporal cortices during word repetition, suggesting that additional processing of the perceptual–phonological attributes of words was taking place. For phonological processing (repetition of nonwords), AD patients (relative to elderly controls) showed decreased activation in the superior and middle temporal gyri, in regions classically associated with phonological knowledge in normal young subjects. At the same time, the AD patients showed greater activation than controls in the inferior temporal gyrus, which is closely related to lexicosemantic knowledge. Enhancement of activity in this region may underlie an abnormal recruitment of lexicosemantic areas because of disrupted access to phonological representations. Overall, these data are consistent with the view that patients with neurodegenerative dementia of the AD type use alternative pathways to process phonological and lexicosemantic information, possibly related to a progressive dedifferentiation of phonological and lexicosemantic neural networks.
Acknowledgements
F. Collette and S. Laureys are Senior Research Associates and S. Majerus is Research Associate at FNRS.
Footnotes
Lexical neighborhood was defined as the number of existing words that differed from the nonword by the substitution, deletion, or addition of a single phoneme [e.g., Vitevitch and Luce, 1999].
REFERENCES
- Belin P,Zatorre RJ,Ahad P ( 2002): Human temporal‐lobe response to vocal sounds. Brain Res Cogn Brain Res 13: 17–26. [DOI] [PubMed] [Google Scholar]
- Binder J ( 2000): The new neuroanatomy of speech perception. Brain 123(Part 12): 2371–2372. [DOI] [PubMed] [Google Scholar]
- Binder J,Price C ( 2001): Functional neuroimaging of language In: Cabeza R,Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. Cambridge: MIT Press; pp 187–252. [Google Scholar]
- Binder JR,Frost JA,Hammeke TA,Rao SM,Cox RW ( 1996): Function of the left planum temporale in auditory and linguistic processing. Brain 119(Part 4): 1239–1247. [DOI] [PubMed] [Google Scholar]
- Binder JR,Frost JA,Hammeke TA,Cox RW,Rao SM,Prieto T ( 1997): Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR,Frost JA,Hammeke TA,Bellgowan PS,Rao SM,Cox RW ( 1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11: 80–95. [DOI] [PubMed] [Google Scholar]
- Binder JR,Frost JA,Hammeke TA,Bellgowan PS,Springer JA,Kaufman JN,Possing ET ( 2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Binder JR,Medler DA,Desai R,Conant LL,Liebenthal E. ( 2005): Some neurophysiological constraints on models of word naming. Neuroimage 27(3): 677–693. [DOI] [PubMed] [Google Scholar]
- Burton M,Small S,Blumstein S ( 2000): The role of segmentation in phonological processing: An fMRI investigation. J Cogn Neurosci 12: 679–690. [DOI] [PubMed] [Google Scholar]
- Burton MW,Locasto PC,Krebs‐Noble D,Gullapalli RP ( 2005): A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage 26: 647–661. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Croot K,Hodges JR,Xuereb J,Patterson K ( 2000): Phonological and articulatory impairment in Alzheimer's disease: A case series. Brain Lang 75: 277–309. [DOI] [PubMed] [Google Scholar]
- Damasio H,Kuljis RO,Yuh W,van Hoesen GW,Ehrhardt J ( 1991): Magnetic resonance imaging of human intracortical structure in vivo. Cereb Cortex 1: 374–379. [DOI] [PubMed] [Google Scholar]
- Damasio H,Grabowski TJ,Tranel D,Hichwa RD,Damasio AR ( 1996): A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- Davis MH,Meunier F,Marslen‐Wilson WD ( 2004): Neural responses to morphological, syntactic, and semantic properties of single words: An fMRI study. Brain Lang 89: 439–449. [DOI] [PubMed] [Google Scholar]
- Demonet JF,Chollet F,Ramsay S,Cardebat D,Nespoulous JL,Wise R,Rascol A,Frackowiak R ( 1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115(Part 6): 1753–1768. [DOI] [PubMed] [Google Scholar]
- Demonet JF,Price C,Wise R,Frackowiak RS ( 1994): Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: A positron‐emission tomography study in normal human subjects. Neurosci Lett 182: 25–28. [DOI] [PubMed] [Google Scholar]
- Desgranges B,Baron JC,de la Sayette V,Petit‐Taboue MC,Benali K,Landeau B,Lechevalier B,Eustache F ( 1998): The neural substrates of memory systems impairment in Alzheimer's disease. A PET study of resting brain glucose utilization. Brain 121(Part 4): 611–631. [DOI] [PubMed] [Google Scholar]
- Dräger B,Jansen A,Bruchmann S,Förster AF,Pleger B,Zwitserlood P,Knecht S ( 2004): How does the brain accommodate to increased task difficulty in word finding? A functional MRI study. Neuroimage 23: 1152–1160. [DOI] [PubMed] [Google Scholar]
- Dronkers NF ( 1996): A new brain region for coordinating speech articulation. Nature 384: 159–161. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ,Friederici AD,Müller K,von Cramon DY ( 2002): fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Fox RJ,Kasner SE,Chatterjee A,Chalela JA ( 2000): Aphemia: An isolated disorder of articulation. Clin Neurol Neurosurg 103: 123–126. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Ashburner J,Frith CD,Poline JB,Heather JD,Frackowiak RSJ ( 1995): Spatial registration and normalisation of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Frison L,Pocock SJ ( 1992): Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 11(13): 1685–1704. [DOI] [PubMed] [Google Scholar]
- Garrard P,Lambon Ralph MA,Watson PC,Powis J,Patterson K,Hodges JR ( 2001): Longitudinal profiles of semantic impairment for living and nonliving concepts in dementia of Alzheimer's type. J Cogn Neurosci 13: 892–909. [DOI] [PubMed] [Google Scholar]
- Gathercole SE,Frankish CR,Pickering SJ,Peaker S ( 1999): Phonotactic influences on short‐term memory. J Exp Psychol Learn Mem Cogn 25: 84–95. [DOI] [PubMed] [Google Scholar]
- Giffard B,Desgranges B,Nore‐Mary F,Lalevee C,Beaunieux H,de la Sayette V,Pasquier F,Eustache F ( 2002): The dynamic time course of semantic memory impairment in Alzheimer's disease: Clues from hyperpriming and hypopriming effects. Brain 125(Part 9): 2044–2057. [DOI] [PubMed] [Google Scholar]
- Glosser G,Kohn SE,Friedman RB,Sands L,Grugan P ( 1997): Repetition of single words and nonwords in Alzheimer's disease. Cortex 33: 653–666. [DOI] [PubMed] [Google Scholar]
- Glosser G,Friedman RB,Kohn SE,Sands L,Grugan P ( 1998): Cognitive mechanisms for processing nonwords: Evidence from Alzheimer's disease. Brain Lang 63: 32–49. [DOI] [PubMed] [Google Scholar]
- Graff‐Radford NR,Damasio AR,Hyman BT,Hart MN,Tranel D,Damasio H,Van Hoesen GW,Rezai K ( 1990): Progressive aphasia in a patient with Pick's disease: A neuropsychological, radiologic, and anatomic study. Neurology 40: 620–626. [DOI] [PubMed] [Google Scholar]
- Grossman M,Payer F,Onishi K,White‐Devine T,Morrison D,D'Esposito M,Robinson K,Alavi A ( 1997): Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer's disease. J Neurol Neurosurg Psychiatry 63: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M,Payer F,Onishi K,D'Esposito M,Morrison D,Sadek A,Alavi A ( 1998): Language comprehension and regional cerebral defects in frontotemporal degeneration and Alzheimer's disease. Neurology 50: 157–163. [DOI] [PubMed] [Google Scholar]
- Grossman M,Koenig P,Glosser G,DeVita C,Moore P,Rhee J,Detre J,Alsop D,Gee J ( 2003): Neural basis for semantic memory difficulty in Alzheimer's disease: An fMRI study. Brain 126(Part 2): 292–311. [DOI] [PubMed] [Google Scholar]
- Grossman M,McMillan C,Moore P,Ding L,Glosser G,Work M,Gee J ( 2004): What's in a name: Voxel‐based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain 127(Part 3): 628–649. [DOI] [PubMed] [Google Scholar]
- Harasty JA,Halliday GM,Kril JJ,Code C. ( 1999): Specific temporoparietal gyral atrophy reflects the pattern of language dissolution in Alzheimer's disease. Brain 122(Pt4): 675–686. [DOI] [PubMed] [Google Scholar]
- Harasty JA,Halliday GM,Xuereb J,Croot K,Bennett H,Hodges JR ( 2001): Cortical degeneration associated with phonologic and semantic language impairments in AD. Neurology 56: 944–950. [DOI] [PubMed] [Google Scholar]
- Hickok G,Poeppel D ( 2000): Towards a functional neuroanatomy of speech perception. Trends Cogn Sci 4: 131–138. [DOI] [PubMed] [Google Scholar]
- Hirono N,Mori E,Ishii K,Imamura T,Tanimukai S,Kazui H,Hashimoto M,Takatsuki Y,Kitagaki H,Sasaki M ( 2001): Neuronal substrates for semantic memory: A positron emission tomography study in Alzheimer's disease. Dement Geriatr Cogn Disord 12: 15–21. [DOI] [PubMed] [Google Scholar]
- Holmes AP,Friston KJ ( 1998): Generalisability, random effects and population inference. Neuroimage 7: 754–754. [Google Scholar]
- Howard D,Patterson K,Wise R,Brown WD,Friston K,Weiller C,Frackowiak R ( 1992): The cortical localization of the lexicons. Positron emission tomography evidence. Brain 115(Part 6): 1769–1782. [DOI] [PubMed] [Google Scholar]
- Hulme C,Maughan S,Brown GDA ( 1991): Memory for familiar and unfamiliar words: Evidence for a long term memory contribution to short term memory span. J Mem Lang 30: 685–701. [Google Scholar]
- Jacquemot C,Pallier C,LeBihan D,Dehaene S,Dupoux E ( 2003): Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. J Neurosci 23: 9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L,Wustenberg T,Scheich H,Heinze HJ ( 2002): Phonetic perception and the temporal cortex. Neuroimage 15: 733–746. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS,Penhune VB,Zatorre RJ ( 2000): Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123: 155–163. [DOI] [PubMed] [Google Scholar]
- Jueptner M,Weiller C ( 1995): Review: Does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 2: 148–156. [DOI] [PubMed] [Google Scholar]
- Just MA,Carpenter PA,Keller TA,Eddy WF,Thulborn KR ( 1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Lattner S,Meyer EM,Friederici AD ( 2005). Voice perception: Sex, pitch, and the right hemisphere. Hum Brain Mapp 24: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC,Lindenberger U ( 1999): Cross‐level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age In: Nilsson LG,Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Toronto: Hogrefe and Huber; pp 104–146. [Google Scholar]
- Majerus S,Collette F,Van der Linden M,Peigneux P,Laureys S,Delfiore G,Degueldre C,Luxen A,Salmon E ( 2002): A PET investigation of lexicality and phonotactic frequency in oral language processing. Cogn Neuropsychol 19: 343–360. [DOI] [PubMed] [Google Scholar]
- Majerus S,Van der Linden M,Mulder L,Meulemans T,Peters F ( 2004): Verbal short‐term memory reflects the sublexical organization of the phonological language network: Evidence from an incidental phonotactic learning paradigm. J Mem Lang 51: 297–306. [Google Scholar]
- Majerus S,Van der Linden M,Collette F,Laureys S,Poncelet M,Degueldre C,Delfiore G,Luxen A,Salmon E ( 2005): Modulation of brain activity during phonological familiarization. Brain Lang 92: 320–331. [DOI] [PubMed] [Google Scholar]
- Mattis S ( 1973): Dementia Rating Scale. Windsor: NFER‐Nelson. [Google Scholar]
- Mazoyer BM,Tzourio N,Frak V,Syrota A,Murayama N,Levrier O,Salamon G,Dehaene S,Cohen L,Mehler J ( 1993): The cortical representation of speech. J Cogn Neurosci 5: 467–479. [DOI] [PubMed] [Google Scholar]
- McKhann G,Drachman D,Folstein M,Katzman R,Price D,Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of health and human services task force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Mummery CJ,Ashburner J,Scott SK,Wise RJ ( 1999): Functional neuroimaging of speech perception in six normal and two aphasic subjects. J Acoust Soc Am 106: 449–457. [DOI] [PubMed] [Google Scholar]
- New B,Pallier C,Brysbaert M,Ferrand L: Lexique 2: A new French lexical database. Behav Res Method Instrument Comput 36: 516–524. [DOI] [PubMed] [Google Scholar]
- Nicholas M,Obler LK,Albert ML,Helm‐Estabrooks N ( 1985): Empty speech in Alzheimier's disease and fluent aphasia. J Speech Hearing Res 28: 405–410. [DOI] [PubMed] [Google Scholar]
- Okada G,Hickok H ( 2006): Left posterior auditory‐related cortices participate both in speech perception and speech production: Neural overlap revealed by fMRI. Brain Lang 98: 112–117. [DOI] [PubMed] [Google Scholar]
- Perani D,Dehaene S,Grassi F,Cohen L,Cappa SF,Dupoux E,Fazio F,Mehler J ( 1996): Brain processing of native and foreign languages. Neuroreport 7: 2439–2444. [DOI] [PubMed] [Google Scholar]
- Peters F,Majerus S,Olivier L,Van der Linden M,Salmon E,Collette F ( 2007): A multi‐component exploration of verbal short‐term storage deficits in normal aging and Alzheimer's disease. J Clin Exp Neuropsychol 29: 405–417. [DOI] [PubMed] [Google Scholar]
- Petersen SE,Fox PT,Posner MI,Mintun M,Raichle ME ( 1988): Positron emission tomographic studies of the cortical anatomy of singleword processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Poeppel D,Guillemin A,Thompson J,Fritz J,Bavelier D,Braun AR ( 2004): Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia 42: 183–200. [DOI] [PubMed] [Google Scholar]
- Poldrack RA,Temple E,Protopapas A,Nagarajan S,Tallal P,Merzenich M,Gabrieli JD ( 2001): Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J Cogn Neurosci 13: 687–697. [DOI] [PubMed] [Google Scholar]
- Price CJ,Wise RJ,Warburton EA,Moore CJ,Howard D,Patterson K,Frackowiak RS,Friston KJ ( 1996): Hearing and saying. The functional neuro‐anatomy of auditory word processing. Brain 119(Part 3): 919–931. [DOI] [PubMed] [Google Scholar]
- Price CJ,Winterburn D,Giraud AL,Moore CJ,Noppeney U ( 2003): Cortical localisation of the visual and auditory word form areas: A reconsideration of the evidence. Brain Lang 86: 272–286. [DOI] [PubMed] [Google Scholar]
- Pugh KR,Shaywitz BA,Shaywitz SE,Constable RT,Skudlarski P,Fulbright RK,Bronen RA,Shankweiler DP,Katz L,Fletcher JM,Gore JC. ( 1996): Cerebral organization of component processes in reading. Brain 119(Part 4): 1221–1238. [DOI] [PubMed] [Google Scholar]
- Scott SK,Blank CC,Rosen S,Wise RJ ( 2000): Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123(Part 12): 2400–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi T,Koyama S,Kakigi R ( 2001): The effect of phonological repetition on cortical magnetic responses evoked by visually presented words. J Cogn Neurosci 16: 1250–1261. [DOI] [PubMed] [Google Scholar]
- Semenza C,Zettin M ( 1989): Evidence from aphasia for the role of proper names as pure referring expressions. Nature 342: 678–679. [DOI] [PubMed] [Google Scholar]
- Simos PG,Breier JI,Fletcher JM,Foorman BR,Castillo EM,Papanicolaou AC ( 2002): Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex 12: 297–305. [DOI] [PubMed] [Google Scholar]
- Specht K,Reul J ( 2003): Functional segregation of the temporal lobes into highly differentiated subsystems for auditory perception: An auditory rapid event‐related fMRI‐task. Neuroimage 20: 1944–1954. [DOI] [PubMed] [Google Scholar]
- St George M,Kutas M,Martinez A,Sereno MI ( 1999): Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain 122: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme Medical Publishers. [Google Scholar]
- Tanji K,Suzuki K,Yamadori A,Tabuchi M,Endo K,Fujii T,Itoyama Y ( 2001): Pure anarthria with predominantly sequencing errors in phoneme articulation: A case report. Cortex 37: 671–678. [DOI] [PubMed] [Google Scholar]
- Thioux M,Pesenti M,Costes N,De Volder A,Seron X ( 2005): Task‐independent semantic activation for numbers and animals. Brain Res Cogn Brain Res 24: 284–290. [DOI] [PubMed] [Google Scholar]
- Tubach JP,Boë LJ ( 1990): Un corpus de transcription phonétique. Constitution et exploitation statistique. Ecoles nationales supérieur des télécommunication. [Google Scholar]
- Uppenkamp S,Johnsrude IS,Norris D,Marslen‐Wilson W,Patterson RD ( 2006): Locating the initial stages of speech‐sound processing in human temporal cortex. Neuroimage 31: 1284–1296. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R,Price C,Wise R,Josephs O,Frackowiak RS ( 1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Vigneau M,Beaucousin V,Herve PY,Duffau H,Crivello F,Houde O,Mazoyer B,Tzourio‐Mazoyer N ( 2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30: 1414–1432. [DOI] [PubMed] [Google Scholar]
- Vitevitch MS,Luce PA ( 1998): When words compete: Levels of processing in perception of spoken words. Psychol Sci 9: 325–329. [Google Scholar]
- Vitevitch MS,Luce PA,Pisoni DB,Auer ET ( 1999): Phonotactics, neighborhood activation, and lexical access for spoken words. Brain Lang 68: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R,Juengling F,Bubrowski P,Jost E,Dykierek P,Talazko J,Huell M ( 2004): Hemispheric asymmetries of hypometabolism associated with semantic memory impairment in Alzheimer's disease: A study using positron emission tomography with fluorodeoxyglucose‐F18. Psychiatry Res 132: 159–172. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ,Belin P ( 2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11: 946–953. [DOI] [PubMed] [Google Scholar]


