Abstract
The ability to quickly decide on the nature of unexpected environmental changes is vital for adaptive behavior. Converging evidence suggests that the orbitofrontal cortex plays an important role in the rapid assignment of motivational significance and goal relevance to environmental stimuli. However, its putative role as a central part of a network involved in the prioritization of attentional selection, particulary when significant environmental changes occur unexpectedly or outside of attentional focus, remains to be established. Therefore, we used functional magnetic resonance imaging with a subsequent psychophysiological interaction analysis to reveal the functional connectivity of the right posterior orbitofrontal cortex (pOFC) in a context, in which subjects had to adjust goal‐directed behavior to behaviorally relevant events presented outside of the current attentional focus. As expected, an increased functional interaction between pOFC and regions involved in the modulation of selective attention (pulvinar nucleus and inferior parietal lobule) and processing of “bottom‐up” salience (substantia nigra) could be observed when unattended, but significant changes were relevant for behavior. Moreover, a positive correlation between level of accuracy and an increased functional connectivity between pOFC and extrastriate cortex suggested that a motivationally‐triggered signal from pOFC may have increased visual processing of the relevant but currently unattended stimulus attribute. These data provide evidence that the interplay between the pOFC and these regions underlies a mechanism by which organisms rapidly achieve voluntary control of attentional resources to deal with behaviorally significant changes that occur outside of current attentional focus. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: orbitofrontal cortex, attention, executive control, psychophysiological interaction, fMRI
INTRODUCTION
The ability to rapidly adjust behavior in response to unexpected environmental change is particularly vital in dangerous situations, in which only rapid, context‐sensitive behavioral reactions guarantee survival. In situations, in which unexpected, but relevant changes occur outside of current attentional focus, it is advantageous for organisms to have the ability to both quickly detect and analyze the nature of the environmental alteration, which is necessary to decide on an appropriate course of action. In a multifarious environment the limited processing capacity of sensory systems strongly challenges the organism's propensity to detect important information [Vuilleumier, 2005; Williams, 2006]. It has thus been assumed that general information processing may be organized by stimulus significance, which is determined by its relevance to the organism's current goals (depending on whether a stimulus is furthering or obstructing the goal; [Compton, 2003]) and/or its relation to the organism's core motivations (i.e., the need to minimize danger and maximize pleasure and reward; [Williams, 2006]). By driving the priority with which a particular stimulus is noticed from background “noise” and is subsequently processed in further detail, stimulus significance ensures that behavior is mainly controlled by relevant information [Williams, 2006].
Because environmental conditions are never static it is necessary that multimodal sensory inputs are constantly monitored and updated for changes in stimulus significance, which allows organisms to flexibly react to significant changes and to maximize the overall benefit. One region that may be predisposed for such a global monitoring function is the orbitofrontal cortex (OFC). Its ability to flexibly code relative incentive value of reinforcers [e.g., Gottfried et al., 2003] and changes in stimulus‐outcome contingencies [e.g., O'Doherty et al., 2003] thereby enables the OFC to provide a constant update of environmental conditions with regard to their motivational significance allowing organisms to breach perseverative responding and achieve rapid context‐sensitive adjustments in goal‐directed behavior [Nobre et al., 1999; see also Elliott and Deakin, 2005; Kringelbach, 2005]. Interestingly, orbitofrontal activations are not at all restricted to consciously perceived stimuli, but have also been observed during the presentation of significant stimuli that were completely extinguished due to extensive unilateral parietal damage [Vuilleumier et al., 2002] or that occurred at irrelevant or ignored locations during execution of spatial attention tasks [Vuilleumier et al., 2001]. This further indicated that even coarse visual input may enable the OFC to evaluate stimuli as motivationally significant, which would be advantageous in situations of danger and threat, as it could facilitate the rapid recognition of unattended, but significant changes. Direct connections between early sensory cortices and the OFC may thereby provide a possible route by which crude (preattentive) information on visual change can be rapidly relayed to OFC for further evaluation, which would also facilitate actual stimulus recognition in higher‐order sensory cortices [Bar et al., 2006; see also Kveraga et al., 2007].
Assuming that the OFC represents a crucial function in the evaluation of the current motivational relevance of environmental change, the aim of this study was to directly assess the functional connectivity of the OFC in a situation of changing task contingencies that had a direct implication for the current behavioral goal. We thereby sought to disentangle the putative role of the OFC in the prioritization of attentional selection and behavioral control, by directly comparing orbitofrontal connectivity in a situation of unexpected visual change that required an immediate behavioral adjustment compared to a situation in which a change in stimulus color had to be ignored. For this purpose, we reanalyzed data from a previous study [Gruber et al., 2009, in which infrequent, affectively neutral color deviants that had been presented outside of current attentional focus required an immediate adjustment of behavior. Our previous study had been able to show that motivationally relevant infrequent events selectively activated the posterior orbitofrontal cortex (pOFC) and regions of a frontoparietal network that is known to be involved in attentional orienting to visual change [e.g., Kiehl et al., 2005; see also Corbetta and Shulman, 2002]. In contrast, infrequent distractor deviants that also occurred in the background of a primary task, but simply had to be ignored, failed to activate these regions. This implied that processing of behaviorally relevant infrequent events must have recurred on a neural mechanism that supposedly influenced (voluntary) attentional selection and allowed a rapid behavioral adjustment upon the unexpected change in behavioral relevance. For this reason, it was expected that during processing of the behaviorally significant infrequent deviants the pOFC should exhibit an increased functional connectivity with regions of the frontoparietal network [Corbetta and Shulman, 2002].
MATERIALS AND METHODS
Subjects
The analysis was based on functional imaging data from the study of Gruber et al. 2009. Because of data loss from one person, the analysis included data from 10 healthy volunteers (5f). Ethical approval was obtained and all subjects gave written informed consent and were paid for participation.
Experimental Design
Participants performed a cued task‐switching paradigm, in which they occasionally had to adjust goal‐directed behavior to infrequent color deviants that were presented outside of the current focus of attention. In each session, participants were required to respond to either the color or the shape of abstract geometric objects. Subjects received a cue prior to each stimulus that informed them about which task to perform. In each trial, one of two different geometric objects was presented, which could appear in one of three different colors (blue, red, or white). The two different objects and two of the colors (red and blue) occurred with normal frequency during the whole experiment and were always mapped to a left or right manual response, respectively. Consequently, most of the stimuli were congruent or incongruent with respect to the response to be given. The third color (white) was presented exclusively in the object task (i.e., it occurred in the currently irrelevant, unattended stimulus dimension). It was introduced to make it possible to systematically vary the motivational/behavioral relevance of an infrequent deviant event that occurred outside of current attentional focus. Accordingly, the task‐switching experiment was subdivided into three consecutive fMRI sessions [Gruber et al., 2009, for a more detailed description], of which only two will be explained here:
In session 1, the white color was presented infrequently and formed the currently irrelevant stimulus dimension that was not mapped to any response. Subjects were informed about the possibility of occurrence of such a deviant event, but were instructed to completely ignore it in the object task. Still, because of its infrequency (salience) it was nevertheless expected that the infrequent distractor would elicit a reflexive orienting reaction, which could be followed by a voluntary redirection of attention towards the relevant stimulus attribute object shape. Prior to session 2, the instruction was changed and subjects were required to respond to the infrequent white color presented in the currently irrelevant stimulus dimension of the object task by pressing a third response button with the ring‐finger of their right hand. Through this simple instruction, the low‐frequency deviants acquired motivational/behavioral relevance.
The trial‐structure was identical for all sessions. The cue was always presented for 500 ms. It was followed by an interstimulus‐interval of 250 ms after which the target‐stimulus was presented for 750 ms. After a blank‐screen delay of 250 ms the next trial began. Behavioral responses were recorded for 1,000 ms measured from target‐onset. All trials were presented in a counterbalanced pseudorandomized order. Sessions 1 and 2 each contained a total of 480 trials with an equal number of color and object tasks half of which were either response congruent or incongruent. In 40 of the object trials the deviant color was presented in the currently irrelevant stimulus dimension. Infrequent deviants thus on average occurred every 12th trial. For the SPM‐model, we further matched deviant trials of interest with a comparison condition that was not infrequent. To achieve this we prespecified 40 of the congruent object trials in each session as “critical congruent trials,” which were located five to six trials apart from the deviant events. From subjects' perspective these trials did not differ from the remaining congruent object trials. Nevertheless, critical congruent trials were matched for (at least) two preceding and two subsequent trial types (trial type transitions), thus ruling out the possibility of confounding effects resulting from temporally correlated trials.
Stimuli were back projected on a translucent screen that the participants viewed through a mirror during the fMRI acquisition. The head was stabilized by small cushions to avoid head movements during scanning. Triggering of the visual stimulation by the scanner impulse during the functional data acquisition and generation of stimuli was conducted through the ERTS Software (Experimental Run Time System, Version 3.11, BeriSoft Cooperation, Frankfurt am Main, Germany).
FMRI Acquisition and Analysis
Imaging was performed on a 3 Tesla Siemens Trio system (Erlangen, Germany) equipped with the standard bird cage head coil. Twenty‐one axial slices parallel to the anterior commissure‐posterior commissure line were obtained in ascending acquisition order (slice thickness = 4 mm; interslice gap = 1 mm) using a gradient‐echo echo planar imaging (EPI) sequence (echo time of 30 ms, a flip angle of 90°; field‐of‐view = 19.2; 64 × 64 matrix; interscan repetition time (TR) = 1,750 ms). A total of 1,280 volumes was acquired. At the start of each scan a blank screen was presented for the duration of 7 TRs to allow time for magnetization to reach steady state. The images acquired during this period were discarded from data‐analysis.
The fMRI data were preprocessed and statistically analyzed with SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing comprised realignment and unwarping, correction for slicetime acquisition differences (reference slice: 11), and spatial normalization to standard stereotactic space (using the Montreal Neurological Institute (MNI) template provided by SPM2; normalized voxelsize: 4 mm × 4 mm × 4 mm). For group analysis, data were further spatially smoothed with a Gaussian kernel (FWHM: 12 mm). In the event‐related single‐subject analysis 12 trial types were modeled as separate conditions for each session. Statistical analyses were carried out using a general linear model which created a design matrix that allowed testing for brain activity changes associated with different experimental conditions presented at different time points during the course of the experiment.
To assess our hypothesis of an orbitofrontal interaction with (posterior) brain regions involved in attentional processing, we employed a psychophysiological interaction analysis [PPI; Friston et al., 1997] in the specific context in which a significant change (i.e., behaviorally relevant infrequent color deviant) occurred outside of current attentional focus. This allowed us to detect regionally specific responses in one brain region in terms of the responses in another brain region during a certain cognitive process (functional connectivity). Accordingly, the first eigenvariate time series (VOI) of the local activation maximum in the right pOFC (MNI‐coordinates: 32, 28, −12; surrounded by a 4 mm sphere), which was selectively observed during processing of infrequent deviants that required a rapid behavioral adjustment [session 2; Gruber et al., 2009], was extracted and deconvolved with the canonical hemodynamic response function (HRF), serving as the physiological factor. This maximum was determined from the results of the random effects analysis (RFX) from our previous study [Gruber et al., 2009], in which this focus had also survived correction for multiple comparisons at a statistical threshold of 0.05, corrected for family‐wise error. The resulting time‐course was then convolved with the psychological vector P of the time t, which was set to 1 if t was the onset (in scans) of an infrequent behaviorally relevant deviant event (session 2), to −1 in case t was the onset of an infrequent irrelevant deviant event (session 1), and to 0 for all other events, like it was done in the direct subtraction‐contrast between behaviorally relevant infrequent deviants (session 2) and irrelevant infrequent deviants [session 1; see Gruber et al., 2009]. Reconvolving the resulting function with the HRF produced a vector X, which was entered as the first regressor in the design matrix of a general linear model. The vector P and the detrended VOI were also convolved with the HRF to form the second and third covariates. The PPI term was thus derived from the product of the activation time course with the psychological factor. The model estimation was performed as a commonly used least‐squares fit. In the t‐contrasts (PPI contrasts) the PPI vector X was computed against implicit baseline, which meant that all parameters except for X, which was set to 1, were set to zero. For group statistics, RFX‐analyses were performed on single subject PPI contrast images, thresholded at P < 0.005, uncorrected.
In a second step, we also tested the hypothesis whether the degree of functional interaction between the right pOFC and other brain regions involved in attentional selection and sensory processing was positively correlated with accuracy levels in the context, in which a deviant event was also relevant for behavior. It was expected that an increased functional coupling between the pOFC and regions of attentional and sensory processing should also increase recognition accuracy and therefore enable a higher number of correct behavioral responses with regard to the infrequent deviant color in the shape task. For this purpose, correlations between the positive PPI (psychophysiological interaction term) and accuracy levels (i.e., percentage of correct responses for relevant oddball stimuli in the shape task) were explored by using the SPM2 simple regression analysis with a significance level of P < 0.005, uncorrected.
RESULTS
As expected, an increased functional interaction between activation in right pOFC and regions involved in the modulation of selective (visual) attention and processing of visual salience was observed, when salient changes in the unattended stimulus‐dimension were relevant for behavior (motivationally significant) compared to a context in which they had to be ignored (cf., Fig. 1; Table I). These regions comprised the inferior parietal lobule (IPL) bilaterally, the left pulvinar nucleus of the thalamus, the left substantia nigra (SN), and the right posterior inferior temporal cortex and adjacent inferior occipital cortex.
Figure 1.
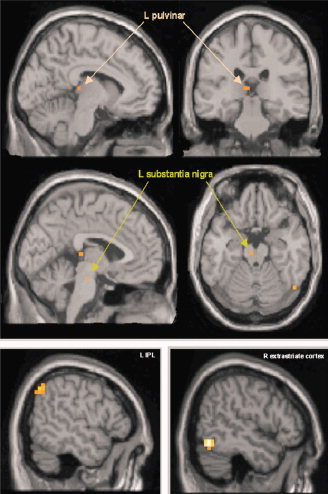
Regions, that showed a positive interaction with pOFC during processing of infrequent behaviorally relevant deviants. Activations superimposed on sections of the standard MNI‐template. Activations were statistically thresholded at P < 0.005, uncorrected. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Brain regions showing a positive interaction with pOFC during processing of infrequent behaviorally relevant deviants
| Region | MNI‐coordinates | Statistical effects (t‐values) |
|---|---|---|
| L inferior parietal lobule | −56, −60, 44 | 4.21 |
| R inferior parietal lobule/ intraparietal sulcus | 44, −48, 56 | 3.17* |
| L pulvinar nucleus | −8, −28, 8 | 3.36 |
| L substantia nigra | −4, −20, −20 | 3.38 |
| R extrastriate cortex (area V4) | 52, −64, −12 | 6.06 |
Coordinates and t‐values of local maxima.
If not indicated otherwise activations are reported at P < 0.005, uncorrected.
P < 0.01, uncorrected.
For exploratory purposes, we also analyzed the negative connectivity of the pOFC (i.e., for the reversed contrast), which did not yield any significant clusters at the applied threshold of P < 0.005, uncorrected.
The regression analysis further revealed a positive correlation between accuracy level and the increased functional interaction between the right pOFC and the cluster in the right extrastriate cortex (MNI‐coordinates: 44, −60, −8; t = 7.69). This means that an increased functional coupling between the pOFC and extrastriate cortex (area V4) during presentation of a behaviorally relevant unattended deviant was associated with increased behavioral recognition accuracy (i.e., the percentage of correct behavioral responses). None of the remaining clusters observed in the PPI‐analysis showed such an association.
DISCUSSION
Human behavior is determined by multiple interwoven systems guiding attentional selection and adaptive behavior. The observation of an increased functional connectivity between the right pOFC and various subcortical and cortical regions, which commonly have been associated with (rapid) sensory processing and the modulation of visual attention [Posner and Petersen, 1990], confirmed our initial hypothesis, that the OFC may be part of a functional network that helps to prioritize (attentional) selection of motivationally significant sensory change, even if the change occurs in the background of current attentional focus. The IPL, pulvinar nucleus, and SN [together with the superior colliculus, which did not show up in our PPI‐analysis, but was nevertheless activated in the relevant subtraction contrast presented in Gruber et al., 2009] have previously been reported to form a circuit that is involved in covert attentional orienting and voluntary guidance of eye movements [Posner and Petersen, 1990]. Sensory responses in these regions can be triggered rapidly and have been shown to scale with the salience (i.e., unexpectedness) and motivationally significance of environmental stimuli, which indicated that these regions may rapidly point visual attention towards stimuli with greater significance for action. For instance, in previous studies the SN exhibited increased activation to surprise‐inducing stimuli, which reflected the degree to which a current sensory state differed from a previous one [Lee et al., 2006; see also Redgrave et al., 1999], and thus appeared to be critical for perceptual decisions that rapidly identify stimuli as both unpredicted and visually salient [Comoli et al., 2003]. Similarly, the pulvinar nucleus of the thalamus also showed selectively enhanced responses to relevant stimuli independent of whether stimuli were consciously perceived or remained unseen, were masked or blurred or had a low‐spatial frequency [see for example a study in monkeys with striatal lesions, Gross, 1991; or the masking study of Williams et al., 2006; see also Vuilleumier, 2005; Ward and Danziger, 2005]. In nonhuman primates, the pulvinar nucleus and the inferior parietal lobule have been observed to respond to internally generated, task‐related changes in attentional state, but not stimulus properties per se, reflecting voluntary modulation of attention and sensory filtering of behaviorally relevant environmental input [Robinson and Petersen, 1992; Salzmann, 1995; see also Kastner and Pinsk, 2005]. Notably, increased activation in the pulvinar nucleus and the OFC has been observed when emotionally significant stimuli (e.g., threat‐related stimuli) were presented outside of current attentional focus or even failed to enter conscious awareness [Morris et al., 1997, 1999; see also Vuilleumier et al., 2003]. Moreover, both orbitofrontal and inferior parietal areas have previously been associated with enhanced orienting of spatial attention towards emotional stimuli [Armony and Dolan, 2002] and were coactivated during voluntary attentional disengagement [e.g., Nobre et al., 1999; see also: Corbetta and Shulman, 2002]. Working in collaboration, in the present study these regions may have thus formed a network that was ideally suited for the regulation (i.e., the enhancement) of selective (visual) attention, which supposedly enabled prioritized visual processing of significant stimulus attributes.
The general role of the OFC has been characterized as that of a processor of motivational significance. It has been proposed that the OFC has the ability to flexibly represent a constantly updated significance‐currency of environmental stimuli, which is integrated with knowledge on current (behavioral) goals and subjective needs [Kringelbach, 2005]. More specifically, bidirectional anatomical connections with sensory cortices may enable the OFC to keep track of mismatches between expected and factually perceived goal‐relevant sensory input that have an implication for behavioral choice [see Barbas and Zikopoulos, 2007; Romanski et al., 1997]. Activation in the OFC has previously been observed when invalid cues violated current expectations [e.g., Nobre et al., 1999] or when reward contingencies changed unexpectedly and required an immediate reversal of stimulus choice [e.g. Cools et al., 2002; Kringelbach and Rolls, 2003; O'Doherty et al., 2003; see also: Elliott and Deakin, 2005; Izquierdo et al., 2004]. Our data show that response accuracy, necessary for the behavioral adjustment following the detection of the unexpected deviant color in the object task, was obviously determined by the degree of increased functional connectivity between pOFC and V4. This suggests an important role for the integrative capacity of the pOFC and related sensory cortices in the inhibition of prepotent responses and rapid reversals of stimulus choice. Our observation of an increase in connectivity between pOFC and sensory cortices indicates that the posterior orbitofrontal contribution in behavioral control processes may be accomplished by an increased interaction with visual cortices, but not by a direct inhibition of motor areas. In fact, the lateral pOFC lacks direct anatomical connections with motor cortices. Instead, “motor output” must pass a frontomedial route, which functions as a “sensory‐visceromotor link” that is critical for the guidance of motivated and reward‐related behaviors [Öngür and Price, 2000]. For these reasons, we would conform with the conclusion initially formulated by O'Doherty et al. [ 2003] that the pOFC may rapidly identify contingency changes that violate previously build expectations, which, according to the present observation, may be accomplished through an increased positive interaction with sensory processing regions. Activity in area V4 of the ventral visual stream has previously been observed to be subject to attentional modulation expressed by enhanced firing rates for currently attended stimuli, which has been interpreted as underlying the ability to identify and remember the properties of a particular object out of the many that may be represented on the retina [Moran and Desimone, 1985]. It is therefore likely that the presently observed interaction could have aided to resolve perceptual ambiguity or could have enhanced feature‐based attention, which would accelerate stimulus evaluation and visual recognition of stimuli in the (attentional) periphery [Bar et al., 2006; Summerfield et al., 2006]. Our observation of an increased functional coupling between pOFC and sensory cortices could therefore be interpreted as reflecting a mechanism by which (visual) attention to stimulus attributes with greater significance for action is rapidly enhanced.
Finally, it is important to note that the observed functional network resembled a circuit, in which the amygdala has been assigned the central role in emotional integration of significant information with attentional selection. This “emotional” circuity was mainly activated during processing of highly significant threat‐ or danger‐related stimuli [e.g. fearful facial expressions; please see Vuilleumier, 2005 for a comprehensive review]. Although it included most of the parts of the network observed in this study, an orbitofrontal response has not been detected in most of these studies [Vuilleumier, 2005]. For this reason, it was somewhat surprising that in the present study we neither observed an increased connectivity between the right pOFC and the amygdala nor did we previously find a significant amygdala activation in the direct subtraction contrast between behaviorally relevant and irrelevant deviants [Gruber et al., 2009]. The fact that our study only involved affectively neutral but nevertheless behaviorally/motivationally significant stimuli may probably explain the absence of activation in the amygdala. There is already evidence in nonhuman primates that activation in the amygdala does not seem to be essential for the actual maintenance and integration of motivationally significant information with information on behavioral responding [Pickens et al., 2003; Winstanley et al., 2004]. In a situation, in which an unexpected visual change represented a reliable signal that indicated the need for a rapid behavioral adjustment (i.e., in an object reversal learning task) visual input alone appeared to be sufficient to rapidly guide behavioral responding [Izquierdo and Murray, 2007a; see also Izquierdo and Murray, 2007b, for review]. Even complete amygdala damage did not interfere with the reversal ability of monkeys during performance of an object‐reversal task, in which the (visual) presence or absence of feedback in form of a food stimulus indicated the current contingency of the stimulus chosen [Izquierdo et al., 2003; Izquierdo and Murray, 2007a]. Instead, damage to the rhinal cortex was found to yield a significant impairment, supposedly due to the cessation of recognition‐related visual input, which was expressed by a failure to correctly recognize the contingency change and adjust behavior accordingly [Murray et al., 1998]. For these reasons, one may assume that the pOFC is more generally involved in processing of changes in sensory stimulation that also directly affect behavioral responding, whereby information on such a motivationally significant visual change does not necessarily need to pass the amygdala. In contrast, the amygdala should be expected to be primarily involved in automatic alerting to threatening stimuli or other emotional stimuli that trigger strong inborn emotional reactions and thus have a direct linkage to the organism's fundamental motivations (i.e., the motivation to avoid harm and maximize overall well‐being).
CONCLUSION
In sum, this study was able to show that the pOFC may be part of an “attentional” circuit that appears to be modulated by motivational significance. Vuilleumier [ 2005] has recently denoted that the OFC, apart from the amygdala, may provide an important route by which emotion (or motivation) may influence attentional systems. The current results provide initial support for this assumption and further specify the function of the observed network as one that appears to be specialized for the processing of (visual) stimuli in the attentional background, which have a direct implication for goal‐directed behavior (i.e., violate previously built expectations and demand a rapid behavioral adjustment). The prioritized selection of unattended but significant features of the environment may thus strongly depend on a functional network consisting of the pOFC and associated sensory systems, which may place unexpected sensory changes in the appropriate context for action.
REFERENCES
- Armony JL,Dolan RJ ( 2002): Modulation of spatial attention by fear‐conditioned stimuli: An event‐related fMRI study. Neuropsychologia 40: 817–826. [DOI] [PubMed] [Google Scholar]
- Bar M,Kassam KS,Ghuman AS,Boshyan J,Schmid AM,Dale AM,Hamalainen MS,Marinkovic K,Schacter DL,Rosen BR,Halgren E ( 2006): Top‐down facilitation of visual recognition. Proc Natl Acad Sci USA 103: 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H,Zikopoulos B ( 2007): The prefrontal cortex and flexible behavior. Neuroscientist 13: 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C,Company T,Tejedor J,Cruz‐Rizzolo RJ,Reinoso‐Suarez F ( 2000): The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex 10: 220–242. [DOI] [PubMed] [Google Scholar]
- Comoli E,Coizet V,Boyes J,Bolam JP,Canteras NS,Quirk RH,Overton PG,Redgrave P ( 2003): A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci 6: 974–980. [DOI] [PubMed] [Google Scholar]
- Compton RJ ( 2003): The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behav Cogn Neurosci Rev 2: 115–129. [DOI] [PubMed] [Google Scholar]
- Cools R,Clark L,Owen AM,Robbins TW ( 2002): Defining the neural mechanisms of probabilistic reversal learning using event‐related functional magnetic resonance imaging. J Neurosci 22: 4563–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M,Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Elliott R,Deakin B ( 2005): Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol 65: 89–116. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Buechel C,Fink GR,Morris J,Rolls E,Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,O'Doherty J,Dolan RJ ( 2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Gross CG ( 1991): Contribution of striate cortex and the superior colliculus to visual function in area MT, the superior temporal polysensory area and the inferior temporal cortex. Neuropsychologia 29: 497–515. [DOI] [PubMed] [Google Scholar]
- Gruber O,Diekhof EK,Kirchenbauer L,Goschke T: Modulation of regional brain activations through the behavioral relevance of low‐frequency events. Neuroimage, submitted for publication. [Google Scholar]
- Izquierdo A,Murray EA ( 2007a): Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci 27: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A,Murray EA ( 2007b): Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann NY Acad Sci 1121: 273–296. [DOI] [PubMed] [Google Scholar]
- Izquierdo A,Suda RK,Murray EA ( 2003): Effects of selective amygdala and orbital prefrontal cortex lesions on object reversal learning in monkeys. Soc Neurosci Abstr 29: 90.92. [Google Scholar]
- Izquierdo A,Suda RK,Murray EA ( 2004): Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24: 7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S,Pinsk MA ( 2005): Visual attention as a multilevel selection process. Cogn Affect Behav Neurosci 4: 483–500. [DOI] [PubMed] [Google Scholar]
- Kiehl KA,Stevens MC,Laurens KR,Pearlson G,Calhoun VD,Liddle PF ( 2005): An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) MRI study of an auditory oddball task. Neuroimage 25: 899–915. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML ( 2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML,Rolls ET ( 2003): Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 20: 1371–1383. [DOI] [PubMed] [Google Scholar]
- Kveraga K,Ghuman AS,Bar M ( 2007): Top‐down predictions in the cognitive brain. Brain Cogn 65: 145–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ,Youn JM,Mary JO,Gallagher M,Holland PC ( 2006): Role of substantia nigra‐amygdala connections in surprise‐induced enhancement of attention. J Neurosci 26: 6077–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J,Desimone R ( 1985): Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784. [DOI] [PubMed] [Google Scholar]
- Morris JS,Friston KJ,Dolan RJ ( 1997): Neural responses to salient visual stimuli. Proc R Soc Lond Ser B Biol Sci 264: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS,Ohman A,Dolan RJ ( 1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96: 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA,Baxter MG,Gaffan D ( 1998): Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci 112: 1291–1303. [DOI] [PubMed] [Google Scholar]
- Nobre AC,Coull JT,Frith CD,Mesulam MM ( 1999): Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci 2: 11–12. [DOI] [PubMed] [Google Scholar]
- O'Doherty J,Critchley H,Deichmann R,Dolan RJ ( 2003): Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci 23: 7931–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D,Price JL. 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Pickens CL,Saddoris MP,Setlow B,Gallagher M,Holland PC,Schoenbaum G ( 2003): Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci 23: 11078–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI,Petersen SE ( 1990): The attention system of the human brain. Ann Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Redgrave P,Prescott TJ,Gurney K ( 1999): Is the short‐latency dopamine response too short to signal reward error? Trends Neurosci 22: 146–151. [DOI] [PubMed] [Google Scholar]
- Robinson DL,Petersen SE ( 1992): The pulvinar and visual salience. Trends Neurosci 15: 127–132. [DOI] [PubMed] [Google Scholar]
- Romanski LM,Giguere M,Bates JF,Goldman‐Rakic PS ( 1997): Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 379: 313–332. [PubMed] [Google Scholar]
- Salzmann E ( 1995): Attention and memory trials during neuronal recording from the primate pulvinar and posterior parietal cortex (area PG). Behav Brain Res 67: 241–253. [DOI] [PubMed] [Google Scholar]
- Summerfield C,Egner T,Greene M,Koechlin E,Mangels J,Hirsch J ( 2006): Predictive codes for forthcoming perception in the frontal cortex. Science 314: 1311–1314. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P ( 2005): How brains beware: Neural mechanisms of emotional attention. Trends Cognit Sci 9: 585–594. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL,Driver J,Dolan RJ ( 2001): Effects of attention and emotion on face processing in the human brain: An event‐related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL,Clarke K,Husain M,Driver J,Dolan RJ ( 2002): Neural response to emotional faces with and without awareness: Event‐related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia 40: 2156–2166. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL,Dolan RJ ( 2003): Reciprocal links between emotion and attention In: Frackowiak RSJ,Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human Brain Function. San Diego: Academic Press; pp 419–444. [Google Scholar]
- Ward R,Danziger S ( 2005): Selective attention and response control following damage to the human pulvinar In: Humphreys GW,Riddoch MJ, editors. Attention in Action: Advances of Cognitive Neuroscience. New York: Psychology Press; pp 325–350. [Google Scholar]
- Williams LM ( 2006): An integrative neuroscience model of “significance” processing. J Integr Neurosci 5: 1–47. [DOI] [PubMed] [Google Scholar]
- Williams LM,Das P,Liddell BJ,Kemp AH,Rennie CJ,Gordon E ( 2006): Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci 26: 9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA,Theobald DE,Cardinal RN,Robbins TW ( 2004): Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24: 4718–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]


