Abstract
Background
General anaesthetics affect loss of consciousness by disrupting information-passing and integration within thalamo-cortical (TC) networks. Feedback cortical connections that carry internally generated signals such as expectation and attention appear more sensitive to anaesthesia than feedforward signals. However, direct evidence for this effect in non-primary cortex is lacking. In addition, direct comparisons between TC core and matrix, and between cortico-cortical (CC) feedforward and feedback responses have not been reported.
Methods
We investigated the disruption of synaptic responses by isoflurane of four distinct afferent pathways to non-primary neocortex. We independently activated TC core and matrix and reciprocal CC (feedforward and feedback) pathways using optogenetic techniques, and compared the relative sensitivity of synaptic responses to isoflurane.
Results
Under control conditions, activation of axon terminals of all pathways evoked postsynaptic currents (recorded extracellularly) and postsynaptic potentials in pyramidal neurones. CC feedback responses were substantially more sensitive to isoflurane (0 to 0.53 mM) compared with TC core, TC matrix, or CC feedforward pathways.
Conclusion
Differential sensitivity of CC feedback synaptic responses to isoflurane in a clinically relevant range suggests a role for disruption of these afferents in the hypnotic effects of anaesthetic agents.
Keywords: anaesthetics, general; channel rhodopsin; consciousness; isoflurane; neocortex; neuronal network; thalamus
Editor's key points.
-
•
Modulation by general anaesthetics of neural pathways connecting the cerebral cortex and thalamus are likely critical for consciousness.
-
•
The effects of isoflurane on synaptic transmission were investigated in mouse brain slices using optogenetic techniques to activate afferent pathways to non-primary cortex.
-
•
Greater sensitivity of cortico-cortical than thalamo-cortical feedback synaptic responses to isoflurane suggests a role for disruption of these afferents in the hypnotic effects of volatile anaesthetics.
The mechanisms of general anaesthetic-induced loss of consciousness (LOC) have profound clinical relevance, and are relevant as well for expanding and unifying basic theories of consciousness.1, 2 Central to these theories is the sharing of information between nodes in the cortico-thalamic network3, 4, 5 carried by thalamo-cortical (TC) and cortico-cortical (CC) feedback and feedforward pathways.6, 7 Studies examining the effects of anaesthetics on the contents of consciousness have focused on this information exchange in particular.8 However, the degree to which anaesthetics selectively modulate these pathways, especially in non-primary cortical areas that are critical for consciousness and particularly sensitive to anaesthetics,9, 10 remains unclear.
Previous noninvasive studies suggest that CC connectivity (especially feedback connectivity) is suppressed by anaesthetics.11, 12, 13, 14 Synaptic responses to intracortical stimulation in primary auditory cortex (A1) slices are suppressed to a greater extent than are responses to stimulation of TC afferents from primary auditory thalamus.15 Less is known about anaesthetic effects on projections of non-primary thalamic nuclei, which are the source of both modulatory (‘matrix’) and information-bearing (‘core’) efferents16 to non-primary cortex. These nuclei receive little direct sensory input but facilitate information transfer between cortical areas via recurrent cortico-thalamocortical loops and may contribute to maintaining awareness.17, 18, 19 Also unknown is whether CC feedforward afferents, which connect directly different levels of the cortical hierarchy, are as robust to anaesthetic effects as primary thalamic feedforward afferents. Here, we activated four distinct afferent thalamo-cortical and cortico-cortical pathways optogenetically and compared the effects of isoflurane on synaptic responses of each in non-primary cortex.
Methods
Expression of channel rhodopsin and brain slice preparation
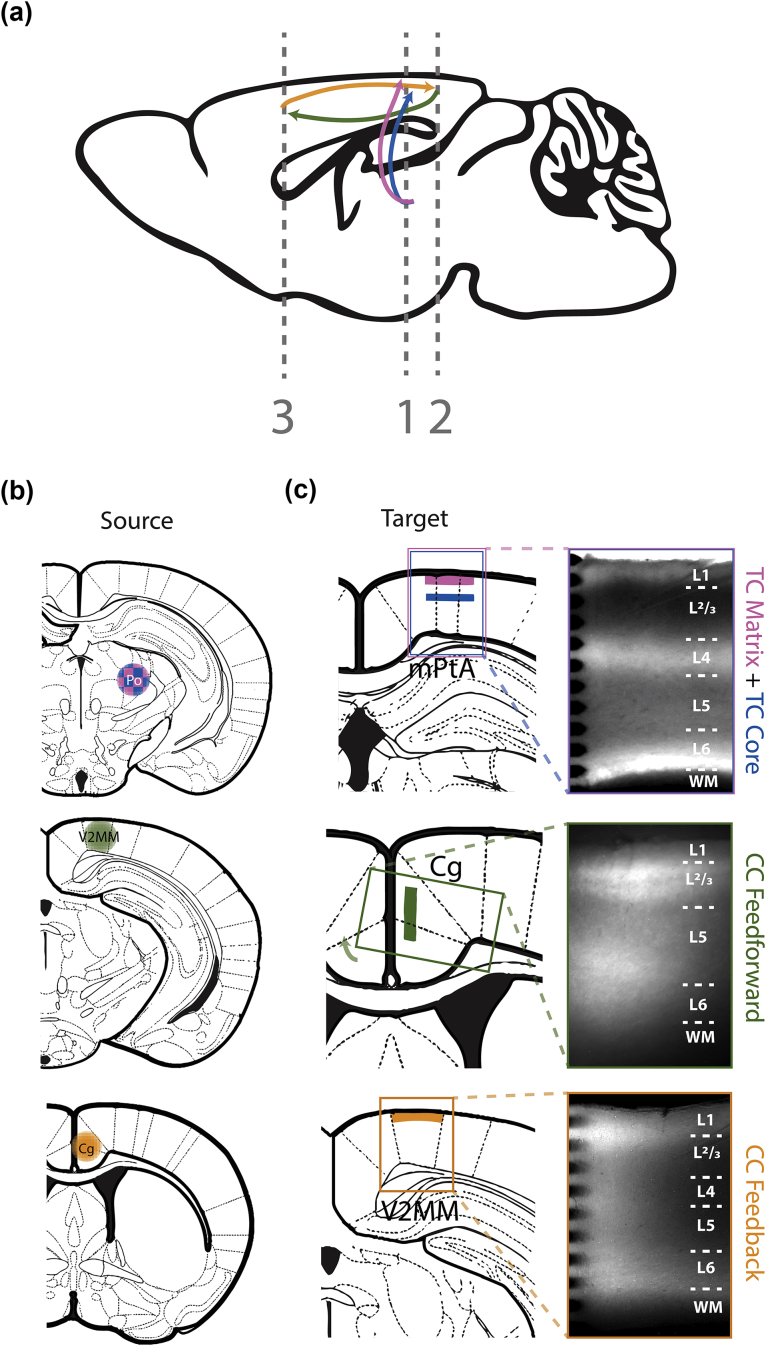
Synaptic pathways were activated optogenetically after expressing excitatory opsins in nerve terminals of specific afferent fibres (Fig. 1a; see Supplementary Methods for additional details). To obtain ChR2 expression in both TC matrix and core projections to non-primary cortex, injections were made in posterior thalamus (Po; Fig. 1b, top), a non-primary thalamic nucleus that contains a combination of matrix and core cells, distinguished here by the laminar distribution of their axon terminals in layers 1 and 4 (L1 and L4; Fig. 1C, top), respectively.16 After injections in Po, brain slice recordings were made in the medial parietal association area (mPtA). To examine reciprocal CC connections, injections were made in either a secondary visual area (V2MM) to label feedforward afferents (Fig. 1b, middle row) or anterior cingulate cortex (Cg) to label feedback afferents (Fig. 1b, bottom row), and recordings were made in Cg (Fig. 1c, middle row) or V2MM (Fig. 1c, bottom row), respectively. Acute coronal brain slices (500 μm thick) were prepared from mPtA, Cg or V2MM ipsilateral to viral injections (see Supplementary Materials for additional details). Responses to afferent stimulation were recorded using a multichannel electrode that spanned the cortical column under control, isoflurane (0–0.53 mM; 1–2 different concentrations, delivered in aqueous phase), and recovery conditions, as described.20 In a subset of slices, intracellular recordings were made from cortical pyramidal neurones using whole-cell patch clamp to compare excitatory postsynaptic potentials (EPSPs) with extracellular responses, as described.21
Fig 1.
Optogenetic activation of four independent afferent pathways in neocortex. (a) A schematic showing projection patterns of four distinct afferent pathways: TC matrix (pink), TC core (blue), CC feedforward (green), and CC feedback (gold). Vertical dashed lines show coronal planes of section for brain slices in (c). (b) A viral vector containing ChR2 and YFP reporter was injected into the Po (top), V2MM (V2MM; middle), or Cg (bottom). Colour scheme of injection is representative of pathway shown in (a). Note that TC core (blue) and TC matrix (pink) cells are interspersed in Po, as reflected by the checkered pattern in the schematic. (C) Atlas (left) and fluorescence (right) images of recording area. Shaded coloured regions in atlas images represent ChR2 and YFP reporter expressed in axon terminals after injections shown in (b), in mPtA (top), Cg (middle), and V2MM (bottom). Only the terminal regions optically stimulated in this report are shaded. Coloured boxes in the atlas images represent the approximate recording area shown in fluorescence images. Layer boundaries are delineated with dotted lines in fluorescence images. The tips of the multichannel recording array are visible on the left-hand side of the fluorescence images in the top and bottom panels. TC, thalamo-cortical; CC, cortico-cortical; Po, posterior thalamus; V2MM, secondary visual area; Cg, anterior cingulate cortex; mPtA, medial parietal association area; WM, white matter.51
Optogenetic stimulation of afferent axon terminals
Synaptic responses were evoked using light stimulation of TC or CC axon terminals expressing ChR2, restricted to the cortical layer with the highest expression of yellow fluorescent protein (YFP). Brief (0.5–2.0 ms) light pulses were delivered at a range of 1–10 different light intensities (1.5–15 mW) randomly interleaved with an inter-stimulus interval between 20 s and 2 min, directed over the cortical column containing the recording array. Synaptic responses were evoked using light stimulation of axon terminals expressing ChR2 via a fibreoptic cable (250 μm diameter; ThorLabs, Newton, NJ, USA) coupled to a TTL-triggered LED (473 nm; Luxeon Star, Lethbridge, AB, Canada) or a patterned illumination device (Polygon400; Mightex, Pleasanton, CA, USA). For animals injected in non-primary thalamus, the light was positioned in mPtA over either L1 (to activate TC matrix afferents) or L4 (to activate TC core afferents). For animals injected in Cg, the light was positioned in V2MM over L1 to activate feedback CC terminals. For animals injected in V2MM, the light was positioned in Cg over L2/3 to activate feedforward CC terminals.
Data analysis
Local field potentials (LFPs) were isolated by applying a bandpass filter at 1–300 Hz (expanded to 1–1000 Hz for latency calculations to avoid signal distortion) and averaged across trials of the same light stimulus intensity. Current source density (CSD)22 was calculated using the spline inverse CSD method23 applied to LFPs across 16 channels, such that negative values represent current sinks (i.e. inward transmembrane currents). Using the channel with the earliest current sink onset, percent block was calculated by dividing the difference in sink amplitude between control and isoflurane conditions by the control sink amplitude. Experiments in which at least partial recovery was not observed were excluded. Intracellular recordings were bandpass filtered at 0.1–2000 Hz and averaged across trials. Latency, amplitude, and half-widths were calculated for both CSD and intracellular recordings for comparison.
Linear mixed-effects model
To evaluate the effect of isoflurane on sink magnitude in each of four afferent pathways, data were fit to a linear mixed-effects model.24 The use of a linear mixed-effects model allows for principled analysis of hierarchical data by including both random and fixed effects in statistical models, such that non-independence among or within samples and experimental groups is considered. Our model included the response variable ‘percent block by isoflurane’, fixed effects of afferent pathway (TC core, TC matrix, CC feedforward, or CC feedback) and isoflurane concentration (0 to 0.53 mM), and random effect of slice (experiment) with random slope. To exclude the possibility that observed effects were simply caused by degradation of responses over time, we included several ‘sham’ experiments (0 mM isoflurane) for each pathway and allowed for random intercepts in the model. A likelihood ratio test was used to compare a model with an interaction term between fixed effects of afferent pathway and concentration of isoflurane to a model with no such interaction; the model was significantly improved by including the interaction (χ2(3)=12.21, P=0.0067). After choosing this model, coefficient estimates were generated for comparison of slopes between afferent pathways using t-tests with Sattherthwaite's method for degrees of freedom.
Results
Synaptic responses under control conditions
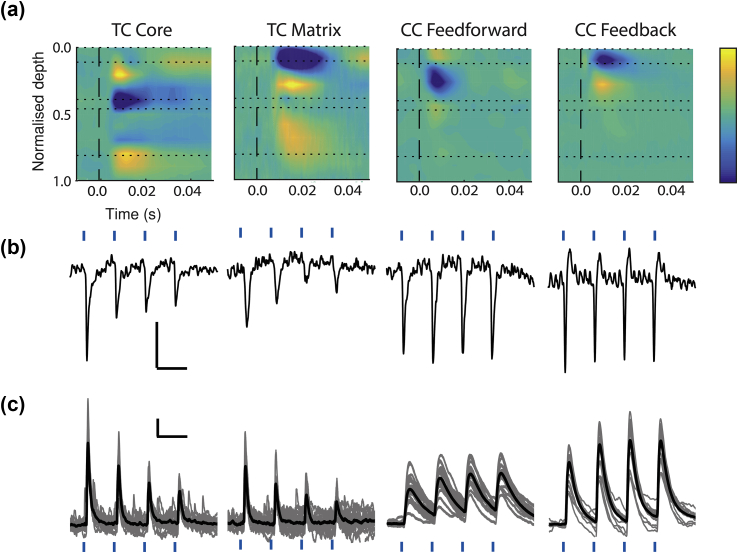
For each afferent pathway, synaptic responses were elicited by directing light over the cortical layer with the highest density of YFP-labelled axon terminals (Fig. 1c). We distinguished TC core vs matrix projections by activating thalamic terminals in cortical L4 and L1, respectively (Fig. 1c, top row). CC feedforward projections terminated in the superficial layers, with heaviest labelling in L2/3 (Fig. 1c, middle row). CC feedback projections terminated in all layers, though labelling density was highest in L1 (Fig. 1c, bottom row), as reported.25, 26 For all afferent pathways, stimuli elicited short-latency (Table 1), putatively monosynaptic current sinks and excitatory postsynaptic potentials (EPSPs) (Fig. 2). The shortest latency current sinks were in L1 and L4 for TC matrix and core projections, respectively, and in L2/3 and L1 for CC feedforward and feedback projections, respectively. Durations of CC feedback synaptic responses were significantly longer than CC feedforward responses, and TC matrix longer than TC core (Table 1), consistent with a postulated modulatory roles of the former. Similar to previous results,27 TC core afferents, but not CC feedforward or feedback afferents, exhibited short-term synaptic depression (Fig. 2b, Table 1). Short-term synaptic depression was also observed in TC matrix responses (Fig. 2b, Table 1), as has been found in non-primary thalamic inputs to L1 of prefrontal cortex28 and L4 of secondary somatosensory cortex.29
Table 1.
Properties of postsynaptic responses. Values are presented as mean (standard deviation). Parameters from extracellular data were calculated using the CSD signal from the channel that showed the earliest onset of the inward-going current after the light stimulus. Half-widths are width of current sink or EPSP signal at half the maximum amplitude. Comparisons of half-widths of the CSD signal were made within TC* and within CC† groups using a standard one-way analysis of variance; TC matrix responses were longer than TC core responses (*F1,41=19.82, P<0.0001) and CC feedback half-widths were longer than CC feedforward (†F1,34=7.70, P=0.009). CC, cortico-cortical; CSD, current source density; EPSP, excitatory postsynaptic potential; TC, thalamo-cortical.
| Latency (ms) | Half-width (ms) | Paired pulse ratio | ||
|---|---|---|---|---|
| TC core | CSD (n=16) | 4.67 (1.85) | 8.27 (3.06)* | 0.64 (0.23) |
| Intracellular (n=7) | 3.57 (1.01) | 30.00 (15.90) | 0.62 (0.22) | |
| TC matrix | CSD (n=23) | 5.11 (1.69) | 12.13 (2.62)* | 0.72 (0.19) |
| Intracellular (n=10) | 4.63 (1.87) | 26.10 (14.27) | 0.61 (0.16) | |
| CC feedforward | CSD (n=8) | 3.02 (1.01) | 7.81 (1.31)† | 1.17 (0.37) |
| Intracellular (n=4) | 3.63 (0.78) | 32.75 (16.46) | 1.04 (0.13) | |
| CC feedback | CSD (n=21) | 4.15 (1.97) | 9.42 (1.77)† | 0.90 (0.31) |
| Intracellular (n=4) | 3.69 (2.29) | 27.49 (14.31) | 1.04 (0.22) | |
Fig 2.
Synaptic responses evoked under control conditions. (a) Representative examples of responses after a single light pulse (473 nm, black dashed line) used to activate ChR2-expressing axon terminals of four afferent pathways to cortex (left to right): TC core, TC matrix, CC feedforward, and CC feedback. CSD colour plots are shown, where current sinks (inward-going transmembrane current) are blue (scale: –2.0 to 2.0 μA mm−3 for TC matrix and CC feedforward, –1.0 to 1.0 μA mm−3 for TC core and CC feedback) and layer boundaries are shown with horizontal dotted lines. (b) CSD signals evoked as in (a), but with a train of four light pulses (blue tick marks) delivered at 10 Hz. Signals shown were isolated from the channel exhibiting the earliest current sink in (a). Horizontal scale bars: 100 ms. Vertical scale bar represents 1.0 μA mm−3 for TC matrix and CC feedforward traces, and 2.0 μA mm−3 for TC core and CC feedback traces. (c) Whole-cell current-clamp recordings from four cortical pyramidal cells demonstrating intracellular analogues of CSDs shown in (b) (not necessarily from same experiment); excitatory postsynaptic potentials were evoked by a train of four light pulses at 10 Hz. Horizontal scale bar: 100 ms. Vertical scale bar represents 0.5 mV for TC matrix, TC core, and CC feedforward traces, and 2.0 mV for CC feedback traces. TC, thalamo-cortical; CC, cortico-cortical; CSD, current source density.
We also recorded intracellular correlates of synaptic responses under control conditions to ensure that extracellular recordings were associated with EPSPs of typical latency and shape. For each stimulus pathway, intracellular recordings from L2/3 and L5 pyramidal cells showed monosynaptic EPSPs after each of four light pulses delivered at 10 Hz (Fig. 2c). Latencies from intracellular recordings were comparable with corresponding parameters from CSD traces. Half-widths of EPSPs were notably longer than those of CSD current sinks (Table 1), as expected from the effect of membrane capacitance on the voltage signal.
Effect of isoflurane on monosynaptic current sink amplitude is pathway-specific
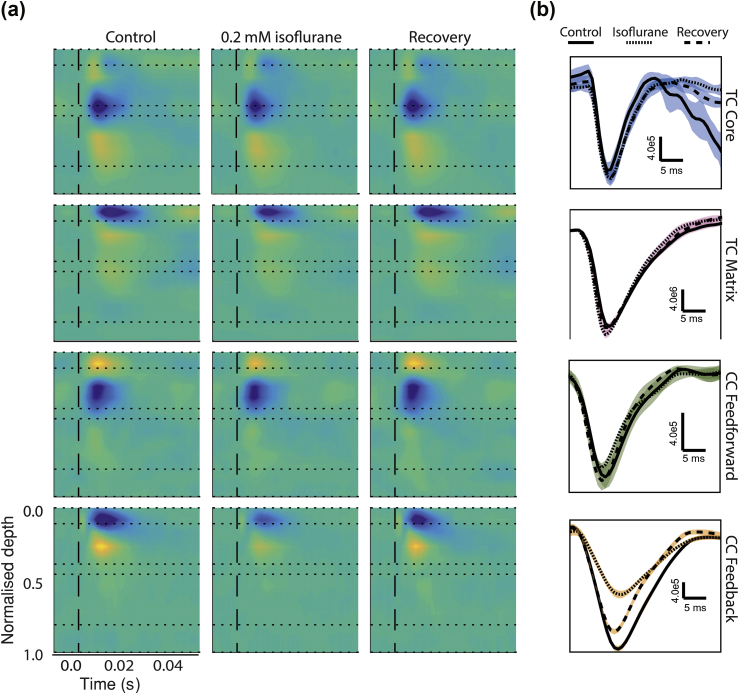
We investigated the anaesthetic sensitivity of the four synaptic pathways by applying isoflurane to brain slices, as described.20 After eliciting synaptic responses to light stimulation under control conditions, isoflurane was applied at 0.0 to 0.53 mM, followed by recovery in regular artificial cerebrospinal fluid (CSF). Sink amplitudes were compared across drug conditions. We observed a striking difference in sensitivity of CC feedback responses compared with the other three pathways (Fig. 3). Current sinks elicited by activation of feedback CC terminals were substantially smaller in the presence of isoflurane compared with control and recovery periods, whereas the amplitudes of CC feedforward and both TC matrix and core responses were largely unaffected. No significant effect of isoflurane on half-widths or latencies was observed for any pathway (Table 1).
Fig 3.
Current sinks evoked in each of four afferent pathways across control, isoflurane, and recovery. (a) CSD colour plots comparing evoked synaptic responses across control (a, left column), 0.2 mM isoflurane (a, middle column), and recovery (a, right column) for representative examples of each of four afferent pathways to cortex, from top to bottom row: TC core, TC matrix, CC feedforward, and CC feedback; colour scale: –2.0 to 2.0 μA mm−3 for TC core and CC feedback, –1.0 to 1.0 μA mm−3 for CC feedforward, and –5.0 to 5.0 μA mm−3 for TC matrix. (b) Signal from the channel with the earliest sink were used to compare synaptic effects across control (solid), 0.2 mM isoflurane (dotted), and recovery (dashed). Shaded regions, which are coloured using colour scheme in Fig. 1, indicate ±1 standard deviation among trials. TC, thalamo-cortical; CC, cortico-cortical; CSD, current source density.
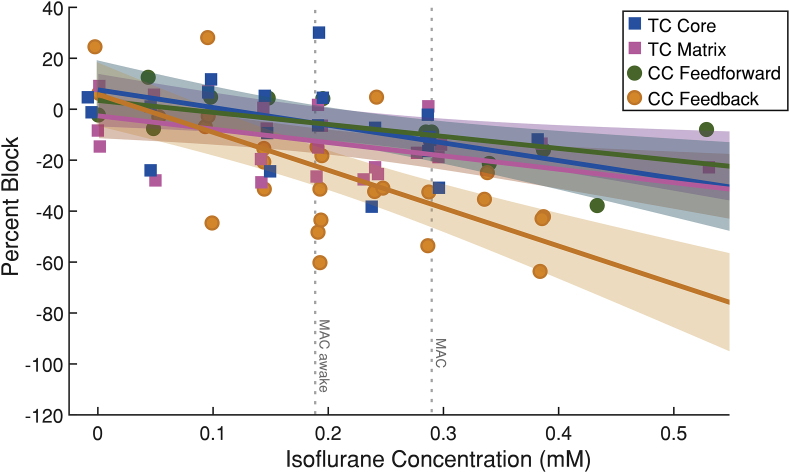
The effect of isoflurane on sink magnitude was compared across slices, pathways, and isoflurane concentrations using a linear mixed-effects model (Fig. 4). Responses from all pathways were reduced to some extent by isoflurane, but CC feedback responses were reduced to the greatest extent (Table 2). Pairwise comparisons of effects on all afferent pathways showed a significantly greater effect of isoflurane on CC feedback synaptic responses compared with TC matrix and CC feedforward pathways (Table 2), where CC feedback responses were suppressed by 14.9% (standard deviation [sd]=2.5%) by 0.1 mM isoflurane (P=8.1×10−8). There was no significant difference in the effect of isoflurane between the TC matrix and TC core pathways (P=0.41).
Fig 4.
CC feedback cortical afferents are preferentially suppressed under isoflurane. Results from individual experiments (individual markers) and model fits (i.e. percent block as a function of concentration of isoflurane; solid lines) with 95% confidence interval (shaded) are shown. Responses evoked by stimulation of CC feedback afferents to cortical layer 1 (gold) are suppressed to a greater extent under isoflurane than CC feedforward (green) and both TC matrix (pink) and core (blue) pathways, especially at and above a concentration corresponding to ∼0.9% isoflurane, the concentration at which loss of consciousness occurs in mice. TC, thalamo-cortical; CC, cortico-cortical.
Table 2.
Comparison of effect of isoflurane on synaptic responses of four distinct afferent pathways to cortex. Slopes of the effect of isoflurane on each pathway with reference to no slope (shown as % block per 0.1 mM isoflurane) are shown, followed by pairwise comparison of effects slopes between any two pathways, represented as the difference between individual slopes. Note that the effect isoflurane on CC feedback responses is significantly greater than CC feedforward and both TC core and matrix responses. CC, cortico-cortical; CI, confidence interval; TC, thalamo-cortical.
| Effect on distinct pathways (% block per 0.1 mM isoflurane) | |||
|
Pathway |
Estimate |
95% CI |
P-value |
| TC core | –7.03 | [–12.2, –2.25] | 0.0059 |
| CC feedforward | –4.17 | [–9.89, 0.91] | 0.14 |
| TC matrix | –5.65 | [–9.88, –1.18] | 0.0076 |
| CC feedback |
–14.9 |
[–20.0, –10.1] |
3.09×10−8 |
| Pairwise comparison of slopes | |||
|
Comparison |
Estimate |
95% CI |
P-value |
| CC feedback–TC core | –7.90 | [–14.9, –1.35] | 0.023 |
| CC feedback–CC feedforward | –10.8 | [–17.4, –3.49] | 0.0045 |
| CC feedback–TC matrix | –9.29 | [–15.9, –3.42] | 0.0040 |
| TC core–TC matrix | –1.38 | [–7.67, 4.71] | 0.64 |
| TC core–CC feedforward | –2.85 | [–11.4, 4.42] | 0.45 |
| CC feedforward–TC matrix | 1.47 | [–5.69, 8.27] | 0.67 |
Discussion
We found that CC feedback synaptic responses in non-primary cerebral cortex were suppressed by isoflurane more than TC matrix, TC core, and CC feedforward responses, complementing our previous study in primary auditory cortex15 and indirect evidence from noninvasive electrophysiology and imaging studies.12, 13, 14 In predictive processing theories of brain function, feedback signals carry internally generated predictions about sensory observations, and feedforward signals carry information about mismatches between those predictions and observations. Conscious perception is the process of adjusting generative model parameters to explain away the sensory signals.6, 7 It is within this context that anaesthetic suppression of CC feedback signals could act to disrupt awareness.8
The molecular mechanisms underlying differential sensitivity to isoflurane are of considerable practical interest. Anaesthetics have been shown to suppress synaptic transmission presynaptically via altering calcium influx, synaptic machinery, and vesicle release,30, 31, 32 with greater sensitivity of glutamatergic compared with gamma aminobutyric acid (GABA)ergic synapses.33, 34 CC and TC synapses differ at the molecular and ultrastuctural level,35 which may contribute to their differential sensitivity to anaesthetics. Future experiments could test the sensitivity of these molecular targets to isoflurane in isolation.
The relative insensitivity of TC matrix responses to isoflurane observed here is surprising. TC matrix afferents overlap with CC feedback terminal fields,26 and may facilitate conscious sensory processing.36, 37 Indeed, activity in TC matrix cells is high during wakefulness and low during non-rapid eye movement (REM) sleep, and activation of matrix thalamic nuclei during sleep promotes rapid awakening in mice and during anaesthesia promotes EEG desynchronisation,38 suggesting a role for these cells in recovery of consciousness after sleep and anaesthesia.17, 39 Our findings are incongruent with the conclusions drawn by Liu and colleagues40 regarding the effects of propofol anaesthesia on functional TC connectivity in human volunteers, which found that functional connectivity between non-specific (i.e. non-primary) thalamic nuclei and a number of non-primary cortical areas is more sensitive to propofol anaesthesia compared with specific thalamic connectivity. Although useful for identifying distinct effects on correlated activity among brain areas, functional connectivity measures cannot distinguish directionality or indirect effects. It is possible that the effects of propofol on TC connectivity observed40 reflect a decrease in synaptic efficacy at cortico-thalamic synapses or indirect effects on connectivity amongst cortical areas, rather than direct effects on the thalamo-cortical connections we investigated here.
Raz and colleagues15 showed that in primary auditory cortex, synaptic responses to electrical stimulation of either L1 or of feedback projections from V2 were preferentially suppressed by isoflurane compared with stimulation of core TC afferents. Because of their overlapping terminal fields, stimulation of L1 could engage both CC feedback and TC matrix afferents simultaneously, whereas activation of V2→A1 projections alone would be exclusively CC feedback; still, the authors found that isoflurane suppressed L1 and V2→A1 responses comparably. In this study, we used optogenetics to activate each afferent pathway independently. We note that the block by isoflurane of L1 or V2→A1 responses reported by Raz and colleagues15 is comparable with the block of CC feedback responses alone that we find here (13.5% decrease in sink amplitude for 0.1 mM isoflurane15 compared with 14.9% decrease in sink amplitude for 0.1 mM isoflurane here), raising the possibility that sensitivity or density of TC matrix afferents varies with cortical region. Thus, the functional roles of TC matrix inputs may also vary at different levels of the cortical hierarchy, taking on a modulatory role in primary sensory cortex, but acting as driving inputs in higher order areas.28, 29
Our work demonstrates the sensitivity of CC feedback connectivity to anaesthetics, and the relative insensitivity of thalamic and cortical feedforward connectivity. Although brain slice preparations are ideally suited to answer specific questions about pharmacological sensitivity of specific groups of synapses, these preparations have several limitations that are relevant to investigation of mechanisms of anaesthesia and features of conscious sensory perception. For example, spontaneous cortical activity is very sensitive to anaesthetic agents,9 but spontaneous activity is already suppressed in brain slice preparations even under control conditions, likely because of the absence of neuromodulators and long-range excitatory connections.41 These limitations require experiments in vivo to determine the extent that neuromodulators, long-range connections and suppression of ongoing activity contribute to LOC under anaesthesia.
Our results present an intriguing question: why is activity in higher-order cortical areas especially sensitive to anaesthetics9, 10 even though synaptic responses of their driving inputs, non-primary TC and feedforward CC afferents are insensitive? This may relate to two distinguishing features of the dynamic brain: activity and connectivity. Our experimental approach of optogenetically activating synaptic terminals in cortical target areas directly tests the effect of isoflurane on connectivity by eliminating the prerequisite for activity of afferent pathways. However, these two features of neural networks are not entirely dissociable. Most anaesthetics (other than ketamine) suppress spiking activity and raise spike thresholds,42, 43, 44, 45, 46, 47 and changes in excitability also contribute to changes in connectivity.48 Hentschke and colleagues20 showed that despite preservation of early TC responses, evoked recurrent activity in brain slices was not sustained in the presence of isoflurane. The distinction between activity and connectivity may also help explain the diversity of results among EEG studies of changes in connectivity under anaesthesia. For example, whereas some investigations have shown preferential suppression of long-range intracortical connectivity,11, 12, 14 others have shown increases.49, 50 It is possible that the latter scenario arises in spite of decreases in synaptic strength when activity becomes synchronised and thus strongly coordinated between regions.
Questions remain as well regarding the relevance of CC feedback connections to consciousness. Studies of the neural basis of consciousness, and investigations of mechanisms of LOC under anaesthesia such as this study, are correlative in nature. Feedback connections are relevant for sensory processing and are particularly sensitive to anaesthetics, but it is unlikely that suppression of feedback inputs alone is responsible for LOC. Demonstrating the contribution of differential sensitivity of feedback connections to anaesthetic LOC will require causal manipulations in vivo that can explicitly link activity and connectivity within cortico-thalamic networks to distinct features of consciousness. How sensitivity of distinct synaptic inputs, as we observe here, synergistically interacts with intrinsic and network-level changes observed during anaesthesia in the intact brain will help elucidate the spatiotemporal relationships within thalamo-cortical networks that subserve consciousness.
Authors' contributions
Study design: CAM, MIB
Data collection: CAM
Data analysis: CAM, BMK, MIB
Manuscript preparation: CAM, BMK, MIB
Acknowledgements
The authors thank Donata Oertel for comments on the manuscript and Sean M. Grady for technical assistance.
Handling editor: H.C. Hemmings Jr
Editorial decision: 22 June 2019
Footnotes
This article is accompanied by an editorial: Role of cortical feedback signalling in consciousness and anaesthetic-induced unconsciousness by G.A. Mashour, Br J Anaesth 2019:123:403–405, doi: 10.1016/j.bja.2019.07.001
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.06.018.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
National Institutes of Health (R01 GM109086 to MIB), and the Department of Anesthesiology, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alkire M.T. Loss of effective connectivity during general anesthesia. Int Anesthesiol Clin. 2008;46:55–73. doi: 10.1097/AIA.0b013e3181755dc6. [DOI] [PubMed] [Google Scholar]
- 2.Mashour G.A. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci Biobehav Rev. 2013;37:2751–2759. doi: 10.1016/j.neubiorev.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crick F., Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–126. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- 4.Llinas R., Ribary U., Contreras D., Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch C., Massimini M., Boly M., Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. 2016;17:307–321. doi: 10.1038/nrn.2016.22. [DOI] [PubMed] [Google Scholar]
- 6.Dehaene S., Changeux J.P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashour G.A., Hudetz A.G. Bottom–up and Top–down mechanisms of general anesthetics modulate different dimensions of consciousness. Front Neural Circ. 2017;11:44. doi: 10.3389/fncir.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nourski K.V., Steinschneider M., Rhone A.E., Kawasaki H., Howard M.A., 3rd, Banks M.I. Auditory predictive coding across awareness states under anesthesia: an intracranial electrophysiology study. J Neurosci. 2018;38:8441–8452. doi: 10.1523/JNEUROSCI.0967-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Lauer K.K., Ward B.D., Rao S.M., Li S.J., Hudetz A.G. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33:2487–2498. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku S.W., Lee U., Noh G.J., Jun I.G., Mashour G.A. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrarelli F., Massimini M., Sarasso S. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci USA. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrouff J., Perlbarg V., Boly M. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Lee U., Ku S., Noh G., Baek S., Choi B., Mashour G.A. Disruption of frontal–parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz A., Grady S.M., Krause B.M., Uhlrich D.J., Manning K.A., Banks M.I. Preferential effect of isoflurane on top–down versus bottom–up pathways in sensory cortex. Front Syst Neurosci. 2014;8 doi: 10.3389/fnsys.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones E.G. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 17.Saalmann Y.B. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci. 2014;8:83. doi: 10.3389/fnsys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillery R.W., Sherman S.M. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 19.Neske G.T. The slow oscillation in cortical and thalamic networks: mechanisms and functions. Front Neural Circ. 2015;9:88. doi: 10.3389/fncir.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hentschke H., Raz A., Krause B.M., Murphy C.A., Banks M.I. Disruption of cortical network activity by the general anesthetic isoflurane. Br J Anaesth. 2017;119:685–696. doi: 10.1093/bja/aex199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause B.M., Murphy C.A., Uhlrich D.J., Banks M.I. PV+ cells enhance temporal population codes but not stimulus-related timing in auditory cortex. Cereb Cortex. 2019;29:627–647. doi: 10.1093/cercor/bhx345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 23.Pettersen K.H., Devor A., Ulbert I., Dale A.M., Einevoll G.T. Current-source density estimation based on inversion of electrostatic forward solution: effects of finite extent of neuronal activity and conductivity discontinuities. J Neurosci Methods. 2006;154:116–133. doi: 10.1016/j.jneumeth.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen M., Hansen T. Statistical analyses of repeated measures in physiological research: a tutorial. Adv Physiol Educ. 2004;28:2–14. doi: 10.1152/advan.00042.2003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S., Xu M., Kamigaki T. Selective attention. Long-range and local circuits for top–down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh S.W., Harris J.A., Ng L. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman S.M., Guillery R.W. On the actions that one nerve cell can have on another: distinguishing "drivers" from "modulators". Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruikshank S.J., Ahmed O.J., Stevens T.R. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viaene A.N., Petrof I., Sherman S.M. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci U S A. 2011;108:18156–18161. doi: 10.1073/pnas.1114828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumgart J.P., Zhou Z.Y., Hara M. Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proc Natl Acad Sci U S A. 2015;112:11959–11964. doi: 10.1073/pnas.1500525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herring B.E., Xie Z., Marks J., Fox A.P. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol. 2009;102:1265–1273. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z., McMillan K., Pike C.M. Interaction of anesthetics with neurotransmitter release machinery proteins. J Neurophysiol. 2013;109:758–767. doi: 10.1152/jn.00666.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westphalen R.I., Desai K.M., Hemmings H.C., Jr. Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth. 2013;110:592–599. doi: 10.1093/bja/aes448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westphalen R.I., Hemmings H.C., Jr. Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. J Pharmacol Exp Ther. 2006;316:216–223. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- 35.Fremeau R.T., Voglmaier S., Seal R.P., Edwards R.H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Castejon C., Nunez A. Cortical neural computation by discrete results hypothesis. Front Neural Circ. 2016;10:81. doi: 10.3389/fncir.2016.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkum M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36:141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Honjoh S., Sasai S., Schiereck S.S., Nagai H., Tononi G., Cirelli C. Regulation of cortical activity and arousal by the matrix cells of the ventromedial thalamic nucleus. Nat Commun. 2018;9:2100. doi: 10.1038/s41467-018-04497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crunelli V., David F., Lőrincz M.L., Hughes S.W. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol. 2015;31:72–80. doi: 10.1016/j.conb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Liu X., Lauer K.K., Ward B.D., Li S.J., Hudetz A.G. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stepanyants A., Martinez L.M., Ferecsko A.S., Kisvarday Z.F. The fractions of short- and long-range connections in the visual cortex. Proc Natl Acad Sci U S A. 2009;106:3555–3560. doi: 10.1073/pnas.0810390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antkowiak B., Helfrichforster C. Effects of small concentrations of volatile anesthetics on action potential firing of neocortical neurons in vitro. Anesthesiology. 1998;88:1592–1605. doi: 10.1097/00000542-199806000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Hentschke H., Schwarz C., Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 44.Hudetz A.G., Vizuete J.A., Imas O.A. Desflurane selectively suppresses long-latency cortical neuronal response to flash in the rat. Anesthesiology. 2009;111:231–239. doi: 10.1097/ALN.0b013e3181ab671e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaese B.H., Ostwald J. Anesthesia changes frequency tuning of neurons in the rat primary auditory cortex. J Neurophysiol. 2001;86:1062–1066. doi: 10.1152/jn.2001.86.2.1062. [DOI] [PubMed] [Google Scholar]
- 46.Zurita P., Villa A.E.P., Deribaupierre Y., Deribaupierre F., Rouiller E.M. Changes of single-unit activity in the cats auditory thalamus and cortex associated to different anesthetic conditions. Neurosci Res. 1994;19:303–316. doi: 10.1016/0168-0102(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 47.Ries C.R., Puil E. Mechanism of anesthesia revealed by shunting actions of isoflurane on thalamocortical neurons. J Neurophysiol. 1999;81:1795–1801. doi: 10.1152/jn.1999.81.4.1795. [DOI] [PubMed] [Google Scholar]
- 48.Friston K.J. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 49.Lee M., Sanders R.D., Yeom S.-K. Network properties in transitions of consciousness during propofol-induced sedation. Sci Rep. 2017;7:16791. doi: 10.1038/s41598-017-15082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy M., Bruno M.A., Riedner B.A. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34 doi: 10.1093/sleep/34.3.283. 283–91A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paxinos G., Franklin K.B.J. 2nd edn. Academic Press; San Diego, CA: 2003. The mouse brain in stereotaxic coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.