Abstract
In the present study, we were interested in distinguishing the cortical representations of within‐modal and cross‐modal divided attention tasks by using functional magnetic resonance imaging. Sixteen healthy male subjects aged between 21 and 30 years underwent two within‐modal (auditory/auditory, visual/visual) and one cross‐modal (auditory/visual) divided attention task, as well as related selective attention control conditions. After subtraction of the corresponding control task the three divided attention tasks, irrespective of sensory modality, revealed significant activation in a predominantly right hemisphere network involving the prefrontal cortex, the inferior parietal cortex, and the claustrum. Under the cross‐modal condition, however, the frontal and parietal activation was more extended and more bilateral and there also was stronger right hemisphere activation of the anterior cingulate cortex and the thalamus. In comparison to the within‐modal conditions additional bilateral frontal and left inferior parietal activation was found for the cross‐modal condition. The supplementary fronto‐parietal, anterior cingulate cortex, and thalamus activation in the auditory/visual condition could be argued to reflect an additional demand for coordination of two ongoing cross‐modal cognitive processes. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: fMRI, divided attention, attention, prefrontal cortex, inferior parietal cortex, thalamus, cingulate cortex, claustrum
INTRODUCTION
In everyday life, the ability to divide attention is indispensable since we attend concurrently to a multitude of different inputs either occurring in the same (within‐modal) or in different (cross‐modal) sensory modalities (e.g. visual and auditory stimuli). Divided attention means that individuals are engaged in two or more different tasks at the same time, they have to divide attention between these tasks and allocate mental resources to each of them. Norman and Shallice [1986] postulated that the coordination of two interfering tasks is controlled by the “Supervisory Attentional System” which they assumed to have its neural correlate in frontal structures. Animal experiments [Goldman‐Rakic, 1987] and craniocerebral injury studies [McDowell et al., 1997; van Zomeren and van den Burg, 1985] as well as studies of patients with a rupture of an aneurysm of the anterior a. communicans [Rousseaux et al., 1996] actually showed that carrying out divided attention tasks is indeed largely dependent on frontal structures. Duncan et al. [1997] distinguished between within‐ and cross‐modal divided attention tasks and found a more restricted attentional capacity within but not between sensory modalities, raising the question whether there are different attentional networks. Unfortunately, functional imaging studies dealing with divided attention until now have concentrated either on within‐modal or on cross‐modal dual tasks but have not compared the two with each other [for a review see Coull, 1998; Mesulam, 1999]. Furthermore, the activated brain regions found in the within‐modal as well as in the cross‐modal studies are inconsistent, probably because of the different stimulus material and/or types of tasks.
One of the groups concerned only with within‐modal stimuli [Madden et al., 1997], investigated differences in visual selective and divided attention tasks in a PET study. Age‐unrelated brain activation for dividing attention was found in occipitotemporal, occipitoparietal, and bilateral prefrontal regions. Besides a right prefrontal involvement, activation of the anterior cingulate gyrus was found in another PET study [Corbetta et al., 1991] dealing with within‐modal divided attention, where subjects had to differentiate between shape, color, and speed of a visual stimulus. In a functional magnetic resonance imaging (fMRI) study on working memory [Bunge et al., 2000] participants performed two tasks (sentence reading and short‐term memory for five words) either separately or concurrently. Also in this study, within‐modal dual‐task performance showed stronger activation in bilateral prefrontal areas when compared with either task performed alone, but no area was activated beyond the single‐tasks' regions. In another within‐modal fMRI study [Koechlin et al., 1999], the posterior dorsolateral prefrontal cortex and the lateral parietal cortex were activated bilaterally during a visual dual‐task where the participants had to differentiate between upper‐ and lower‐case letters.
Among the authors studying cross‐modal tasks, Johannsen et al. [1997] investigated sustained and divided attention in normal elderly humans with visual and vibrotactile stimuli. Under the divided attention condition, the PET data revealed right hemisphere activation in inferior parietal and prefrontal regions and thalamic activation in the left hemisphere. In an fMRI study by Szameitat et al. [2002], the analysis of the dual‐task/single‐task subtraction revealed that cortical areas along the inferior frontal sulcus, the middle frontal gyrus and the intraparietal sulcus were involved in cross‐modal dual‐task performance. Subjects were given both auditory and visual three‐choice response tasks performed either separately as single tasks or concurrently as a dual‐task, for which an increased difficulty level led to stronger bilateral activation in the regions described. In another fMRI study, Loose et al. [2003] assessed cross‐modal (visual/auditory) divided attention and selective attention tasks in healthy male subjects. In comparison to the selective attention conditions, the divided attention paradigm evoked additional left prefrontal activation, which the authors suggested to be crucial in the execution of controlled processing when attention is divided between two sources of cross‐modal information.
While several of these findings seem highly relevant and interesting, no clear activation pattern can be extracted for either the within‐modal or the cross‐modal experiments, which led us to our study trying to differentiate between networks involved in within‐modal and cross‐modal divided attention tasks.
According to Wickens' Multiple Resources Theory [1984], different types of tasks (e.g. verbal vs. nonverbal, visual vs. auditory, and manual vs. vocal) rely on different processing resources. Therefore, processing two tasks simultaneously might be more difficult if they take hold of the same pool of resources. Furthermore, one can draw the conclusion that processing two tasks presented simultaneously in the same sensory modality (e.g. two visual tasks) should call for higher top‐down control and thus for stronger frontal activation than two tasks presented in different modalities and tapping their individual resources.
The purpose of this fMRI study was to distinguish the differences in cortical representations of within‐modal (auditory/auditory; visual/visual) and cross‐modal (auditory/visual) divided attention tasks and to analyze the management of attentional resources. If the comparison of the tasks would reveal stronger frontal activation for the within‐modal conditions this would be in line with Wickens' multiple resources model. On the other hand, authors of task switching studies prefer the hypothesis that switching between two sensory modalities has higher demands on cognitive flexibility than switching within one modality [Adcock et al., 2000; Dove et al., 2000]. Thus, the results of our cross‐modal condition could corroborate either Wickens' or the “task switchers” hypothesis.
METHODS
Subjects
Sixteen male healthy right‐handed subjects, mean age 25.2 years (range 21–30 years), participated in the study. No subject had a history of psychiatric or neurological disorder nor a history of head trauma. All subjects gave written consent according to the declaration of Helsinki. The study was approved by the Ethics Committee of the University Hospital of Aachen and by federal authorities.
Experimental Design and Procedure
The participants underwent an fMRI study consisting of three divided attention conditions (DAC) and four selective attention control conditions (SAC) presented in randomized order (Fig. 1). The DACs consisted of two within‐modal (auditory/auditory, visual/visual) and one cross‐modal task (auditory/visual). In the auditory/auditory DAC, subjects listened to high pitched (1,500 Hz) and low pitched (200 Hz) tones presented in alternating order (SOA 1,500 ms, stimulus duration 1,000 ms). Thirty six percent of the tones changed their pitch after 500 ms presentation time either from 1500 to 750 Hz or from 200 to 400 Hz. Participants had to respond if they heard a consecutive sequence (probability 50%) of two descending high pitched or two ascending low pitched tones. In the second within‐modal DAC (visual/visual), circles and squares were presented in alternating order (SOA 1,500 ms, stimulus duration 1,000 ms). Thirty six percent of the objects changed their dimension after 500 ms presentation time. The subjects had to respond if they saw two increasing circles or two decreasing squares in sequential order. In the cross‐modal auditory/visual DAC, squares and low pitched tones were presented alternately (SOA 1,500 ms, stimulus duration 1,000 ms). In 36% of the presented stimuli, the size of the squares decreased or the low pitched tones raised their pitch. Participants had to respond if they saw two decreasing squares or if they heard two ascending low pitched tones in sequential order. Because of the fact that under the auditory/auditory DAC the two different auditory stimuli could not be presented simultaneously, we had to choose a fast alternating presentation of the stimuli under all conditions to keep the tasks comparable. The task nevertheless is a divided attention task, because the information in both “channels” has to be kept in working memory and continuously updated to make the decision whether there were two consecutive changes (1‐back condition) in either of the two sequences. Thus, the experiment utilizes a real “dual task paradigm” taken to be essential for divided attention setups. A high level of supervisory attentional control sensu [Norman and Shallice, 1986] respectively a high level of central executive control in working memory [Baddeley, 1993] is necessary to cope with our tasks.
Figure 1.
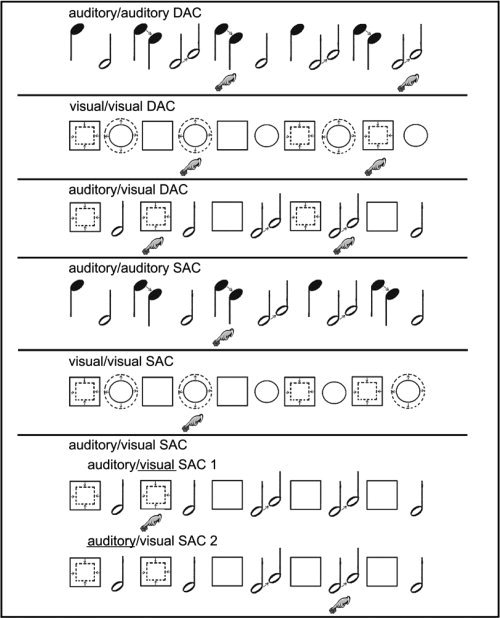
Schematic examples of the three divided attention (DAC) and four selective attention conditions (SAC). The “finger on the button” shows where the participants had to respond.
The selective attention tasks differed from the divided attention tasks just by the instructions given to the participants. Thus, changes in brain activity were caused only by a different attentional state and not by physical variations of the stimuli. During the performance of the auditory/auditory SAC, participants had to attend only to the high pitched tones and ignore the low pitched ones. In the visual/visual SAC subjects had to observe only the circles and ignore the squares. Finally, the auditory/visual SAC is a compound of two sessions. In the first session (SAC1) the volunteers had to attend to the visual stimuli while ignoring the auditory ones in the other session (SAC2) and vice versa. One must point out that the subjects in the SAC had to press the response button half as frequently as under the DAC, with the risk of motor activation being stronger in the DAC. In the rest periods of all sessions, participants had to keep their eyes open while watching a black screen. To familiarize the subjects with the task, the experiment was explained and practised outside the scanner. Auditory stimuli were presented via fMRI suitable headphones (Commander XG, Resonance Technologies, LA) and visual stimuli were presented via MR‐compatible LCD goggles (VisuaStim XGA, Resonance Technology, LA). The subjects responded by a right‐hand thumb key press. Each of the seven sessions was embedded into a typical box‐car design with six periods of rest‐activation alternations (rest period 27.9 s, 9 scans each; activation period 46.5 s, 15 scans each).
Image Acquisition
All measurements were conducted using a whole body Philips Gyroscan NT 1.5 Tesla MRI (Philips Medical Systems, Nederland B.V.) with a standard head coil. After orienting the axial slices in the anterior‐posterior commisure (AC‐PC), plane functional images were acquired using a T2*‐weighted echo planar imaging (EPI) sequence with a repetition time (TR) of 3,100 ms, an echo time (TE) of 50 ms, and a flip angle (FA) of 90 degrees. In total 1,008 volumes were collected, consisting of 34 contiguous slices with a thickness of 3.5 mm measured with whole brain coverage. A 64 × 64 matrix with a field of view (FOV) of 220 mm was used yielding an effective voxel size of 3.4375 × 3.4375 × 3.5 mm3. Head motion was minimized by using Velcro straps and foam padding.
Image Analysis
Functional images were preprocessed and statistically analyzed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). Images were realigned to correct for motion. Translation and rotation correction did not exceed 2 mm and 1.7°, respectively, for any of the participants. Images were spatially normalized into the anatomical space of the MNI brain template (Montreal Neurologic Institute) to accommodate intersubject variation in brain anatomy and to allow pixel‐by‐pixel averaging across subjects with a voxel size of 4 × 4 × 4 mm3 in the x, y and, z dimensions. These functional images were smoothed using a Gaussian filter of 8 × 8 × 8 mm3 to increase signal‐to‐noise ratio in the images. Random effects statistical analysis was performed at an intensity threshold of P = 0.001 uncorrected for all complex contrasts with an extent threshold of k = 5 voxels and all contrasts being inclusively masked by the minuend with P = 0.001 uncorrected to eliminate deactivations of the subtrahend becoming significant because of the subtraction (see results). Finally, coordinates of activations were transformed from MNI to Talairach space [Talairach and Tournoux, 1988] using the matlab function mni2tal.m implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
RESULTS
Behavioral Data
The mean accuracy level of performance in the auditory/auditory DAC as well as in the auditory/visual DAC was 95.7%, in the visual/visual DAC 96.5%. Numerically, even higher levels were achieved for the selective attention conditions with a rate of 98.4% correct answers in the auditory/auditory SAC and the auditory/visual SAC 2 as well as 99.2% in the visual/visual SAC and 99.6% in the auditory/visual SAC 1. (Fig. 2). The seven tasks, however, were compared by paired t‐tests and showed no significant differences for the level of accuracy (P > 0.05).
Figure 2.
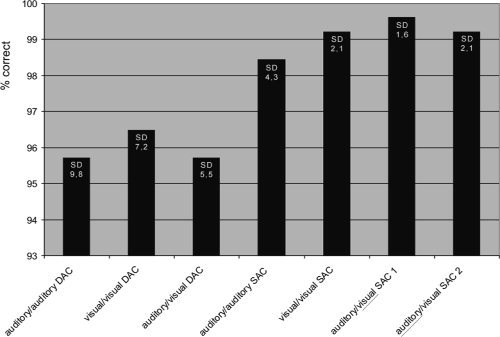
Mean% correct responses and their standard deviations (SD) in the three divided attention conditions auditory/auditory DAC, visual/visual DAC, auditory/visual DAC and the four selective attention conditions auditory/auditory SAC, visual/visual SAC, auditory/visual SAC1 (responses only to visual stimuli) as well as auditory/visual SAC2 (responses only to auditory stimuli).
Functional Magnetic Resonance Imaging
All DAC's were contrasted with their adjacent control tasks (SAC's) to discriminate structures that respond stronger to divided attention tasks. The mean image of the contrasts “auditory/auditory DAC vs. auditory/auditory SAC” and “visual/visual DAC vs. visual/visual SAC” (Fig. 3, Table I) displays typical activation of within‐modal divided attention tasks (“within‐modal DAC‐SAC”), irrespective of sensory modality. This contrast revealed right hemisphere only activation in the precentral gyrus, the middle frontal gyrus, the claustrum, and the inferior parietal lobule. On the other hand, the cross‐modal contrast “auditory/visual DAC vs. auditory/visual SAC” showed significant bilateral activation in the middle and superior frontal gyrus, the inferior and superior parietal lobule, as well as right hemisphere activation in the inferior frontal gyrus, the cingulate gyrus, claustrum, the precentral gyrus, insula, thalamus, the lateral globus pallidus, and finally left superior temporal gyrus activation (Fig. 3, Table II). In contrast, subtracting the DACs from their related SACs left no significant activation at all.
Figure 3.
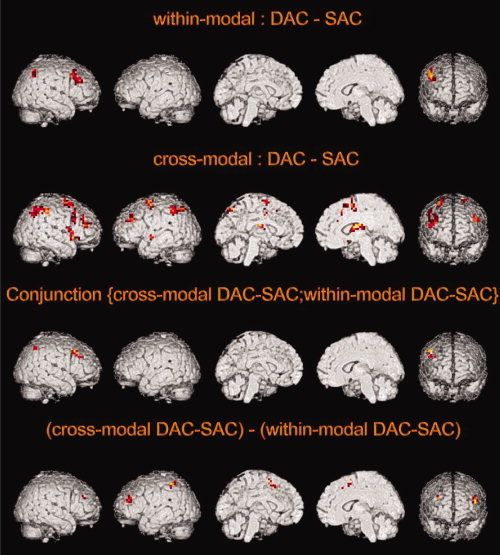
Functional activation map of the divided attention (DAC) minus selective attention (SAC) contrasts. Group‐averaged t‐maps (P < 0.001, uncorrected, inclusively masked by the minuend, mask: P < 0.001 uncorrected) for the contrasts: within‐modal (DAC‐SAC): mean of the contrasts auditory/auditory (DAC‐SAC) and visual/visual (DAC‐SAC); cross‐modal (DAC‐SAC): auditory/visual (DAC‐SAC); conjunction (cross‐modal DAC‐SAC; within‐modal DAC‐SAC): conjunction of the contrasts auditory/auditory (DAC‐SAC), visual/visual (DAC‐SAC), and auditory/visual (DAC‐SAC); (cross‐modal DAC‐SAC)—(within‐modal DAC‐SAC): auditory/visual (DAC‐SAC) contrasted by the mean of auditory/auditory (DAC‐SAC) and visual/visual (DAC‐SAC). The contrast (within‐modal DAC‐SAC)—(cross‐modal DAC‐SAC) left no significant activation.
Table I.
Mean significant differences in brain regions during the within‐modal divided attention tasks auditory/auditory and visual/visual compared with the adjacent auditory and visual selective attention control‐conditions
| Within‐modal: DAC‐SAC | |||||
|---|---|---|---|---|---|
| Brain region | Talairach coordinates | ||||
| x | y | z | z‐value | Voxels | |
| R precentral gyrus (BA 9) | 44 | 21 | 39 | 3.43 | 35 |
| R middle frontal gyrus (BA 9) | 44 | 32 | 28 | 3.15 | |
| R middle frontal gyrus (BA 10) | 36 | 36 | 24 | 3.09 | |
| R inferior parietal lobule (BA 40) | 36 | −52 | 39 | 3.28 | 22 |
| R claustrum | 32 | 12 | 3 | 3.11 | 5 |
P < 0.001 uncorrected; k = 5.
Table II.
Significant differences in brain regions during the cross‐modal divided attention task compared with the mean of the two related selective attention control‐conditions
| Cross‐modal: DAC‐SAC | |||||
|---|---|---|---|---|---|
| Brain region | Talairach coordinates | ||||
| x | y | z | z‐value | Voxels | |
| R claustrum | 32 | 8 | −4 | 4.73 | 67 |
| R precentral gyrus (BA 44) | 55 | 8 | 11 | 3.82 | |
| R insula (BA 13) | 44 | 12 | 3 | 3.78 | |
| R inferior frontal gyrus (BA 47) | 36 | 27 | −8 | 3.31 | |
| R middle frontal gyrus (BA 9/10/46) | 28 | 32 | 24 | 4.58 | 38 |
| R superior frontal gyrus (BA 10) | 36 | 51 | 20 | 3.29 | |
| L frontal lobe (sub‐gyral) (BA 6) | −24 | −1 | 55 | 4.52 | 45 |
| L superior frontal gyrus (BA 6) | −20 | 7 | 62 | 4.13 | |
| R inferior parietal lobule (BA 40) | 36 | −52 | 43 | 4.52 | 101 |
| R superior parietal lobule (BA 40) | 36 | −52 | 50 | 4.22 | |
| R thalamus | 4 | −11 | 19 | 4.38 | 54 |
| R lentiform nucleus (lateral globus pallidus) | 24 | −15 | 4 | 3.63 | |
| R middle frontal gyrus (BA 6) | 24 | 14 | 55 | 4.27 | 58 |
| R superior frontal gyrus (BA 6) | 20 | 3 | 66 | 3.37 | |
| L superior parietal lobule (BA 7) | −16 | −63 | 51 | 4.27 | 65 |
| L inferior parietal lobule (BA 40) | −44 | −44 | 46 | 4.06 | |
| R cingulate gyrus (BA 32) | 12 | 22 | 43 | 4.07 | 6 |
| L superior temporal gyrus (BA 22) | −48 | −4 | −3 | 3.75 | 8 |
| L middle frontal gyrus (BA 9/46) | −40 | 33 | 35 | 3.69 | 20 |
| R middle frontal gyrus (BA 8/9) | 44 | 21 | 28 | 3.48 | 17 |
P < 0.001 uncorrected; k = 5.
Furthermore, a conjunction analysis of the presented within‐ and cross‐modal DAC vs. SAC contrasts revealed a right hemisphere activation network consisting of the precentral gyrus, the superior frontal gyrus, the claustrum as well as the inferior and superior parietal lobule (Fig. 3, Table III). Finally, the subtraction of the mean within‐modal activations (“within‐modal DAC‐SAC”) from the cross‐modal ones (“cross‐modal DAC‐SAC”) left activation in the right middle frontal gyrus, the left superior, medial and middle frontal gyrus as well as the left inferior parietal lobule (Fig. 3, Table IV). The reversed contrast left no significant activation at all.
Table III.
Brain regions identified by the conjunction analysis of the two within‐modal contrasts “auditory/ auditory DAC‐SAC” and “visual/visual DAC‐SAC” and the cross‐modal contrast “auditory/visual DAC‐SAC”
| Conjunction {cross‐modal DAC‐SAC; within‐modal DAC‐SAC} | |||||
|---|---|---|---|---|---|
| Brain region | Talraich coordinates | ||||
| x | Y | z | z‐value | Voxels | |
| R precentral gyrus (BA 9) | 44 | 21 | 39 | 3.38 | 11 |
| R inferior parietal lobule (BA 40) | 40 | −52 | 50 | 3.10 | 5 |
| R superior parietal lobule (BA 7) | 32 | −56 | 51 | 3.95 | |
| R claustrum | 32 | 12 | 3 | 3.02 | 2 |
| R superior frontal gyrus (BA 9) | 36 | 36 | 28 | 2.96 | 1 |
P < 0.001 uncorrected; k = 0.
Table IV.
Significant differences in brain regions during the cross‐modal divided attention task “auditory/visual DAC‐SAC” compared with the within‐modal divided attention tasks “auditory/auditory DAC‐SAC” and “visual/visual DAC‐SAC”
| (Cross‐modal DAC‐SAC)—(within‐modal DAC‐SAC) | |||||
|---|---|---|---|---|---|
| Brain region | Talairach coordinates | ||||
| x | y | z | z‐value | Voxels | |
| L superior frontal gyrus (BA 9) | 0 | 7 | 55 | 4.19 | 15 |
| L medial frontal gyrus (BA 8) | −4 | 18 | 47 | 3.40 | |
| R middle frontal gyrus (BA 9) | 32 | 36 | 24 | 3.68 | 7 |
| L inferior parietal lobule (BA 40) | −48 | −44 | 54 | 3.46 | 9 |
| L middle frontal gyrus (BA 46) | −44 | 40 | 16 | 3.34 | 12 |
P < 0.001 uncorrected; k = 5.
DISCUSSION
In the present study we wanted to distinguish the cortical representations of within‐modal (auditory/auditory; visual/visual) vs. cross‐modal (auditory/visual) DAC by fMRI.
The three DACs did not differ behaviorally with respect to the accuracy of performance (95.7%–96.5%), which means that Wickens' behavioral predictions concerning within‐modal tasks being more difficult than cross‐modal tasks could not be confirmed by the behavioral data of our experiment. This finding may be due to a rather low overall difficulty level of the three DACs, although all participants subjectively rated the auditory/visual task being easier than either of the within‐modal conditions.
After subtraction of the related SAC, all DAC's revealed significant activation in the right prefrontal cortex, in the right inferior and superior parietal cortex, and in the right claustrum. The fronto‐parietal activations seem to be characteristic for divided attention tasks and for most other kinds of executive attentional processing irrespective of sensory modality [Pessoa et al., 2003], whereas the claustrum is considered to be involved especially in cross‐modal processing [Calvert, 2001; Ettlinger and Wilson, 1990; Hadjikhani and Roland, 1998]. Crick and Koch [2005] see an analogy for the role of the claustrum as a conductor coordinating a group of players in an orchestra. Although the claustrum displays the global maximum of activation in our cross‐modal DAC, it is also significantly activatedin the within‐modal DAC's which brings us to the assumption that the claustrum conducts also “different players with the same instruments.” In other words, the claustrum plays an important role in coordinating two tasks simultaneously irrespective if they are presented in the same or in two different sensory modalities.
According to Wickens' multiple resources theory, performing two tasks simultaneously in the same sensory modality should be more difficult than cross‐modal dual tasks. Therefore, one might assume that simultaneous execution of two tasks which are presented in the same sensory modality should call for a higher top‐down control than a dual task with cross‐modal shifting. Thus, under the within‐modal conditions a stronger frontal activation might be hypothesized than under the cross‐modal condition. The results however, revealed exactly the opposite pattern: the DAC vs. SAC contrasts showed a much more extended frontal activation for the cross‐modal condition. Contrasted with the within‐modal DACs, the cross‐modal DAC led to additional activation in the middle frontal gyrus bilaterally as well as to left hemisphere medial and superior frontal, and inferior parietal activation. One plausible explanation of these results might be that the supplementary fronto‐parietal activations in the cross‐modal DAC possibly reflect the greater demand for sensory coordination of two ongoing cross‐modal cognitive processes. This is in accordance with the results of the cross‐modal divided attention study of Loose et al. [2003].
Furthermore, we demonstrated enhanced right hemisphere anterior cingulate activation in the cross‐modal DAC. The cingulate gyrus is known to play an important role in attentional processing [Posner and Rothbart, 1998] and in more recent attentional taxonomies it is proposed to be the most central part of an “executive attention” network [Fernandez‐Duque and Posner, 2001; Fernandez‐Duque et al., 2000]. Banati et al. [2000] as well as Laurienti et al. [2003] showed that the anterior cingulate cortex is especially involved in executing cross‐modal tasks. Although many studies report increased activity of the cingulate cortex as a result of increased task difficulty [Banati et al., 2000; Buckner et al., 1996; Klingberg and Roland, 1997], the comparable level of accuracy of our three DACs makes it more probable that the cingulate cortex activation in the auditory/visual condition of our experiment is caused by the cross‐modal character of the task and not by a general increase of difficulty level under this condition. Furthermore, the cross‐modal DAC significantly activated right hemisphere thalamic structures, which are known to correlate with activations of the anterior cingulate cortex [Mottaghy et al., 2006; Paus et al., 1997; Sturm et al., 2004] in attention tasks. The thalamus is considered to operate as a gating system among the anterior cingulate, the dorsolateral prefrontal cortex, the inferior parietal cortex, and the noradrenergic brain stem part of the reticular activation system [Guillery et al., 1998; Steriade et al., 1986; Sturm et al., 1999; Stuss and Benson, 1986; Yingling and Skinner, 1975]. The increased activation of the anterior cingulate gyrus and the thalamus in the cross‐modal DAC thus reflects the higher demands on the gating system when the concurrent supply of activation needed for simultaneous processing of two tasks in different sensory modalities has to be controlled.
CONCLUSION
All three DACs lead to activations in the right prefrontal cortex, in the right inferior and superior parietal cortex as well as in the right claustrum. These areas might delineate the common network involved in distributing or dividing attention between two different tasks irrespective of sensory modality. In line with recent cross‐modal divided attention studies, there were additional bilateral middle and superior frontal activations in the cross‐modal rather than in the within‐modal DACs reflecting the greater demand for coordination of attentional resources for tasks performed simultaneously in two sensory modalities and thus a higher need for top‐down processing of attention [Corbetta and Shulman, 2002]. This notion is corroborated by the right hemisphere anterior cingulate cortex and thalamus activations in the cross‐modal auditory/visual DAC, reflecting the higher demand for controlling activation under this condition.
REFERENCES
- Adcock RA,Constable RT,Gore JC,Goldman‐Rakic PS ( 2000): Functional neuroanatomy of executive processes involved in dual‐task performance. Proc Natl Acad Sci USA 97: 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD ( 1993): Working memory or working attention? In: Baddeley AD,Weiskrantz L, editors. Attention: Selection, Awareness and Control. A tribute to Donald Broadbent. Oxford: Oxford University Press. [Google Scholar]
- Banati RB,Goerres GW,Tjoa C,Aggleton JP,Grasby P ( 2000): The functional anatomy of visual‐tactile integration in man: A study using positron emission tomography. Neuropsychologia 38: 115–124. [DOI] [PubMed] [Google Scholar]
- Buckner RL,Raichle ME,Miezin FM,Petersen SE ( 1996): Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci 16: 6219–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA,Klingberg T,Jacobsen RB,Gabrieli JD ( 2000): A resource model of the neural basis of executive working memory. Proc Natl Acad Sci USA 97: 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA ( 2001): Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex 11: 1110–1123. [DOI] [PubMed] [Google Scholar]
- Corbetta M,Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M,Miezin FM,Dobmeyer S,Shulman GL,Petersen SE ( 1991): Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT ( 1998): Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343–361. [DOI] [PubMed] [Google Scholar]
- Crick FC,Koch C ( 2005): What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 360: 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A,Pollmann S,Schubert T,Wiggins CJ,Von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Duncan J,Martens S,Ward R ( 1997): Restricted attentional capacity within but not between sensory modalities. Nature 387: 808–810. [DOI] [PubMed] [Google Scholar]
- Ettlinger G,Wilson WA ( 1990): Cross‐modal performance: Behavioural processes, phylogenetic considerations and neural mechanisms. Behav Brain Res 40: 169–192. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Duque D,Posner MI ( 2001): Brain imaging of attentional networks in normal and pathological states. J Clin Exp Neuropsychol 23: 74–93. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Duque D,Baird JA,Posner MI ( 2000): Executive attention and metacognitive regulation. Conscious Cogn 9: 288–307. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS ( 1987): Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr 6: 299–309. [DOI] [PubMed] [Google Scholar]
- Guillery RW,Feig SL,Lozsadi DA ( 1998): Paying attention to the thalamic reticular nucleus. Trends Neurosci 21: 28–32. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N,Roland PE ( 1998): Cross‐modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. J Neurosci 18: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen P,Jakobsen J,Bruhn P,Hansen SB,Gee A,Stodkilde‐Jorgensen H,Gjedde A ( 1997): Cortical sites of sustained and divided attention in normal elderly humans. Neuroimage 6: 145–155. [DOI] [PubMed] [Google Scholar]
- Klingberg T,Roland PE ( 1997): Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Brain Res Cogn Brain Res 6: 1–8. [DOI] [PubMed] [Google Scholar]
- Koechlin E,Basso G,Pietrini P,Panzer S,Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ,Wallace MT,Maldjian JA,Susi CM,Stein BE,Burdette JH ( 2003): Cross‐modal sensory processing in the anterior cingulate and medial prefrontal cortices. Hum Brain Mapp 19: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose R,Kaufmann C,Auer DP,Lange KW ( 2003): Human prefrontal and sensory cortical activity during divided attention tasks. Hum Brain Mapp 18: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ,Turkington TG,Provenzale JM,Hawk TC,Hoffman JM,Coleman RE ( 1997): Selective and divided visual attention: Age related changes in regional cerebral blood flow measured by H215O PET . Hum Brain Mapp 5: 389–409. [DOI] [PubMed] [Google Scholar]
- McDowell S,Whyte J,D'Esposito M ( 1997): Working memory impairments in traumatic brain injury: Evidence from a dual‐task paradigm. Neuropsychologia 35: 1341–1353. [DOI] [PubMed] [Google Scholar]
- Mesulam MM ( 1999): Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM,Willmes K,Horwitz B,Muller HW,Krause BJ,Sturm W ( 2006): Systems level modeling of a neuronal network subserving intrinsic alertness. Neuroimage 29: 225–233. [DOI] [PubMed] [Google Scholar]
- Norman DA,Shallice T ( 1986): Attention to action: Willed and automatic control of behaviour In: Davidson RJ,Schwartz GE, Shapiro D, editors. Consciousness and Self‐Regualtion. New York: Plenum Press. [Google Scholar]
- Paus T,Zatorre RJ,Hofle N,Caramanos Z,Gotman J,Petrides M,Evans aA ( 1997): Time‐related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci 9: 392–408. [DOI] [PubMed] [Google Scholar]
- Pessoa L,Kastner S,Ungerleider LG ( 2003): Neuroimaging studies of attention: From modulation of sensory processing to top‐down control. J Neurosci 23: 3990–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI,Rothbart MK ( 1998): Attention, self‐regulation and consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux M,Godefroy O,Cabaret M,Benaim C,Pruvo JP ( 1996): Analysis and course of cognitive deficits after rupture of aneurysms of the anterior communicating artery. Rev Neurol 152: 678–687. [PubMed] [Google Scholar]
- Steriade M,Domich L,Oakson G ( 1986): Reticularis thalami neurons revisited: Activity changes during shifts in states of vigilance. J Neurosci 6: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W,de Simone A,Krause BJ,Specht K,Hesselmann V,Radermacher I,Herzog H,Tellmann L,Muller‐Gartner HW,Willmes K ( 1999): Functional anatomy of intrinsic alertness: Evidence for a fronto‐parietal‐thalamic‐brainstem network in the right hemisphere. Neuropsychologia 37: 797–805. [DOI] [PubMed] [Google Scholar]
- Sturm W,Longoni F,Fimm B,Dietrich T,Weis S,Kemna S,Herzog H,Willmes K ( 2004): Network for auditory intrinsic alertness: A PET study. Neuropsychologia 42: 563–568. [DOI] [PubMed] [Google Scholar]
- Stuss DT,Benson DF ( 1986): The Frontal Lobes. New York: Raven Press. [Google Scholar]
- Szameitat AJ,Schubert T,Muller K,Von Cramon DY ( 2002): Localization of executive functions in dual‐task performance with fMRI. J Cogn Neurosci 14: 1184–1199. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotactic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- van Zomeren AH,van den Burg W ( 1985): Residual complaints of patients two years after severe head injury. J Neurol Neurosurg Psychiatry 48: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens CD ( 1984): Processing resources in attention In: Parasuraman R,Davies DR, editors. Varieties of Attention. New York: Academic Press. [Google Scholar]
- Yingling CD,Skinner JE ( 1975): Regulation of unit activity in nucleus reticularis thalami by the mesencephalic reticular formation and the frontal granular cortex. Electroencephalogr Clin Neurophysiol 39: 635–642. [DOI] [PubMed] [Google Scholar]


