Abstract
Objective: Poor decision‐making is a hallmark of addiction, whether to substances or activities. Performance on a widely used test of decision‐making, the Iowa Gambling Task (IGT), can discriminate controls from persons with ventral medial frontal lesions, substance‐dependence, and pathological gambling. Positron emission tomography (PET) studies indicate that substance‐dependent individuals show altered prefrontal activity on the task. Here we adapted the IGT to an fMRI setting to test the hypothesis that defects in ventral medial and prefrontal processing are associated with impaired decisions that involve risk but may differ depending on whether substance dependence is comorbid with gambling problems. Method: 18 controls, 14 substance‐dependent individuals (SD), and 16 SD with gambling problems (SDPG) underwent fMRI while performing a modified version of the IGT. Result: Group differences were observed in ventral medial frontal, right frontopolar, and superior frontal cortex during decision‐making. Controls showed the greatest activity, followed by SDPG, followed by SD. Conclusion: Our results support a hypothesis that defects in ventral medial frontal processing lead to impaired decisions that involve risk. Reductions in right prefrontal activity during decision‐making appear to be modulated by the presence of gambling problems and may reflect impaired working memory, stimulus reward valuation, or cue reactivity in substance‐dependent individuals. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: substance abuse, pathological gambling, decision‐making, fMRI, prefrontal cortex
INTRODUCTION
The Iowa Gambling Task (IGT) is a widely used instrument for assessing decision‐making under uncertainty [Bechara et al., 1994]. On this task, patients with medial and orbitofrontal lesions, pathological gamblers, and a subset of substance dependent individuals demonstrate preferences for short‐term gains at the risk of larger net losses [Bechara et al., 1994, 2000; Manes et al., 2002; Petry, 2001a, b]. Positron emission tomography (PET) studies during decision‐making using the IGT [Ernst et al., 2002] have yielded increased cerebral blood flow in ventral medial frontal, orbitofrontal, and cingulate cortex, areas frequently implicated in risky decision making. Compared with that of controls, these regions are also differentially affected in substance dependent individuals. Bolla et al. found dose‐related reductions in right orbitofrontal and dorsolateral prefrontal cortex in abstinent marijuana users compared with that of controls [Bolla et al., 2005]. Reductions in right dorsolateral prefrontal cortex were also found in abstinent cocaine users [Bolla et al., 2003]. Overall, these studies suggest that dysfunction of the right prefrontal neural network may underlie the poor decisions characteristic of substance users.
The extent to which separate brain regions can be linked to different components of the decision‐making process such as stimulus, decision, motor action, anticipation, and feedback response is limited by the temporal resolution of PET (∼1 min). In contrast, higher temporal resolution of functional MRI (fMRI) allows modeling of these components separately as recently demonstrated by Fukui et al. Medial frontal activity was observed during risky anticipation (high‐risk minus low‐risk selections) [Fukui et al., 2005]. While the paradigm in this fMRI study mimicked the original IGT, the lack of baseline scans where no decision was made but the task was identical in all other respects precluded evaluation of the decision‐making process per se.
Previous studies have also been limited by potentially confounding effects of the gambling nature of the task. Inherent in many decision‐making tasks that involve risk, including the IGT, are sensory gambling cues that, by themselves, may induce prefrontal activity in the absence of a required decision [Crockford et al., 2005]. This is important to consider because pathological gambling is highly comorbid with substance abuse. A survey of nearly 35,000 respondents revealed an odds ratio ranging from 3 to 6 between pathological gambling and drug abuse [Petry et al., 2005]. Thus, it is not clear whether the altered pattern of prefrontal activity observed in substance dependenceindividuals is related to substance abuse or reflects comorbid gambling problems.
In the current study, we address several of these limitations. First, we utilize fMRI to achieve higher temporal resolution than earlier PET studies using the IGT. Second, we carefully characterize our subject population to differentiate between individuals with substance dependence only when compared with those with substance dependence and gambling problems. Although projections from ventral tegmental area to the nucleus accumbens are well known to underlie acute positive drug reinforcement, research suggests that other structures such as the prefrontal cortex are likely to be critical in the development of drug addiction. Thus, our a priori hypothesis was that compared to controls,substance‐dependent individuals would show decreased ventral medial prefrontal activity during decision making that involved risk. We also predicted that, among substance‐dependent individuals, presence of gambling problems would be associated with greater prefrontal activity as a result of the gambling cues inherent in the IGT.
METHODS
Participants
56 participants were recruited (16 controls, 20 participants with substance dependence without pathological gambling (SD), and 20 participants with substance dependence and pathological gambling (SDPG)). Groups were matched for age, gender, and ethnicity. SD and SDPG were recruited from the Addiction Research and Treatment Service at UCDHSC, a long‐term residential treatment service. Substance dependent individuals had experienced serious substance abuse problems in the past and were required to be abstinent a minimum of 2months. Urine toxicology screening for opiates, stimulants, and marijuana was conducted at the time of MR scanning. SDPG were defined as having a South Oaks Gambling Screen (SOGS) scores ≥5 [Lesieur and Blume, 1987]. SD and controls had SOGS scores ≤1. All participants provided written informed consent approved by the Colorado Multiple Institutional Review Board.
Tests and Interviews
The following were administered to all participants:
Iowa Gambling Task Modified for fMRI
We used a modified version of the Iowa Gambling Task (IGT) [Bechara et al., 1994] appropriate for an fMRI setting, which avoids some confounds of the original task. As in the IGT, there were four decks of cards. Two of the decks (“good decks”) were associated with low payouts and low penalties, resulting in an overall net gain. The other two decks (“bad decks”) were associated with large payouts and large penalties resulting in an overall net loss. For each trial, participants were shown four decks, along with the instructions “Play or Pass” printed under one of the four decks. Participants decided whether to Play or Pass on each trial and pressed a button to indicate the response. If the subject chose Play, a monetary outcome was shown on the screen, and this number was added to the running total. Thus, in contrast to the IGT, the computer selected the card, rather than the subject, ensuring that differences in search strategy across the decks did not confound performance. In addition, participants received a single monetary outcome on all trials in which “Play” was chosen (e.g., +$100 or −$50), rather than receiving a constant reward and occasional punishment.
Participants began the task with a hypothetical $2,000 and were told they could earn $10.00 real dollars if they had more than $2,000 at the end of the task. Participants completed 64 “learning” trials while anatomical images were acquired, followed by 96 trials during functional scanning. Each trial was 14 s (4 s decision, 4 s outcome, 6 s during which participants viewed a “+” to allow the hemodynamic response to return close to baseline before the next trial). The first two scans (4 s) corresponding to the decision component comprised an event.
Decision condition
The subject was asked to decide whether to Play or Pass the card by pressing the appropriate button, followed by the outcome associated with that deck.
No decision condition
The task was identical to the decision condition except that participants were explicitly told which button to press (e.g., press Play), followed by the outcome associated with that deck. Participants were informed that although they would continue to win and lose money even if they did not want to make a Play response, they would still be able to learn about the payoff schedule of the decks to benefit later trials.
MR Image Acquisition
Images were acquired with a 3T GE whole body MR system (General Electric, Milwaukee, WI) using a standard quadrature head coil. A high‐resolution 3D T1‐weighted anatomical scan was acquired, followed by the functional scans using gradient‐echo echo‐planar imaging (EPI) (TR = 2,000, TE = 35, FA = 77, 642 matrix, 240 mm2 FOV, axial slices angled parallel to the AC‐PC line, 4 mm thick, 0 mm gap). Two runs of 337 scans per run were acquired. A set of inversion recovery images (IR‐EPI) (TR = 3,000, TE = 35, TI = 505, FA = 90) acquired at the same resolution and position as the functional images was used as an intermediate step in the spatial normalization process described later.
fMRI Data Analysis
Motion correction, spatial normalization, model specification and estimation, and statistical inference were performed with SPM2.
Preprocessing
After excluding the first seven volumes for saturation effects, images were motion‐corrected and resliced. Eight participants were excluded for head motion exceeding 2 mm, resulting in good quality data on 48 participants (14 control, 16 SD, 18 SDPG). EPI images were normalized to the Montreal Neurological Institute (MNI) template using a three‐part method that included an intermediate IR‐EPI dataset. EPI images were coregistered to the IR‐EPI series. The coregistered IR‐EPI images were segmented into gray matter, white matter, and CSF. The segmented gray matter images were normalized to the MNI gray matter template. Transformation parameters were then applied to the coregistered EPI series. Compared with normalizing functional gradient echo EPI directly to the EPI template, the three‐part method resulted in better normalization particularly in the orbitofrontal regions. A 6‐mm FWHM Gaussian smoothing kernel was applied to the normalized images.
First level fixed‐effects model specification
Data were analyzed using two‐stage mixed effects model. At the first level, two predictors were used to model fMRI data. The first predictor sought to ascertain which brain regions were activated when an individual made a decision as compared to when no decision was required. A hybrid block‐event related design was used in which the first two scans corresponding to the decision component of each trial comprised an event. The second predictor sought to ascertain which brain regions were more sensitive to wins or losses. This was a pure event‐related model that retrospectively extracted the fMRI response immediately following either a win or loss outcome for each trial. Trials of wins were compared with that of losses and vice versa, regardless of whether the individual made the decision. The third predictor was similar to the one used by previous investigators [Fukui et al., 2005] which sought to ascertain whether brain activity differed among groups during the contrast of risky > safe decks. In all cases, the modeled fMRI signal was convolved with the hemodynamic response function. Low frequency noise was removed using a high pass filter with cutoff of 128 s.
Brain activity for predictors 1 and 2 (2nd level mixed effects)
Predictor 1 (decision > no decision): A 1 sample t‐test of the parameter estimate was performed on all participants. Statistical maps were corrected for multiple comparisons using family‐wise error correction, and set at a threshold of P < 0.05 and minimum 50 contiguous voxels (∼0.5 cm3). Brain regions, Brodmann area, MNI coordinate, t‐value, and P‐values were reported for local maxima.
Predictor 2 (wins > losses or losses > wins): Because the total number of events for each subject was relatively low (∼80), we lacked power to analyze this condition with multiple comparisons correction. Therefore, statistical maps were set at a lower threshold of P < 0.001 and a spatial extent of 30 voxels.
Group difference in brain activity for predictors 1 and 2 (2nd level mixed effects)
Predictor 1.
Differences among the three groups for predictor 1 (decision > no decision) were analyzed using a second level one‐way ANCOVA with education and IQ as covariates. Maps were set at a threshold of P < 0.005 (F > 6, df = 2, 43) uncorrected for multiple comparisons and a spatial extent of 30 voxels. Brain regions, Brodmann area, MNI coordinate, and size of fMRI effect (β) was reported for each local maxima. Post‐hoc comparisons were made using Tukey's test.
Predictor 2.
We did not perform a second level ANOVA for the second predictor due to insufficient power.
Post‐hoc test of group differences in risky versus safe decisions
Regions identified as significantly different across groups for predictor 1 were selected for subsequent analysis using an event‐related model that compared the decision to play cards from risky (A, B) versus safe (C,D) decks. IQ and education were entered as covariates.
Analysis of Behavioral Data
Performance
In our version of the IGT, 160 trials were presented to the participant, half of which consisted of Decision and the other half passive or No decision. The 80 Decision trials were divided into five time periods. Therefore, each deck was viewed four times per time period (4 decks × 4 views × 5 time periods = 80) for the Decision condition. The net score (difference between number of times good versus bad cards were played) was calculated for each time period. A 3 (group) × 5 (time) repeated measures ANOVA on the net score was analyzed for main effects of group, time, and group by time interaction, after covarying for IQ and education.
RESULTS
Subject Characteristics
There was no difference between SDPG and SD in the number of substances on which they met criteria for dependence (CIDI‐SAM ≥3) (SDPG vs. SD, 3.8 (1.7) vs. 3.0 (1.0)). There was a trend for higher tobacco dependence among SDPG compared with SD and controls (P = 0.06, Pearson χ 2). Controls were more educated, had a higher IQ, and scored lower on measurements of impulsivity and sensation‐seeking compared with SD and SDPG (Table I). Although the groups were slightly unbalanced for gender, this was not significant (χ 2 = 2.98, 2 df).
Table I.
Characteristics of the study population
| SDPG (n = 20) | SD (n = 20) | Control (n = 16) | |
|---|---|---|---|
| Age | 35 (7) | 35 (7) | 37 (9) |
| Gender (M/F) | 12/8 | 10/10 | 5/11 |
| Education | 11 (2) | 11 (3) | *14 (2) |
| Ethnicity | |||
| Caucasian | 14 | 16 | 12 |
| African American | 3 | 0 | 2 |
| Hispanic | 3 | 2 | 2 |
| SOGSa | *10.7 (4.4) | 0.2 (0.4) | 0.1 (0.3) |
| Mean numberb | 3.8 (1.7) | 3.1 (1.0) | na |
| % Substance depc | |||
| Cocaine | 75% | 75% | na |
| Alcohol | 70% | 70% | na |
| Amphetamine | 70% | 60% | na |
| Cannabis | 55% | 60% | na |
| Opiates | 40% | 25% | na |
| Hallucinogens | 25% | 5% | na |
| Sedative | 15% | 5% | na |
| Club drugs | 0% | 10% | na |
| Inhalants | 0% | 0% | na |
| Phencyclidine | 0% | 0% | na |
| Tobacco | ***30% | 0% | 6.3% |
| IQ | 100 (11) | 103 (12) | **113 (9) |
| Impulsivity | 76 (15) | 73 (12) | *55 (8) |
| IMP‐SSd | 13 (6) | 12 (5) | ***7 (5) |
Data are mean (SD).
South Oaks Gambling Screen (SOGS) (Lesieur and Blume, 1987).
Mean number = Mean number of substances on which participants met criteria for dependence (CIDI‐SAM ≥3).
Substance dep, % of participants who met criteria for dependence for that drug.
Impulsivity and sensation seeking (IMP‐SS).
****P = 0.06, Pearson χ 2.
P < 0.005, control vs. SDPG, control vs. SD.
P < 0.01, control vs. SDPG, control vs. SD.
P < 0.05, control vs. SDPG, control vs. SD.
In‐Magnet Behavioral Data
Performance
Repeated measures ANOVA on net score revealed a significant main effect of time (F = 3.6, df = 4, P = 0.01). An increase in net score over time was consistent with learning. There was no main effect of group or interaction between group and time. Compared to SD, controls and SDPG had a higher net score on all but the second time block, but this was not significant. A partial η2 which describes the proportion of variability attributed to the factor time (linear model) for each group was 0.21, 0.14, 0.08 for control, SDPG, and SD, respectively (see Fig. 1).
Figure 1.
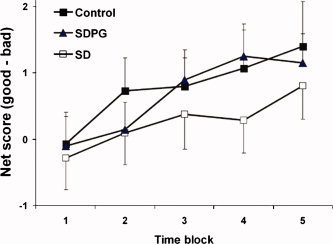
Performance on modified Iowa Gambling Task. All participants increased net score over time consistent with learning. Controls and SDPG had higher net scores than SD, but the difference was not statistically significant (data are mean ± SEM).
Predictor 1: Brain Activity During Decision‐Making
Group average.
Second‐level analysis of all participants (n = 48) revealed significant activity in right orbitofrontal, bilateral ventral lateral frontal/anterior insula, anterior cingulate, ventral medial frontal (BA 25), ventral striatum, parietal, and occipital lobes during decision‐making (Table II, Fig. 2).
Table II.
Regions of brain activity during “decision > no decision” across all participants (n = 48)
| Region | MNI coord | t | P | ||
|---|---|---|---|---|---|
| Right orbitofrontal (BA10) | 25 | 58 | −8 | 6.2 | 0.002 |
| Right anterior cingulate (BA 32) | 6 | 36 | 26 | 8.6 | 0.000 |
| Left anterior cingulate (BA 32) | −6 | 32 | 36 | 8.9 | 0.000 |
| Right ventral lateral frontal/anterior insula (BA 47) | 34 | 22 | −4 | 7.5 | 0.000 |
| 44 | 18 | 2 | 6.8 | 0.000 | |
| Left ventral lateral frontal/anterior insula (BA 47) | −30 | 24 | −8 | 6.5 | 0.001 |
| Left ventral medial prefrontal (BA 25) | −12 | 16 | −16 | 7.1 | 0.000 |
| Left nucleus accumbens | −8 | 10 | −8 | 6.6 | 0.001 |
| Right subgenual anterior cingulate (BA 25/11) | 12 | 20 | −10 | 6.0 | 0.005 |
| Left parietal (BA 7) | −28 | −66 | 44 | 6.1 | 0.003 |
| Right parietal (BA 7) | 34 | −64 | 40 | 6.1 | 0.003 |
| Left occipital (BA 18) | −26 | −90 | −4 | 6.8 | 0.000 |
| −24 | −88 | −12 | 6.2 | 0.002 | |
P‐values are corrected for multiple comparisons.
Figure 2.
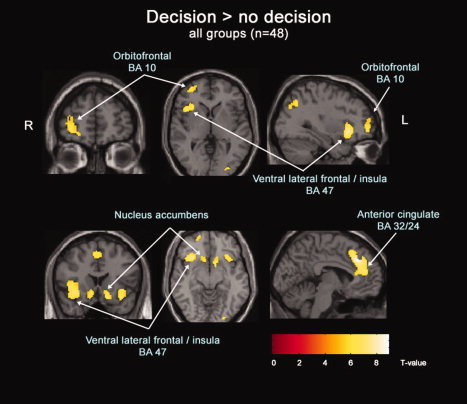
Second level analysis of brain regions active during decision‐making compared with no‐decision making. Statistical maps were corrected for multiple comparisons using family‐wise‐error and were set at a threshold P < 0.005.
Group differences.
After controlling for IQ and education, brain activity differed across groups in the ventral medial frontal (BA 25/11) (F 2,43 = 11.3, P < 0.001), right frontopolar (BA 10) (F 2,43 = 9.2, P < 0.001), and right superior frontal gyri (BA 9/10) (F 2,45 = 12.4, P < 0.001) (see Fig. 3). Post‐hoc comparisons showed that controls had greater ventral medial prefrontal activity than all substance dependent persons. Controls and SDPG had greater right superior frontal and frontopolar activity compared with SD (Table III, Fig. 4).
Figure 3.
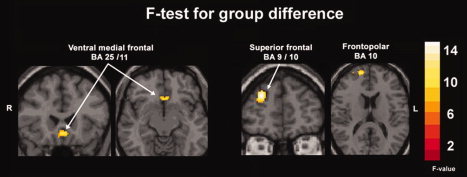
Second level ANCOVA statistical maps showing brain regions significantly different across three groups during decision‐making were ventral medial prefrontal, right superior frontal, right frontopolar and were set at threshold F > 6 (P < 0.005). Images are shown in radiological convention (right is on the left). Education and IQ were entered as covariates.
Table III.
Brain regions where activity differed across group during decision‐making
| Region | MNI | F (df 2,43) | fMRI effect size (β) | ||||
|---|---|---|---|---|---|---|---|
| Control | SDPG | SD | |||||
| Ventral medial frontal (BA 25/11) | −2 | 24 | −8 | 11.3 | 0.78* | −0.35 | −0.33 |
| Right superior frontal (BA 9/10) | 28 | 48 | 30 | 12.4 | 0.47 | 0.39 | −0.65*** |
| Right frontopolar (BA 10) | 18 | 58 | 14 | 9.2 | 0.28 | 0.32 | −0.52** |
SD, substance‐dependent; SDPG, substance‐dependent with gambling problems; BA, Brodmann area. IQ and education were entered as covariates.
Control vs. SD, control vs. SDPG, P < 0.005.
SD vs. control, SD vs. SDPG, P < 0.001.
SD vs. control, SD vs. SDPG, P < 0.005.
Figure 4.
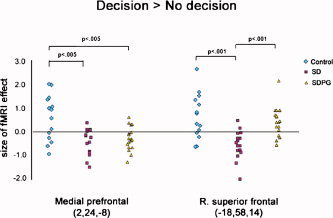
Size of fMRI effect during decision‐making for ventral medial frontal and right orbitofrontal regions (MNI coordinates shown) was largest in controls, followed by SDPG, followed by SD. Data represent size of fMRI effect (β) for individuals. Data were analyzed using ANOVA and post‐hoc Tukey's, controlling for education and IQ.
Predictor 2: Brain Activity During Wins Versus Losses and Losses Versus Wins
Group average.
For the condition of wins greater than losses, medial frontal lobe regions were activated. For the condition of losses greater than wins, lateral frontal regions showed greater activity (Fig. 5, P < 0.001, uncorrected for multiple comparisons). There was insufficient power to detect brain activity after correcting for multiple comparisons (total events ∼80 per subject).
Figure 5.
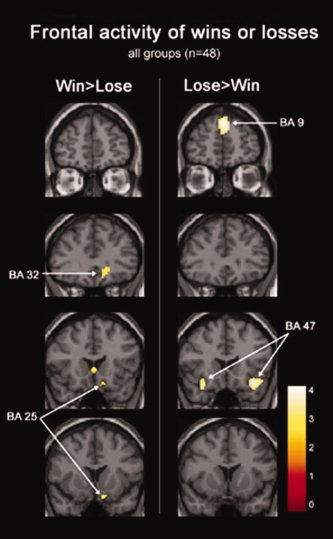
Second level event‐related analysis of brain activity during the experience of winning or losing. Medial inferior orbitofrontal regions (BA 25 (10, 14, −20) and BA 32 (16, 34, −6)) were more active during wins versus losses, whereas lateral inferior orbitofrontal regions (BA 47/12 (34, 20, −16) were more active during losses versus wins. Superior medial frontal (BA 9 (0, 52, 38)) was also more active during losses vs. wins. Color bar corresponds to t‐value.
Group differences.
There were no significant group differences for predictor 2.
Post‐Hoc Test of Group Differences in Risky Versus Safe Decisions
There was a significant group difference in ventral medial frontal activity when deciding to play risky as compared to safe decks (F = 4.5, df = 2, P = 0.02). Post‐hoc analysis showed that SDPG activated this region more than controls (parameter estimates: −64 ± 0.66, −0.27 ± 0.58, 0.16 ± 0.65, mean ± SD for controls, SD, SDPG, respectively). There were no group differences in right prefrontal regions.
DISCUSSION
Brain Activity During Decision‐Making Using the Modified IGT
Our study indicated that the brain regions activated during decision‐making involving risk, when compared with passively watching the computer make decisions on our modified version of the IGT, included orbitofrontal cortex, ventral medial frontal, ventrolateral frontal/anterior insula, anterior cingulate, ventral striatum, parietal, and occipital lobes. As such, our results are consistent with lesion [Bechara et al., 1994; Clark et al., 2003; Manes et al., 2002; Saver and Damasio, 1991], PET [Bolla et al., 2003, 2005; Ernst et al., 2002; Fishbein et al., 2005; London et al., 2000; Rogers et al., 1999a, b], and fMRI [Cohen et al., 2005; Elliott et al., 2000] studies implicating orbitofrontal cortex in reward‐related decision‐making processes. Our version of the modified gambling task appears to engage the same general neural circuitry as the standard gambling task.
It should be acknowledged that because in our modified IGT, the computer determines the deck that will be played, rather than the participant doing so, anticipation precedes as well as follows the decision. As such, the two components are not easily separated in our paradigm. Our primary objective was to examine the process in total, rather than specifically isolate anticipation from other components of decision making and to avoid having participants feel pressured to make a “split‐second” decision, which would introduce an element of randomness.
Orbitofrontal and ventral medial prefrontal cortex
Our results are consistent with previous PET studies of the IGT [Bolla et al., 2003, 2005; Ernst et al., 2002] in yielding prefrontal, anterior cingulate, and parietal lobe activity. fMRI studies have shown orbitofrontal cortex (OFC) activity during decision‐making involving guesswork [Elliott et al., 1999], uncertainty and risk [Cohen et al., 2005], and encoding stimulus reward value [O'Doherty, 2004; O'Doherty et al., 2001b]. The OFC activity we observed is within BA10 and more dorsal than that reported in most imaging studies of decision‐making where it lies closer to BA 10/11. Our local maxima are similar, however, to that of Rogers et al., who also used a decision‐making task invoking risk and conflict [Rogers et al., 1999b]. In this context, it is interesting to note that impairment on the IGT was, in fact, greater for patients with dorsal prefrontal lesions compared with orbitofrontal lesions [Manes et al., 2002], suggesting a prominent role of more dorsal areas of prefrontal cortex in decision making. The activity was most prominent on the right side which is consistent with previous work showing preferential right orbitofrontal prefrontal activity during decision‐making [Elliott et al., 1999; Ernst et al., 2002] and a right laterality effect in lesion studies [Clark et al., 2003]. Implications of ventral medial prefrontal cortex vmPFC activity are discussed below in the section on group differences.
Anterior cingulate cortex
We observed activity in anterior cingulate cortex (ACC), consistent with previous PET studies using the IGT [Ernst et al., 2002], but in contrast to studies using the Rogers Decision‐making task (RDMT) which involves choosing between likely, but small risk and reward, or unlikely, but large risk and reward [Rogers et al., 1999a]. A major difference between the tasks is that the IGT involves learning. These findings support the notion that ACC is involved in stimulus‐reinforcement learning and performance monitoring, especially when predictability is low and rate of errors is high. From the viewpoint of a naïve subject, outcomes in the RDMT are more predictable than in the IGT because the probability of reward is explicitly given on every trial. ACC has also been implicated in reward expectancy [Shidara and Richmond, 2002], high risk decision making [Cohen et al., 2005], and self‐selected responses [Elliott et al., 1999].
Ventral lateral frontal/anterior insula
Activity in the ventral lateral frontal regions reported here (BA 47) is almost identical in location to that observed by Rogers et al. in their PET study [Rogers et al., 1999b]. It has been postulated that this region mediates retrieval or is involved in inhibitory function.
Nucleus accumbens
The nucleus accumbens within the ventral striatum has long been implicated in drug reinforcement and reward anticipation. In animals trained in cocaine self‐administration, neurons in the nucleus accumbens fire prior to the lever press and are thought to reflect anticipation [Carelli and Deadwyler, 1996]. Knutson et al. has shown that anticipation of increasing rewards activates ventral striatum in healthy participants [Knutson et al., 2001]. Given that our paradigm examined anticipation along with decision, it is possible that the activity we observed in the nucleus accumbens was related to the anticipation or unpredictability of reward.
Occipital/parietal
Increased activation of occipital and parietal activation during decision‐making could reflect greater visual attention or resource allocation [Banich, 2004] necessitated by having to actively make a decision rather than passively watching the computer do so. Superior parietal activity has been reported in previous studies of decision‐making [Rogers et al., 1999b].
Group Differences in Brain Activity During Decision‐Making
The key finding of this study was that, compared with controls, substance‐dependent individuals showed lower ventral medial frontal activity, supporting the a priori hypothesis that this sample group would show altered ventral medial frontal activity during decisions involving risk. In addition, we observed less right prefrontal activity during decision‐making in nongambling substance abusers compared with gambling substance abusers or controls. Later, we discuss the group differences observed for these regions.
Ventral medial prefrontal cortex/infragenual ACC
We found that compared with controls both groups with substance dependence showed less activation of this region with the maximal group difference at the border of BA 25 and 11. The ventral medial prefrontal cortex (VMPFC), which consists of infra‐ and prelimbic cortex, is not a clearly defined structure [Milad and Quirk, 2002] and has been considered to include both BA 11 (Paulus et al., 2002; Schnyer et al., 2005) and BA 25 (also called infragenual anterior cingulate) [London et al., 2004]. Hence, our findings are consistent with impairment on the IGT found in patients with bilateral VMPFC lesions [Bechara et al., 1994] and support a hypothesis that defects in ventral medial frontal processing lead to impaired decisions that involve risk.
BA 25 and 11 are both implicated in drug addiction. Medial BA 11 may mediate motivation, reward salience, and impulse control, whereas BA 25 may mediate affective state, emotional reactivity, visceromotor function, and drug craving [Volkow et al., 2005]. Also consistent with our findings, Bolla et al. found lower medial frontal activity in heavy compared with light marijuana users and in both groups compared with controls, although the differences compared with controls were more lateral within BA 11 [Bolla et al., 2005]. Further evidence of abnormal function in BA 25 in substance users is lower glucose metabolism observed during a continuous performance task [London et al., 2004]. It is unlikely that group differences in brain activity were due to differences in performance, since any group differences in behavior were slight, at best. The fact that substance‐dependent individuals (gambling and non‐gambling) had lower VMPFC activity during decision‐making, despite similar performance, suggests that fMRI may be a more sensitive probe of VMPFC dysfunction in this group that, by definition, takes greater risks despite adverse consequences.
Retrospective, event‐related modeling of the decision to play risky compared with safe decks revealed greater ventral medial/infragenual activity in gamblers with SD compared with that in controls. The finding would be consistent with a heightened visceral reaction during the selection and anticipation of risky cards as would be expected in a gambler. Further studies will be necessary to confirm the finding given the overall low power.
Right anterior prefrontal (frontopolar BA 10, superior frontal BA 9/10)
In this region there was less activity for nongambling substance abusers than the other two groups. For the purposes of this discussion, we will consider the two peaks (frontopolar and superior frontal) together as they are near each other and credibly localize to BA 10 since the activity was at the border of superior BA 10. Consistent with our findings, Paulus has reported reduced right prefrontal and dorsolateral frontal activity during a two‐choice prediction task in methamphetamine users compared with that in controls [Paulus et al., 2002, 2003]. Using PET imaging, Bolla et al. reported lower activity in right prefrontal cortex in abstinent marijuana users compared with that in controls during decision making using the IGT. The local maximum in their study (20, 40, 33) is very close to ours (28, 48, 30). Northoff et al. found that performance on the IGT could be predicted by activity during an affective judgment task at a peak (16, 66, 8) close to the one at which we observe group differences (18, 58, 14), suggesting that this region in our task may be involved in affective judgments [Northoff et al., 2006]. The altered prefrontal activity in substance dependent individuals that we observed was generally more anterior and dorsal than the orbitofrontal cortex, which has been implicated in drug addiction. Several studies suggest that orbitofrontal cortex dysfunction in drug users may reflect deficits in reward and risk assessment, motivation, emotion, craving, and goal‐directed behavior [Kalivas and Volkow, 2005; London et al., 2000]. It is uncertain why the local maximum in our study was more dorsal than OFC, although our results are consistent with a study showing that patients with dorsal prefrontal lesions were more impaired on the IGT than those with orbitofrontal lesions, independent of lesion volume [Manes et al., 2002]. In general, less consideration has been given to defining the function of the rostral prefrontal (frontopolar) as compared with orbitofrontal. In contrast to these studies, increased right prefrontal activity was seen in cocaine addicts compared to controls performing the IGT [Bolla et al., 2003]. Further work will be needed to explain these differences.
Among substance users, the presence of gambling problems was associated with greater right prefrontal activity, possibly reflecting hypersensitivity to gambling cues. Pathological gamblers showed greater orbitofrontal and right DLPFC activity compared with controls during visual gambling‐related cues [Crockford et al., 2005]. Another possibility is that the higher right prefrontal activity reflects better stimulus‐reinforcement learning by the SDPG group. In fact, similar to controls, they learned to differentiate the good and bad decks slightly faster than nongambling SD. A third possible explanation of the difference among substance users is that prefrontal cortex may act to bias responses based on previously experienced rewards and punishments [Frank and Claus, 2006]. That activity was lowest in the SD suggests that this group is less able to maintain reward and/or punishment information from trial to trial, and thus may be less sensitive to that particular type of information during decisions.
Feedback Response to Wins Compared With Losses
Kringelbach and Rolls suggest two distinct functional patterns within the orbitofrontal cortex: a medial‐lateral trend representing value of the reinforcers and a posterior‐anterior trend representing complexity. Medial regions appear to be more sensitive to reward while lateral regions more sensitive to punishment [Kringelbach and Rolls, 2004]. Abstract reinforcers (e.g., monetary contingencies) tended to activate anterior orbitofrontal cortex while primary reinforces (e.g., taste or smell) tended to activate posterior orbitofrontal cortex. Consistent with the medial‐lateral distinction of reinforcement value, we observed a nonsignificant trend of inferior medial activity in ‘Win > loss’ and lateral activity in ‘loss > win’ [Kringelbach and Rolls, 2004; O'Doherty et al., 2001].
We did not find significant activation in ventral striatum during reward as has been shown by others [Breiter et al., 2001; Knutson et al., 2000]. There are several possible reasons. First, there were relatively few punishment trials which may have led to poor estimates of the model and low power. Second, in the analysis of wins > losses, we included wins and losses in both the Decision and No Decision condition. Thus, it is conceivable that expectations were different during the No Decision trials, and activity in the striatum was washed out. Third, since our trial onset time was not jittered with respect to the TR, it is possible that the hemodynamic response function was not modeled precisely at the correct time with regard to the outcome response, thus reducing the sensitivity for small activations. As our primary aim was to investigate group differences in neural processes during decision‐making not necessarily the response to outcome, our findings of no activity in nucleus accumbens are not necessarily contrary to previous studies.
Behavioral Performance on Modified IGT
There were no differences in the net score (difference in choices from good versus bad decks) between controls and substance‐dependent individuals. Although most studies find lower performance in substance‐dependent individuals compared with that in controls we did not. As shown by Bolla et al., such differences in behavioral performance are not necessary to observe differences in brain activation. In that study, individuals addicted to cocaine had differences in prefrontal activity compared to controls in the absence of significant differences in performance on IGT [Bolla et al., 2003]. The ability of IGT to discriminate between substance users and controls in adolescents has not been as consistent as in the adult population [Aklin et al., 2005; Ernst et al., 2003; Lejuez et al., 2003].
Our modified version differs from the original IGT in several ways, which may help to explain why we did not observe significant group differences in performance. In the original task, the subject is free to select any of four decks of cards, whereas in the modified version a deck is presented to the subject who then decides to play or pass, thus assuring that participants make a decision about each deck an equal number of times. Second, to provide a suitable baseline for fMRI analysis, a No decision block was included in which the subject is told whether to Play or Pass. Third, in the IGT, trials are self‐paced and presented in quick succession whereas in the fMRI version, cards were presented at a fixed rate and each trial was followed by 6 s of fixation. The effect sizes (η2) for the behavioral data suggests that SD performed worst, while controls and SDPG performed similarly well. In spite of these differences, we feel the task is comparable to the original IGT and the fMRI analysis benefits from the added constraints. The performance mimics those of the classic IGT task. Over time, participants learn to select cards from the low reward but good decks as compared to high reward but bad decks. In the IGT, it is reasonable to assume that within every trial, participants consider (not necessarily consciously) potential gains and losses (or an overall expected valence [Busemeyer and Stout, 2002]) associated with each card, and that cards with higher expected valence are more likely to be chosen. Similarly, when participants are required to make a Play/Pass decision in our task, the cognitive process of comparing the previous gains and losses is likely engaged. The decision is made if a particular threshold for expected valence is exceeded. One could argue that the decks in the IGT are approached in a similar fashion, where each expected valence is assessed and a Play/Pass (Go/NoGo; Approach/Avoid) choice is made for each deck. Thus, although the pace is different, the decision process is likely to be very similar in the original and modified IGT. The modified task remains complex (no subject was able to articulate a strategy for deck selection) with low predictability, features thought to be essential for eliciting emotional input into the decision. Presenting a single outcome rather than allowing a free choice eliminates the problem of not knowing whether the subject is more focused on gain or loss. Using this modification, Peters and Slovic found that affective reactivity correlated in predicted ways with performance (e.g., positive reactivity was associated with attraction to high‐gain decks while negative reactivity was associated with avoidance of high‐risk decks) [Peters and Slovic, 2003]. Thus, while the modified tasks is not the same as the original, there is evidence that affectivity is involved in the decision.
Our finding that SDPG was similar to or better than SD is inconsistent with that of Petry et al., who found that gambling substance users were more impaired on IGT compared with nongambling substance users [Petry, 2001b]. In that study, the degree of impairment was not overwhelming, with the gambling substance users showing only a mild preference for deck B. Small samples and a nonsignificant difference in females may explain the behavioral findings. In a previous study, female controls showed lower performance compared with female substance users, whereas male controls showed better performance compared with male substance users [Bechara and Martin, 2004; Stout et al., 2005]. Therefore, the proportionately large number of female controls may contribute to the lack of significant group differences in behavior.
Limitations
A limitation of this study was the difference in education and IQ between the controls and substance dependent individuals. It was difficult to recruit controls of similar IQ and education level because the potential controls often turned out to have a history of drug use. Group effects were significant after controlling for education, IQ, and gender using ANCOVA. The lack of neuropsychological tests of affect, memory, and attention is a limitation as group differences in brain activity may reflect group differences in these functions. Regarding the diagnosis of pathological gambling, the SOGS [Lesieur and Blume, 1987] is a screening instrument and not a diagnostic assessment. Nonetheless, a score of five or greater identifies the presence of gambling problems.
CONCLUSION
Despite these limitations, the present study suggests that impaired decision‐making in chronic substance users may be mediated by abnormalities in ventral medial frontal processing. The decrease in right prefrontal activity in substance dependent individuals as compared to those whose substance dependence is comorbid with pathological gambling may reflect differences in stimulus reward valuation, cue‐reactivity, or the ability to maintain information on recent gain or loss.
Acknowledgements
The authors wish to thank Ken Gaipa and Julie Miller from the Addiction Research Treatment Service for their support and Nicole Johnson for assistance in recruitment.
Presented at the Organization for Human Brain Mapping 2006, Florence, Italy.
REFERENCES
- Aklin WM,Lejuez CW,Zvolensky MJ,Kahler CW,Gwadz M ( 2005): Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behav Res Ther 43: 215–228. [DOI] [PubMed] [Google Scholar]
- Banich M ( 2004): Cognitive Neuroscience and Neuropsychology, 2nd ed Houghton Mifflin Co., Boston, MA, 252–285. [Google Scholar]
- Bechara A,Martin EM (2004): Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology 18: 152–162. [DOI] [PubMed] [Google Scholar]
- Bechara A,Damasio AR,Damasio H,Anderson SW ( 1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A,Tranel D,Damasio H ( 2000): Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 (Part 11): 2189–2202. [DOI] [PubMed] [Google Scholar]
- Bolla KI,Eldreth DA,London ED,Kiehl KA,Mouratidis M,Contoreggi C,Matochik JA,Kurian V,Cadet JL,Kimes AS,Funderburk FR,Ernst M ( 2003): Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision‐making task. Neuroimage 19: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI,Eldreth DA,Matochik JA,Cadet JL ( 2005): Neural substrates of faulty decision‐making in abstinent marijuana users. Neuroimage 26: 480–492. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Aharon I,Kahneman D,Dale A,Shizgal P ( 2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 619–639. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR,Stout JC ( 2002): A contribution of cognitive decision models to clinical assessment: Decomposing performance on the Bechara gambling task. Psychol Assess 14: 253–262. [DOI] [PubMed] [Google Scholar]
- Carelli RM,Deadwyler SA ( 1996): Dual factors controlling activity of nucleus accumbens cell‐firing during cocaine self‐administration. Synapse 24: 308–311. [DOI] [PubMed] [Google Scholar]
- Clark L,Manes F,Antoun N,Sahakian BJ,Robbins TW ( 2003): The contributions of lesion laterality and lesion volume to decision‐making impairment following frontal lobe damage. Neuropsychologia 41: 1474–1483. [DOI] [PubMed] [Google Scholar]
- Cohen MX,Heller AS,Ranganath C ( 2005): Functional connectivity with anterior cingulate and orbitofrontal cortices during decision‐making. Brain Res Cogn Brain Res 23: 61–70. [DOI] [PubMed] [Google Scholar]
- Cottler LB,Schuckit MA,Helzer JE,Crowley T,Woody G,Nathan P,Hughes J ( 1995): The DSM‐IV field trial for substance use disorders: Major results. Drug Alcohol Depend 38: 59–69. [DOI] [PubMed] [Google Scholar]
- Crockford DN,Goodyear B,Edwards J,Quickfall J,El Guebaly N ( 2005): Cue‐induced brain activity in pathological gamblers. Biol Psych 58: 787–795. [DOI] [PubMed] [Google Scholar]
- Elliott R,Rees G,Dolan RJ (1999):Ventromedial prefrontal cortex mediates guessing.Neuropsychologia 37: 403–411. [DOI] [PubMed] [Google Scholar]
- Elliott R,Friston KJ,Dolan RJ ( 2000): Dissociable neural responses in human reward systems. J Neurosci 20: 6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M,Bolla K,Mouratidis M,Contoreggi C,Matochik JA,Kurian V,Cadet JL,Kimes AS,London ED ( 2002): Decision‐making in a risk‐taking task: A PET study. Neuropsychopharmacology 26: 682–691. [DOI] [PubMed] [Google Scholar]
- Ernst M,Grant SJ,London ED,Contoreggi CS,Kimes AS,Spurgeon L ( 2003): Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry 160: 33–40. [DOI] [PubMed] [Google Scholar]
- Fishbein DH,Eldreth DL,Hyde C,Matochik JA,London ED,Contoreggi C,Kurian V,Kimes AS,Breeden A,Grant S ( 2005): Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res 23: 119–136. [DOI] [PubMed] [Google Scholar]
- Frank MJ,Claus ED ( 2006): Anatomy of a decision: Striato‐orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev 113: 300–326. [DOI] [PubMed] [Google Scholar]
- Fukui H,Murai T,Fukuyama H,Hayashi T,Hanakawa T ( 2005): Functional activity related to risk anticipation during performance of the Iowa gambling task. Neuroimage 24: 253–259. [DOI] [PubMed] [Google Scholar]
- Kalivas PW,Volkow ND ( 2005): The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry 162: 1403–1413. [DOI] [PubMed] [Google Scholar]
- Knutson B,Westdorp A,Kaiser E,Hommer D ( 2000): FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12: 20–27. [DOI] [PubMed] [Google Scholar]
- Knutson B,Adams CM,Fong GW,Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21(RC159): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML,Rolls ET ( 2004): The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol 72: 341–372. [DOI] [PubMed] [Google Scholar]
- Lejuez CW,Aklin WM,Zvolensky MJ,Pedulla CM ( 2003): Evaluation of the balloon analogue risk task (BART) as a predictor of adolescent real‐world risk‐taking behaviours. J Adolesc 26: 475–479. [DOI] [PubMed] [Google Scholar]
- Lesieur HR,Blume SB ( 1987): The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am J Psychiatry 144: 1184–1188. [DOI] [PubMed] [Google Scholar]
- London ED,Ernst M,Grant S,Bonson K,Weinstein A ( 2000): Orbitofrontal cortex and human drug abuse: Functional imaging. Cereb Cortex 10: 334–342. [DOI] [PubMed] [Google Scholar]
- London ED,Simon SL,Berman SM,Mandelkern MA,Lichtman AM,Bramen J,Shinn AK,Miotto K,Learn J,Dong Y,Matochik JA,Kurian V,Newton T,Woods R,Rawson R,Ling W ( 2004): Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61: 73–84. [DOI] [PubMed] [Google Scholar]
- Manes F,Sahakian B,Clark L,Rogers R,Antoun N,Aitken M,Robbins T ( 2002): Decision‐making processes following damage to the prefrontal cortex. Brain 125: 624–639. [DOI] [PubMed] [Google Scholar]
- Milad MR,Quirk GJ ( 2002): Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74. [DOI] [PubMed] [Google Scholar]
- Northoff G,Grimm S,Boeker H,Schmidt C,Bermpohl F,Heinzel A,Hell D,Boesiger P ( 2006): Affective judgment and beneficial decision making: Ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp 27: 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP ( 2004): Reward representations and reward‐related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol 14: 769–776. [DOI] [PubMed] [Google Scholar]
- O'Doherty J,Kringelbach ML,Rolls ET,Hornak J,Andrews C ( 2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4: 95–102. [DOI] [PubMed] [Google Scholar]
- Patton JH,Stanford MS,Barratt ES ( 1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51: 768–774. [DOI] [PubMed] [Google Scholar]
- Paulus MP,Hozack N,Frank L,Brown GG,Schuckit MA ( 2003): Decision making by methamphetamine‐dependent subjects is associated with error‐rate‐independent decrease in prefrontal and parietal activation. Biol Psychiatry 53: 65–74. [DOI] [PubMed] [Google Scholar]
- Paulus MP,Hozack NE,Zauscher BE,Frank L,Brown GG,Braff DL,Schuckit MA ( 2002): Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine‐dependent subjects. Neuropsychopharmacology 26: 53–63. [DOI] [PubMed] [Google Scholar]
- Peters E,Slovic P ( 2003): The springs of action: Affective and analytical information processing in choice. Pers Soc Psychol 26: 1465–1475. [Google Scholar]
- Petry NM ( 2001a): Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol 110: 482–487. [DOI] [PubMed] [Google Scholar]
- Petry NM ( 2001b): Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend 63: 29–38. [DOI] [PubMed] [Google Scholar]
- Petry NM,Stinson FS,Grant BF ( 2005): Comorbidity of DSM‐IV pathological gambling and other psychiatric disorders: Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 66: 564–574. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation ( 1999). Wechsler Abbreviated Scale of Intelligence; San Antonio, TX. [Google Scholar]
- Rogers RD,Everitt BJ,Baldacchino A,Blackshaw AJ,Swainson R,Wynne K,Baker NB,Hunter J,Carthy T,Booker E,London M,Deakin JF,Sahakian BJ,Robbins TW ( 1999a): Dissociable deficits in the decision‐making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan‐depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339. [DOI] [PubMed] [Google Scholar]
- Rogers RD,Owen AM,Middleton HC,Williams EJ,Pickard JD,Sahakian BJ,Robbins TW ( 1999b): Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19: 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL,Damasio AR ( 1991): Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia 29: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Schnyer DM,Nicholls L,Verfaellie M ( 2005): The role of VMPC in metamemorial judgments of content retrievability. J Cogn Neurosci 17: 832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M,Richmond BJ ( 2002): Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science 296: 1709–1711. [DOI] [PubMed] [Google Scholar]
- Stout JC,Rock SL,Campbell MC,Busemeyer JR,Finn PR ( 2005): Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav 19: 148–157. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Wang GJ,Ma Y,Fowler JS,Wong C,Ding YS,Hitzemann R,Swanson JM,Kalivas P ( 2005): Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine‐addicted subjects but not in controls: Relevance to addiction. JNeurosci 25: 3932–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]


