Abstract
Understanding the neurobiological substrates of self‐recognition yields important insight into socially and clinically critical cognitive functions such as theory of mind. Experimental evidence suggests that right frontal and parietal lobes preferentially process self‐referent information. Recognition of one's own face is an important parameter of self‐recognition, but well‐controlled experimental data on the brain substrates of self‐face recognition is limited. The goal of this study was to characterize the activation specific to self‐face in comparison with control conditions of two levels of familiarity: unknown unfamiliar face and the more stringent control of a personally familiar face. We studied 12 healthy volunteers who made “unknown,” “familiar,” and “self” judgments about photographs of three types of faces: six different novel faces, a personally familiar face (participant's fraternity brother), and their own face during an event‐related functional MRI (fMRI) experiment. Contrasting unknown faces with baseline showed activation of the inferior occipital lobe, which supports previous findings suggesting the presence of a generalized face‐processing area within the inferior occipital‐temporal region. Activation in response to a familiar face, when contrasted with an unknown face, invoked insula, middle temporal, inferior parietal, and medial frontal lobe activation, which is consistent with an existing hypothesis suggesting familiar face recognition taps neural substrates that are different from those involved in general facial processing. Brain response to self‐face, when contrasted with familiar face, revealed activation in the right superior frontal gyrus, medial frontal and inferior parietal lobes, and left middle temporal gyrus. The contrast familiar vs. self produced activation only in the anterior cingulate gyrus. Our results support the existence of a bilateral network for both perceptual and executive aspects of self‐face processing that cannot be accounted for by a simple hemispheric dominance model. This network is similar to those implicated in social cognition, mirror neuron matching, and face‐name matching. Our findings also show that some regions of the medial frontal and parietal lobes are specifically activated by familiar faces but not unknown or self‐faces, indicating that these regions may serve as markers of face familiarity and that the differences between activation associated with self‐face recognition and familiar face recognition are subtle and appear to be localized to lateral frontal, parietal, and temporal regions. Hum. Brain Mapping, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: self‐face recognition, face recognition, consciousness, social cognition, self‐awareness, right hemisphere, social cognitive neuroscience
INTRODUCTION
There is mounting evidence that the capacity for self‐recognition is distinct from familiar face‐recognition [Keenan et al., 2000] and exists only in higher primates [Gallup, 1970]. It has also been argued that self‐referent processing takes place predominantly in the right hemisphere (RH) [Craik et al., 1999; Keenan et al., 2003; Platek et al., 2004; but see Turk et al., 2002] and is prerequisite for a mature theory of mind [Gallup, 1982; Keenan et al., 2003; Povinelli et al., 1999]. To date the intrahemispheric substrates of self‐face recognition have not been fully characterized nor have studies utilized personally familiar, gender‐matched facial stimuli as a control.
Multiple lines of evidence from studies of patients with RH lesions or hemispheric disconnection syndromes indicate that RH function is critical to recognizing one's own face. In postcommissurotomy patients, the galvanic skin response (GSR) to pictures of the self presented to the RH was twice as large as when the faces were presented to the left hemisphere (LH) [Preilowski, 1977] and the RH has been shown to be capable of identifying self‐face as accurately as the LH [Sperry et al., 1979]. Split‐brain patients, asked to discriminate self from familiar faces, respond faster with their left hand, also suggesting an RH advantage [Keenan et al., 2004]. Patients presented with and image of self‐face morphed with a famous face during intracarotid amobarbitol (IAT/WADA) evaluations failed to recognize their own face in the morphed image when the RH was anesthetized [Keenan et al., 2001]. Patients who perceive their reflection in a mirror as that of an imposter (“mirror sign”) have abnormal RH neuropsychological function, further supporting the critical role of RH function in self‐face recognition [Breen, 1999; Breen et al., 2001; Feinberg, 2001; Spangenberg et al., 1999].
Data from more recent behavioral and neuroimaging studies support the findings of the earlier lesion studies. Right‐handed subjects asked to identify self, famous, and novel faces with a button press responded to self‐face, but not famous or novel faces, faster with their left than their right hand. Assuming contralateral motor control, this finding supports the hypothesis of RH dominance in self‐face processing [Keenan et al., 1999, 2000; Platek and Gallup, 2002; Platek et al., 2003a, b, 2004]. Although most data support RH predominance for self‐processing, Turk et al. [ 2002] found LH dominance for self‐face recognition in a split‐brain patient.
Overall, the behavioral and lesion data points to an RH dominance in self‐face processing; however, the functional imaging data on the intrahemispheric substrates of self‐face recognition is less conclusive. Both O15 PET and BOLD fMRI studies have revealed a pattern of activation that was frequently bilateral and varied in its spatial distribution.
In two fMRI studies, Kircher et al. [ 2000, 2001] found differential activation in left prefrontal and middle temporal cortex when comparing self‐face to unknown faces and right middle temporal gyrus and left inferior frontal and parietal lobe activation when contrasting self with subjects' romantic partner. Sugiura et al. [ 2000] investigated passive and active recognition of one's own face in nine subjects using O15 PET. When comparing active and passive viewing of one's own face, activation in left fusiform gyrus, right supramarginal gyrus, left putamen, and right hippocampus was reported. A direct contrast of active discrimination of self‐face from foil faces with passive viewing of self‐face showed activation in right inferior and medial frontal gyri and right anterior cingulate gyrus. This study did not dissociate the effect of familiarity and self‐face recognition. More recently, Platek et al. [ 2004] contrasted fMRI response to self‐face and a familiar famous face and found activation in right superior frontal gyrus. Kircher et al. [ 2001] attempted to match familiarity by using photographs of a subject's romantic partner and found greater bilateral activation including left inferior frontal gyrus, middle temporal gyrus, supramarginal and inferior parietal lobe, and right insula and hippocampus [see also Kircher et al., 2000]. Sugiura et al. [ 2004] compared brain activity during exposure to self‐face, the familiar face of a personal friend, and a learned face. The self‐face condition was associated with activation in the right occipitotemporal junction, frontal operculum, posterior cingulate, and left fusiform gyrus.
So far, consensus regarding the neural substrates of self‐face recognition has not been achieved. A potential source of disparate findings is the variability in control conditions, particularly on the dimension of familiarity leading to patterns representing a combination of activity related to self‐, as well as general face recognition, target recognition, and emotional processing, each of which has been shown to invoke distinct neural networks [e.g., Herzmann et al., 2004; Mohr et al., 2002]. For example, studies that used familiar famous [Platek et al., 2004; Sugiura et al., 2004; but see Herzmann et al., 2004] or romantic partner [Kircher et al., 2001] faces as contrast stimuli did not quantify or control the degree of familiarity. Moreover, romantic partner face may invoke activity related not only to familiarity but also to the emotional and reward value of these stimuli. Another source of across‐study variability could be the paradigm design. All neuroimaging studies of self‐face to date have employed either blocked or slow event‐related paradigm designs and therefore have limited ecological and experimental validity and suffer from diminished power to detect functional correlates of behavior [D'Esposito et al., 1999]. Furthermore, studies that employ morphed facial stimuli, purportedly to reduce habituation, results in a “mismatch between the internal representation of the face and the externally presented version” [Kircher et al., 2000, p. 134]. As a result, these studies may have tapped cognitive processes related to kin recognition/self‐referent phenotype matching [Platek et al., 2004, 2005].
The goal of the present study was to identify the brain regions specifically involved in self‐face recognition when controlling for familiarity. Improvements in experimental design include the use of six novel distracter faces matched for age, gender, ethnicity, and level of education, and quantifying the duration and quality of familiarity of nonself faces. Additionally, we employed a fast event‐related design at high field strength (3 T). We hypothesized that: 1) contrasting distracter faces with the null condition (scrambled face) would result in significant activation of the “face areas” (e.g., fusiform gyrus and inferior occipital gyrus) [Kanwisher et al., 1997; Rossion et al., 2003]; 2) familiar face compared to unknown face would show reduced activity in the amygdala [Schwartz et al., 2003; Rossion et al., 2003]; and 3) self‐face contrasted with personally familiar faces would reveal greater activation in right prefrontal and parietal areas [e.g., Lou et al., 2004; Platek et al., 2004; Seger et al., 2004] and medial structures such as the precuneus and medial frontal lobes [Northoff and Bermpohl, 2004].
SUBJECTS AND METHODS
Subjects
Twelve healthy (screened for drug use, physical, neurological and psychiatric history, and contraindications with MRI) right‐handed male University of Pennsylvania undergraduate students (mean age = 19.36, SD = 0.5) were recruited in triads that consisted of the subject and two comembers of the subject's university fraternity (i.e., fraternity brothers). The study was approved by the local Institutional Review Board at the University of Pennsylvania. Participants were paid US$50 for their participation. Recruitment was restricted to males in order to maintain uniformity among our sample.
Photographs
Prior to scanning, all subjects were photographed using a 5 megapixel color digital camera (Nikon Coolpix 5000) under uniform lighting conditions and matched for luminance using Adobe PhotoShop (San Jose, CA) (see Fig. 1). For the photograph, individuals were instructed to maintain a neutral facial expression. For each triad, one image of a fraternity brother was randomly selected as the familiar face control. Pictures of unknown (unfamiliar) individuals were obtained by taking pictures of 40 students from a different university, matched for multiple demographic indices (e.g., age, ethnicity, level of education). Six unknown (unfamiliar) faces were randomly selected from the database of distracter faces for inclusion as distracter faces in each subject's task. All images were presented in color (self‐face was presented mirror‐reversed; i.e., as they would appear in a mirror).
Figure 1.

Example screen shot of the experimental paradigm.
Familiarity Scale
In order to control for the degree of familiarity with the faces, participants completed a familiarity scale for each familiar and distracter face. Parameters of familiarity were: length of acquaintance (0 = less than 1 month, 2 = since college, 4 = since high school, 6 = since junior high school, 8 = since grade school, 10 = all your life); frequency of interaction (0 = less than once per year, 2.5 = annually, 5 = monthly, 7.5 = weekly, 10 = daily); like/dislike (–5 = intensely dislike, –3.5 = dislike, –2 = moderately dislike, 0 = neither like nor dislike, 2 = moderately like, 3.5 = like, 5 = intensely like); and trust/distrust (–5 = intensely distrust, –3.5 = distrust, –2 = moderately distrust, 0 = neither trust nor distrust, 2 = moderately trust, 3.5 = trust, 5 = intensely trust).
fMRI Procedures
Imaging data were acquired using blood oxygenation level‐dependent (BOLD) imaging [Bandettini et al., 1992] on a clinically approved 3 T Siemens Trio Scanner (Iselin, NJ). A 5‐min magnetization‐prepared, rapid acquisition gradient echo image (MPRAGE) was acquired for anatomic overlays of functional data and spatial normalization [Talairach and Tournoux, 1988]. BOLD imaging used a 33‐slice whole‐brain, single‐shot gradient‐echo (GE) echo‐planar (EPI) sequence (TR/TE = 2000/21 ms, FOV = 240 mm, matrix = 64 × 64, slice thickness/gap = 4/0 mm). This delivered a nominal voxel resolution of 3.75 × 3.75 × 4 mm.
Paradigm Design
The study was designed to allow direct contrast between BOLD responses to self‐faces and personally familiar faces. A fast event‐related sequence consisting of 308 trials, in a 7‐min session, was employed. Subjects responded to four conditions: self‐face, familiar face, six unknown (distracter) faces (see Fig. 1), and a null condition (luminance matched scrambled face with central fixation point) (see Fig. 1). Six unknown distracters were used to limit the probability that subjects would simply habituate to the unknown face and make responses without first having to make an evaluative decision. Self‐face and familiar face appeared 30 times each; each distracter face appeared 10 times (total 60), and the null condition was presented 188 times. Each face was presented for 500 ms with an interstimulus interval ranging from 1.5–16 s. Subjects were asked to make “self,” “known,” or “unknown” responses as quickly and as accurately as possible using a three‐button fiber optic response pad (fORP, Current Designs). A practice session was given prior to scanning to familiarize them with the response pad and procedures.
Image Analysis
Functional MRI (fMRI) data were preprocessed in SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Images were motion‐corrected to the median image using b‐spline interpolation (4° of freedom), slice time‐corrected, highpass‐filtered (100 s), and spatially smoothed (8 mm FWHM, isotropic). The median functional and anatomical volumes were coregistered then transformed into the standard anatomical space (T1 MNI template) using the trilinear interpolation [Ashburber and Friston, 1999].
Subject‐level statistical analyses were performed using the general linear model in SPM2. The three condition events were modeled using a canonical hemodynamic response function. Contrast maps were obtained through the following linear contrasts of event types: unknown face vs. scrambled face, familiar face vs. unknown face, and self‐face vs. familiar face. Group‐level random effects analyses were accomplished by entering whole brain contrasts into one‐sample t‐tests and resulting SPM{T} maps were transformed to unit normal distribution SPM{Z} maps [Friston et al., 1994, 1995]. A significance threshold based on spatial extent using a height of z ≥ 3.82 and cluster probability P = 0.05 [Forman et al., 1995] was applied to the effects of interest and surviving voxels were retained for further analyses. These contrasts are presented at an uncorrected P‐value of 0.001 requiring a minimum of 8 voxels [Worsley et al., 1996].
RESULTS
Familiarity Ratings
Fraternity brothers' pictures were rated as familiar with uniform duration of acquaintance (mean = 2 years ± 0.00). All subjects reported high rates of interaction (9.9 ± 0.32), likeableness (4.0 ± 0.67), and trust (3.9 ± 0.88) for fraternity brothers, indicating high personal familiarity. No subject reported familiarity with any of the distracter faces.
Behavioral Responses
Subjects correctly classified faces as self, familiar, or unknown 80% or more of the time, which was significantly better than chance (P < 0.01). Subjects were slightly better at identifying unknown distracter faces than self or familiar faces, χ2(2) = 14.829, P < 0.001 (see Fig. 2). A repeated measures ANOVA revealed a difference in reaction time to face stimuli as well (F(2,20) = 13.197, P < 0.001). Post‐hoc analysis revealed that subjects responded significantly faster to the unknown distracter faces than to either self or familiar faces (P < 0.001; see Fig. 3). Reaction time to self and familiar face did not differ.
Figure 2.
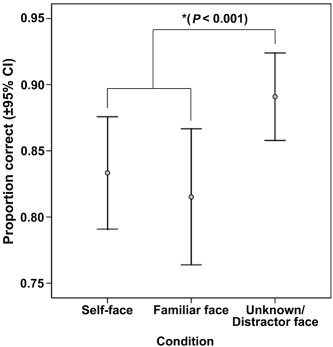
Mean proportion correct (±95% confidence interval) identification of faces.
Figure 3.
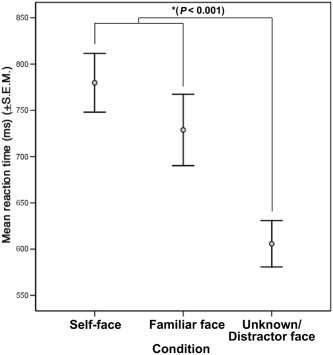
Mean reaction time (ms) (±SEM) for each face stimulus.
fMRI Data
BOLD signal associated with distracter faces was significantly higher than the signal associated with the null (scrambled face) control condition in the inferior occipital gyrus, frontal lobes, and anterior cingulate (e.g., BA 47, 32, 9, 10, 6), precuneus, and posterior cingulate gyrus (Fig. 4a, Table I).
Figure 4.
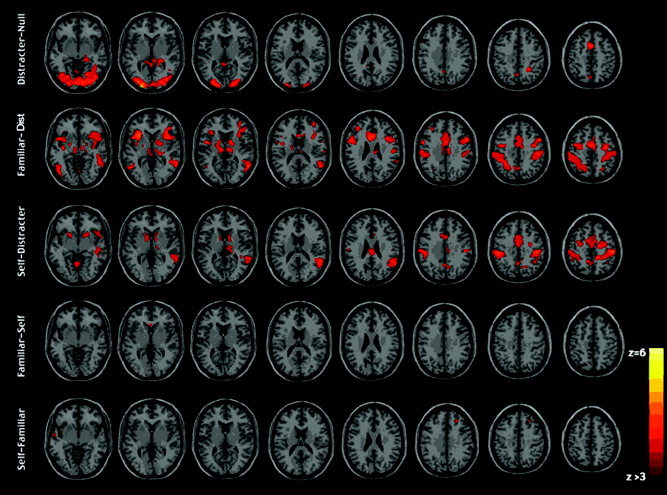
a: Significant activation when contrasting an unknown face (distracter) with scrambled face luminance matched crosshair control stimuli. b: Significant activation associated with familiar face recognition when contrasted to the unknown distracter face. c: Significant activation when contrasting self‐face with unknown distracter face. (a–c cluster detection corrected to P < 0.001, cluster P < 0.05). d: Significant activation when contrasting familiar face with self‐face. e: Self‐face with familiar face (d,e corrected for spatial extent using height threshold P < 0.001, minimum of 8 voxels cluster size; no cluster detection or statistical correction). Images are displayed in neurological convention.
Table I.
Local Maxima of CBF change during distracter minus null contrast corrected at P = 0.001 and minimum cluster size at P = 0.05
| Region (BA) | Hemisphere | x | y | z | Size | Z‐score |
|---|---|---|---|---|---|---|
| Inferior occipital gyrus (18) | R | 30 | −84 | −6 | 10178 | 6.07 |
| Medial frontal gyrus (32) | L | 0 | 10 | 48 | 743 | 4.25 |
| Parahippocampal gyrus (27) | R | 24 | −34 | 0 | 696 | 4.15 |
| Precuneus (7) | R | 26 | −48 | 42 | 161 | 4.07 |
| Cingulate gyrus (31) | L | −8 | −44 | 30 | 410 | 3.84 |
| Middle frontal gyrus (6) | R | 36 | −4 | 56 | 236 | 3.71 |
When familiar faces were contrasted with unknown faces, significant increases in activity over baseline were found in the insula (BA 13), middle temporal gyrus (BA 22), inferior parietal lobule (BA 40), and the middle frontal gyrus (BA 6,10). There was no activation in the temporal‐occipital face region that survived statistical thresholding (see Fig. 4b, Table II).
Table II.
Local maxima of CBF change during familiar minus distracter contrast, corrected at P = 0.001 and minimum cluster size P = 0.05
| Region (BA) | Hemisphere | x | y | z | Size | Z‐score |
|---|---|---|---|---|---|---|
| Sub‐gyral (21) | R | 42 | −6 | −14 | 11202 | 5.59 |
| Insula (13) | L | −32 | 18 | 4 | 2099 | 5.36 |
| Cerebellum | L | −20 | −58 | −36 | 5038 | 5.20 |
| Middle temporal gyrus (22) | R | 50 | −46 | 0 | 1532 | 5.19 |
| Inferior pareital lobule (40) | L | −50 | −38 | 44 | 2825 | 4.44 |
| Middle frontal gyrus (6) | L | −40 | −2 | 44 | 1226 | 4.4 |
| Middle frontal gyrus (10) | L | −34 | 40 | 22 | 198 | 4.17 |
Contrasting self‐face with the distracter face activated right postcentral gyrus (BA 2), supramarginal gyrus (BA 40), and superior temporal gyrus (BA 22), and left cerebellum and bilateral lentiform nucleus (see Fig. 4c, Table III).
Table III.
Local maxima of CBF change during self minus distracter contrast corrected at P = 0.001 and cluster at P = 0.05
| Region (BA) | Hemisphere | x | y | z | Size | Z‐score |
|---|---|---|---|---|---|---|
| Postcentral gyrus (2) | R | 54 | −22 | 48 | 9777 | 4.82 |
| Cerebellum | L | −22 | −76 | −26 | 4394 | 4.70 |
| Supramarginal gyrus (40) | R | 58 | −48 | 24 | 1619 | 4.63 |
| Lentiform nucleus | L | −12 | 8 | −2 | 337 | 4.20 |
| Lentiform nucleus | R | 12 | 8 | −2 | 352 | 4.05 |
| Thalamus | R | 16 | −28 | 10 | 213 | 3.70 |
| Superior temporal gyrus (22) | R | 56 | 12 | −6 | 197 | 3.63 |
Contrasting familiar face with self‐face revealed significant activation in only the anterior cingulate (BA 24) (see Fig. 4d, Table IV), whereas contrasting self‐face with familiar face revealed significant activation in the right superior frontal gyrus (BA 21), medial frontal gyrus (BA 11), inferior parietal lobule (BA 39), and left middle temporal gyrus (BA 21) (see Fig. 4e, Table V).
Table IV.
Local Maxima of CBF change during familiar minus self contrast, uncorrected P = 0.001 and minimum cluster size = 8
| Region (BA) | Hemisphere | x | y | z | Size | Z‐score | P (uncorr) |
|---|---|---|---|---|---|---|---|
| Anterior cingulate (24) | L | −2 | 24 | −2 | 62 | 3.80 | < 0.001 |
Table V.
Local maxima of CBF change during self minus familiar contrast, uncorrected P = 0.001 and minimum cluster size = 8
| Region (BA) | Hemisphere | x | y | z | Size | Z‐score | P (uncorrected) |
|---|---|---|---|---|---|---|---|
| Middle temporal gyrus (21) | L | −52 | 4 | −16 | 29 | 4.36 | < 0.001 |
| Superior frontal gyrus (9) | R | 26 | 34 | 34 | 80 | 4.29 | < 0.001 |
| Medial frontal gyrus (11) | R | 6 | 48 | −12 | 27 | 3.96 | < 0.001 |
| Inferior parietal lobule (39) | R | 50 | −62 | 40 | 9 | 3.63 | < 0.001 |
| Middle temporal gyrus (21) | L | −58 | −6 | −4 | 25 | 3.57 | < 0.001 |
| Superior frontal gyrus (6) | R | 20 | 16 | 56 | 22 | 3.33 | < 0.001 |
| Middle frontal gyrus (6) | R | 26 | −12 | 46 | 8 | 3.31 | < 0.001 |
DISCUSSION
Consistent with our hypothesis, we found that novel face processing is subserved by neural networks that include both the “face areas” of the brain [Halgren et al., 1999; Kanwisher et al., 1997], such as the inferior occipital gyrus, as well as regions in the right middle temporal gyrus and bilateral frontal lobes. This activation pattern is in agreement with existing literature [Kanwisher et al., 1997] and confirms the robustness of our paradigm.
Contrasting familiar face with unknown face revealed activation in the right middle temporal lobe and left middle frontal gyrus and inferior parietal lobe. Contrary to what we predicted, we did not find activation of the “face areas” to familiar faces. In agreement with existing literature [Rossion et al., 2003], discrimination between familiar and unfamiliar faces was subserved by a different set of neural substrates. In contrast to existing literature [Schwartz et al., 2003], which showed amygdala activation to novel faces, neither novel nor familiar face activated the amygdala in our study.
And finally, contrasting self‐face with unknown face revealed activation in the right postcentral, supramarginal, and superior temporal gyri. In direct contrast between self and familiar face, we found activation in a set of bilateral substrates that included the right superior frontal gyrus, inferior parietal and medial frontal lobes, and left middle temporal gyrus. These data are particularly compelling because our study used a rigorous control procedure for measuring familiarity with the familiar face stimuli and our behavioral data suggest that discrimination between self and familiar was more cognitively challenging than classifying unknown faces as unknown (see Figs. 2, 3).
The right superior frontal gyrus and inferior parietal lobe activation to self‐face is consistent with the right hemisphere/frontal‐parietal model of self‐awareness [Decety and Chaminade, 2003; Gallup et al., 2003; Jackson and Decety, 2004; Keenan et al., 2001, 2003a, b; Lou et al., 2004; Platek et al., 2004; Sugiura et al., 2000] and our working hypotheses. Additionally, our findings of activation to self‐face in the left middle temporal gyrus and right medial frontal lobe indicate that the network responsible for self‐face recognition is more extensive than previously thought. The left temporal and bilateral medial activation (BA 6, 9, 11, 21, 39) is consistent with the hypothesis that self‐processing is part of a social cognitive system (i.e., theory of mind) related to broader levels of self‐awareness [Gallup, 1982; Haxby et al., 2000; Iacoboni et al., 1999]. A left hemisphere model of self‐awareness [Kircher et al., 2000; Turk et al., 2002] has been proposed as an alternative to the prevailing RH model; however, our results may be used to reconcile the left and right hemisphere models of self‐awareness and supports a more complex bilateral network [Kircher et al., 2001] for both perceptual and executive aspects of self‐face processing that cannot be reduced to a simplistic hemispheric dominance model. The fact that familiar minus self only activated the anterior cingulate may be related to the emotional‐attentional demands associated with responding to personally familiar faces [Fichtenholtz et al., 2004].
Our data suggest that discrimination between self and other, and specifically between self and familiar other, activates right prefrontal cortex and inferior parietal lobe. Frontal lobe activation is likely important for active discrimination between self and other [Sugiura et al., 2000], while parietal lobe activation might be the result of representing self‐face as part of a general awareness of one's own body [Jackson and Decety, 2004], but this demands further investigation. We hypothesize [see also Gallup, 1982] that activation with self should mobilize neural resources needed to engage in social cognitive processes such as theory of mind (i.e., medial prefrontal lobe) [e.g., Fletcher et al., 1995; Gallagher et al., 2000; Platek et al., 2004] and other self‐referent processing (e.g., self‐referent adjective classification) [Fossatti et al., 2004], which may also involve aspects of the mirror neuron system (e.g., inferior parietal lobe) [Rizzolatti et al., 1996; Rizzolatti and Craighero, 2004]. The left anterior temporal lobe activation associated with self‐face recognition may be a result of one or both of two possible processes associated with this region. First, our task may have tapped verbal labeling of names to faces (see Fig. 1) [e.g., Gorno‐Tempini et al., 1998]. Second, the anterior temporal lobes have also been implicated in social cognitive processes, such as theory of mind [Baron‐Cohen et al., 1999; Platek et al., 2004].
Our study had several limitations. The sample size was powered to demonstrate group effects, but insufficient to examine correlates of individual differences in the BOLD response to self‐face presentations. It was also limited to males, which precludes generalization of our interpretations to females, but also indicates an important direction for future research. We limited our sample to males to maintain uniformity of our sample. Previous studies included subjects' romantic partners as controls for familiarity [Kircher et al., 2001] and mixed gender samples [Platek et al., 2004]. Because the existence of sex differences in self‐processing has not been experimentally characterized, inclusion of mixed sex samples may have produced confounded results. The use of only one self and familiar face, but six novel distracter faces, may have also affected our results and may account for the increased activation in the self‐distracter and familiar‐distracter conditions. However, as a way of increasing our confidence that subjects were making real unknown classifications of the unknown stimulus faces, as opposed to employing a simpler strategy (e.g., fixation on one pixel), we used six unknown distracters. It appears from our behavioral data that this procedure might have increased the cognitive demand on discriminating self from familiar other (see Figs. 2, 3). Future research could investigate the role of varied numbers of distracters on behavioral performance and neurocognitive activation.
In conclusion, we found a robust activation specific to self‐face recognition in a cohort of fraternity brothers rigorously matched and controlled for the length and quality of interpersonal acquaintance and multiple demographic indices. Our findings extend the existing hypotheses of the functional neuroanatomy of self‐awareness and indicate that a distributed bilateral set of substrates encompassing right frontal and parietal regions as well as left temporal and medial frontal lobes is responsible for self‐awareness in humans. This is true also for the unique processing of familiar faces. It is important to note that the self vs. familiar and familiar vs. self contrasts produced activation maps primarily in frontal regions. This finding is consistent with the notion that the capacity to process complex information about self and others is computed by recently evolved neural substrates [Gallup, 1998]. This notion is speculative and demands cross‐species investigation, which is currently under way in a number of laboratories. Additionally, we found that general (i.e., distracter vs. null) and familiar face recognition are subserved by substrates that are different from those involved in self‐recognition, suggesting that self‐face recognition is at least somewhat unique from processing other types of facial information and stimuli.
REFERENCES
- Ashburber J, Friston KJ (1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron‐Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC (1999): Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 11: 1891–1898. [DOI] [PubMed] [Google Scholar]
- Breen N (1999): Misinterpreting the mirrored self. Paper presented at the Association for the Scientific Study of Consciousness, London, Ontario, Canada.
- Breen N, Caine D, Coltheart M (2001): Mirrored‐self misidentification: two case of focal onset dementia. Neurocase 7: 239–254. [DOI] [PubMed] [Google Scholar]
- Craik IM, Moroz T, Moscovitch M, Stuss DT, Wincour G, Tulving E, Kapur S (1999): In search of the self: a positron emission tomography study. Psychol Sci 10: 26–34. [Google Scholar]
- Decety J, Chaminade T (2003): When the self represents the other: a new cognitive neuroscience view on psychological identification. Conscious Cogn 12: 577–596. [DOI] [PubMed] [Google Scholar]
- Feinberg TE (2001): Altered egos: how the brain creates the self. New York: Oxford University Press. [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS (2004): Emotion‐attention network interactions during a visual odd‐ball task. Cogn Brain Res 20: 67–80. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD (1995): Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 57: 109–128. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 210–220. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD (2000): Reading the mind in cartoons and stories: an fMRI study of 'theory of mind' in verbal and nonverbal tasks. Neuropsychologia 16: 814–821. [DOI] [PubMed] [Google Scholar]
- Gallup GG Jr (1970): Chimpanzees: self‐recognition. Science 167: 86–87. [DOI] [PubMed] [Google Scholar]
- Gallup GG Jr (1982): Self‐awareness and the emergence of mind in primates. Am J Primatol 2: 237–248. [DOI] [PubMed] [Google Scholar]
- Gallup GG Jr (1997) On the rise and fall of self‐conception in primates In: Snodgrass JG, Thompson RL, editors. The self across psychology: self‐recognition, self‐awareness, and the self‐concept Ann N Y Acad Sci 818: 73–82. [DOI] [PubMed] [Google Scholar]
- Gallup GG Jr, Anderson JR, Platek SM (2003): Self‐awareness, social intelligence, and schizophrenia In: David AS, Kircher T, editors. The self and schizophrenia: a neuropsychological perspective. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gorno‐Tempini ML, Price CJ, Josephs O, Vendenberghe R, Cappa SF, Kapur N, Frackowiak RS, Tempini ML (1998): The neural systems sustaining face and proper‐name processing. Brain 121: 2103–2118. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dale AM, Sereno MI, Tootell RBH, Marinkovich K, Rosen B (1999): Location of human face‐selective cortex with respect to retinotopic areas. Hum Brain Mapp 7: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Decety J (2004): Motor cognition: a new paradigm to study self‐other interactions. Curr Opin Neurobiol 14: 259–263. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP, McCutcheon B, Freund S, Gallup GG Jr, Sanders G, Pascual‐Leone A (1999): Left hand advantage in a self‐face recognition task. Neuropsychologia 37: 1421–1425. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Freund S, Hamilton RH, Ganis G, Pascual‐Leone A (2000): Hand response differences in a self‐face identification task. Neuropsychologia 38: 1047–1053. [DOI] [PubMed] [Google Scholar]
- Keenan JP, McCutcheon B, Pascual‐Leone A (2001a): Functional magnetic resonance imaging and event related potentials suggest right prefrontal activation for self‐related processing, Brain Cogn 47: 87–91. [Google Scholar]
- Keenan JP, Nelson AM, Pascual‐Leone A (2001b): Self‐recognition and the right hemisphere. Nature 409: 305. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Gallup GG Jr, Falk D (2003a): The face in the mirror: the search for the origins of consciousness. New York: HarperCollins/Ecco. [Google Scholar]
- Keenan JP, Wheeler MA, Ewers M (2003b): The neuropsychology of self In: David AS, Kircher T, editors. The self and schizophrenia: a neuropsychological perspective. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Keenan JP, Wheeler MA, Platek SM, Lardi G, Lassonde M (2004): Self‐face recognition in a callosotomy patient. Eur J Neurosci 18: 2391–2395. [DOI] [PubMed] [Google Scholar]
- Kircher T, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brummer M, Simmons A, Williams SC, Bartels M, David AS (2000): Towards a functional neuroanatomy of self‐processing: effects of faces and words. Cogn Brain Res 10: 133–144. [DOI] [PubMed] [Google Scholar]
- Kircher T, Senior C, Phillips ML, Rabe‐Hesketh S, Benson P, Bullmore ET, Brammer M, Simmons A, Bartels M, David AS (2001): Recognizing one's own face. Cognition 78: 1–15. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupian M, Keenan JP, Nowak M, Kjaer TW, Sackheim HA, Lisanby SH (2004): Parietal cortex and representation of the mental self. Proc Natl Acad Sci U S A 101: 6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Gallup GG Jr (2002): Self‐face recognition is affected by schizotypal personality traits. Schizophr Res 57: 311–315. [DOI] [PubMed] [Google Scholar]
- Platek SM, Critton SR, Myers TE, Gallup GG Jr (2003a): Contagious yawning: the role of self‐awareness and mental state attribution. Cogn Brain Res 17: 223–227. [DOI] [PubMed] [Google Scholar]
- Platek SM, Myers TE, Critton SR, Gallup GG Jr (2003b): A left‐hand advantage for self‐description and the effects of schizotypal personality traits. Schizophr Res 65: 147–151. [DOI] [PubMed] [Google Scholar]
- Platek SM, Thomson JW, Gallup GG Jr (2004a): An integrated and intermodal self: cross modal self‐recognition. Conscious Cogn 13: 197–210. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG Jr, Mohamed FB (2004): Where am I? The neurological correlates of self and other. Cogn Brain Res 19: 114–122. [DOI] [PubMed] [Google Scholar]
- Platek SM, Raines DM, Gallup GG Jr, Mohamed FB, Thomson JW, Myers TE, Panyavin IS, Levin SL, Davis JA, Fonteyn LCM, Arigo DR (2005): Reactions to children's faces: males are more affected by resemblance and so are their brains. Evol Human Behav 25: 394‐405. [Google Scholar]
- Povinelli DJ, Bierschwale D, Cech G (1999): Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. Br J Dev Psychol 17: 37–60. [Google Scholar]
- Preilowski B (1977): Self‐recognition as a test of consciousness in left and right hemisphere of “split‐brain” patients. Act Nerv Super (Praha) 19(Suppl 2): 343–344. [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L (2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996): Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M (2003): The functionally defined right occipital and fusiform “face areas” discriminate from visually familiar faces. Neuroimage 19: 877–883. [DOI] [PubMed] [Google Scholar]
- Spangenberg K, Wagner A, Bachman T (1998): Neuropsychological analysis of a case of abrupt onset following a hypotensive crisis in a patient with vascular dementia. Neurocase 4: 149–154. [Google Scholar]
- Sperry R, Zaidel E, Zaidel D (1979): Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia 17: 153–166. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Okada K, Kato T, Nakamura A, Hatano K, Itoh K, Kojima S, Fukuda H (2000): Passive and active recognition of one's own face. Neuroimage 11: 36–48. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1998): Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral mapping. New York: Thieme. [Google Scholar]
- Turk DJ, Heatherton TF, Kelley WM, Funnel MG, Gazzaniga MS, Macrae CM (2002): Mike or me? Self‐recognition in a split‐brain patient. Nat Neurosci 5: 841–842. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]


