Abstract
Twin studies suggest that variation in human brain volume is genetically influenced. The genes involved in human brain volume variation are still largely unknown, but several candidate genes have been suggested. An overview of structural Magnetic Resonance (brain) Imaging studies in twins is presented, which focuses on the influence of genetic factors on variation in healthy human brain volume. Twin studies have shown that genetic effects varied regionally within the brain, with high heritabilities of frontal lobe volumes (90–95%), moderate estimates in the hippocampus (40–69%), and environmental factors influencing several medial brain areas. High heritability estimates of brain structures were revealed for regional amounts of gray matter (density) in medial frontal cortex, Heschl's gyrus, and postcentral gyrus. In addition, moderate to high heritabilities for densities of Broca's area, anterior cingulate, hippocampus, amygdala, gray matter of the parahippocampal gyrus, and white matter of the superior occipitofrontal fasciculus were reported. The high heritability for (global) brain volumes, including the intracranium, total brain, cerebral gray, and white matter, seems to be present throughout life. Estimates of genetic and environmental influences on age‐related changes in brain structure in children and adults await further longitudinal twin‐studies. For prefrontal cortex volume, white matter, and hippocampus volumes, a number of candidate genes have been identified, whereas for other brain areas, only a few or even a single candidate gene has been found so far. New techniques such as genome‐wide scans may become helpful in the search for genes that are involved in the regulation of human brain volume throughout life. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: brain volume, heritability, MRI, polymorphisms, twins, human
INTRODUCTION
Although showing an impressive prenatal growth, the human brain is not completed at birth. There is considerable brain growth during childhood. Interestingly, our brain is not fully matured after adolescence either. Evidence is accumulating of dynamic changes taking place in the human brain throughout life. Such lifetime dynamic changes in the human brain might allow us to optimally adapt our lives to our environments and experiences. Evidence is also accumulating that brain structure is under considerable genetic influence. The mechanisms by which interaction between genes and environment occur and dynamics of brain structure and its association with brain functioning still remain unknown. However, twin and family studies and newly evolving genetic approaches start to give us a glimpse as to which genes and (interacting) environmental influences are shaping our brains.
In a recent study, head circumference was measured in healthy children at different ages. Compared to measures at birth (mean, SD was 34.9, 1.1 cm), head circumference was found to increase with more than 30% in the first year (46.6, 1.3 cm); between 1 and 4 years of age with another 9% (50.9, 1.4 cm); and between 4 and 8 years with an additional 4% (53.4, 1.4 cm) [Gale et al., 2006]. Magnetic resonance imaging (MRI) studies have shown that at 6 years of age, total cerebral volume has reached 95% of its adult volume [Giedd et al., 1999]. Considering that a medium‐large adult head size is estimated at 58 cm and expected to remain stable, the volumetric findings are quite compatible with a small increase in head circumference after childhood. Within the brain, however, dynamic changes continue from childhood into adulthood. In the landmark studies of a cohort of children between 4 and 20 years of age, using MRI, it was shown that gray matter and white matter both increase in volume until early adolescence. In early adolescence, gray matter volume starts to decrease, except for gray matter of the temporal lobe, which was found to increase into late adolescence [Giedd et al., 1999]. Overall, white matter volume continues to increase [Giedd et al., 1999; Paus et al., 1999]. Thus, while overall brain size has almost reached its adult size by 6 years of age, the gray/white matter ratio decreases in adolescence and into adulthood [for reviews, see Durston et al., 2001; Paus, 2005; Toga et al., 2006].
In adulthood, brain volume continues to change. Cross‐sectional volumetric studies consistently showed a steady decrease in total brain volume, predominantly due to decrease in gray matter [Allen et al., 2005; Hulshoff Pol et al., 2002a; Raz et al., 2004]. Density mapping of gray matter revealed that the trajectories of age‐effects varied over the cortex, with visual, auditory, and limbic cortices showing a more linear pattern of aging than the frontal and parietal neocortices [Sowell et al., 2002]. Longitudinal studies have confirmed that indeed several of the age‐associated changes in brain volumes are probably nonlinear. White‐matter volume was found to increase until age 45 before it starts to decrease [Bartzokis et al., 2001]. In a 5‐year MRI follow‐up study, we recently observed continued growth in total brain volume in a proportion of a subgroup of healthy adult subjects until 40 years of age, suggesting that at least in a subpopulation of adults, brain growth continues into adulthood [Van Haren et al., in press]. Thus, in adulthood, significant dynamic changes in brain structure continue to take place, with continued growth of white matter and nonlinear decreases in total brain volume and gray matter volumes and densities.
Brain structure as we measure it macroscopically using MRI, and the dynamic changes therein, have a functional relevance. In healthy subjects, the level of intellectual functioning has been positively associated with whole brain, gray, and white‐matter volumes [Posthuma et al., 2002; Thompson et al., 2001]. More focally, several brain areas were found to be correlated with intelligence [Frangou et al., 2004; Haier et al., 2004; Reiss et al., 1996]. Interestingly, in a recent study, it was shown that the trajectory changes in cortical thickness throughout adolescence are associated with the level of intelligence [Shaw et al., 2006]. It is important to know which genes are involved in brain structure and if common genes are involved in both brain structure and cognitive functioning so as to possibly explain their association.
Studies in quantitative genetics describe the decomposition of (observed) phenotypic variance into genetic and environmental sources. Genetically related individuals are studied to disentangle these sources of phenotypic variance. Heritability is the proportion of genetic variance over the total variance. Environmental variance can be decomposed into environmental variance shared by members of a family (common environment) or nonshared variance, which is unique to a certain individual (unique environment). To determine the relative contribution of genetic, common, and unique environmental influences on variation in brain structures, the (extended) twin model is a particularly powerful approach [Posthuma and Boomsma, 2000]. More specifically, heritability estimates of brain structure are usually based on data from monozygotic twin pairs (MZ, who are nearly always genetically identical) and dizygotic twin pairs (DZ, who share on average 50% of their segregating genes). If for a certain brain measure monozygotic twin pairs resemble each other more closely than dizygotic twin pairs, it can be inferred that variation of the brain measure is heritable. However, in addition to genetic influences, common (or shared) environmental influences may play a role in explaining resemblances. The presence of shared environmental factors is suggested when correlations in DZ twins are larger than half of the MZ correlation [Boomsma et al., 2002]. A first impression of the importance of unique environmental factors is obtained from the extent to which MZ twins do not resemble each other.
To estimate contributions of additive genetic (A) effects, common (or shared) environmental (C), and unique environmental effects (E) to variation in a phenotype, structural equation modeling (SEM) is increasingly used in twin studies. SEM is a more advanced method, which, in contrast to the Falconer method, is capable of explicitly testing whether genetic or environmental factors contribute significantly to explaining individual differences. In other words, SEM allows for parameter estimations, while the Falconer (correlational) method merely allows parameter calculation. In extended twin‐studies, the siblings, or sometimes other relatives of twins, are included in the study design. This increases the statistical power to detect the influences of common environmental influences shared by members from the same family.
We review the findings from (extended) twin studies on normal human brain structure and discuss the impact of these findings on future studies into specific genes and environmental factors that are involved in the continuing development of our brains throughout life.
METHODS
A PubMed indexed search was carried out with a limitation for human subjects and the following keywords: (brain volume) or (white/gray matter) and (twin) or (heritability). For inclusion, papers had to be written in English, and use structural MRI or computer tomography (CT). These included volumetric MRI (both global and focal measures), voxel‐based morphometry (VBM) and diffusion tensor imaging (DTI) (for information on white‐matter integrity). Case studies or qualitative studies were not included. If available, information on the number of subjects, average age and age range of the sample, type of analysis (Structural Equation Modeling or Falconer method), and heritability estimates with their 95% confidence intervals were extracted from the papers.
RESULTS
Heritability of Brain Volumes
A total of 14 twin studies measuring brain volume have been carried out, of which 11 were analyzed using SEM and another 3 used the Falconer method for calculating heritability. Brain structure in healthy MZ and DZ twin pairs was first quantitatively studied using CT [Reveley et al., 1984] (Table I). In this study, it was found that lateral ventricle variation was mostly explained by genetic factors. Later studies, using MRI, found high heritability estimates of global brain measures including intracranial volume (>81%) [Baaré et al., 2001; Carmelli et al., 1998; Pfefferbaum et al., 2000] and total brain volume (66–97%) [Baaré et al., 2001; Bartley et al., 1997; Pennington et al., 2000; Wright et al., 2002]. The first twin‐sibling study to measure the genetic contributions to variation in global gray and white matter found heritability estimates of 82% for gray matter and 88% for white matter [Baaré et al., 2001]. The volumes of each cerebral hemisphere showed 65% heritability [Geschwind et al., 2002]. For variation in cerebellar volume, a heritability of 88% was reported [Posthuma et al. 2000]. Area measurements of the corpus callosum revealed heritability estimates between 79 and 94% [Pfefferbaum et al., 2000; Scamvougeras et al., 2003]. In a study that did not include DZ twin pairs, MZ twin pair correlations were high (>0.90 for cerebellum, total brain, gray and white matter, and >0.75 for caudate nucleus, putamen, thalamus and cortical depth) when compared with a healthy comparison group, indicating an upper limit of heritability [White et al., 2002].
Table I.
Twin studies on human brain volumes
| Authors | Subjects | Age in years (range) | Brain region | Heritability (A) in % (95% CI) |
|---|---|---|---|---|
| Reveley et al. [1984]a | 18 MZ, 18 DZ | NA | LV | 82%–85% (NA) |
| Bartley et al. [1997] | 10 MZ (6 M), 9 DZ (3 M) | MZ:31 (19–54) | TB | 94 % (NA) |
| DZ: 23 (18–29) | Gyral patterns | 7–17% (NA) | ||
| Carmelli et al. [1998] | 74 MZ, 71 DZ (M) | 68–79 years | IC | 91 % (NA) |
| Pennington et al. [2000]a | RD 25 MZ (12 M), 23 DZ (16 M) | RD MZ 17.1, DZ: 16.8 | TB | 97% (NA) |
| Non‐RD: 9 MZ (4 M), 9 DZ (4 M) | Non‐RD MZ 19.4, DZ 18.7 | Neocortex | 56% (NA) | |
| Subcortex | 70% (NA) | |||
| Pfefferbaum et al. [2000] | 45 MZ, 40 DZ (M) | MZ 72.2 | IC | 81% (72–90) |
| DZ 71.4 | CC | 79% (69–89) | ||
| 68–78 | LV | 79% (55–100) | ||
| Baaré et al. [2001] | 54 MZ (33 M) | MZM: 31.2, MZF: 34.1, DZM: 30.3, DZF: 30.6, OS: 30.3 sibs: 29 | IC | 88% (82–92) |
| 58 DZ (17 M, 21 OS) | (19–69) | TB | 90% (85–93) | |
| 34 sibs | GM | 82% (73–88) | ||
| WM | 87% (80–91) | |||
| LV | C: 59% (47–69), E: 41% (31–53) | |||
| 15 MZ, 18 DZ | 75.7 ± 2.7 years | Size CC | 5:1 (NA) | |
| Microstructure CC | 3:1 (NA) | |||
| (DTI) | (relative proportion A:E) | |||
| Sullivan et al. [2001] | See Pfefferbaum et al. [2000] | See Pfefferbaum et al. [2000] | HIP | 40% (NA) |
| Thompson et al. [2001]a | 10 MZ (5 M), 10 DZ (5 M) | 48.2 (±3.4) | Middle frontal | 90–95% (NA) |
| Sensorimotor and anterior temporal cortices asymmetry heritability for Broca's and Wernicke's (Cortical thickness) | ||||
| Eckert et al. [2002]a | 27 MZ, 12 DZ (all M) | MZ: 6.9–16.4, DZ: 6.1–15.0 | Planum temporale asymmetry | NA (NA) |
| Geschwind et al. [2002] | 72 MZ | MZ: 72.3 | Cerebral hemispheres | 65% (NA) |
| 67 DZ (M) | DZ: 71.8 | Cerebral asymmetry | LHS: More C influence | |
| (68–78) | LH/RH handedness | LH: less genetic control | ||
| Posthuma et al. [2000] | See Baaré et al. [2001] | See Baaré et al. [2001] | CB | 88% (81–92) |
| Wright et al. [2002]b | 10 MZ (6 m), 10 DZ (4 m) | MZ: 31 (19–54) | TB | 66% (17–100) |
| DZ: 23 (18–29) | LV | C: 48%(0–97), E:50% (32–84) | ||
| CB | 63%, E: 22% (NA) | |||
| Ventrolateral FR, cingulate, anterior & superior and transverse temp, retro‐ splenium | 58–73% (NA) | |||
| White et al. [2002]b | 12 MZ (6 m), | MZ: 24.5 ± 7.2 | TB, GM, WM, CB | r > 0.90 |
| 12 control pairs (6 m) | Controls: 24.4 ± 7.2 | CAU, PUT, THAL cortical depth | r > 0.75 | |
| No DZ | Low correlations | |||
| (MZ corr) | ||||
| Scamvougeras et al. [2003] | 14 MZ, 12 DZ | MZ: 16–41, DZ: 18–32 | CC | 94% (NA) |
| Pfefferbaum et al. [2004] | 34 MZ, 37 DZ. | T1: 68–80 years | CC (T1) | 89% (NA) |
| T2: 72–84 years | CC (T2) | 92% (NA) | ||
| 4 year longitudinal | LV (T1) | 92% (NA) | ||
| Follow‐up | LV (T2) | 88% (NA) | ||
| Hulshoff Pol et al. [2006]b | See Baaré et al. [2001] | See Baaré et al. [2001] | WM (SOF, CC, CST) | 69–82% (NA) |
| GM MFL, SFL, STL, CING, PARAHIP, AMYG, OCC | 55–85% (NA) | |||
| Wallace et al. [2006] | 90 MZ (52 M, 38 F), | MZ: 11.9 | TB | 89% (67–92) |
| 37 DZ (22 M, 15 F) | DZ: 10.9 | GM | 82% (50–87) | |
| (5–19) | WM | 85% (56–90) | ||
| FR, TEMP, PAR | 77–88% (50–90) | |||
| CB | 49% (13–83) | |||
| LV | 31% (0–67), C: 24% (0–58), E: 45% (33–60) |
Parts of this table will also be published in Peper JS, Zwiers MP, Boomsma D, Kahn RS, Hulshoff Pol HE. Human brain volume: what's in the genes? In: Handbook of Behavior Genetics (Y Kim, editor). New York: Springer. In press.
A, additive genetic; AMYG, amygdala; C, common environment; CAU, caudate; CB, cerebellum; CC, corpus callosum; CI, confidence interval; CING, cingulate cortex; CST, corticospinal tract; DTI, diffusion tensor imaging; DZ, dizygotic; DZF, dizygotic female; DZM, dizygotic male; E, unique environment; F, female; FR, frontal lobe; GM, gray matter; HIP, hippocampus; IC, intracranial volume; LH, left handed; LV, lateral ventricles; M, male; MFL, medial frontal lobe; MZ, monozygotic; MZF, monozygotic female; MZM, monozygotic male; NA, not available; OCC, occipital lobe; occ‐front‐temp, occipito‐fronto temporal; OS, opposite sex; PAR, parietal lobe; PARAHIP, parahippocampal gyrus; PUT, putamen; RD, reading disability; RH, right handed; Sibs, siblings; SOF, superior orbitofrontal; TB, total brain; TEMP, temporal lobe; THAL, thalamus; SFL, superior frontal lobe; STL, superior temporal lobe; WM, white matter.
Falconer method of heritability.
Voxel‐based morphometry.
In the only published twin‐study to date in children consistent with previous adult studies, additive genetic effects accounted for a substantial portion of variability in nearly all brain regions with the notable exception of the cerebellum [Wallace et al., 2006].
A number of global brain areas seem to be mainly under environmental control. The overall gyral patterning of the cortex was found to be under environmental control [Bartley et al., 1997; Eckert et al., 2002]. Moreover, common and unique environmental factors explained the individual variation in lateral ventricle volumes [Baare et al., 2001; Wright et al., 2002]. However, individual differences in lateral ventricle size were mainly of genetic origin in a study consisting of elderly subjects [Pfefferbaum et al., 2000, 2004]. Although the volume of the cerebellum was found to be mainly under influence of genes in adults [Posthuma et al., 2000; Wright et al., 2002], in children, it was found to be largely under common (30%) and unique environmental (21%) control [Wallace et al., 2006]. In the only study to date measuring heritability of hippocampus volume, moderate heritability of 40% was found [Sullivan et al., 2001].
Heritability of Regional Brain Areas Using VBM
Three studies have examined possible genetic effects on more specific brain areas using VBM and cortical thickness measures. Two of these studies were analyzed with SEM. One study additionally used a path analysis on the results [Wright et al., 2002], and one study used a stringent correction of multiple comparisons [Hulshoff Pol et al., 2006] according to the Random Field Theory [Worsley et al., 1996].
Cortical thickness of middle frontal, sensorimotor, and anterior temporal cortices, as well as Wernicke's region was found to be particularly influenced by genetic factors [Thompson et al., 2001]. VBM revealed high heritabilities for paralimbic structures and temporal/parietal neocortical areas [Wright et al., 2002]. Furthermore, brain density of the medial and superior frontal, superior temporal, and occipital gray matter (Fig. 1) and connecting white matter of the superior occipitofrontal fasciculus and corpus callosum (Fig. 2) were particularly influenced by genetic factors [Hulshoff Pol et al., 2006]. Unique environmental factors influenced vast gray matter and white matter areas surrounding the lateral ventricles (up to 50%) [Hulshoff Pol et al., 2006].
Figure 1.
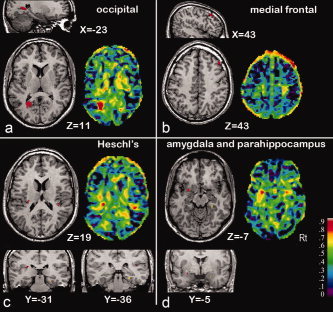
Genetically influenced focal gray matter density brain areas. Heritability estimates of gray matter density in focal brain areas in healthy adult humans are shown for the significance level thresholded A‐map superimposed on axial and sagittal sections through the magnetic resonance image of the standardized reference brain (left) and for the complete A‐map (right). Data are based on a study in 258 monozygotic and dizygotic twin‐pairs and their siblings from 112 Dutch families. For genetic analyses, structural equation modeling and voxel‐based morphometry was used. (From Hulshoff Pol et al., J Neurosci 2006, 26, 10235–10242, © Society for Neuroscience, reproduced by permission.)
Figure 2.
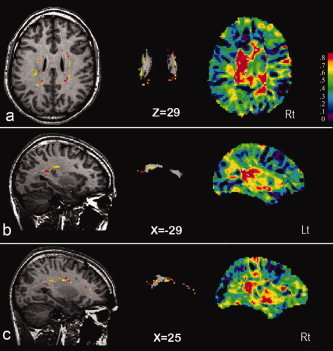
Genetically influenced focal white‐matter density brain areas superimposed on the histologically defined map of the superior occipitofrontal fascicle. Heritability of white‐matter density in focal brain areas in healthy adult humans is shown for the significance level thresholded A‐map superimposed on axial and sagittal sections in the left (Lt) and right (Rt) hemisphere through the magnetic resonance image of the standardized reference brain (left) and superimposed on the histologically defined map of the occipitofrontal superior fascicle (middle) (reproduced with kind permission of Drs. K. Zilles, K. Amunts and U. Burgel). The complete A‐maps are shown on the right. (From Hulshoff Pol et al., J Neurosci 2006, 26, 10235–10242, © Society for Neuroscience, reproduced by permission.)
Heritability of Changes in Brain StructureWith Age
In the only study to date that measured heritability estimates of changes in brain volumes over time, genetic contributions to variability in intracranial volume, corpus callosum, and lateral ventricles were found to be high in healthy elderly [Pfefferbaum et al., 2000] and remained high at longitudinal follow‐up of 4 years [Pfefferbaum et al., 2004].
Heritability of Brain Structure and the Association with Brain Function
Total brain volume, gray matter, and white matter are positively correlated with general intelligence, and these structures and verbal and performal intelligence share a common genetic origin [Posthuma et al., 2002]. However, this finding does not necessarily mean that genes influence focal brain structures in the same manner throughout the brain. Moreover, it does not necessarily mean that a common genetic origin with general intelligence is shared by all structures throughout the brain. In a study involving monozygotic and dizygotic twin pairs, frontal gray matter volume and intelligence were positively correlated [Thompson et al., 2001]. Since this correlation was more prominent in monozygotic when compared with dizygotic twins, this finding suggested that frontal lobe volume and intelligence share genetic factors, although it was unresolved whether this association shares a common genetic origin [Thompson et al., 2001; Toga and Thompson, 2004]. In a study involving monozygotic and dizygotic twin pairs and their singleton siblings, using structural equation modelling, verbal, and performal intelligence were found to share a common genetic origin with an anatomical neural network involving the frontal, occipital, and parahippocampal gray matter and connecting white matter of the superior occipitofrontal fascicle, and the corpus callosum [Hulshoff Pol et al., 2006].
DISCUSSION
On the basis of the twin and family studies, it can be inferred that variation in overall human brain volume is highly heritable. The large influence of genes on human brain volume seems to be already present in childhood. Moreover, variation in brain volumes remains to be largely explained by genetic factors, even in old age. Adult twin‐studies showed high heritability estimates for volumes of specific structures and for overall brain size in adulthood (between 66 and 97%). Also, both variations in volumes of global gray and global white matter are largely determined by genes. However, individual differences in lateral ventricle volume are mainly explained by environmental factors, suggesting that its surrounding brain tissue is at least partly influenced by environmental factors. Genetic effects were shown to vary regionally within the brain, with high heritabilities of frontal lobe volumes (90–95%), moderate estimates in the hippocampus (40–69%), and environmental factors influencing several medial brain areas. Heritability estimates of focal brain structures based on VBM and cortical thickness studies revealed high heritabilities for densities of medial frontal cortex, Heschl's gyrus, and postcentral gyrus. In addition, moderate heritabilities for densities of hippocampus, amygdala, gray matter of the parahippocampal gyrus, and white matter of the superior occipitofrontal fasciculus were reported in one or more studies. Areas, which show a high heritability for volume, emphasize the relevance of these brain areas when searching for genes influencing brain structure.
Significant heritabilities for total brain volume and gray and white‐matter volumes were consistently found across studies, with most studies finding high heritabilities. Estimates varied between 65 and 97%. Focally, moderate to high heritabilities of the bilateral medial frontal cortex, bilateral Heschl's gyrus, and left postcentral gyrus were consistently found across studies [Hulshoff Pol et al., 2006; Thompson et al., 2001; Wright et al., 2002]. Furthermore, the posterior cingulate was found to be heritable in two studies [Hulshoff Pol et al., 2006; Wright et al., 2002], underscoring the relevance of these brain areas when searching for genes influencing brain structure and function. Broca's language area, ventrolateral prefrontal cortex, and anterior cingulate gyrus, superior frontal cortex, occipital (striate and extrastriate) cortex, the anterior cingulate, amygdala, and parahippocampal gyrus were found to be significantly heritable in single studies. These single findings were at least partly due to differences in methodology used between studies in healthy adult twin samples [for a discussion see Hulshoff Pol et al., 2006]. Notwithstanding differences in findings, the overlapping results are quite promising. It seems that the individual variation in morphology of areas involved in attention, language, visual, and possibly emotional processing, as well as in sensorimotor processing are strongly genetically influenced. Unique environmental factors influenced the lateral ventricles in the majority of studies [see Pfefferbaum et al. (2000, 2001) for high heritabilities) and brain tissue surrounding the lateral ventricles (up to 50% in Hulshoff Pol et al., 2006]. This suggests that medially, some focal brain regions are probably largely influenced by (unique) environmental influences.
To which extent genetic and environmental factors influence the age‐related changes in brain structure is an important question. So far, only one study measured the stability of genetic influences onto changes in brain structure over time. In elderly twin‐subjects, high heritability estimates were found to remain stable after a 4‐year interval [Pfefferbaum et al., 2004]. Thus, at this point, it can only be inferred from separate twin‐cohorts with different age‐ranges that a high heritability for global brain volumes seems to be present throughout life, including the intracranium, total brain, cerebral gray, and white matter. A possible exception may have to be made for the cerebellum, which revealed high heritability estimates in adult twin‐samples, but was found to have a low heritability estimate in a childhood twin‐sample. Whether the extent of genetic and environmental influences changes with age remains to be investigated. The only study to date suggests that in the elderly the relative influences of genes and environmental factors is quite stable and largely determined by genes (80%).
Importantly, the high heritability of brain volume is functionally relevant. For instance, the association between brain volumes and intelligence was found to be of genetic origin [Posthuma et al., 2002]. Moreover, the association between frontal gray matter volume and intelligence was suggested to be due to genetic factors [Thompson et al., 2001; Toga and Thompson, 2004]. Recently, the association of intelligence with gray matter of the frontal and occipital lobes, the parahippocampus and connecting white matter was found to be influenced by genes common to brain structure and intelligence [Hulshoff Pol et al., 2006]. These findings demonstrate that a common set of genes may cause the association between brain structure and cognitive functions. However, in elderly twins, the associations between frontotemporal brain volumes and executive function were found to be because of common environmental influences shared by twins from the same family [Carmelli et al., 2002]. These results point to the possibility that overlapping sets of genes or common environmental influences cause variation in two distinct phenotypes. However, other causal models are also consistent with the findings. It might be, for example, that a higher level of cognitive functioning leads a person to select an environment that also increases brain size. The genetic influence on brain size then simply reflects the genetic influences on cognition. Thus, the specific mechanism, pathways, and genes that are involved in human brain morphology and its association with cognitive functions remain elusive.
Considering the high heritabilities for global brain volumes and particular focal brain densities and thicknesses, the search for genes that are involved in brain growth, aging, and brain structure maintenance is important. Such knowledge can help us understand normal developmental and age‐associated changes in individual variation in brain functioning. Moreover, it enhances our knowledge of individual variation in brain functioning and facilitates the interpretation of the morphological changes found in psychiatric disorders such as schizophrenia. Also, it allows future efforts to find particular genes responsible for brain structures to be concentrated in areas that are under considerable genetic influence [Hulshoff Pol et al., 2006]. A genetic approach to find genes involved in brain structure that has been applied in several studies is that of diseases with a clear genetic etiology. A review of brain‐imaging studies in Huntington's disease, Down syndrome, Williams syndrome, and Velocardiofacial syndrome, revealed, besides disease specific brain changes, decreases in total brain, white matter, and hippocampus volumes, irrespective of the genes and/or chromosomes involved. This suggests that many genes are probably involved in the individual variation of these measures [Peper et al., in press]. Another genetic approach that may aid us in our quest to find genes involved in individual brain variation is the study of polymorphisms of specific genes in healthy subjects. A polymorphism is defined as the existence of multiple alleles of a gene within a population. It is a naturally occurring variation in the sequence of genetic information on a segment of DNA among individuals. Those variations are considered normal and should not be confused with true mutations, which are alterations of the original genetic material, often being harmful.
The few studies on polymorphisms in healthy subjects have revealed associations with brain volumes or densities. For example, Val/met (i.e., valine/methionine amino acids) variant carriers of the Brain Derived Neurotrophic Factor (BDNF)‐gene (a gene involved in reducing the amount of naturally occurring neuronal cell death) were found to have a reduced size of the prefrontal cortex [Pezawas et al., 2004] and hippocampus compared to val/val carriers [Bueller et al., 2006; Pezawas et al., 2004; Szeszko et al., 2005]. In addition, in met‐BDNF carriers, a negative relation was found between volume of the dorsolateral prefrontal cortex and age, which was not present in the val‐BDNF carriers [Nemoto et al., 2006]. A study of allelic variants of the Apolipoprotein (ApoE)‐gene—thought to be involved in cell growth and regeneration of nerves—showed that healthy elderly subjects who were homozygous for the Epsilon4 allele, i.e., e4–e4 genotype, had smaller hippocampal volumes than subjects heterozygous for that allele and than e4 noncarriers [Lemaitre et al., 2005; Lind et al., 2006]. Also, the presence of a single ApoE‐epsilon4 allele was associated with an increased rate of hippocampal volume loss in healthy women [Cohen et al., 2001]. Two variants of the X‐linked monoamine oxidase A‐gene (MAOA) were recently associated with brain volumes in healthy subjects. The low‐expression variant predicted volume reductions in cingulate gyrus, amygdala, insula, and hypothalamus, whereas the high expression variant was associated with changes in orbitofrontal volume [Meyer‐Lindenberg et al., 2006]. Overall, studying polymorphisms in healthy subjects yield valuable information on specific genes that may be involved in brain volume. However, as it is a newly developing area of research, the robustness of the findings needs to be pointed out and therefore replication is warranted.
One has to keep in mind that there are certain limitations with respect to the currently reviewed twin‐studies. First, because of small sample sizes of a number of studies, assumptions on the contribution of genetic effects in these studies have to be interpreted with some caution. Although the high heritabilities in themselves require smaller sample sizes than for traits, which are characterized by a smaller contribution of genetic effects, the main drawback is the lack of power to test for the influence of common environment. Testing an AE model versus a CE model depends on larger sample sizes. Unfortunately, in MRI‐research, because of costs and complexity, this is currently not feasible. Also, models testing for interaction between genes or between genetic and environmental factors are complicated because of a lack of power. One might consider, for these situations that aim to go beyond simply establishing heritability, to include other relatives and to use for example an “extended twin design” in which siblings of twins are also tested [Posthuma and Boomsma, 2000]. Another limitation is that in some studies, there is a large variability in age of the sample. This complicates generalizability of findings, as with age brain volumes undergo dynamic changes. Also, with age, the influences of genes and environmental factors on human brain structure may change. Therefore, in homogeneous age groups, more accurate heritabilities can be provided. In addition, there are some limitations of the twin‐design in general. First, in the classical twin design, it is generally assumed that with respect to the trait under study, MZ and DZ twin‐pairs are treated alike, i.e., the “Equal environment Assumption (EEA).” It seems unlikely that with respect to the development of the brain, there are environmental treatments that make MZ twins more alike than DZ twins. In studies, in which the EEA has been tested for other traits, by using, for example, the influence of actual and perceived zygosity on trait similarity, no violation of EEA was found for a range of psychological traits [Kendler et al., 1993; see also Martin et al., 1997].
Finally, it has been argued that the twin method may yield an inflated estimation of heritabilities compared to family and/or adoption studies. On the other hand, family studies might give lower heritability estimations as subjects with different ages within families are compared. Thus, when taking these factors into careful consideration, estimates of genetic influences are probably quite accurate [Martin et al., 1997]. Indeed, findings on brain volume in twins could be generalized to the general (singleton) population in a study comparing twins and singleton siblings, birth order, and zygosity, particularly after correcting for head size or intracranial volume [Hulshoff Pol et al., 2002b].
Without specific knowledge of candidate genes, linkage studies are now employed with the goal to localize a gene that influences a phenotype. This approach can be used when genetic marker data (based on DNA polymorphisms of known location in the genome) are available in extended families or in sibling pairs. Linkage studies are often called a‐theoretical (“blind” search for genes) in contrast to association studies, which require knowledge of candidate genes [Vink and Boomsma, 2002]. Linkage studies require data collection in related individuals (e.g., siblings or large pedigrees). A newly emerging field of genetic research is the study of epigenetics. Epigenetics comprises mechanisms of inheritance, which are not the consequences of changes in DNA structure. They affect gene transcription, with environmental factors acting as modulators or inducers of epigenetic factors. One such (important) factor is DNA‐methylation [see Santos et al., 2005]. The genome‐wide pattern of DNA‐methylation was found to be more alike within monozygotic young than in monozygotic adult and elderly twin pairs [Fraga et al., 2005], although recent studies of DNA‐methylation profiling did not observe an association with age [Eckhart et al. 2006; Heijmans et al., 2007]. It is important to investigate which environmental factors have an influence on the expression of genes (as found in DNA‐methylation). Additionally, the study of interaction between genes and environmental factors is warranted. Furthermore, the simultaneous effects of multiple genes and possibly the interaction among genes, also needs investigation as the high heritability of a complex quantitative phenotype such as brain volume cannot be explained by a single‐gene polymorphism.
Taken together, MRI studies in twins indicate that, given the basic additive genetic model, overall brain volume in adulthood is highly heritable. Twin studies carried out in large and more homogenous samples, analyzed with advanced quantitative genetic methods are needed to test for influences of genetic, common, and unique environmental factors or interactions between genetic and environmental influences. Since brain volume changes dynamically throughout life, longitudinal twin studies in childhood as well as in adulthood are needed to investigate the stability of genetic (and environmental) influences onto functional neural networks in human brain. New brain‐imaging methods, such as DTI‐fiber tracking and (resting state) functional MRI, allow study of the heritability of neural networks underlying brain functioning. Such new MRI methods, in coherence with new genetic approaches, will enable us to further disentangle which genes and environmental factors and interactions therein influence human brain structure throughout life.
Parts of this review will also be published in Peper JS, Zwiers MP, Boomsma D, Kahn RS, Hulshoff Pol HE: Human brain volume: What's in the genes? In: Kim Y, editor. Handbook of Behavior Genetics. New York: Springer (in press).
REFERENCES
- Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM ( 2005): Magnetic resonance imaging of cerebellar‐prefrontal and cerebellar‐parietal functional connectivity. Neuroimage 28: 39–48. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, van Haren NE, van Oel CJ, Kahn RS ( 2001): Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex 11: 816–824. [DOI] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR ( 1997): Genetic variability of human brain size and cortical gyral patterns. Brain 120 (Part 2): 257–269. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J ( 2001): Age‐related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry 58: 461–465. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L ( 2002): Classical twin studies and beyond. Nat Rev Genet 3: 872–882. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez‐Hassan D, Burmeister M, Zubieta JK ( 2006): BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry 59: 812–815. [DOI] [PubMed] [Google Scholar]
- Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, Miller BL ( 1998): Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 29: 1177–1181. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Reed T, DeCarli C ( 2002): A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiol Aging 23: 413–420. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T ( 2001): Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology 57: 2223–2228. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H ( 2001): Anatomical MRI of the developing human brain: What have we learned? J Am Acad Child Adolesc Psychiatry 40: 1012–1020. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Molloy EA, Blumenthal J, Zijdenbos A, Giedd JN ( 2002): The epigenesis of planum temporale asymmetry in twins. Cereb Cortex 12: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, Burton J, Cox TV, Davies R, Down TA, Haefliger C, Horton R, Howe K, Jackson DK, Kunde J, Koenig C, Liddle J, Niblett D, Otto T, Pettett R, Seemann S, Thompson C, West T, Rogers J, Olek A, Berlin K, Beck S ( 2006): DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet 38: 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestart ML, Heine‐Suner D, Cigudosa JC, Urioste M, Benitez J, Boix‐Chornet M, Sanchez‐Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M ( 2005): Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 102: 10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SC ( 2004): Mapping IQ and gray matter density in healthy young people. Neuroimage 23: 800–805. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Bredow M, Martyn CN ( 2006): The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 118: 1486–1492. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D ( 2002): Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA 99: 3176–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL ( 1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2: 861–863. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT ( 2004): Structural brain variation and general intelligence. Neuroimage 23: 425–433. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Kremer D, Tobi E, Boomsma DI, Slagboom PE ( 2007): Heritable rather than age‐related environmental factors underlie variation in DNA methylation of the human IGF2/H19 locus. Hum Mol Genet 16: 547–554. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, van Haren NE, van der Tweel I, Staal WG, Baare WF, Kahn RS ( 2002a): Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry 159: 244–250. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Posthuma D, Baare WF, de Geus EJ, Schnack HG, van Haren NE, van Oel CJ, Kahn RS, Boomsma DI ( 2002b): Twin‐singleton differences in brain structure using structural equation modelling. Brain 125: 384–390. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins L, Evans AC, Amunts K, Burgel U, Zilles, de Geus EJ, Boomsma DI, Kahn RS ( 2006): Genetic contributions to human brain morphology and intelligence. J Neurosci 26: 10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ ( 1993): A test of the equal—Environment assumption in twin studies of psychiatric illness. Behav Genet 23: 21–27. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B ( 2005): No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage 24: 1205–1213. [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, Adolfsson R, Cruts M, Sleegers K, Van BC, Nyberg L ( 2006): Reduced hippocampal volume in non‐demented carriers of the apolipoprotein E epsilon4: Relation to chronological age and recognition memory. Neurosci Lett 396: 23–27. [DOI] [PubMed] [Google Scholar]
- Martin N, Boomsma D, Machin G ( 1997): A twin‐pronged attack on complex traits. Nat Genet 17: 387–392. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Buckholtz JW, Kolachana B, Hariri R, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR ( 2006): Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103: 6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, Kunugi H ( 2006): The Val66Met polymorphism of the brain‐derived neurotrophic factor gene affects age‐related brain morphology. Neurosci Lett 397: 25–29. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2005): Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 9: 60–68. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC ( 1999): Structural maturation of neural pathways in children and adolescents: In vivo study. Science 283: 1908–1911. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, Filley CM, Galaburda A, DeFries JC ( 2000): A twin MRI study of size variations in human brain. J Cogn Neurosci 12: 223–232. [DOI] [PubMed] [Google Scholar]
- Peper JS, Zwiers MP, Boomsma D, Kahn RS, Hulshoff Pol HE: Human brain volume: What's in the genes? In: Kim Y, editor. Handbook of Behavior Genetics. New York: Springer; (in press). [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer‐Lindenberg A, Weinberger DR ( 2004): The brain‐derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 24: 10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D ( 2000): Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiol Aging 21: 63–74. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D ( 2004): Morphological changes in aging brain structures are differentially affected by time‐linked environmental influences despite strong genetic stability. Neurobiol Aging 25: 175–183. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI ( 2000): A note on the statistical power in extended twin designs. Behav Genet 30: 147–158. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJC, Neale MC, Pol HEH, Baare WEC, Kahn RS, Boomsma D ( 2000): Multivariate genetic analysis of brain structure in an extended twin design. Behav Genet 30: 311–319. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI ( 2002): The association between brain volume and intelligence is of genetic origin. Nat Neurosci 5: 83–84. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD ( 2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25: 377–396. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB ( 1996): Brain development, gender and IQ in children. A volumetric imaging study. Brain 119: 1763–1774. [DOI] [PubMed] [Google Scholar]
- Reveley AM, Reveley MA, Chitkara B, Clifford C ( 1984): The genetic basis of cerebral ventricular volume. Psychiatry Res 13: 261–266. [DOI] [PubMed] [Google Scholar]
- Santos KF, Mazzola TN, Carvalho HF ( 2005): The prima donna of epigenetics: The regulation of gene expression by DNA methylation. Braz J Med Biol Res 38: 1531–1541. [DOI] [PubMed] [Google Scholar]
- Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF ( 2003): Size of the human corpus callosum is genetically determined: An MRI study in mono and dizygotic twins. Neurosci Lett 338: 91–94. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J ( 2006): Intellectual ability and cortical development in children and adolescents. Nature 440: 676–679. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL ( 2002): Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol 44: 4–16. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D ( 2001): Heritability of hippocampal size in elderly twin men: Equivalent influence from genes and environment. Hippocampus 11: 754–762. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz‐Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK ( 2005): Brain‐derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry 10: 631–636. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold‐Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW ( 2001): Genetic influences on brain structure. Nat Neurosci 4: 1253–1258. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM ( 2004): Genetics of brain structure and intelligence. Annu Rev Neurosci 28: 1–23. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER ( 2006): Mapping brain maturation. Trends Neurosci 29: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans RG, Carati I, Rais M, Kahn RS: Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- Vink JM, Boomsma DI ( 2002): Gene finding strategies. Biol Psychol 61: 53–71. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Eric SJ, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN ( 2006): A pediatric twin study of brain morphometry. J Child Psychol Psychiatry 47: 987–993. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P ( 2002): Brain volumes and surface morphology in monozygotic twins. Cereb Cortex 12: 486–493. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET ( 2002): Genetic contributions to regional variability in human brain structure: Methods and preliminary results. Neuroimage 17: 256–271. [DOI] [PubMed] [Google Scholar]


