Abstract
Cognitive procedural learning is characterized by three phases (cognitive, associative, and autonomous), each involving distinct processes. We performed a behavioral study and a positron emission tomography (PET) activation study using the Tower of Toronto task. The aim of the behavioral study was to determine cognitive predictors for the length of each of the three learning phases, in order to preselect subjects for the PET study. The objective of the second study was to describe the cerebral substrates subtending these three phases. Contrasted with a reference (motor) task, the cognitive phase activated the prefrontal cortex, cerebellum, and parietal regions, all of which became less active as learning progressed. The associative phase was characterized by the activation of the occipital regions, right thalamus, and caudate nucleus. During the autonomous phase, new regions were involved, including the left thalamus and an anterior part of the cerebellum. These results, by employing a direct comparison between phases, provide the first evidence of the involvement and the time course of activation of different regions in each learning phase, in accordance with current models of cognitive procedural learning. The involvement of a frontoparietal network suggests the use of strategies in problem solving during the cognitive phase. The involvement of the occipital regions during the associative and autonomous phase suggests the intervention of mental imagery. Lastly, the activation of the cerebellum during the autonomous phase is consistent with the fact that performance in this phase is determined by psychomotor abilities. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: procedural memory, Tower of Toronto, Tower of Hanoi, learning, prefrontal cortex, cerebellum, parietal cortex, thalamus, occipital cortex, PET
INTRODUCTION
Procedural memory as defined by Cohen and Squire [1980] is the memory system in charge of the encoding, storage, and retrieval procedures that underlie motor, verbal, or cognitive skills. Procedural learning is the process whereby a procedure is encoded in procedural memory and is measured as an improvement in the speed and accuracy of performances on a task with practice. Abilities are assessed by means of learning tasks involving a motor, verbal, or cognitive procedure (e.g. rotor test, mirror reading, and Tower of Hanoi (TH) task, respectively). Procedural learning has to be distinguished from implicit learning, which is often assessed on the basis of serial reaction times. During implicit learning, the subject acquires information without realizing it, through an unconscious sensitivity to the regularity. Conversely, during procedural learning, the subject provides a voluntary effort of learning. This kind of learning is observed when a subject learns to drive, when a sportsman learns to execute a perfect movement or when someone learns to calculate. It is only when the subject has become sufficiently skilful to solve the task that he or she “loses control” of its execution.
We can improve our understanding of this phenomenon by combining two complementary approaches: cognitive psychology and neuroimaging. Previous researches into cognitive procedural learning have suggested that skills undergo three characteristic phases (cognitive, associative, and autonomous), and the role played by cognitive processes in the learning of complex procedures (or intersystemic collaboration) has been theorized in the ACT model [Adaptive Control of Thoughts; Anderson, 2000]. During the cognitive phase, learning requires the intervention of various cognitive functions. Strategies based on declarative memory and working memory are needed to generate a new cognitive procedure that will be automated. During the associative phase, as the skill improves with practice, it undergoes significant changes, including considerable reductions in cognitive involvement. The final, autonomous phase does not require the intervention of cognitive functions, and is essentially characterized by the intervention of procedural memory per se. Ackerman [1988; see also Ackerman and Cianciolo, 2000] carried out a series of studies designed to locate the three phases of procedural learning postulated in the ACT model, via specific cognitive determinants of individual differences during skill acquisition. Eight experimental manipulations were used to evaluate the cognitive ability demands associated with different levels of information‐processing complexity and consistency. Subjects practiced a choice discrimination reaction time task (i.e. they were instructed to press a number‐pad key that corresponded to the two‐letter position on the screen; for ex: LM = Lower row – Middle row), consistent mapping procedures and an air traffic controller simulation (also used to show generalization for a complex task). The consistence of the tasks thus allowed the development of proficiency through the three phases described in the ACT model. Ackerman [1988] found that during the cognitive phase, performance levels were associated with general intelligence. In this phase, improvements and individual differences were quite considerable. During the associative phase, performance levels were related to perceptual processing and variability between subjects decreased. Lastly, in the autonomous phase, individual differences in performance were only determined by psychomotor functions. In summary, these models show that learning a new cognitive procedure requires processes which are highly controlled in the (initial) cognitive phase while more automatic processes are only required in the (final) autonomous phase. Indeed, the autonomous phase does not require the intervention of the cognitive functions involved in the cognitive phase and is essentially characterized by the intervention of procedural memory per se [Anderson, 2000]. More recently, in our laboratory, Beaunieux et al. [2006] confirmed for the first time the existence of three qualitatively different learning phases (cognitive, associative, and autonomous) using the Tower of Toronto task (TT task) that is an isomorphous version of the TH task. In a first experiment, 100 subjects were submitted to 40 massed trials of the TT task and nonprocedural cognitive tasks designed to assess the cognitive processes underlying automation of the cognitive procedure. This study demonstrated that healthy young subjects need to complete the TT task at least 20 times before entering the autonomous phase and confirmed the involvement of nonprocedural cognitive components (intelligence, working memory, and episodic memory) during the first two phases of cognitive procedural learning. These findings showed that procedural memory only becomes functionally independent in the autonomous phase. In the same paper, Beaunieux et al. [2006] conducted a second experiment in order to study the effect of distributed learning conditions on the dynamics of procedural learning in 50 different subjects. This second experiment confirmed the previous results but showed that distributed learning slows down the process of cognitive procedural learning.
Functional brain imaging studies of procedural learning have mainly looked at motor [Doyon et al., 2002, 2003; Grafton et al., 1992; Sakaï et al., 1998] or verbal [Kassubek et al., 2001] procedures and have revealed how brain activation changes over the course of learning. The fMRI study conducted by Sakaï et al. [1998], which focused on the acquisition of a motor procedure, showed a shift in brain activation. Frontal areas were mainly activated during the early learning stage, whereas involvement of the parietal cortex increased during the more advanced stage. These authors suggested, with reference to Fitts [1964] and Anderson [1982], that these results might reflect the transition from the cognitive phase to the autonomous one. Moreover, Sakai et al. [1998] showed that some cerebral substrates involved in the associative phase corresponded to those involved in both the cognitive and autonomous phases.
Although no research has yet been conducted into the cerebral substrates involved in the three phases of cognitive procedural learning, several assumptions can be inferred from previous neuroimaging findings. By examining the impact of age‐related changes in regional brain volumes and cognitive resources on the acquisition of a cognitive skill, Head et al. [2002] showed that at the early stage of TH problem learning (corresponding to the first trial), speed, and efficiency were correlated with both prefrontal cortex volume and working memory. These results are consistent with cognitive studies [Ackerman, 1988]. However, the eight TH trials administered in Head's study were not sufficient to assess the other phases, notably the autonomous phase.
Fincham et al. [2002] sought to find out which areas of the brain underwent changes in activity during the solution of an adapted version of the five‐disk TH task. In effect, when solving this problem, some moves are more difficult than others and require more planning. To monitory the strategy used by the subjects, they were taught beforehand to apply a specific strategy. This training provided a means of knowing when participants set themselves goals in the performance of the task and how many goals they set at a time (1, 2, or 3 steps to reach the goal). After training, participants solved novel problems during event‐related fMRI scanning. The activation sensitive to the number of planning steps concerned the frontal, parietal (angular and precuneus), premotor, and subcortical regions. Anderson et al. [2005] used the same task and a very similar methodology, albeit less restrictive for the subjects. Although Fincham et al. [2002] slowed the pace of problem‐solving to one move every 16 s, (in the absence of restrictions, participants make one move approximately every 2 s.), Anderson et al. [2005] excluded the artificial delays inserted into the problem‐solving. These authors showed that parietal activity increased and motor region activity decreased at points that require considerable intellectual engagement and concluded that activity in the parietal region offers some potential for predicting when a person is planning.
RATIONALE OF THE STUDY
The aim of our study was not to identify the brain structures that are responsible for solving the TT, but to highlight the substrates involved during the discovery and automation of the task procedure. We performed two complementary studies using the TT task in order to extend our knowledge of the neural substrates of cognitive procedural learning: a behavioral study and a positron emission tomography (PET) activation study. The aim of the behavioral study was to determine predictors for the length of the three learning phases in order to select subjects for the PET study. The length of the learning phase had to be broadly similar for all our selected subjects, so that they would not reach the associative phase either too early or too late, thereby allowing two recordings of brain activity to be carried out for each phase of the PET study. This cognitive study included two experiments: the first in order to identify the best predictors of the length of the cognitive and associative phases [from the 100 subjects of the first experiment of Beaunieux et al., 2006] and the second (with 27 different subjects) in order to check the efficacy of these predictors.
Beaunieux et al. [2006] had used the TT in order to further categorize the three learning phases, analyzing the cognitive determinants of procedural performance levels (for each trial in the learning process). The present behavioral study was designed to answer another question. We sought to highlight the cognitive components responsible for the length of each phase (and not for the performance level of each trial). These results would then enable us to establish predictors for the length of these three phases and would be used thereafter as criteria for selecting subjects for the PET study.
The PET activation study was intended to highlight the specific neural substrates of each phase during cognitive procedural learning. Given that this protocol requires subjects to move disks, the PET technique was more appropriate than fMRI. A computerized version of the TT was not used because the motor component of this kind of learning is crucial and determines performance levels in the autonomous phase.
In the light of previous behavioral and neuroimaging studies, we assumed that cognitive procedural learning would involve different cerebral structures as learning progressed. Likely areas included the frontal lobe, the parietal regions, and the cerebellum, but we were above all interesting in finding out which learning phase these structures contributed to the most and whether the patterns of activation matched established models of cognitive psychology.
BEHAVIORAL STUDY: PREDICTORS OF PROCEDURAL PERFORMANCE
The aim of this study was to find cognitive indicators of the length of the three TT learning phases for each subject. In effect, there is a degree of heterogeneity in procedural performances during learning. Some subjects learn more quickly than others and thus do not reach the same learning phase after an identical number of trials. Accordingly, we performed two complementary experiments in order to identify cognitive predictors of the length of the cognitive and associative phases of the TT procedure (Experiment 1) and to check the efficacy of these predictors (Experiment 2).
Experiment 1: Highlight Predictors of the Length of the Phases
Subjects
Subjects in this first experiment came from the study by Beaunieux et al. [2006] and corresponded to 100 unpaid volunteers (50% males), between 18 and 35 years old (mean age = 21.8; SD = 3.9). Their mean educational level, assessed by the Mill Hill Scale [Deltour, 1998], was 33.9/44 (SD = 3.9). All subjects were screened by a health questionnaire for any history of neurological or psychiatric conditions, head trauma, and alcohol or drug abuse. Because the procedural task involves the processing of colors, participants were also screened for color blindness using the Ishihara Test [Ishihara, 1997]. Lastly, we made sure that none of the participants were familiar with the TT problem.
Materials and design
Procedure.
The experimental protocol featured two sessions with a one‐week interval. The first session concerned the procedural learning of the TT task. Subjects were asked to perform 40 trials, i.e. 8 blocks of 5 trials. To assess the cognitive processes determining the length of the learning phases, a set of complementary cognitive tasks was added in the second session.
Procedural task: The TT problem. The TT task consisted of a rectangular base and three pegs. Four different‐colored disks were used: one black, one red, one yellow, and one white. The TT disks were initially stacked on the leftmost peg, with the darkest one at the bottom and the lightest one on top. The task consisted in rebuilding this configuration on the rightmost peg, obeying the following two rules: only one disk may be moved at a time, and a darker disk may never be placed on top of a lighter one. We also added a rule that was, in fact, a cue for the subject: begin by putting the white disk on the middle peg. This instruction was given in order to avoid a probably random choice by the subjects and to help them to find more quickly the optimal solution in order to observe an earlier automation. These rules were read out to the subjects and explained through examples of authorized and unauthorized moves. All the instructions were printed on a sheet of paper placed near the subject. They were required to solve 8 blocks of 5 consecutive trials, with a 5‐min break between each block. The TT device was connected to a computer that recorded the completion time and the number of moves per trial for each subject. The minimum number of moves for the 4‐disk TT task is 15. As we gave a clue for the first move, this move (and the time it took) was not taken into account. The optimum solution was thus 14 moves.
Complementary cognitive tasks [for further details, see Beaunieux et al., 2006]. With reference to the literature, we took into account five nonprocedural cognitive functions thought to play a part in cognitive procedural learning. On the basis of Ackerman's studies [1988], we assessed nonverbal intelligence, perceptual processing, and psychomotor functions. We also measured the efficiency of episodic memory and executive functions.
Nonverbal intellectual functions were assessed using two subtests of the Wechsler Adult Intelligence Scale [WAIS‐III; Wechsler, 2001 French version]: Block Design and Matrix Reasoning. These tests assessed nonverbal intelligence abilities. Episodic memory was assessed using an abridged form of the California Verbal Learning Test (CVLT), together with the Digit Symbol‐Coding (pairing) and Digit Symbol‐Coding (free recall) tests. Executive functions (inhibition, flexibility, planning, and working memory) were assessed using the last subtest of the Stroop Test [Stroop, 1935], the Trail Making test [Reitan, 1958], an adapted version of the Tower of London [Shallice, 1982], and a Simple/Choice Reaction Time test. Slave systems of the working memory were assessed by means of two span tests: the WAIS‐III digit span [Wechsler, 2001] and the visuospatial span test from the BEM 144 [Signoret, 1991]. The ability to handle information in working memory was also measured, using the Letter Number Sequencing test taken from the WAIS‐III. Perceptual processing was measured by means of two subtests of the WAIS‐III: Digit Symbol‐Coding and Symbol Search. We also selected the “Word” and “Color” perceptual tasks of the Stroop Test [Stroop, 1935] and the BAMS‐T [Lahy, 1978]. To assess psychomotor abilities, we asked the subjects to carry out two disk transfer tasks. The aim was to transfer 4 disks (one by one) from the leftmost peg to the middle peg, then to the rightmost peg and finally back to the leftmost one. The only instruction we gave was to use only one hand. The total transfer time was recorded (for 12 moves). This transfer task was performed twice (before and after the procedural learning of the TT task). The same transfer task was performed with the Tower of London task. An average transfer time was calculated on the basis of these recorded times.
Statistical analyses
Delimitation of the three learning phases.
The delimitation of the three phases was done for each individual subject, using the number of moves per trial. The cognitive phase corresponded to the search for the best solution, characterized by numerous errors. The subject remained in the cognitive phase until s/he had found the optimum solution (i.e. 14 movements). The length of the cognitive phase therefore corresponded to the number of trials during which the subject failed to find the optimum solution. The associative phase started with the discovery of this optimum solution, i.e. when the subject solved the problem in 14 moves for the first time. The procedure was not completely mastered during the associative phase. Sometimes, the subject solved the problem in 14 movements, sometimes in 15 or 16. Some errors were always present, especially at the difficult points in the problem. Accordingly, the length of the associative phase corresponded to the number of trials during which the subject solved the procedure in 14 moves or near this optimum solution. Lastly, the autonomous phase was characterized by a succession of optimum resolutions. This phase started when the subject was able to provide the optimum solution to the TT task five times in a row, i.e. in exactly 14 moves each time. However, we deemed that the subject was allowed to make one error during the autonomous phase, i.e. one extra move among 70 consecutive moves (= 5 trials of 14 moves).
To obtain a more accurate indicator than the number of trials, we also took into account the length of the phases (in seconds). The length of one phase thus corresponded to the sum of the time of resolution of each trial that was expressed in seconds. Stepwise regressions were performed, entering all the complementary cognitive tasks as independent variables (predictors) and the length of the phases as the dependent variable (i.e. the variable to be predicted) in an attempt to track the best predictive measures. We thus carried out a linear regression (forward stepwise) analysis for the cognitive phase and another for the associative phase. The length of the autonomous phase corresponded to the number of trials remaining once the subject had left the associative phase and was thus determined by the length of the first two.
Results
Mean length of the learning phases.
Overall, the 100 subjects presented a cognitive phase lasting 6.8 trials (SD = 7.4), an associative phase lasting 10 trials (SD = 8.8), and an autonomous phase lasting 23.2 trials (SD = 11.7). In other words, the cognitive phase covered trials 1–7, the associative phase trials 8–17, and the autonomous phase trials 18–40.
Phase length predictors.
In the forward stepwise regression analysis of the length of the cognitive phase, the Block design was the first variable to be included in the model (P < 0.0001). The CVLT was the second variable (overall significance at this second step: P < 0.01), followed by the accuracy and speed scores on the BAMS‐T (P < 0.005) and lastly by the simple reaction time (P < 0.048). These tasks explained 37.7% of the variance of the length of the cognitive phase. For the length of the associative phase, the accuracy score on the BAMS‐T was the first variable to be included in the model (P < 0.005). The scores on the choice reaction time (P < 0.007) and the Letter number sequencing (P < 0.048) were the two last tasks to be included. These tasks explained 15.5% of the variance of the length of the associative phase.
Comments
Results showed that the cognitive phase extended from trial 1 to trial 7, whereas the associative phase went from trial 8 to 17. The regression analysis showed that the length of the cognitive phase was mainly determined by nonverbal intelligence, episodic memory, and attention. These predictors are consistent with other studies [Ackerman, 1987; Beaunieux et al., 2006] conducted using a different methodology. Using correlation analyses, these studies identified cognitive determinants of performance levels (for each trial in the learning process) that were similar to ours. This similarity in results can be explained by the link that exists between performance levels and the length of each phase, the latter being a consequence of the former. In our study, the length of the associative phase was determined by attention, perceptual processing, and working memory (central executive). In line with Woltz [1988] and Ackerman [1987], who highlighted the contribution of working memory and perceptual processing to performance levels during the associative phase, our results showed that working memory capacities and perceptual processing were the cognitive components that best explained the variance in the length of the associative phase. In short, this study successfully established cognitive predictors for the length of each learning phase. The scores on the Block design, CVLT, and BAMS‐T make it possible to predict the length of the cognitive phase. Similarly, the scores on the BAMS‐T, the Choice reaction time and the Letter number sequencing allow us to predict the length of the associative phase. Lastly, by predicting the length of these two first phases, it is possible to select subjects who will reach the autonomous phase, by excluding subjects whose cognitive and associative phases would be too long.
Experiment 2: Checking the Efficacy of the Predictors
The aim of this second experiment was to check the efficacy of the predictors previously advanced with a new group of subjects.
Subjects
Twenty‐seven healthy young volunteers (mean age = 19.9, SD = 1.32) were included in the study. Their mean educational level, assessed by the Mill Hill Scale [Deltour, 1998], was 34.19/44 (SD = 2.97). The inclusion criteria were identical to those of the preceding study.
Methodology
First, the cognitive tests retained as predictors for the cognitive phase (Block design, CVLT, BAMS‐T, and Simple reaction time) were proposed to the subjects. To predict the length of the associative phase, the BAMS‐T, the choice reaction time, and the SLC were added. For each subject we then predicted the length of the two first learning phases according to these cognitive tests. One week later subjects were asked to perform 40 trials of the TT under the PET camera (vide infra).
Initially, a Student's t‐test was performed to compare the length of the predicted phases with those really observed. This analysis was made for the cognitive and the associative phases. This comparison was initially carried out in a global way (i.e. on the average lengths of the group). Then, we were interested in the length of the individual phases. From the length of the predicted phases, we decided if each subject would be “appropriate” or not for a PET protocol, using the following criteria of selection: the length of the cognitive phase had to be performed between 180 and 800 s. The minimum length was fixed at 180 s because we wished to acquire two measures of each phase and that a phase requires 90 s of acquisition. With regard to the associative phase, its length had to be performed between 180 and 400 s. This second phase is generally shorter than the first. Finally, we checked that these “selected” subjects would have been correctly chosen by examining their real performance. In the same way, we ensured that the subjects that did not present an adequate learning ability were really excluded by our predictors.
Results
Globally, the results showed that there was no significant difference between the average length of the predicted phases and those really measured. The comparison between the predictive length of the cognitive phase (mean = 430 s) and the real length (mean = 378 s) showed no significant difference (t = 0.86; P > 0.40). With regard to the associative phase, there was no significant difference between the predicted length (mean = 289 s) and the observed phase (mean = 270 s; t = 0.40; P > 0.69).
Thus, the individual analysis of the results enabled us to select subjects and to check if the selection was correct. Among the 27 subjects, we would have selected 12 subjects according to the predicted phases and excluded 15 subjects. The selected subjects (75%) presented adequate phases for inclusion in a PET protocol. Concerning the excluded subjects, 46% showed effectively too short or too small phases as predicted.
Comments
The predictors were not efficient at 100% and the selection lead to a strict selection that excluded “adequate subjects.” These exclusions can be explained by the relatively low percentage of explanation of the predictors (37.7% for cognitive phase and 15.5% for associative phase). But our principal aim was to exclude “extreme” subjects and this experiment showed that these subjects were well detected by the predictors. Thus, we concluded that these predictors were reliable for inclusion in the PET study.
PET ACTIVATION STUDY: CEREBRAL SUBSTRATES OF THE THREE LEARNING PHASES
Materials and Methods
Subjects
Twelve additional healthy subjects (who had not undergone the behavioral study; six women and six men; mean age = 22.4 years, range from 18 to 35 years (SD = 2.5)) took part in this study. They were selected on the basis of the cognitive predictors established during the previous study and the equation of the linear regression obtained for each phase. The length of each phase was calculated using the following equation: Y (length of the phase in seconds) = origin + (β) task 1 + (β) task 2 etc… The magnitude of the coefficients β allows one to compare the relative contribution of each independent variable in the prediction of the dependent variable. The length of the cognitive phase was calculated by this equation: Y (length of the cognitive phase) = 4528.17 + (−25.06) block design + (−57.23) CVLT + (−1092.62) Accuracy score on the BAMS‐T + (−588.59) Speed score on the BAMS‐T + (−2.30) Simple reaction time. Similarly, we estimated the length of the associative phase using the following equation: Y (length of the associative phase) = 1023.06 + (−486.04) Accuracy score on the BAMS‐T + 38.286 Choice reaction time + (−26.346) Letter number sequencing. For a single PET acquisition, each subject had to perform the task for 90 s. As the PET activation protocol featured two scans per learning phase, each phase had to last at least 180 s. For this reason, the 12 subjects included in the PET protocol all presented a predicted length of a minimum of 180 s and a maximum of 700 s. The protocol was approved by the regional ethics committee, and all the subjects gave informed written consent before participating. The inclusion criteria were identical to those of the preceding study and were supplemented by criteria specific to structural MRI and PET scans. All were right‐handed, as determined by the Edinburgh questionnaire [Oldfield, 1971].
PET Activation Paradigm
During this PET activation paradigm, subjects were placed in a supine position on an adjustable table. Experimental tasks were performed with the right arm and an intravenous catheter was placed in the left arm for the administration of H2O15. The height of the table and the position of the TT problem were adjusted for each subject to achieve the most comfortable position. A mirror was positioned above the subject's head, to allow him/her to see the TV screen, which in turn was placed in front of the scanner. The movements carried out by the subject were filmed and shown on the TV screen. The subjects were submitted to the same procedural learning process as in the first study (performing 40 TT trials). The reference task of this PET paradigm was very similar to the transfer task described above. The subject had to move the TT disks (one by one, regardless of their color) from the leftmost peg to the middle one, then to the rightmost one. Then, they had to move the disks (two by two) from the rightmost peg, to the middle one, then to the leftmost one. Subjects were trained to perform this easy procedure before the experiment started.
Each subject underwent 12 consecutive scans (injection of H2O15) during a single PET session lasting 2 h, including 2 repetitions of 4 different experimental conditions and 4 resting scans. The activation paradigm began with two scans at rest. The first condition corresponded to the motor reference task. After two scans during this reference task, the rules of the TT problem were explained to the subjects, who then had to try and find the solution. The two “cognitive acquisitions” corresponded to trials 1–2 and 3–5 (Fig. 1). The following three trials were performed without any PET scans. The 2 “associative acquisitions” took place between trials 9–13 and 14–17. Subjects then performed 13 trials of the TT problem without any PET scans. For the 2 “autonomous acquisitions,” the subjects were scanned during trials 31–35 and 36–40. Lastly, 2 resting scans were performed. In summary, the conditions were performed in the following order: Rest, Rest, Reference, Reference, Cognitive, Cognitive, Associative, Associative, Autonomous, Autonomous, Rest, and Rest. The purpose of the scans at rest was to compare brain activation before and after learning (data not shown in the present paper).
Figure 1.
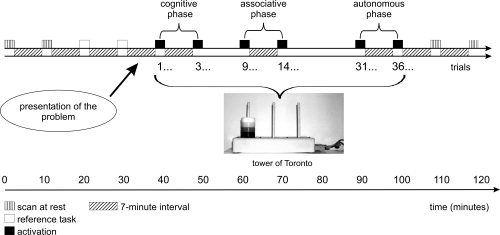
Experimental protocol.
Data Acquisition
Behavioral data acquisition
As in the previous study, the following two variables of the TT problem were recorded (i.e. number of moves and time per trial).
PET data acquisition
Measurements of the regional distribution of radioactivity were performed with a Siemens ECAT HR+ PET camera with full‐volume acquisition allowing the reconstruction of 63 planes. Transmission scans were obtained with a 68Ga source prior to emission scans. The duration of each scan was 90 s. Approximately 5 mCi of H2O15 was administered as a slow bolus in the left antecubital vein using an automatic infusion pump. Each experimental condition began 15 s before data acquisition and continued until scan completion. This process was repeated for each of the 12 scans, for a total injected dose of ∼70 mCi. The interval between injections was 6 min 40 s. The subject's head was aligned transaxially to the orbitomeatal line using a laser beam. The position of the head was checked with the laser beam prior to each injection.
Image handling and transformations
All calculations and image transformations were performed on Unix System workstations. First of all, the 8 scans of each subject were realigned, using AIR 3.0 software. For subsequent data analysis, we used Statistical Parametric Mapping software (SPM 99, Welcome Department of Cognitive Neurology) implemented in the MATLAB environment. The images were nonlinearly transformed into a standard space, i.e. the MNI PET template of SPM 99. They were smoothed using a 12‐mm Gaussian filter. As the images were scaled to an overall CBF grand mean of 50 ml/100g/min, we refer to “adjusted rCBF” hereafter. We used a grey matter threshold of 80% of the whole brain mean and covariates were centered before inclusion in the design matrix.
Data Analysis
Behavioral data analysis
To study the learning effect, the behavioral data obtained with the TT was initially processed using a repeated‐measures analysis of variance. Thus, to verify the decrease in variability with learning, we also calculated a Coefficient of Variation (CV) for each trial (CV = standard deviation/mean).
PET scan analysis
Conjunction analysis.
To identify the cerebral substrates involved in all three learning phases, we used a conjunction analysis based on the recently proposed “valid conjunction inference with the minimum statistic” [Nichols et al., 2005]. In this test, each comparison in the conjunction is individually significant, which corresponds to the valid test for a “logical AND.”
Subtraction analyses for each learning phase.
First, to pinpoint the cerebral structures associated with each learning phase, we carried out three planned comparisons of means: Cognitive vs. Reference, Associative vs. Reference, and Autonomous vs. Reference. Only significant increases are reported for these comparisons.
Second, in each peak of significant activity in each these three contrasts, we have also extracted the level of activity for each condition (reference, cognitive, associative, and autonomous). The activation level of these regions was then submitted to a repeated‐measures analysis of variance in order to observe the time course of activation during learning (repetition factor: phases). Post‐hoc analyses were conducted using Fisher's LSD test.
Finally, we contrasted the experimental conditions themselves in order to compare the regions more activated during the cognitive versus the autonomous phase, but also between the cognitive versus associative and between the associative versus autonomous phases. For all these comparisons we used the “masking” routine of SPM99 (function inclusive) to search only for rCBF increases related to learning effect (for example: cognitive phase versus associative phase) in the areas found to be activated in the contrast condition (for example: cognitive phase–reference task).
For the PET scan comparisons, a proportional scaling model was used and analysis was performed on a voxel‐by‐voxel basis. The results of the t statistic (SPM {t}) were then turned into a normal standard distribution (SPM {z}). The significant cut‐off was set at the P < 0.001 uncorrected for multiple comparisons. Anatomical/cytoarchitechtonic location of significant activation was based on the SPM 99 MNI template. All the coordinates listed in the sections below are the SPM 99 coordinates.
Correlational analyses
Lastly, to study the link between activation and performance levels, we assessed the correlations between the activation level of the regions involved in the cognitive phase and the mean times of the TT trials performed during this phase. Only significant correlations (P < 0.05) were retained. Because of the lack of variability characterizing the associative and autonomous phases, we did not assess correlation for these two phases.
RESULTS
Behavioral Data: Assessing Cognitive Procedural Learning
First, in terms of moves, the results showed a significant effect of trial repetition (F(39, 390) = 2.77; P < 0.0001). There was an overall decrease in the number of moves needed to solve the problem across the 40 trials.
The analysis of variance carried out on the data for time per trial (Fig. 2a) showed a trial effect on mean performance levels (F(39, 390) = 12.19; P < 0.0001). The subjects improved their performances with practice, their completion times decreasing across the skill acquisition trials. The analysis of the coefficients of variation showed greater variability at the start of learning (Fig. 2b).
Figure 2.
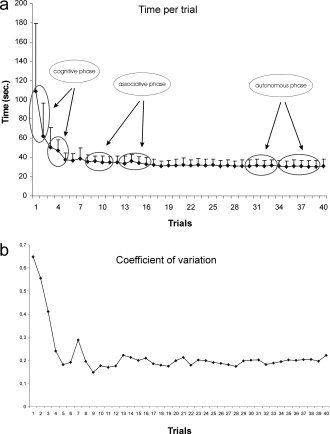
Performance trends in terms of time (a) and CV (b) per trial in the TT task (12 subjects).
PET Data
PET scan comparisons
For technical reasons, for one subject, one “cognitive acquisition” of the two obtained was excluded from the analyses.
Conjunction analysis.
The conjunction analysis revealed that only the right frontal cortex was activated in all three learning phases (cf. Table I).
Table I.
Brain regions common to all three learning phases vs. reference tasks
| Anatomical region | Z score | Cluster extent (k) | % | Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Frontal Sup R | (BA 11) | 4.14 | 315 | 32.70 | 28 | 60 | 2 |
| Frontal Sup Orb R | 30.16 | ||||||
| Frontal Mid Orb R | 15.27 | ||||||
| Frontal Mid R | 15.26 | ||||||
Areas significantly activated at P < 0.001 uncorrected for multiple comparison. Anatomical location of significant activation and approximate Brodmann's areas was as described in the Materials and Method section Stereotaxic coordinates shown here are those listed in SPM99.
Subtraction analyses for each learning phase.
Using the t‐test, three planned comparisons of means were carried out between each of the three learning phases and the reference task (motor task). Significant increases are reported for all these comparisons (Tables II, III, IV and Fig. 3).
Table II.
Brain regions activated during the cognitive phase vs. reference task
| Anatomical region | Z score | Cluster extent (k) | % cluster | Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cognitive phase vs. reference | |||||||
| Frontal Mid Orb R | (BA 11/10) | 6.28 | 1,558 | 24.84 | 26 | 56 | −12 |
| Frontal Mid R | 34.21 | ||||||
| Frontal Sup Orb R | 16.69 | ||||||
| Cerebellum Crus 1 R | 6.26 | 2,160 | 45.14 | 44 | −72 | −30 | |
| Cerebellum Crus 2 R | 23.84 | ||||||
| Cerebellum Crus 2 L | 5.91 | 3,288 | 42.64 | −38 | −70 | −36 | |
| Cerebellum Crus 1 L | 32.42 | ||||||
| Precuneus L | (BA 7/19) | 5.49 | 1,539 | 36 | −10 | −72 | 44 |
| Parietal Sup L | 19.49 | ||||||
| Parietal Inf L | 17.48 | ||||||
| Occipital Mid L | 13.39 | ||||||
| Frontal Mid L | (BA 10) | 5.23 | 1,058 | 35.26 | −30 | 52 | 10 |
| Frontal Sup Orb L | 19.85 | ||||||
| Frontal Mid Orb L | 17.01 | ||||||
| Frontal Sup L | 11.44 | ||||||
| Angular R | (BA 7/39/19) | 5.01 | 1,889 | 48.49 | 36 | −66 | 44 |
| Parietal Inf R | 12.39 | ||||||
| Parietal Sup R | 11.81 | ||||||
| Occipital Mid R | 10.75 | ||||||
| Anterior cingulate gyrus R | (BA 24) | 5.01 | 816 | 47.55 | 6 | 36 | 14 |
| Anterior cingulate gyrus L | 26.35 | ||||||
| Frontal Sup medial L | 14.34 | ||||||
| Frontal Inf Tri R | (BA 45/46) | 4.58 | 896 | 14.84 | 46 | 32 | 28 |
| Frontal Mid R | 75.89 | ||||||
| Insula R | (BA 47) | 4.41 | 196 | 62.76 | 38 | 22 | −10 |
| Frontal Inf Orb R | 36.22 | ||||||
| Frontal Mid L | (BA 9) | 4.28 | 145 | 87.59 | −30 | 10 | 50 |
| Precentral L | 12.41 | ||||||
Areas significantly activated at P < 1.001 uncorrected for multiple comparison. Anatomical location of significant activation and approximate Brodmann's areas was as described in the Materials and Method section Stereotaxic coordinates shown here are those listed in SPM99.
Table III.
Brain regions activated during the associative pharse vs. reference task
| Anatomical region | Z score | Cluster extent (k) | % cluster | Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Associative phase vs. reference | |||||||
| Calcarine R | (BA 17/18) | 5.32 | 1,041 | 39.83 | 6 | −88 | −2 |
| Lingual R | 24.70 | ||||||
| Calcarine L | 15.35 | ||||||
| Frontal Mid R | (BA 11/10/46) | 5.29 | 800 | 51.50 | 30 | 62 | 2 |
| Frontal Sup R | 17.62 | ||||||
| Frontal Sup Orb R | 15.75 | ||||||
| Thalamus R | 4.82 | 170 | 50.59 | 14 | −10 | 18 | |
| Caudate R | 20.59 | ||||||
| Frontal Sup Orb R | (BA 11) | 4.66 | 563 | 41.56 | 16 | 38 | −26 |
| Rectus R | 23.80 | ||||||
| Occipital Mid L | (BA 19) | 4.18 | 255 | 58.82 | −28 | −68 | 34 |
| Parietal Inf L | 23.92 | ||||||
| Cerebelum Crus 1 R | 4.03 | 191 | 53.93 | 52 | −60 | −40 | |
| Cerebelum Crus 2 R | 46.07 | ||||||
Table IV.
Brain regions activated during the associative pharse vs. reference task
| Anatomical region | Z score | Cluster extent (k) | % clustera | Coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Autonomous phase vs. reference | |||||||
| Calcarine L | (BA 17/18) | 6.55 | 5137 | 20.03 | 4 | −90 | −4 |
| Calcarine R | 18.10 | ||||||
| Lingual R | 16.64 | ||||||
| Lingual L | 8.82 (21.62%) | ||||||
| Cerebelum 6 R | 8.62 (24.68%) | ||||||
| Cerebelum 4 5 R | 4.63 (27.64%) | ||||||
| Vermis 6 | 2.61 (36.12%) | ||||||
| Vermis 7 | 1.97 (52.06%) | ||||||
| Frontal Mid Orb R | (BA 11/10) | 5.91 | 605 | 20.99 | 22 | 56 | −10 |
| Frontal Sup Orb R | 37.02 | ||||||
| Frontal Sup R | 24.30 | ||||||
| Thalamus L | 4.83 | 647 | 53.01 | −16 | −30 | 4 | |
| Thalamus R | 15.15 | ||||||
| Frontal Mid L | (BA 11/10) | 4.42 | 330 | 33.33 | −26 | 52 | −8 |
| Frontal Mid L | 23.94 | ||||||
| Frontal Sup Orb L | 22.42 | ||||||
| Frontal Sup L | 18.48 | ||||||
| Rectus R | (BA 11) | 4.23 | 249 | 53.01 | 2 | 44 | −20 |
| Rectus L | 16.47 | ||||||
| Frontal Mid R | (BA46) | 4.09 | 133 | 100 | 42 | 50 | 8 |
In brackets the percentage of the region included in the cluster (only for regions where % cluster is <10%).
Figure 3.
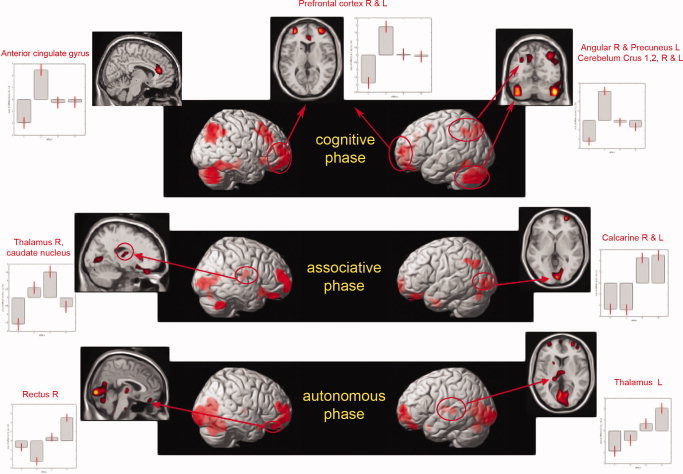
PET scan comparisons: brain activation of the three phases of cognitive procedural learning (TT task). Significantly activated regions at the threshold of P < 0.001 uncorrected for multiple comparisons and time course of activation across learning. The plots represent the relative contribution of the different conditions of our paradigm, according to the “effects of interests” for selected peaks. The first column corresponds to the reference condition. The 2nd, 3rd, and 4th columns correspond respectively to the cognitive, associative, and autonomous phases.
Cognitive phase versus reference
The cognitive phase was associated with extensive activation of the right frontal cortex (BA11/10, BA 45/46), right and left cerebellum (posterior), left precuneus (BA7/19) and right angular region (BA 39/7/19), anterior cingulate gyrus, and finally with left frontal regions (BA9,10) (cf. Table II).
Associative phase versus reference
A comparison of the associative phase with the reference task showed bilateral activation of the calcarine and lingual regions (BA 17/18), right middle and right orbitofrontal region (BA11/10/46), right thalamus and right caudate nucleus, left occipital region (BA 19), and right posterior cerebellum (cf. Table III).
Autonomous phase versus reference
A comparison of the autonomous phase with the reference task revealed bilateral activation of the calcarine and lingual regions (BA 17/18), right cerebellum (anterior), bilateral superior and middle frontal areas (BA11/10), and left thalamus. (cf. Table IV)
The activation level of these regions was then submitted to a repeated‐measures analysis of variance in order to observe the time course of activation during learning (cf. Fig. 3). All these regions presented a principal effect of learning (P < 0.05). Concerning the cognitive phase, the post‐hoc analyses showed that all regions (bilateral prefrontal and parietal regions, bilateral cerebellum, and right anterior cingulate gyrus) were significantly more implicated during the cognitive phase than during the two other phases (P < 0.05). For all these regions, there was no significant difference between the associative and autonomous phases. These results were also observed in the analyses contrasting the cognitive phase and the two other ones. These comparisons of the experimental conditions were reported in Figure 4. The contrast cognitive–associative (Fig. 4b) revealed a decrease in the right and left cerebellum, the anterior cingulate gyrus, the right angular, the left precuneus, and finally in the right orbitofrontal cortex. The contrast cognitive–autonomous (Fig. 4a) revealed a decrease in the same regions as well as one additional region, the left inferior parietal cortex.
Figure 4.
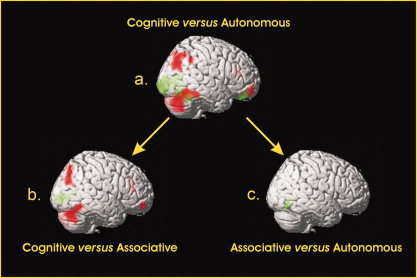
Brain comparisons between the three phases. The figure represents the 6 contrasts of the experimental conditions. In red: areas of significantly (P < 0.001, uncorrected) higher activation in early compared to later phases of learning (i.e. cognitive–autonomous, cognitive–associative, and associative–autonomous). In green: areas of significantly higher activation in late compared to earlier phases of learning (i.e. autonomous–cognitive, associative–cognitive, and autonomous–associative).
Concerning the comparison of the associative phase with the reference task, one region corresponding to the right thalamus and the right caudate nucleus tended to be more activated during the associative phase than during the cognitive (one‐tailed, P < 0.06) and the autonomous (P< 0.003) phases. One region, the right calcarine, was more activated than during the cognitive phase (P < 0.0001), but no differences were reported with the autonomous phase. This latter result was also observed in the contrast associative–cognitive showing an increase of activation in the right calcarine region (Fig. 4b).
Concerning the comparison of the autonomous phase with the reference task, regions such as the left thalamus and the right rectus increased with learning and were significantly more activated during this last phase (P < 0.05 and P < 0.054 for the left thalamus). The contrast autonomous phase–cognitive phase revealed an increase in activation in the right calcarine, right rectus, and left thalamus (Fig. 4a). Finally, the contrast autonomous‐associative revealed an increase in activation in the right lingual region (Fig. 4c), whereas the contrast associative‐autonomous did not reveal any decrease.
Correlational analyses
We analyzed the correlations between the different regions highlighted during the cognitive phase and the mean time taken to solve the TT. In the case of the cognitive phase (trials 1 and 2), results revealed negative correlations with the right angular region (r = −0.77; P < 0.05; Fig. 5). In other words, the quicker a subject solved the problem, the more this region was activated.
Figure 5.
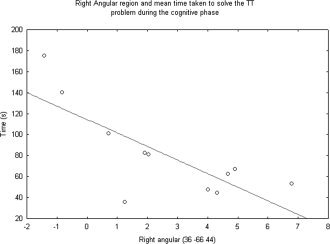
Correlation between mean time taken to solve the trials of the TT problem during the cognitive phase (trials 1 and 2) and activation of the right angular region (36−66.44).
DISCUSSION
Our aim was to study cognitive procedural learning using a double approach, associating both behavioral and neuroimaging studies. The behavioral study established the cognitive predictors for the length of each learning phase: the cognitive phase was mainly determined by nonverbal intelligence, episodic memory, and attention, whereas the length of the associative phase was determined by attention, perceptual processing, and working memory. These predictors are consistent with cognitive models of procedural learning and validate the criteria we used to delimit the learning phases on an individual basis. Thanks to these predictors, we were able to select suitable subjects for the PET protocol and the results of this first study also helped us to interpret our neuroimaging findings.
The behavioral results of the PET study revealed a significant effect of training on the ability to solve the TT problem (in term of moves and time). Overall, our results are consistent with imaging studies of noncognitive procedural learning, suggesting that learning involves neuronal changes in frontal and parietal areas, the cerebellum, and the basal ganglia [Hikosaka et al., 2002; Poldrack and Gabrieli, 2001]. The conjunction analysis showed that only one region (orbitofrontal cortex) remained active throughout all the learning phases. However, during the cognitive phase, ANOVA of the activation level of the frontal regions underwent a significant effect of learning, i.e. a significant reduction. Activation of the orbitofrontal area has previously been observed by Beauchamp et al. [2003], in subjects asked to solve various Tower of London problems. In its early learning phase, this task involves the same cognitive processes as the TT such as planning. In their study, during the acquisition of this cognitive skill, decreases were observed in the activity of the orbitofrontal and frontopolar regions, which have been shown to be involved in complex cognitive processes such as decision‐making and guessing. According to Damasio [1994], the orbitofrontal cortex is involved in decision‐making processes and, more specifically, allows inappropriate responses to be excluded on the basis of previous experience and, accordingly appropriate strategies to be implemented. The orbitofrontal region is also thought to play a role in the encoding of information in memory [Frey and Petrides, 2000]. Using the presentation of visual information in different conditions that varied in their encoding demands, these authors showed that as encoding increased across the different conditions, there was a corresponding increase in activity in the right orbitofrontal cortex. This region seems to be linked to the amount of effort allocated to the task. In our study, we can assume that the increased activity of the right orbitofrontal (predominant) region during the cognitive phase was due to the load allocated to solving the problem a load that was greater at the beginning of the learning process than at the end. The subject had to encode new information, such as the rules of the problem and the goal they had to reach. They also had to encode the correct moves in order to find the optimum procedure. In the light of our hypotheses, and more specifically with reference to Sakaï et al. [1998], we expected to find a decrease in frontal activation with learning, due to the disengagement of the cognitive components needed to solve the problem.
The results of our study also confirmed the existence of three distinctive phases during the learning of a cognitive procedure, by demonstrating the involvement of different brain regions in each learning phase. The cognitive phase was associated with extensive activation of the right and left orbitofrontal cortex, right and left cerebellum, left precuneus and right angular region, and anterior cingulate gyrus. Long regarded as the centre of motor activities, the cerebellum is now thought to play a role in many cognitive activities, too [Thach, 1998]. Van Mier and Petersen [2002] found a significantly close correlation between decreases in cerebellar activation and decreases in errors while practicing a motor learning task. Moreover, the regions specifically observed in our study were the left areas Crus 1 and Crus 2. Located in the posterior lobe of the cerebellum (and more particularly in the neocerebellum), these areas are more closely associated with the cognitive role of the cerebellum [Papathanassiou, 2002; Schmahmann and Caplan, 2006]. We can assume that the activation of the left cerebellum during the cognitive phase was related to cognitive processes and, more specifically, to error detection and the correction of inappropriate moves. The activation of the parietal areas (angular and precuneus) observed at the start of learning was similar to that observed in studies using the Tower of London [Baker et al., 1996; Newman et al., 2003; Schall et al., 2003]. This type of activation is thought to stem from the visuospatial, attentional, or verbal working memory demands of this type of task. The activation of these two parietal regions has also been observed during a modified version of the TH [Anderson et al., 2005; Fincham et al., 2002] and attributed to planning load. The first TT learning phase is indeed characterized by planning necessary when subjects are trying to develop optimal strategies to solve the problem. This hypothesis is supported by the negative correlation we found between the activation level of the right angular region and the time taken to solve the initial TT trials. The quicker a subject solved the problem (i.e. the more efficiently), the higher this region's level of activation. We can thus suppose that the anterior cingulate gyrus implicated only during the cognitive phase was due to the attentional load required by the task at the beginning of the learning.
The associative phase showed activation of the right prefrontal regions (BA 46/11/10), right caudate nucleus, bilateral calcarine and lingual regions (BA 17/18), and right thalamus.
This phase was characterized by the involvement of the BA 11, but significantly less so than the cognitive phase). Like in the cognitive phase, the cluster extended to the BA 10 and 46 frontal areas. Numerous neuroimaging studies of working memory and problem‐solving have highlighted the activation of these prefrontal areas [Cabeza and Nyberg, 2000; Curtis et al., 2000], suggesting the intervention of working memory. During the associative phase, subjects had to associate short sequences of the procedure discovered during the cognitive phase and maintain them in working memory. Like Woltz [1988], who demonstrated the involvement of working memory in the two first learning phases, and in line with the results of our first study suggesting that working memory can serve as a predictor for the associative phase, we can assume that subjects used always working memory during this second learning phase to hold the different subgoals in memory. The associative phase was also associated with the activation of specific areas, notably the right caudate nucleus. Neuropsychological studies of Huntington's disease and Parkinson's disease have revealed impaired learning of motor skills [Heindel et al., 1989], perceptual skills [Martone et al., 1984], and cognitive skills [Saint‐Cyr et al., 1988; Vakil and Herishanu‐Naaman, 1998]. Similarly, Mochizuki‐Kawai et al. [2004] showed that patients with subcortical degeneration (Parkinson's disease) exhibit deficits in the retention of newly acquired motor skills, whereas patients with cortical degeneration (Alzheimer's disease) are able to retain newly acquired motor skills for at least 3 months. These authors concluded that the striatum plays an important role as a motor consolidation system. Neuroimaging studies have associated basal ganglia activity with motor skill learning [Seitz et al., 1990] and cognitive skill learning [Poldrack et al., 1999]. These authors suggest “that the role of the striatum in skill learning may be analogous to the role proposed for the hippocampus in episodic memory. It serves to maintain cortical representations until they can be ingrained by the local cortical mechanism.” Accordingly, the involvement of the caudate nucleus in our study could reflect the acquisition (and maintenance) of a cognitive procedure. During this second phase, we observed the activation of the thalamus. This region has been found to play a role in either motor or nonmotor learning in several studies [Grafton et al., 1992; Poldrack et al., 1998] but its precise role has yet to be elucidated. The associative phase was also characterized by the bilateral activation of the calcarine and lingual regions (BA 17 and BA 18), both of which are involved in the processing of visual information. A series of experiments by Kosslyn et al. [1993, 1995, 1996] provided evidence of similarities between visual perception and visual imagery by showing increased blood flow in areas 17 and 18 during imagery. “Imagery can be defined as manipulating sensory information that comes not from the sense, but from memory” [Cabeza and Nyberg, 2000]. We can assume that, at this stage of learning, subjects began to use mental imagery to anticipate suitable future moves while making the preceding ones. According to Denis [1985], this second phase of learning is characterized by the development and control of mental images. With additional practice, i.e. when the subjects reach the autonomous phase, these images become clearer. This theory could explain the involvement of these occipital regions during the autonomous phase.
Lastly, the autonomous phase revealed activation of the right cerebellum, calcarine and lingual regions (BA 17/18), bilateral superior and middle frontal areas (BA 11/10), and left thalamus. In the case of the cerebellum, the areas that were activated and amplified with learning were anterior to those involved during the cognitive phase and are known to be responsible for regulating movements. These data could correspond to the increased involvement of the psychomotor abilities observed at the end of procedural TT learning [Beaunieux et al., 2006]. The right cerebellum was activated at the same time as the left thalamus and right rectus. This new activation could be interpreted in the light of studies of consolidation. Over the course of 3 months, Takashima et al. [2006] looked at how consolidation (in declarative memory) affects the neural correlates of memory retrieval. Results showed that hippocampal activity for correct confident recognition decreased, whereas activity in a ventral medial prefrontal region increased. Similarly, in our study we found that the regions where activation decreased with learning (right frontal, caudate nucleus, and parietal regions) were supplemented by new regions involved in consolidation. The prefrontal activations with those of the cerebellum, at the end of the learning, may reflect the involvement of a cortico‐cerebellar system in the maintaining of the cognitive ability. They can also be due to the constraints of the PET protocol implying a minimum of control of the procedure, even if the subjects had reached the autonomous phase of the learning. Although the subjects have attained the autonomous phase, we can suppose that they not “fully automated” the procedure and automation of the procedure could be reinforced with more practice.
Our results are consistent with the transition described by Sakai et al. [1998]. The results of this latter study suggest that the acquisition of visuomotor sequences requires frontal activation, whereas the retrieval of visuomotor sequences requires parietal activation: a finding that might reflect the transition from the declarative stage to the procedural one. In the present study, we observed a transition from the frontoparietal regions to the occipital and thalamic regions. We may also interpret these findings as a transition from the declarative stage (cognitive phase) to the procedural one (autonomous phase). The implication of different regions involved between these two studies might be explained by the different nature of the tasks (visuomotor sequence learning versus cognitive procedural learning).
We also performed complementary analyses (data not shown) in order to take into account the execution speed, since the moves are slower at the beginning than at the end of the learning. Thus, we added speed scores (i.e. number of moves divided by the total time per trials) as a nuisance variable in the PET analyses. The PET results were very similar to those obtained without this nuisance variable and we may conclude that the rCBF measures were not influenced by the execution speed of the moves.
Overall, our results fit the cognitive models of cognitive procedural learning. The involvement of the frontoparietal network could reflect the cognitive engagement (strategies in problem solving and planning) associated with the cognitive phase. The activation of the right caudate nucleus during the associative phase may have been temporary, corresponding to the acquisition of the correct procedure. Although this activation may be very brief, the neuropsychological data of patients with striatal dysfunctions show that it is crucial. Lastly, the activation of the cerebellum (anterior part) during the autonomous phase is consistent with the well‐known role of this region in procedural memory and the fact that psychomotor abilities determine performance levels. Our results also revealed that the most important changes in cerebral activation happened between the cognitive and associative phases (i.e. at the beginning of the learning phase when procedural performance improvement is the most important).
Acknowledgements
We thank D. Luet, M.H. Noël, O. Tirel, and the staff of Cyceron, the MRC Cyclotron Unit for their assistance.
REFERENCES
- Ackerman PL ( 1987): Individual differences in skill learning: An integration of psychometric and information processing perspectives. Psychol Bull 102: 1, 13–27. [Google Scholar]
- Ackerman PL ( 1988): Determinants of individual differences during skill acquisition: Cognitive abilities and information processing. J Exp Psychol Gen 117: 288–318. [Google Scholar]
- Ackerman PL, Cianciolo AT ( 2000): Cognitive, perceptual‐speed, and psychomotor determinants of individual differences during skill acquisition. J Exp Psychol Appl 6: 259–290. [DOI] [PubMed] [Google Scholar]
- Anderson JR ( 1982): Acquisition of cognitive skill. Psychol Rev 89: 369–406. [Google Scholar]
- Anderson JR ( 2000): Skill acquisition In: Anderson JR, Learning and Memory: An Integrated Approach, 2nd ed, pp. 304–337, New York: Wiley. [Google Scholar]
- Anderson JR, Albert MV, Fincham JM ( 2005): Tracing problem solving in real time: fMRI analysis of the subject‐paced Tower of Hanoi. J Cogn Neurosci 17: 1261–1274. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RSJ, Robbins TW ( 1996): Neural systems engaged by planning: A PET study of the Tower of London task. Neuropsychologia 34: 515–526. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JAD, Doyon J ( 2003): Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage 20: 1649–1660. [DOI] [PubMed] [Google Scholar]
- Beaunieux H, Hubert V, Witkowski T, Pitel AL, Rossi S, Danion JM, Desgranges B, Eustache F ( 2006): Which processes are involved in cognitive procedural learning? Memory 14: 521–539. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Neural bases of learning and memory: Functional neuroimaging evidence. Curr Opin Neurol 13: 415–421. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR ( 1980): Preserved learning and retention of pattern‐analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 210: 207–210. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Zald DH, Pardo JV ( 2000): Organization of working memory within the human prefrontal cortex: A PET study of self‐ordered object working memory. Neuropsychologia 38: 1503–1510. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1994): Descartes' error and the future of human life. Sci Am 271: 144. [DOI] [PubMed] [Google Scholar]
- Deltour JJ ( 1998): Echelle de vocabulaire de Mill Hill. Adaptation française [Mill Hill Vocabulary Scale. French‐language adaptation]. Paris: EAP. [Google Scholar]
- Denis M ( 1985): Visual imagery and the use of mental practice in the development of motor skills. Can J Appl Sport Sci 10: 4S–16S. [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG ( 2002): Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhume V, Ungerleider LG ( 2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41: 252–262. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, Van Veen V, Stenger VA, Anderson JR ( 2002): Neural mechanisms of planning: A computational analysis using event‐related fMRI. Proc Natl Acad Sci USA 99: 3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM ( 1964): Perceptual‐motor skill learning In: Melton AW, editor. Categories of Human Learning. New York: Academic Press; pp 253–285. [Google Scholar]
- Frey S, Petrides M ( 2000): Orbitofrontal cortex, a key prefrontal region for encoding information. Proc Natl Acad Sci USA 97: 8723–8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RSJ, Phelps ME ( 1992): Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci 12: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Raz N, Gunning‐Dixon F, Williamson A, Acker JD ( 2002): Age related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychol Aging 17: 72–84. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shrults CW, Walicke PA, Butters N ( 1989): Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. J Neurosci 9: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakaÿ K, Nakahara H ( 2002): Central mechanisms of motor skill learning. Curr Opin Neurobiol 12: 217–222. [DOI] [PubMed] [Google Scholar]
- Ishihara S. 1997. Tests for Color Blindness. Tokyo: Kanehara Shuppan. [Google Scholar]
- Kassubek J, Schmidtke K, Kimmig H, Lücking CH, Greenlee MW ( 2001): Changes in cortical activation during mirror reading before and after training: An fMRI study of procedural learning. Brain Res Cogn Brain Res 10: 207–217. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Maljkovic V, Weise SB, Chabris CF, Hamilton SE, Rauch SL, Buonanno FS ( 1993): Visual mental imagery activates topographically organized visual cortex: PET investigations. J Cogn Neurosci 5: 263–287. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Alpert NM ( 1995): Topographical representations of mental images in primary visual cortex. Nature 378: 496–498. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Rauch SL, Alpert NM ( 1996): Individual differences in cerebral blood flow in area 17 predict the time to evaluate visualized letters. J Cogn Neurosci 8: 78–82. [DOI] [PubMed] [Google Scholar]
- Lahy JM. ( 1978): Test d'Attention Concentrée [Concentrated Attention Test]. Paris: EAP. [Google Scholar]
- Martone M, Butters N, Payne FM, Becker JT, Sax DS ( 1984): Dissociation between skill learning and verbal recognition in amnesia and dementia. Arch Neurol 41: 965–970. [DOI] [PubMed] [Google Scholar]
- Mochizuki‐Kawai H, Kawamura M, Hasegawa Y, Mochizuki S, Oeda R, Yamanaka K, Tagaya H ( 2004): Deficits in long‐term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia 42: 1858–1863. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Sashank V, Just MA ( 2003): Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high level perception. Neuropsychologia 41: 1668–1682. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D ( 2002): L'anatomie du cervelet In: Houdé O, Mazoyer B, Tzourio‐Mazoyer N, editors. Cerveau et psychologie. Introduction à L'imagerie cérébrale anatomique et fonctionnelle. Paris: PUF; pp 137–158. [Google Scholar]
- Poldrack RA, Gabrieli JDE ( 2001): Characterizing the neural mechanisms of skill learning and repetition priming. Evidence from mirror reading. Brain 124: 67–82. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JDE ( 1998): The neural basis of visual skill learning: An fMRI study of mirror reading. Cereb Cortex 8: 1–10. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JDE ( 1999): Striatal activation during acquisition of a cognitive skill. Neuropsychology 13: 564–574. [DOI] [PubMed] [Google Scholar]
- Reitan RM ( 1958): Validity of the Trail Making test as an indicator of organic brain damage. Percept Motor Skills 8: 271–276. [Google Scholar]
- Saint‐Cyr JA, Taylor AE, Lang AE ( 1988): Procedural learning and neostriatal dysfunction in man. Brain 111: 941–959. [DOI] [PubMed] [Google Scholar]
- Sakaï K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B ( 1998): Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci 18: 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall U, Johnston P, Lagopoulos J, Jüptner M, Jentzen W, Thienel R, Dittmann‐Balçar A, Bender S, Ward PB ( 2003): Functional brain maps of Tower of London performance: A positron emission tomography and functional magnetic resonance imaging study. Neuroimage 20: 1154–1161. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D ( 2006): Cognition, emotion and the cerebellum. Brain 129: 290–292. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE, Bohm C, Greitz T, Stone‐Elander S ( 1990): Motor learning in man: A positron tomographic emission study. Neuroreport 1: 57–66. [DOI] [PubMed] [Google Scholar]
- Shallice T ( 1982): Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Signoret JL ( 1991): Batterie d'efficience mnésique (BEM 144). Paris: Elsevier. [Google Scholar]
- Stroop J ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G ( 2006): Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA 103: 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT ( 1998): What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci 2: 331–337. [DOI] [PubMed] [Google Scholar]
- Vakil E, Herishanu‐Naaman S ( 1998): Declarative and procedural learning in Parkinson's disease patients having tremor or bradykinesia as the predominant symptom. Cortex 34: 611–620. [DOI] [PubMed] [Google Scholar]
- Van Mier H, Petersen SE ( 2002): Role of the cerebellum in motor cognition. Ann NY Acad Sci 978: 334–353. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 2001): Wechsler Adult Intelligence Scale, 3rd ed. Paris: EAP. (WAIS III), French version. [Google Scholar]
- Woltz DJ ( 1988): An investigation of the role of working memory in procedural skill acquisition. J Exp Psychol Gen 117: 319–331. [Google Scholar]


