Abstract
Depression has been associated with dysfunctional executive functions and abnormal activity within the anterior cingulate cortex (ACC), a region critically involved in action regulation. Prior research invites the possibility that executive deficits in depression may arise from abnormal responses to negative feedback or errors, but the underlying neural substrates remain unknown. We hypothesized that abnormal reactions to error would be associated with dysfunctional rostral ACC activity, a region previously implicated in error detection and evaluation of the emotional significance of events. To test this hypothesis, subjects with low and high Beck Depression Inventory (BDI) scores performed an Eriksen Flanker task. To assess whether tonic activity within the rostral ACC predicted post‐error adjustments, 128‐channel resting EEG data were collected before the task and analyzed with low‐resolution electromagnetic tomography (LORETA) using a region‐of‐interest approach. High BDI subjects were uniquely characterized by significantly lower accuracy after incorrect than correct trials. Mirroring the behavioral findings, high BDI subjects had significantly reduced pretask gamma (36.5–44 Hz) current density within the affective (rostral; BA24, BA25, BA32) but not cognitive (dorsal; BA24′, BA32′) ACC subdivision. For low, but not high, BDI subjects pretask gamma within the affective ACC subdivision predicted post‐error adjustments even after controlling for activity within the cognitive ACC subdivision. Abnormal responses to errors may thus arise due to lower activity within regions subserving affective and/or motivational responses to salient cues. Because rostral ACC regions have been implicated in treatment response in depression, our findings provide initial insight into putative mechanisms fostering treatment response. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: cingulate cortex, treatment response, depression, emotion, Eriksen Flanker task, source localization, anhedonia, low resolution electromagnetic tomography, gamma activity
INTRODUCTION
In the past decade there has been a surge of interest toward attaining a better understanding of the neural underpinnings and neuropsychological deficits of depression. Emerging from this research is a link between depression and wide‐ranging neuropsychological deficits, particularly in attention, memory, and problem‐solving tasks. Impairment in executive functions, which are critically needed for flexible problem solving, action monitoring, and adaptive behavioral modification, may be one of the core issues underlying cognitive deficits in depression [Austin et al., 2001; Veiel, 1997]. In line with this assumption, executive dysfunctions 1) have been reported in a wide range of patients, including depressed outpatients [Porter et al., 2003] and young patients with dysphoria [Channon and Green, 1999]; 2) predicted poor response to pharmacological treatment [Dunkin et al., 2000]; and 3) were still observed after symptom recovery [Beats et al., 1996; Paradiso et al., 1997].
Substantial progress in cognitive neuroscience has delineated an executive control system centered on the anterior cingulate cortex (ACC) and prefrontal cortex (PFC) critically implicated in governing information processing and response selection in situations requiring adaptive and flexible task performance, such as planning, error monitoring and correction, and response inhibition [Miller and Cohen, 2001; Posner and Dehaene, 1994]. In depression, abnormalities in this distributed network have been described [Davidson et al., 2002], highlighting candidate neural markers of executive dysfunctions that could lead to impaired problem solving, maladaptive behavioral regulation, and abnormal error monitoring. With respect to the ACC, structural [Ballmaier et al., 2004], neurochemical [Auer et al., 2000; Mirza et al., 2004], and functional [Beauregard et al., 1998; Bremner et al., 2004; George et al., 1997; Kumari et al., 2003; Mitterschiffthaler et al., 2003; Okada et al., 2003] abnormalities have been reported in depression.
In order to understand the potential role of ACC dysfunction in depression, it is important to stress that this region is anatomically and functionally heterogeneous, and that “affective” and “cognitive” subdivisions have been described [Bush et al., 2000; Devinsky et al., 1995; Vogt et al., 1995]. Accordingly, the affective subdivision 1) encompasses rostral and ventral areas of the ACC (areas 25, 32, 33, and rostral area 24); 2) has extensive connections with limbic and paralimbic structures; and 3) has been implicated in regulating visceral, autonomic, and emotional responses to stressful behavioral and emotional events. Conversely, the dorsal cognitive subdivision of the ACC (1) includes caudal area 24′, 32′ and the cingulate motor area; (2) has reciprocal connections with the dorsolateral PFC, parietal cortex, and supplementary motor areas; and (3) is involved in detection of response conflict and processing of cognitively demanding information. Echoing this anatomical data, recent neuroimaging studies of executive functions have uncovered important functional specialization within the ACC (Fig. 1A). Specifically, increased activation within dorsal ACC regions extending to the pre‐SMA has been observed during conflict monitoring, which can be conceptualized as the online monitoring of conflict between concurrent processes or response competition [Botvinick et al., 2001; Bunge et al., 2002; Bush et al., 2003; Carter et al., 1998; Casey et al., 2000; Fassbender et al., 2004; Hazeltine et al., 2003; Kerns et al., 2004; MacDonald et al., 2000; Ruff et al., 2001]. In contrast, activation in more rostral and inferior regions of the ACC has been observed after error commission [Braver et al., 2001; Garavan et al., 2003; Kiehl et al., 2000; Laurens et al., 2003; Menon et al., 2001; Rubia et al., 2003; Ullsperger and von Cramon, 2001]. Data from recent event‐related potentials (ERP) studies lends further support to the notion that dorsal ACC activity may index conflict monitoring that is dissociable from error detection and emotional evaluation of errors mediated by the rostral ACC [Luu et al., 2003; van Veen and Carter, 2002]. Collectively, these data suggest that, in depression, dysfunction in specific regions of the ACC could be associated with distinct functional impairments.
Figure 1.
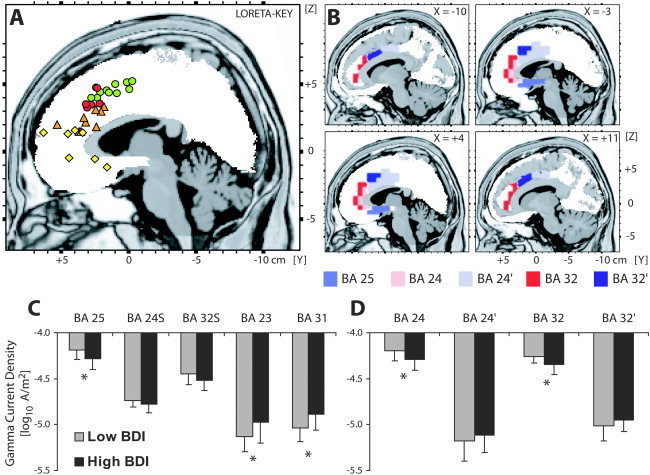
A: Summary of neuroimaging studies highlighting functional specialization within the ACC. Green and red circles denote locations of increased activation during increased conflict monitoring and interference effects [Braver et al., 2001; Bush et al., 2003; Carter et al., 1998, 2000; Fassbender et al., 2004; Kerns et al., 2004; Kiehl et al., 2000; Laurens et al., 2003; MacDonald et al., 2000; Menon et al., 2001; Ruff et al., 2001]. The red circles denote activations associated with the compatibility (Eriksen) or conflict‐adaptation (Gratton) effects in studies using the Eriksen task [Botvinick et al., 2001; Bunge et al., 2002; Casey et al., 2000; Hazeltine et al., 2003; van Veen et al., 2001]. Orange triangles denote activations during error commission, predominantly during go/nogo tasks [Braver et al., 2001; Fassbender et al., 2004; Garavan et al., 2003; Kiehl et al., 2000; Laurens et al., 2003; Menon et al., 2001; Rubia et al., 2003; Ullsperger and von Cramon, 2001]. Yellow diamonds denote the locations of rostral ACC regions that have been linked to treatment response in depression [Buchsbaum et al., 1997; Davidson et al., 2003; Mayberg et al., 1997; Pizzagalli et al., 2001; Saxena et al., 2003; Smith et al., 1999; Wu et al., 1999]. Note that this region is pregenual, i.e., slightly more inferior and anterior than the one implicated in error processing. B: Location and extent of various ACC subdivisions as defined by the Structure‐Probability Maps [Lancaster et al., 1997] and displayed on the LORETA template. Coordinates in mm (MNI space); origin at anterior commissure; (X) = left(–) to right(+); (Y) = posterior(–) to anterior(+); (Z) = inferior(–) to superior(+). C: Mean (and SD) gamma current density within five general ACC regions for low (n = 16) and high (n = 17) BDI subjects. D: Mean (and SD) gamma current density within the affective and cognitive ACC subdivisions for low (n = 16) and high (n = 17) BDI subjects. *Group differences (P < 0.05).
Interestingly, recent neuropsychological studies suggest that executive deficits in depression may be partially due to abnormal responses to negative feedback or oversensitivity to errors and perceived failure [Beats et al., 1996; Elliott et al., 1996, Elliott et al., 1997b; Murphy et al., 2003; Steffens et al., 2001; but see Purcell et al., 1997 and Shah et al., 1999]. In one of these first studies, Beats et al. [1996] found that elderly depressed patients solved the same amount of problems as controls during a planning task, but once a mistake was committed a rapid deterioration of performance was observed [see also Steffens et al., 2001]. Elliott et al. [1996, Elliott et al. 1997b] replicated and extended these findings by showing that abnormal response to negative feedback was (1) correlated with depression severity, (2) specific to depression, and (3) still present after clinical recovery, suggesting that it may be a trait‐like marker of depression. Finally, Murphy et al. [2003]] found that misleading negative feedback disrupted patients' performance in visual discrimination and reversal tasks.
Although these findings invite the possibility that deficits in performance monitoring and abnormal responses to errors may represent a critical link between cognitive deficits and negative affect in depression, little is known about the underlying mechanisms and brain substrates. Thus, abnormal response to errors and negative feedback may arise due to dysfunctional performance monitoring and controlled response strategies for improving performance. Based on the neuroimaging literature reviewed above, these impairments could be linked to dysfunctions in the cognitive (dorsal) subdivision of the ACC. Alternatively, disrupted performance after errors may reflect abnormal affective reactions to internal or external cues signaling that one's own behavior was inappropriate; in this case, dysfunction in affective (rostral) ACC subdivision could be expected.
As an initial attempt to better characterize abnormal reactions to errors in depression, a modified version of the Eriksen Flanker task [Eriksen and Eriksen, 1974] was used in the present study to assess conflict monitoring and post‐error behavioral adjustments in subjects with elevated levels of depressive symptoms. The rationale for using the Eriksen task was twofold. First, prior studies using this or similar speeded‐response tasks have shown that performance can be dissected into different subcomponents, including behavioral adjustments during or after high‐conflict (incompatible) trials, as well as after errors. For example, subjects typically slow down their reaction time (RT) and improve their accuracy on trials following errors, suggesting that they utilize errors to monitor and improve their performance [Laming, 1968; Rabbitt, 1966b]. Second, and more importantly, neuroimaging studies have shown that dorsal and rostral ACC regions are primarily recruited during conflict monitoring and error detection, respectively, elicited by the Eriksen or similar speeded tasks [Botvinick et al., 1999; Casey et al., 2000; Hazeltine et al., 2003; Kerns et al., 2004] (Fig. 1A).
In addition to testing whether elevated levels of depressive symptoms in a nonclinical sample would modulate conflict monitoring and post‐error behavioral adjustments, the second aim of this study was to investigate whether resting electroencephalographic (EEG) activity within specific territories of the ACC assessed before the Eriksen task predicted behavioral performance, and whether specific dysfunction in such ACC regions would explain performance abnormalities in subjects with elevated depressive symptoms. Due to our a priori hypotheses regarding the ACC, EEG analyses were restricted to the theta (6.5–8 Hz) and gamma (36.5–40 Hz) band. This choice was justified by animal and human findings showing that: (1) the ACC is critically implicated in the generation and/or modulation of theta activity [Asada et al., 1999; Gevins et al., 1997; Ishii et al., 1999]; and (2) theta and gamma activity are functionally coupled [Bragin et al., 1995; Burgess and Ali, 2002; Duzel et al., 2003; Penttonen et al., 1998; Hajos et al., 2003; Fell et al., 2003; Schack et al., 2002] such as gamma bursts occur within periods of the theta phase [Buzsaki, 1996, for review]. Since both animal and human studies have also demonstrated links between ACC function and theta oscillations [Feenstra and Holsheimer, 1979; Nishida et al., 2004; Pizzagalli et al., 2003; Talairach et al., 1973] as well as between theta and gamma rhythms [Cape and Jones, 2000; Chrobak and Buzsaki, 1998; Mann and Paulsen, 2005] during task‐free, resting periods, the focus on these EEG bands was warranted.
SUBJECTS AND METHODS
Participants
Forty‐eight female subjects were recruited from the Introductory Psychology pool at the University of Wisconsin–Madison. Subjects were selected based on their score on the Beck Depression Inventory (BDI) [Beck et al., 1961], which was administered to 1,495 students. Control subjects were required to have a BDI score of six or less (low BDI group; n = 22), whereas subjects with elevated depressive symptoms were required to have a BDI of 18 or above1 (high BDI group; n = 26). On the day of the experiment (on average, 30.35±19.42 days postscreening), high and low BDI subjects were required to have a BDI score of ≥12 and ≤4, respectively. Among the 48 subjects identified at the prescreening, 11 failed to meet the BDI criteria at the day of the experiment, and three had data loss for the Eriksen task due to equipment malfunctions. This resulted in a final sample of 17 subjects with high BDI scores (19.94 ± 6.75, range: 12–38) and 17 subjects with low BDI scores (1.53 ± 1.23, range: 0–4). For one low BDI subject, EEG data were lost due to technical problems. Participants were right‐handed [Chapman and Chapman, 1987] and between the ages of 18 and 22. Subjects provided written informed consent to a protocol approved by the Human Subjects Committee of the University of Wisconsin, and received course credit for their participation.
Procedure
After obtaining written informed consent, the state forms of the Positive and Negative Affect Schedule [PANAS; Watson et al., 1988] and State Trait Anxiety Inventory [STAI; Spielberger et al., 1970] were administered to assess pre‐task levels of affect. Next, preparations for the EEG recording were made. Following standard procedures, EEG recording consisted of eight contiguous 1‐min trials (four with eyes open and four with eyes closed), alternating according to an order counterbalanced across subjects. After the EEG recording, instructions for the Eriksen task and a practice block were provided and subjects subsequently performed 10 blocks of the Eriksen task (without EEG recording).2 After the task, subjects filled out the BDI, the state forms of the PANAS and STAI, the trait form of the PANAS, the Mood and Anxiety Symptom Questionnaire [MASQ; Watson et al., 1995], and a post‐task questionnaire assessing subjects' experience with the task. Subjects were then debriefed and compensated.
Task
A modified version of the Eriksen Flanker task was used [Eriksen and Eriksen, 1974]. Each trial started with the presentation of a warning cue (a line) for 1,000 ms in the center of the screen, directly above where a target letter was to be presented. Immediately thereafter, one of four equiprobable letter strings (HHHHH, SSSSS, SSHSS, and HHSHH) was presented. The participants' task was to decide via button press whether the center letter in the string (the target letter) was an “H” or “S.” The target‐key press assignment was counterbalanced across subjects. In so‐called compatible trials, the target letter was the same as the distracter letters (i.e., SSSSS, HHHHH), whereas in incompatible trials, the target and distracter letters were different (i.e., SSHSS, HHSHH). Trials were separated by 1,000 ms (ITI), and each participant completed 10 blocks (50 trials/block), separated by a short break, in which subjects received feedback about their performance (the number of correct responses in a particular block and the cumulative correct responses). The task lasted about 25 min.
Unlike the original Eriksen task, our task included a stimulus degradation function to increase task difficulty and thus elicit more errors. Task parameters (e.g., level of degradation) were first piloted on 18 independent subjects. The center letter (target) degraded after each trial depending on the subject's performance according to the formula: [(Block Score + 1)/(Trial in Block + 15)], where Block Score is the running total correct score in a given block, and Trial in Block is the trial number in the block (range: 1–50). Therefore, if a participant maintained a high accuracy on the task (high Block Score), the stimulus was degraded more than if the participant maintained a lower accuracy.
Apparatus
The Eriksen task was run on a PowerMac 6214CD using PsyScope software [Cohen et al., 1993]. A 128‐channel EEG was recorded using the Geodesic Sensor Net system (Electrical Geodesic, Eugene, OR). The sampling rate was 250 Hz (16 bit precision; bandwidth: 0.01–100 Hz), and the vertex electrode (Cz) served as recording reference. Amplifier gains and zeros were measured prior to each recording session. Following standard procedures [e.g., Luu et al., 2003; Tucker et al., 2003], impedances were kept below 50 KΩ.
Data Reduction and Analyses
Behavioral data
In addition to analyzing overall RT and accuracy, compatibility (Eriksen), post‐error adjustment (Laming/Rabbit), and conflict‐adaptation (Gratton) effects were computed, since these variables have been previously associated with conflict monitoring and post‐error behavioral adjustments as well as ACC activation. Specifically, neuroimaging studies have shown that the compatibility [Botvinick et al., 1999; Bunge et al., 2002; Hazeltine et al., 2003; van Veen et al., 2001] and conflict‐adaptation [Botvinick et al., 1999; Kerns et al., 2004] effects recruit the dorsal ACC, whereas error of commissions are typically associated with rostral ACC activation [e.g., Kiehl et al., 2000; Menon et al., 2001; Ullsperger and von Cramon, 2001] (Fig. 1A).
The Compatibility (Eriksen) effect refers to longer RTs and lower accuracies for incompatible than compatible trials [Eriksen and Eriksen, 1974] due to high response competition between the distracters and the center stimulus in an incompatible trial. Accordingly, this effect was computed as: [RTIncompatible trials – RTCompatible trials] and [AccuracyCompatible trials – AccuracyIncompatible trials]. The post‐error adjustment (Laming) effect reflects longer RT but higher accuracy on trials following incorrect than correct responses [Laming, 1968], suggesting that subjects utilize errors to monitor and adjust their performance. Thus, this effect was computed as: [RTAfter incorrect trials – RTAfter correct trials] and [AccuracyAfter incorrect trials – AccuracyAfter correct trials]. Finally, the Conflict‐adaptation (Gratton) effect reflects shorter RT and higher accuracy for incompatible trials following incompatible trials than incompatible trials following compatible trials [Gratton et al., 1992], likely due to increased recruitment of cognitive control during the preceding incompatible trial. Thus, this effect was computed as: [RTIncompatible trials following compatible trials – RTIncompatible trials following incompatible trials] and [AccuracyIncompatible trials following incompatible trials – AccuracyIncompatible trials following compatible trials].
Finally, to exclude the possibility that the post‐error adjustment and conflict adaptation effects affected each other, control analyses, deconfounding the putative overlap between these effects, were performed. To this end, for the post‐error adjustment effect, analyses were restricted to trials subsequent to incompatible trials; for the conflict adaptation effect, analyses of preceding compatibility were restricted to post‐correct trials.
Baseline EEG data
After gain and zero calibration to microvolts, data were converted to Matlab‐compatible format (MathWorks, Natick, MA). Using in‐house software, EEG channels were first automatically checked for values greater than ±200 μV dynamic range and time frames exceeding this threshold were marked as artifact. Second, a zero‐phase 60 Hz notch filter was used to remove power noise. Third, the 128‐channel EEG data were manually scored for eye movement, blink, muscle, and other artifacts. Channels with artifacts exceeding more than 50% of the 1‐min recording were marked as corrupted (3.30% of the channels) and were later interpolated using a spline interpolation method [Perrin et al., 1989]. Low and high BDI subjects did not differ in the number of sensors requiring interpolation (4.44 ± 3.52 vs. 3.53 ± 2.68, t(31) = 0.84, P > 0.40). Next, all available artifact‐free 2048‐ms EEG epochs from the eyes‐closed trials were extracted (high BDI: 33.29 ± 23.47 vs. low BDI: 31.31 ± 22.00; t(31) = 0.25, P > 0.80), and subjected to standard spectral analyses via Discrete Fourier Transform (DFT) using a boxcar window [Brillinger, 1981]. As in our prior studies in clinical depression [e.g. Pizzagalli et al., 2001], only EEG data extracted from the eyes‐closed trials were used for the analyses to (1) avoid potential artifacts deriving from eye movements, blinks, etc.; and (2) increase comparability across studies.
Subsequently, Low‐Resolution Electromagnetic Tomography [LORETA; Pascual‐Marqui et al., 1994, 1999] was used to estimate the three‐dimensional intracerebral current density distribution of the theta (6.5–8 Hz) and gamma (36.5–44 Hz) EEG band. For the theta band, the 6.5–8 Hz frequency range was selected based on prior factor analysis EEG studies of resting EEG data [Kubicki et al., 1979]. This distributed source localization technique has recently received important cross‐modal validation from studies combining LORETA with functional MRI (fMRI) [Mulert et al., 2004; Vitacco et al., 2002], structural MRI [Worrell et al., 2000], and PET [Pizzagalli et al., 2004; but see Gamma et al., 2004]. LORETA estimates location(s) of electrical source activity by assuming similar activation among neighboring neuronal sources (i.e., no assumption is made about the number of generating sources). This core assumption is implemented by computing the “smoothest” of all possible activity distributions. In the present study, a three‐shell spherical head model [Ary et al., 1981] and EEG electrode coordinates derived from cross‐registrations between spherical and realistic head geometry [Towle et al., 1993] were utilized, which were both registered to the digitized MRI available from the Brain Imaging Centre, Montreal Neurologic Institute [MNI305; Evans et al., 1993; Collins et al., 1994].3 According to the digitized MNI probability atlases, the solution space was restricted to cortical gray matter and hippocampi and included 2,394 voxels (voxel dimension: 7 mm3). After conversion from MNI to Talairach coordinates [Brett et al., 2002], the Structure‐Probability Maps atlas [Lancaster et al., 1997] was used to identify voxels belonging to distinct ACC subdivisions. Based on prior functional and anatomical data [Bush et al., 2000; Devinsky et al., 1995; Paus, 2001; Vogt et al., 1995], voxels belonging to the affective (BA25: 17 voxels, 5.83 cm3; BA24: 12 voxels, 4.12 cm3; BA32: 25 voxels, 8.58 cm3) and cognitive (BA32′: 20 voxels, 6.86 cm3; BA24′: 48 voxels, 16.46 cm3) ACC subdivision were identified (Fig. 1B). To test the regional specificity of putative differences, posterior cingulate regions were also investigated (BA23: 12 voxels, 4.12 cm3; BA31: 45 voxels, 15.44 cm3).
LORETA computes current density as the linear, weighted sum of the scalp electrical potentials and then squares this value for each voxel to yield power of current density (unit: amperes per square meter, A/m2). Before statistical analyses, for every subject and every band, the LORETA solution was normalized to a total power of 1 and log‐transformed for normalization purposes. For each cluster, activity was finally averaged across the voxels.
Statistical Analyses
Self‐report measures
For the state PANAS scale, a mixed analysis of variance (ANOVA) was run with Time (pre‐, post‐task), PANAS Scale (positive, negative) as repeated measures, and Group (low vs. high BDI subjects) as the between‐subject factor. For the trait PANAS scale, a 2 (PANAS Scale) × 2 (Group) ANOVA was performed. For the STAI, a 2 (Time) × 2 (Group) ANOVA was run. For the MASQ, a 4 (MASQ subscales) × 2 (Group) ANOVA was performed. For the sake of brevity, only effects involving Group are reported.
Behavioral data
Mixed ANOVAs were run separately for RT and accuracy variables with Condition and Group as factors. To assess compatibility effects, the performance for compatible vs. incompatible trials was entered as the repeated measure. To investigate post‐error adjustments, the performance following a correct or incorrect response was analyzed. Finally, to test conflict‐adaptation effects, the performance for incompatible trials following a compatible vs. incompatible trials was considered.
To test whether group differences were further modulated by self‐report measures of mood, Pearson correlation and hierarchical regression analyses between the MASQ scores and behavioral performance (compatibility, post‐error adjustment, and conflict‐adaptation effects) were performed. To limit the number of tests performed in these analyses only the MASQ scores were used as predictors. The MASQ was selected because it allows for an independent assessment of depressive and anxious symptoms, thus providing a test of the specificity of putative findings
Baseline EEG data
For the LORETA data, a mixed ANOVA analyses with Brodmann Area (BA; BA25, BA24S, BA32S, BA23, BA31) as repeated measure and Group as the between‐subject factor was performed for each band separately. Note that “BA 24S” (= BA24+BA24′) and “BA32S” (= BA32 + BA32′) refer to the sums of the respective affective and cognitive subdivisions. To directly test putative group differences in cognitive vs. affective ACC subdivisions, a 3‐way ANOVA with BA (BA24, BA32), ACC subdivision (affective, cognitive), and Group as factors was run. Finally, hierarchical regression analyses were run separately for low and high BDI subjects to test whether 1) resting activity in the affective ACC subdivision predicted individual differences in post‐error adjustments after controlling for the activity in the cognitive ACC subdivision; and 2) activity in the cognitive ACC subdivision predicted compatibility or conflict‐adaptation effects after controlling for the affective ACC subdivision.
RESULTS
Self‐Report of Mood and Affect
For both the state and trait form of the PANAS, the Group × PANAS scale interaction was significant (both F(1,32) > 13.40, P < 0.001), reflecting significantly higher negative affect but significantly lower positive affect in high BDI subjects (Table I) at both the pre‐ and post‐task assessment. Similarly, for the STAI only the Group main effect was significant (F(1,32) = 13.62, P < 0.001), due to higher state anxiety in high BDI subjects at both assessments. For the MASQ, higher scores in high BDI subjects, particularly in the two depression subscales, resulted in both the Group and the Group × MASQ subscale effects reaching significance (both F(1,32) > 23.50, P < 0.001).
Table I.
Self‐report measures and behavioral performance in the Eriksen Task for low (N = 17) and high (N = 17) BDI subjects
| Low BDI* | High BDI* | t | P | |
|---|---|---|---|---|
| Self‐report measures | ||||
| BDI | 1.53 ± 1.23 | 19.94 ± 6.75 | 11.06 | 0.001 |
| PANAS‐trait | ||||
| PA | 34.47 ± 4.99 | 26.06 ± 4.96 | −4.93 | 0.001 |
| NA | 16.24 ± 2.33 | 24.94 ± 6.53 | 5.17 | 0.001 |
| PANAS‐statea | ||||
| PA | 28.03 ± 5.25 | 23.21 ± 5.25 | −2.68 | 0.015 |
| NA | 13.91 ± 0.92 | 16.09 ± 2.53 | 3.33 | 0.005 |
| STAI statea | 28.82 ± 4.76 | 36.96 ± 7.74 | 3.69 | 0.001 |
| MASQ | ||||
| GDA | 14.24 ± 3.25 | 24.18 ± 8.71 | 4.41 | 0.001 |
| AA | 19.35 ± 3.46 | 31.88 ± 11.91 | 4.17 | 0.001 |
| GDD | 17.12 ± 4.00 | 34.82 ± 8.29 | 7.93 | 0.001 |
| AD | 43.82 ± 5.84 | 75.00 ± 11.76 | 9.79 | 0.001 |
| Eriksen task | ||||
| Overall performanceb | ||||
| RT (ms) | 508.32 ± 52.88 | 502.68 ± 59.65 | −0.29 | 0.77 |
| Accuracy | 0.84 ± 0.046 | 0.85 ± 0.039 | 0.48 | 0.64 |
| Compatibility (Eriksen) effectc | ||||
| RT (ms) | 6.06 ± 20.04 | 9.41 ± 24.49 | 0.44 | 0.66 |
| Accuracy | −0.12 ± 0.11 | −0.09 ± 0.11 | 1.04 | 0.31 |
| Post‐error (Laming) adjustmentd | ||||
| RT (ms) | 20.76 ± 19.10 | 26.59 ± 17.56 | 0.93 | 0.36 |
| Accuracy | 0.01 ± 0.052 | −0.02 ± 0.030 | −2.05 | 0.048 |
| Conflict‐adaptation (Gratton) effecte | ||||
| RT (ms) | 0.59 ± 8.02 | 2.62 ± 16.00 | 0.47 | 0.64 |
| Accuracy | −0.00 ± 0.03 | 0.01 ± 0.05 | 1.09 | 0.28 |
Values are expressed as mean ± SD.
For the state PANAS and STAI scales, values are averaged between the pre‐ and post‐task assessment because no significant effects involving Time (pre‐ vs. post‐task) emerged.
Mean RT and accuracy (averaged across trial types).
Compatibility effect: [RTIncompatible trials − RTCompatible trials]; [AccuracyCompatible trials − AccuracyIncompatible trials].
Post‐error adjustment effect (including both compatible and incompatible trials): [RTAfter incorrect trials − RTAfter correct trials]; [AccuracyAfter incorrect trials − AccuracyAfter correct trials].
Conflict‐adaptation effect (including both post‐correct and post‐error trials): [RTIncompatible trials following compatible trials − RTIncompatible trials following incompatible trials]; [AccuracyIncompatible trials following incompatible trials − AccuracyIncompatible trials following compatible trials].
Note: BDI: Beck Depression Inventory [Beck et al., 1961]; PANAS: Positive and Negative Affect Schedule [PA: positive affect, NA: negative affect; Watson et al., 1988]; STAI: State Trait Anxiety Inventory [State form; Spielberger et al., 1970]; MASQ: Mood and Anxiety Symptom Questionnaire [Watson et al., 1995; GDA: General Anxiety; AA: Anxious Arousal; GDD: General Depression; AD: Anhedonic Depression].
Behavioral Data
Overall performance
As shown in Table I, no differences emerged in overall RT (t(32) = −0.29, P > 0.70) or accuracy (t(32) = 0.48, P > 0.60) scores, suggesting no general dysfunctions (e.g., attention, motivation) in high BDI subjects. In line with these findings, low and high BDI subjects did not differ in the amount (mean: 0.51 ± 0.02 vs. 0.51 ± 0.02) or variance (standard deviation (SD): 0.16 ± 0.01 vs. 0.16 ± 0.01) of stimulus degradation, which was contingent upon global performance in the task (t(32) < 0.04, P > 0.90).
Compatibility effect: Performance for compatible vs. incompatible trials
For RT, the ANOVA revealed a nearly significant effect of Condition (F(1,32) = 4.06, P = 0.052). As expected from prior studies [Eriksen and Eriksen, 1974], RT was longer for incompatible (508.85 ± 49.02 ms) than compatible (501.12 ± 66.18 ms) trials. The main effect of Group and the Group × Condition interaction were not significant (F < 0.19). Thus, high and low BDI subjects did not differ in their relative RT slowing during incompatible trials (compatibility effect: t(32) = 0.44, P > 0.60; Table I).
For accuracy, the ANOVA showed a significant effect of Condition (F(1,32) = 32.15, P < 0.01), which resulted from unexpectedly higher accuracy for incompatible (0.90 ± 0.060) than compatible (0.80 ± 0.076) trials. As above, the main effect of Group and the interaction were not significant (F < 1.1). Thus, high and low BDI subjects did not differ in their relative accuracy adjustments during incompatible trials (compatibility effect: t(32) = 1.04, P > 0.30; Table I).
Post‐error adjustment effect: Performance following correct vs. incorrect responses
For RT, only the main effect of Condition (F(1,32) = 56.65, P < 0.001) was significant (all F < 0.90). As in prior studies [Laming, 1968; Rabbit, 1966a], this effect was due to significantly longer RT for trials following incorrect (524.79 ± 64.13 ms) than correct (501.12 ± 55.24 ms) responses. Thus, the two groups did not differ in RT slowing after errors (post‐error adjustment: t(32) = 0.44; Table I).
For accuracy, the only significant effect emerging was the Group × Condition interaction (F(1,32) = 4.22, P < 0.05; all other F < 0.80), suggesting that high and low BDI subjects differed in their post‐error adjustment effect (Table I). Critically, the Group × Condition interaction was confirmed also in the control analyses in which the post‐error adjustment effect was restricted to trials subsequent to incompatible trials (F(1,32) = 7.66, P < 0.009; all other F < 0.95). As shown in Figure 2A, high BDI subjects had significantly lower accuracy after incorrect than correct trials (t(16) = 2.41, P < 0.03), an effect that was not present in low BDI subjects (t(16) = −1.43, P > 0.15). Moreover, high BDI subjects had significantly lower accuracy after incorrect trials than low BDI subjects (t(32) = −2.22, P < 0.04), whereas the two groups did not differ in their accuracy after correct trials (t(32) = 0.55, P > 0.50). On an individual level (Fig. 2B), 12 of the 17 high BDI subjects [binomial probability P(12/17) < 0.05] but only 5 of the 17 low BDI subjects [P(5/17) < 0.05] showed a negative post‐error adjustment effect (= Accuracyafter incorrect trial – Accuracyafter correct trials), χ = 5.77, P < 0.05.
Figure 2.
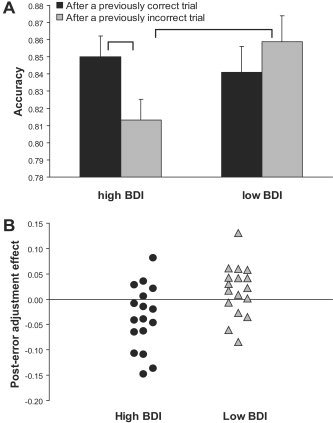
A: Mean accuracy (and SE) after incorrect and correct trials for high BDI (n = 17) and low BDI (n = 17) subjects. B: Individual scores for the post‐error adjustment (Laming) effect [AccuracyAfter incorrect trial – AccuracyAfter correct trials] for low (gray triangles) and high (dark circles) BDI subjects. To eliminate potential overlap between the post‐error adjustment and conflict adaptation effects, values were derived from trials subsequent to incompatible trials.
Conflict‐adaptation effect: Performance for incompatible trials following compatible vs. incompatible trials
For both RT and accuracy, no significant results emerged (all F < 1.19, all P > 0.25). Additionally, no significant effects emerged when the control analyses of preceding compatibility were restricted to post‐correct trials (all F(1,32) < 1.77, all P > 0.19). Thus, high and low BDI subjects did not differ in their behavioral adjustment following incompatible trials (conflict‐adaptation effect: t(32) < 1.10, P > 0.30; Table I).
Correlations Between Behavioral Measures and the Self‐Report Measures of Mood
For high BDI subjects, Pearson correlations were run between behavioral measures (compatibility, post‐error adjustment, and conflict‐adaptation effects) and self‐report measures of mood (MASQ subscales). (For low BDI subjects, the restricted range on the self‐report measures prevented these analyses.) When considering RT, no significant correlations emerged (all P > 0.12). When considering accuracy, the post‐error adjustment effect was negatively correlated with the Anhedonic Depression score (r = −0.58, P < 0.015; Fig. 3A) and with the General Depression score (r = −0.50, P < 0.05; Fig. 3B), whereas no reliable correlations emerged when considering the Anxious Arousal (r = −0.16, ns) or General Anxiety Scores (r = −0.27, ns). No significant correlations emerged between the MASQ scores and the compatibility (all |r| < 0.34, all P > 0.17) or the conflict‐adaptation (all |r| < 0.32, all P > 0.21) effect.
Figure 3.
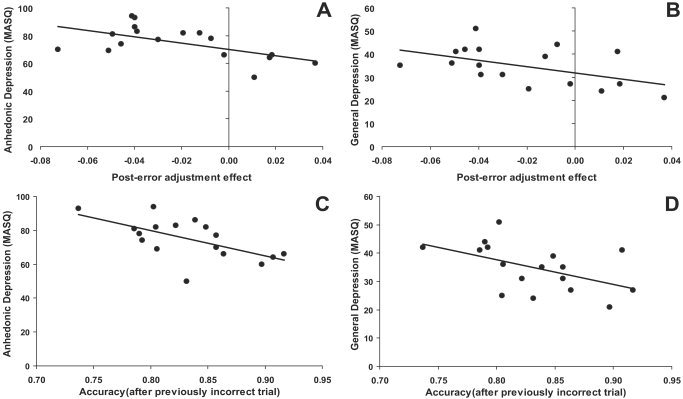
Scatterplot and Pearson's correlation between (A) the post‐error adjustment (Laming) effect and the MASQ Anhedonic Depression scores; (B) the post‐error adjustment (Laming) effect and the MASQ General Depression scores; (C) the mean accuracy after incorrect trials and the Anhedonic Depression scores; and (D) the mean accuracy after incorrect trials and the General Depression scores. High BDI subjects only (n = 17).
Because the post‐error adjustment effect is computed as [Accuracyafter incorrect trial – Accuracyafter correct trials], these correlations suggest that the higher the depressive symptoms, the lower the accuracy after incorrect trials compared to correct trials. Consistent with this interpretation, for high BDI subjects a negative correlation was observed between the Anhedonic Depression score and the accuracy after incorrect (r = −0.61, P < 0.01; Fig. 3C) but not correct (r = −0.31, P > 0.20) trials. The correlations involving incorrect and correct trials were significantly different (Z = −1.94, P < 0.05) [Meng et al., 1992]. Similarly, for high BDI subjects a significant negative correlation emerged between the General Depression score and accuracy after incorrect (r = −0.50, P < 0.05; Fig. 3D) but not correct (r = −0.24, P > 0.30) trials. Yet these correlations were not significantly different (Z = −1.60, ns).
To assess the specificity of the link between depressive symptoms and diminished post‐error adjustment effect, hierarchical regression analyses predicting the post‐error adjustment effect (calculated with accuracy scores) were performed. In the first, trait negative affect (NA) score was entered in a first step followed by the Anhedonic Depression score. In the second analysis, the MASQ General Anxiety scores were entered first, Anxious Arousal scores entered second, and Anhedonic Depression scores were entered last. The third analyses tested whether the Anhedonic Depression scores predicted the post‐error adjustment effect after controlling for General Anxiety, Anxious Arousal, and trait NA scores. Results showed that the Anhedonic Depression scores continued to explain unique variance in the post‐error adjustment effect when controlling for trait NA scores, ΔR 2 = 0.27, ΔF(1,14) = 6.72, P < 0.025, both MASQ anxiety scales, ΔR 2 = 0.27, ΔF(1,13) = 5.59, P < 0.035, or all three measures of anxiety/negative affect, ΔR 2 = 0.25, ΔF(1,12) = 5.08, P < 0.045.
Baseline EEG Data
ANOVA analyses
Contrary to our prediction, the ANOVA conducted on theta current density with Brodmann Area (BA25, BA24S, BA32S, BA23, BA31) and Group as factors revealed no significant effects involving Group (all F < 2.69, all P > 0.10). For gamma, the main effect of Brodmann Area (F(4,124) = 194.81, P < 0.001), and most importantly, the Group × Brodmann Area interaction (F(4,124) = 6,21, Greenhouse‐Geisser ϵ = 0.35, P < 0.01) were significant. Post‐hoc t‐tests (Fig. 1C) revealed that, compared to low BDI subjects, high BDI subjects had higher gamma current density in the posterior cingulate cortex (BA23: t(31) = 2.20, P < 0.035; BA31: t(31) = 2.66, P < 0.015) but lower gamma current density in BA25 (t(31) = −2.50, P < 0.020); groups did not differ in BA24S (t(31) = −1.46, P > 0.15) or BA32S (t(31) = −1.63, P > 0.10). Highlighting the frequency specificity of the results, identical 2 × 2 × 2 ANOVAs performed on delta (1.5–6 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12 Hz), beta1 (12.5–18 Hz), beta2 (18.5–21 Hz), and beta3 (21.5–30 Hz) revealed no reliable effects involving Group (all F < 2.03, all P > 0.15).
To investigate with more precision whether dysfunctional gamma activity was localized to cognitive or affective subdivisions of the ACC, a 3‐way ANOVA with BA (BA24, BA32), ACC subdivision (affective, cognitive), and Group as factors was run. Apart from the main effects of BA and ACC subdivision and a significant BA × ACC subdivision interaction (all F(1,31) > 22.53, all P < 0.001), the only other significant result was the Group × ACC subdivision interaction (F(1,31) = 7.49, P < 0.010). Post‐hoc tests (Fig. 1D) clarified that, compared to low BDI subjects, high BDI subjects had significantly lower gamma activity in the affective (BA24: t(31) = −2.88, P < 0.007; BA32: t(31) = −2.19, P < 0.040) but not cognitive (BA24′: P > 0.15; BA32′: P > 0.30) subdivisions of the ACC. As above, analogous 3‐way ANOVAs run separately for delta, theta, alpha1, alpha2, beta1, beta2, or beta3 current density revealed no reliable effects involving Group (all F < 2.69, P > 0.10).
Regression analyses
For low BDI subjects, the first analysis predicted the post‐error adjustment effect (calculated with accuracy scores), with gamma current density in the cognitive ACC subdivisions (BA24′, BA32′) entered as the first predictor followed by gamma current density in the affective ACC subdivisions (BA24). As expected, the post‐error adjustment effect was not significantly predicted by gamma current density in the cognitive subdivisions (BA24′: β = −0.25; BA32′: β = 0.07; ns). However, pre‐task gamma activity within the affective subdivision of the ACC (BA24) was a significant predictor of the post‐error adjustment effect even after removing the variance associated with gamma activity within BA24′ and BA32′ (β = 0.68; ΔR 2 = 0.31, ΔF(1,12) = 5.72, P < 0.035), as shown in Figure 4. For high BDI subjects, the relation between BA24 gamma activity and the post‐error adjustment effect was reversed. Thus, gamma within the affective subdivision of the ACC was a nearly significant negative predictor of this effect (β = −0.51; ΔR 2 = 0.20, ΔF(1,13) = 3.36, P = 0.090) after removing the variance associated with gamma activity within BA24′ (β = 0.20, ns) and BA32′ (β = −0.18, ns).
Figure 4.
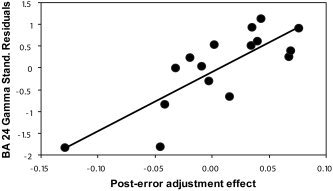
Scatterplot and regression slope between the post‐error adjustment (Laming) effect and (standardized) residuals of the gamma current density in the affective ACC subdivision (BA24) after removing variance associated with the cognitive ACC subdivision (BA24′ and BA32′). The Pearson's correlation between the post‐error adjustment effect and BA24 gamma residuals is r = 0.81, P < 0.005. Low BDI subjects only (n = 16).
For both the low and high BDI subjects, regression analyses testing whether activity in the cognitive ACC subdivision predicted the compatibility or conflict‐adaptation effects (calculated with both RT and accuracy scores) after controlling for the activity in the affective ACC subdivision were nonsignificant (all ΔF(1,13) < 2.17, all P > 0.15).
Theta‐gamma functional coupling
Apart from BA25 (r = 0.24, ns), all correlations between theta and gamma current density within a given cluster were significant (BA23: r = 0.53; BA24: r = 0.59; BA31: r = 0.51; BA32: r = 0.51; all P < 0.002; n = 33).
DISCUSSION
The present study had two related objectives. First, using an experimental task allowing us to independently assess conflict monitoring and behavioral adjustments after errors, we aimed to investigate with more precision putative mechanisms underlying executive dysfunctions in subjects with elevated depressive symptoms. Second, we tested whether resting EEG activity within specific territories of the ACC assessed before the task predicted performance on the Eriksen task, and more importantly, whether specific dysfunctions in such ACC regions would explain task performance deficits in subjects with elevated depressive symptoms.
The following findings emerged. First, unlike subjects with low BDI scores, subjects with elevated depressive symptoms showed lower accuracy in trials following errors than trials following correct responses (i.e., they had a significantly reversed post‐error adjustment effect); compared to low BDI subjects, they also had significantly lower accuracy after incorrect, but not correct, trials. Further highlighting the specificity of this finding, high and low BDI subjects did not differ in their overall RT and accuracy scores, suggesting the absence of general dysfunctions in high BDI subjects. The two groups also did not differ in their behavioral adjustments during or following high‐conflict (incompatible) trials (Eriksen and Gratton effects), suggesting that high BDI subjects had intact conflict monitoring abilities and were able to recruit cognitive control to overcome high‐conflict situations. Second, among the high BDI subjects the post‐error adjustment effect was negatively correlated with the Anhedonic Depression and the General Depression scores of the MASQ. These results suggest that the higher the depressive symptoms, the lower the accuracy after errors. Regression analyses further clarified that Anhedonic Depression scores continued to explain unique variance in the post‐error adjustment effect even after controlling for various measures of anxious symptoms and distress, suggesting that the findings were specific to depressive symptoms and not due to general psychopathology. Third, mirroring the behavioral findings of abnormal responses to errors despite intact conflict monitoring abilities, high BDI subjects had significantly reduced EEG gamma current density before the task within affective (rostral; BA24, BA25, BA32) but not cognitive (dorsal; BA24′, BA32′) subdivisions of the ACC. Fourth, for low, but not high, BDI subjects, pre‐task resting gamma activity within the affective ACC subdivision (BA24) was a significant predictor of the post‐error adjustment effect even after controlling for gamma activity within the cognitive ACC subdivisions. This latter finding extends a recent report that gamma band response over frontocentral regions during an unrelated task predicted later performance in a variety of neuropsychological tests probing the frontal lobe, including working memory and executive functioning (Karakaş et al., 2003). Finally, consistent with animal and human findings of functional coupling between theta and gamma activity [Bragin et al., 1995; Burgess and Ali, 2002; Duzel et al., 2003; Fell et al., 2003; Hajos et al., 2003; Penttonen et al., 1998; Schack et al., 2002] and reports of concurrent oscillations at theta and gamma frequencies in various limbic/prelimbic regions [Chrobak and Buzsaki, 1998; Fellous and Sejnowski, 2000; Fischer et al., 2002], these two EEG rhythms were positively and significantly correlated within the ACC.
Physiologically, decreased resting gamma current density in subjects with elevated depressive symptoms can be interpreted as a marker of decreased tonic activity in rostral ACC regions. This interpretation is based on a broad range of evidence, including human intracortical EEG findings of: (1) increased gamma activity during various mental processes [perception: Rodriguez et al., 1999; learning: Miltner et al., 1999; motor responses: Crone et al., 1998; memory: Fell et al., 2001]; (2) dose‐dependent decreases of gamma activity during anesthesia [Uchida et al., 2000]; and (3) systematic decreases in gamma activity throughout the sleep/wake cycle (highest during wakefulness, intermediate during REM sleep, and lowest during slow wave sleep [Gross and Gotman, 1999]). Consistent with the notion that gamma is a direct indicator of activation, a recent study using concurrent EEG and PET measurements found that the gamma band had the highest number of positive correlations between current density and glucose metabolism [Oakes et al., 2004]. From a functional perspective, gamma oscillations are assumed to reflect large‐scale integration of and synchrony among widely distributed neurons [Konig et al., 1995; Mann and Paulsen, 2005]. Accordingly, decreased resting gamma activity may highlight putative dysfunctional coupling and connectivity of underlying neuronal networks leading to impaired rostral ACC function, and, ultimately, to post‐error adjustment deficits.
Candidate Mechanisms Underlying Abnormal Response to Errors in Depression
Behavioral studies have consistently shown that humans utilize errors and negative feedback to monitor their performance and adjust behavior accordingly [Laming, 1968; Rabbitt, 1966a, b]. In a seminal study, Rabbitt [1966b] found that subjects can detect and correct errors without receiving any external feedback concerning their performance, indicating that internal monitoring is sufficient to correct behavioral responses.4 Together with prior findings of abnormal responses to negative feedback and oversensitivity to errors in clinical depression [Beats et al., 1996; Elliott et al., 1996, 1997b; Murphy et al., 2003; Steffens et al., 2001], the present findings suggest that such adaptive processes following errors are impaired in depression. Specifically, depressed and dysphoric subjects appear to be less efficient in utilizing the information conveyed by errors to monitor and guide subsequent performance. Unlike prior studies, however, the present findings offer initial insight about putative mechanisms underlying abnormal response to errors. Thus, whereas prior studies could not disentangle whether abnormal responses to errors was due to inefficiency in monitoring performance vs. abnormal affective reactions to perceived failure, the present behavioral and EEG findings suggest that the latter mechanism may be responsible. That is, at least in a nonclinical population of subjects with elevated depressive symptoms, conflict monitoring and dorsal ACC regions subserving this function were intact, whereas behavioral adjustments after errors and rostral ACC activity known to subserve error detection were dysfunctional.
Although the behavioral and EEG data nicely converge toward this interpretation, it is important to stress that the present version of the Eriksen task with target degradation was only partially successful in triggering conflict monitoring. Indeed, with the exception of the classic finding of RT slowing for incompatible trials, the compatibility (Eriksen) and conflict‐adaptation (Gratton) effects were not significant, representing a main limitation of the present study. One possibility is that the target degradation used in this task required greater resources for visual perception at the expense of cognitive control, making the task not sensitive enough for triggering conflict monitoring. Moreover, the balanced (50%/50%) proportion of compatible and incompatible trials may have also prevented the development of a powerful prepotent responses leading to increased conflict. These methodological limitations could explain the lack of correlation between activity in the cognitive ACC subdivision and the Eriksen/Gratton effects. In sum, whereas the present behavioral and EEG findings suggest that affective reactions to errors and their underlying neural correlates were dysfunctional in subjects with elevated depressive symptoms, future studies utilizing tasks capable of eliciting simultaneously strong conflict monitoring and error commission will be required for testing the precise mechanisms underlying executive dysfunction in depression. More importantly, studies measuring brain activity while participants are engaged in similar paradigms will be needed for conclusive interpretations about links between ACC dysfunction and abnormal responses to errors. Specifically, to more directly assess brain mechanisms underlying behavioral adjustments after errors in depression, it would be particularly interesting to investigate two event‐related potential (ERP) components that have been implicated in action monitoring, the error‐related negativity (ERN) and feedback‐related negativity (FRN). These negative‐going ERP waveforms occur maximally over frontocentral scalp regions after error commission and error feedback, respectively. Critically, rostral ACC regions have been implicated in their generation [e.g., van Veen and Carter, 2002], possibly through oscillatory processes in the theta range [Gehring and Willoughby, 2004; Luu et al., 2004]. Based on the present EEG findings as well as prior evidence emphasizing a functional coupling between theta and gamma oscillations (see above), it would be interesting to assess the putative role of gamma oscillations in the generation of the ERN and FRN. Consistent with our findings of abnormal error processing in high BDI subjects, ERN and FRN have been recently found to be abnormal in depression [Ruchsow et al., 2004; Tucker et al., 2003].
Finally, we note that, in the present study, no formal clinical interviews (e.g., Structured Clinical Interview for the DSM‐IV, SCID) or detailed personality evaluations were performed to assess current psychopathology, family history of psychopathology, medication use, and personality traits. Because family history of psychopathology and personality traits (e.g., neuroticism) may partially contribute to individual differences observed between high and low BDI subjects, future studies should assess these important variables.
Limitations notwithstanding, the present findings indicate—we believe for the first time—that abnormal reactions to errors in depression are associated with reduced tonic activity within the rostral ACC, a brain region known to be implicated in error processing. More generally, the finding that post‐error adjustments were predicted by resting activity within the rostral ACC sheds new light on brain substrates underlying individual differences in action monitoring. Collectively, the present and prior findings [Elliott et al., 1997b; Murphy et al., 2003] suggest that abnormal responses to perceived failure (errors) may be an important link between cognition and emotion affecting behavioral performance in depression that warrants further empirical inquiry.
Functional Role of the Rostral ACC
Intriguingly, this selective impairment in adjusting behavioral performance immediately after committing an error was accompanied by decreased baseline (i.e., pre‐task) activity in ventral and rostral areas of the ACC (BAs 24/25/32) in the absence of differences in more dorsal regions (BA24′/BA32′). In light of its role in monitoring conflicting response demands, detecting errors, and evaluating the emotional significance of events, the ACC has been considered a site of convergence and integration between affective and cognitive processes [Bush et al., 2000; Mayberg, 1997; Davidson et al., 2002]. The rostral ACC, in particular, by acting as an interface between limbic/prelimbic and frontal regions, is assumed to integrate salient affective and cognitive information, such as that derived from error processing, and modulate attentional processes within more dorsal (cognitive) subdivision accordingly. In depression, such convergence of affective and cognitive processes as well as executive functions governing adaptive task performance may be dysfunctional, since decreased ACC activity has been reported during resting periods [Bench et al., 1993; Drevets et al., 1997; Mayberg et al., 1994], executive tasks [Bremner et al., 2004; George et al., 1997; Okada et al., 2003], and affective manipulations [Beauregard et al., 1998; Kumari et al., 2003; Mitterschiffthaler et al., 2003]. Thus, translating a large neuroimaging literature to the present findings, decreased rostral ACC activity in subjects with elevated depressive symptoms points to disturbed affective and/or motivational responses to errors [Bush et al., 2000; Gehring and Willoughby, 2002; Luu et al., 2003; Tucker et al., 2003; Whalen et al., 1998] and provides a possible physiological explanation for prior neuropsychological findings of abnormal responses to negative feedback or oversensitivity to errors and perceived failure [Beats et al., 1996; Elliott et al., 1996, 1997b; Murphy et al., 2003; Steffens et al., 2001].
Interestingly, in addition to decreased gamma activity within the rostral ACC, high BDI subjects had increased gamma activity within the posterior cingulate cortex (BAs 23/31), a finding, however, that was not hypothesized. Based on reports of increased posterior cingulate/retrosplenial activation during (1) trauma‐related experiences [Fischer et al., 1996]; (2) somatic arousal [Critchley et al., 2000b]; and (3) processing of arousing stimuli [Critchley et al., 2000a], posterior cingulate hyperactivity in high BDI subjects may be associated with increased negative affect and somatic arousal. Notably, in clinical depression the posterior cingulate cortex has been found to be hypoactive during resting periods [e.g., Mayberg et al., 2000; Pizzagalli et al., 2002] and cognitive challenges [Elliott et al., 1997a], a pattern that normalized after symptom remission [Martin et al., 2001; Mayberg et al., 2000]. In light of these findings, posterior cingulate hypoactivity may thus be a state marker of clinical depression, particularly in patients with deficient autonomic/somatic arousal; subjects with elevated levels of depressive symptoms, on the other hand, may show increased posterior cingulate activity, potentially due to elevated physiological arousal and increased negative affect. Future studies are needed to test these conjectures.
Potential Relevance to Mechanisms Underlying Treatment Response in Major Depression
Of note, prior findings in clinically depressed patients have shown that increased rostral ACC activity before treatment was a predictor of treatment response [Awata et al., 2002; Davidson et al., 2003; Mayberg, 1997; Pizzagalli et al., 2001; Saxena et al., 2003; Wu et al., 1999] (Fig. 1). Based on evidence suggesting that the affective (rostral) ACC subdivision is critical for assessing the presence of possible conflicts between the current functional state of the organism and cues that are motivationally and emotionally salient, we speculated that rostral ACC hyperactivation in treatment responders might reflect an increased sensitivity to affective conflict that may eventually foster treatment response [Davidson et al., 2002; Pizzagalli et al., 2001]. Successful affective conflict monitoring would in turn lead to a call for further processing and recruitment of additional cognitive control (likely provided by dorsolateral PFC regions) to resolve the conflict. The present finding of a lawful relation between resting rostral ACC activity and post‐error behavioral adjustments is not only consistent with this speculation but provides initial insight into putative mechanisms underlying treatment response. As a result, we propose that, in eventual treatment responders, rostral ACC hyperactivity may foster the individual's ability to monitor the outcome of actions and adjust behavior when expected outcomes are violated. In treatment‐nonresponders, adaptive action monitoring relying on the rostral ACC may be dysfunctional, particularly after negative outcomes. Future studies in clinical samples are clearly needed to test this hypothesis and the precise functional significance of rostral ACC hyperactivity, particularly since the rostral ACC regions associated with treatment response and error detection only partially overlap (Fig. 1A).
In summary, cognitive theories of depression [Beck et al., 1979] have suggested that people vulnerable to depression show enduring negative cognitive schemata and negative processing biases. Depressed and dysphoric subjects, particularly those with dysfunctional attitudes, have been found to display: amplification of the significance of failure [Wenzlaff and Grozier, 1988]; difficulty in suppressing thoughts associated with failure [Conway et al., 1991]; increased self‐focus after failure feedback [Greenberg and Pyzszczynski, 1986]; and increased depressed mood after occurrence of a negative event [Abela and D'Alessandro, 2002] or negative social feedback [Henriques and Leitenberg, 2002]. Abnormal affective/motivational response to errors or perceived failures, as shown in the present study, is not only consistent with these findings but deepens our understanding of candidate mechanisms and brain correlates underlying negative processing biases in depression.
Acknowledgements
This study was supported by the NIMH (to R.J.D.), the Swiss National Research Foundation (to D.A.P.), the NIH (to D.A.P.), and the William F. Milton Fund (to D.A.P.). We thank Megan Zuelsdorff and Allison Jahn for assistance, John Koger and Larry Greischar for software development, and Kyle Ratner, Pearl Chiu, and Avram Holmes for comments on early versions of the manuscript. We also thank three anonymous reviewers for constructive criticisms.
Footnotes
A BDI score of 12 is the cutoff point for mild depression [Kendall et al., 1987]. For the initial screening, a more stringent cutoff of 18 was used in order to increase the likelihood that the subjects in the high BDI group would still report elevated depressed symptoms at the experimental session.
No EEG was recorded during the Eriksen Flanker task because the present study was designed to investigate (1) behavioral differences in conflict monitoring and error processing in subjects differing in depressive symptoms; and (2) relations between tonic, task‐free ACC activity and individual differences in conflict monitoring and error processing.
Although the LORETA version used in the present study received important cross‐modal validation through fMRI and PET [e.g., Mulert et al., 2004; Pizzagalli et al., 2004; Vitacco et al., 2002], it is important to stress that a number of factors likely weaken the spatial resolution of this approach. In future studies, the use of (1) a more complex head model (rather than a three‐shell spherical head model); (2) individual anatomical scans (rather than a general brain template); and (3) individual digitization of electrode positions (rather than general positions), is expected to improve the spatial resolution of LORETA.
Consistent with this notion, high BDI subjects showed in the present study a negative correlation between post‐error adjustments (calculated with accuracy scores) and scores on the post‐task questionnaire items, “How well do you think you did on the task” (from 1, “very well” to 5, “very poor”; ρ = −0.65, n = 17, P < 0.005) and “How satisfied are you with your performance” (from 1, “very satisfied” to 5, “very unsatisfied”; ρ = −0.68, n = 17, P < 0.005). Thus, the bigger the dissatisfaction with own performance and the more negative evaluation of own performance, the lower the post‐error adjustments. These findings suggest that the subjects were aware of committing errors.
REFERENCES
- Abela JRZ, D'Alessandro DU (2002): Beck's cognitive theory of depression: a test of diathesis‐stress and casual mediation components. Br J Clin Psychol 41: 111–128. [DOI] [PubMed] [Google Scholar]
- Ary JP, Klein SA, Fender DH (1981): Location of sources of evoked scalp potentials: corrections for skull and scalp thicknesses. IEEE Trans Biomed Eng 28: 447–452. [DOI] [PubMed] [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M (1999): Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neurosci Lett 274: 29–32. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F (2000): Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 47: 305–313. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM (2001): Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178: 200–206. [DOI] [PubMed] [Google Scholar]
- Awata S, Konno M, Kawashima R, Suzuki K, Sato T, Matsuoka H, Fukuda H, Sato M (2002): Changes in regional cerebral blood flow abnormalities in late‐life depression following response to electroconvulsive therapy. Psychiatry Clin Neurosci 56: 31–40. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A (2004): Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI‐based parcellation of the prefrontal cortex. Am J Psychiatry 161: 99–108. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R (1996): Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med 26: 591–604. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E (1998): The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport 9: 3253–3258. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961): An inventory for measuring depression. Arch Gen Psychiatry 4: 53–63. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G (1979): Cognitive therapy and depression. New York: Guilford Press. [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ (1993): Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med 23: 579–590. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001): Conflict monitoring and cognitive control. Psychol Rev 108: 624–652. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G (1995): Gamma (40‐100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15: 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A (2001): Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS (2004): Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 161: 637–645. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM (2002): The problem of functional localization in the human brain. Nat Rev Neurosci 3: 243–249. [DOI] [PubMed] [Google Scholar]
- Brillinger DR (1981): Time series: data analysis and theory. New York: McGraw‐Hill. [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C (1997): Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 41: 15–22. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazaltine E, Scanlon MD, Rosen AC, Gabrieli JDE (2002): Dissociable contributions of prefrontal cortex and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Ali L (2002): Functional connectivity of gamma EEG activity is modulated at low frequency during conscious recollection. Int J Psychophysiol 46: 91–100. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA (2003): The multi‐source interference task: validation study with fMRI in individual subjects. Mol Psychiatry 8: 60–70. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (1996): The hippocampo‐neocortical dialogue. Cereb Cortex 6: 81–92. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE (2000): Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep‐wake state. Eur J Neurosci 12: 2166–2184. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000): Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A 97: 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH (2000): Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A 97: 8728–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S, Green PS (1999): Executive function in depression: the role of performance strategies in aiding depressed and non‐depressed participants. J Neurol Neurosurg Psychiatry 66: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP (1987): The measurement of handedness. Brain Cogn 6: 175–183. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G (1998): Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci 18: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J (1993): PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput 25: 257–271. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Conway M, Howell A, Giannopoulos C (1991): Dysphoria and thought suppression. Cogn Ther Res 15: 153–166. [Google Scholar]
- Critchley HD, Daly E, Phillips M, Brammer M, Bullmore E, Williams S (2000a): Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 9: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ (2000b): Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci 8: 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP (1998): Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event‐related synchronization in the gamma band. Brain 121: 2301–2315. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K (2002): Depression: perspectives from affective neuroscience. Annu Rev Psychol 53: 545–574. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH (2003): The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160: 64–75. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, Raichle ME (1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386: 824–827. [DOI] [PubMed] [Google Scholar]
- Dunkin J, Leuchter AF, Cook IA, Kasl‐Godley JE, Abrams M, Rosenberg‐Thompson S (2000): Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord 60: 13–23. [DOI] [PubMed] [Google Scholar]
- Duzel E, Habib R, Schott B, Schoenfeld A, Lobaugh N, McIntosh AR, Scholz M, Heinze HJ (2003): A multivariate, spatiotemporal analysis of electromagnetic time‐frequency data of recognition memory. Neuroimage 18: 185–197. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES (1996): Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med 26: 975–989. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ (1997a): Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 27: 931–942. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel, ES (1997b): Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry 63: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW (1974): Effects of noise letters upon identification of a target letter in a nonsearch task. Percept Psychophys 16: 143–149. [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993): 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE Nuclear Sci Symp Med Imaging Conf 95: 1813–1817. [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H (2004): A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20: 132–143. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CEE, Fernandez G (2001): Human memory formation is accompanied by rhinal‐hippocampal coupling and decoupling. Nat Neurosci 4: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G (2003): Rhinal‐hippocampal theta coherence dring declarative memory formation: interaction with gamma synchronization? Eur J Neurosci 17: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ (2000): Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus 10: 187–197. [DOI] [PubMed] [Google Scholar]
- Feenstra BW, Holsheimer J (1979): Dipole‐like neuronal sources of theta rhythm in dorsal hippocampus, dentate gyrus and cingulate cortex of the urethane‐anesthetized rat. Electroencephalogr Clin Neurophysiol 47: 532–538. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wik G, Fredrikson M (1996): Functional neuroanatomy of robbery re‐experience: affective memories studied with PET. Neuroreport 7: 2081–2086. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Wittner L, Freund TF, Gahwiler BH (2002): Simultaneous activation of gamma and theta network oscillations in rat hippocampal slice cultures. J Physiol (Lond) 539: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamma A, Lehmann D, Frei E, Iwata K, Pascual‐Marqui RD, Vollenweider FX (2004): Comparison of simultaneously recorded [H2(15)O]‐PET and LORETA during cognitive and pharmacological activation. Hum Brain Mapp 22: 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA (2003): A midline dissociation between error‐processing and response‐conflict monitoring. Neuroimage 20: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2002): The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279–2282. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2004): Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; p 14–20. [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM (1997): Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). J Neuropsychiatry Clin Neurosci 9: 55–63. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D (1997): High‐resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7: 374–385. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E (1992): Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen 121: 480–506. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Pyzszczynski T (1986): Persistent high self‐focus after failure and low self‐focus after success: The depressive self‐focusing style. J Pers Soc Psychol 50: 1039–1044. [DOI] [PubMed] [Google Scholar]
- Gross DW, Gotman J (1999): Correlation of high‐frequency oscillations with the sleep‐wake cycle and cognitive activity in humans. Neuroscience 94: 1005–1018. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Robinson DD, Yu JH, Hajos‐Korcsok E (2003): Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo‐hippocampal system. Neuropsychopharmacology 28: 857–864. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD (2003): Material‐dependent and material‐independent selection processes in the frontal and parietal lobes: an event‐related fMRI investigation of response competition. Neuropsychologia 41: 1208–1217. [DOI] [PubMed] [Google Scholar]
- Henriques G, Leitenberg H (2002): An experimental analysis of the role of cognitive errors in the development of depressed mood following negative social feedback. Cogn Ther Res 26: 245–260. [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M (1999): Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 10: 675–679. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Bekci B, Erzengin ÖU (2003): Early gamma response in human neuroelectric activity is correlated with neuropsychological test scores. Neurosci Lett 340: 37–40. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Hollon SD, Beck AT, Hammen CL (1987): Issues and recommendations regarding use of the Beck Depression Inventory. Cogn Ther Res 11: 289–299. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS (2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: an event‐related fMRI study. Psychophysiology 37: 216–223. [PubMed] [Google Scholar]
- Konig P, Engel AK, Singer W (1995): Relation between oscillatory activity and long‐range synchronization in cat visual cortex. Proc Natl Acad Sci U S A 92: 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki S, Hermann WM, Fichte K, Freund G (1979): Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmacopsychiatry 12: 237–245. [DOI] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, Brammer MJ, Poon L, Simmons A, Williams SC, Checkley SA, Sharma T (2003): Neural abnormalities during cognitive generation of affect in treatment‐resistant depression. Biol Psychiatry 54: 777–791. [DOI] [PubMed] [Google Scholar]
- Laming D (1968): Information theory of choice‐reaction times. London: Academic Press. [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC (1997): Automated labeling of the human brain—a preliminary report on the development and evaluation of a forward‐transformed method. Hum Brain Mapp 5: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Ngan ETC, Bates AT, Kiehl KA, Liddle PF (2003): Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain 126: 610–622. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C (2003): Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci 14: 47–53. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S (2004): Frontal midline theta and the error‐related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol 115: 1821–1835. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O (2005): Mechanisms underlying gamma ('40 Hz') network oscillations in the hippocampus—a mini‐review. Prog Biophys Mol Biol 87: 67–76. [DOI] [PubMed] [Google Scholar]
- Martin SD, Martin E, Rai SS, Richardson MA, Royall R (2001): Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry 58: 641–648. [DOI] [PubMed] [Google Scholar]
- Mayberg HS (1997): Limbic‐cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN Jr (1994): Paralimbic hypoperfusion in unipolar depression. J Nucl Med 35: 929–934. [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT (1997): Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA (2000): Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843. [DOI] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, Rubin DB (1992): Comparing correlated correlation coefficients. Psychol Bull 111: 172–175. [Google Scholar]
- Menon V, Adelman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Braun C, Arnold M, Witte H, Taub E (1999): Coherence of gamma‐band EEG activity as a basis for associative learning. Nature 397: 434–436. [DOI] [PubMed] [Google Scholar]
- Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, Rose M, Moore GJ, Rosenberg DR (2004): Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry 43: 341–348. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Suckling J, Poon L, Simmons A, Andrew C, Sharma T (2003): Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport 14: 177–182. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, Juckel G, Hegerl U (2004): Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time‐course of brain activity in target detection. Neuroimage 22: 83–94. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ (2003): Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med 33: 455–467. [DOI] [PubMed] [Google Scholar]
- Nishida M, Hirai N, Miwakeichi F, Maehara T, Kawai K, Shimizu H, Uchida S (2004): Theta oscillation in the human anterior cingulate cortex during all‐night sleep: an electrocorticographic study. Neurosci Res 50: 331–341. [DOI] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Davidson RJ (2004): Functional coupling of simultaneous electrical and metabolic activity in the human brain. Hum Brain Mapp 21: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N (2003): Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 47: 21–26. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG (1997): Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis 185: 748–754. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Michel CM, Lehmann D (1994): Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18: 49–65. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M (1999): Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic‐naive, first‐episode, productive schizophrenia. Psychiatry Res Neuroimag 90: 169–179. [DOI] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G (1998): Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci 10: 718–728. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand D, Echallier JF (1989): Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol 72: 184–187. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Pascual‐Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ (2001): Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158: 405–415. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Pascual‐Marqui RD, Davidson RJ (2002): Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biol Psychiatry 52: 73–85. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Davidson RJ (2003): Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology 40: 939–949. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ (2004): Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry 9: 393–405. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH (2003): Neurocognitive impairment in drug‐free patients with major depressive disorder. Br J Psychiatry 182: 214–220. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S (1994): Attentional networks. Trends Neurosci 17: 75–79. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C (1997): Neuropsychological function in young patients with unipolar major depression. Psychol Med 27: 1277–1285. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA (1966a): Error correction time without external error signals. Nature 212: 438. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA (1966b): Errors and error correction in choice‐response tasks. J Exp Psychol 71: 264–272. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Lachaux GN, Martinerie J, Renault B, Varela FL (1999): Perception's shadow: long distance synchronization of human brain activity. Nature 397: 430–433. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E (2003): Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 20: 351–358. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Gron G, Spitzer M, Kiefer M (2004): The effect of erroneous responses on response monitoring in patients with major depressive disorder: a study with event‐related potentials. Psychophysiology 41: 833–840. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Woodward TS, Laurens KR, Liddle PF (2001): The role of the anterior cingulate cortex in conflict processing: evidence from reverse stroop interference. Neuroimage 14: 1150–1158. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR (2003): Differential brain metabolic predictors of response to paroxetine in obsessive‐compulsive disorder versus major depression. Am J Psychiatry 160: 522–532. [DOI] [PubMed] [Google Scholar]
- Schack B, Vath N, Petsche H, Geissler HG, Moller E (2002): Phase‐coupling of theta‐gamma EEG rhythms during short‐term memory processing. Int J Psychophysiol 44: 143–163. [DOI] [PubMed] [Google Scholar]
- Shah PJ, O'Carroll RE, Rogers A, Moffoot AP, Ebmeier KP (1999): Abnormal response to negative feedback in depression. Psychol Med 29: 63–72. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ (1999): Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry 156: 683–689. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushere RE (1970): Manual of the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Steffens DC, Wagner HR, Levy RM, Horn KA, Krishnan KR (2001): Performance feedback deficit in geriatric depression. Biol Psychiatry 50: 358–363. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas‐Ferrer M, Bonis A, Szikla G, Rusu M (1973): The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol 34: 45–52. [DOI] [PubMed] [Google Scholar]
- Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, Cakmur R, Frank SA, Spire JP (1993): The spatial location of EEG electrodes: locating the best‐fitting sphere relative to cortical anatomy. Electroencephalogr Clin Neurophysiol 86: 1–6. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C (2003): Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol 112: 667–678. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nakayama H, Maehara T, Hirai N, Arakaki H, Nakamura M, Nakabayashi T, Shimizu H (2000): Suppression of gamma activity in the human medial temporal lobe by sevoflurane anesthesia. Neuroreport 11: 39–42. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Falkenstein M (2004): Errors, conflicts, and the brain. Current opinions on performance monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience. [Google Scholar]
- Ullsperger M, von Cramon DY (2001): Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2002): The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77: 477–482. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001): Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Veiel HO (1997): A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol 19: 587–603. [DOI] [PubMed] [Google Scholar]
- Vitacco D, Brandeis D, Pascual‐Marqui R, Martin E (2002): Correspondence of event‐related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp 17: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995): Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 359: 490–506. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA (1995): Testing a tripartite model. I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 104: 3–14. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Grozier SA (1988): Depression and the magnification of failure. J Abnorm Psychol 97: 90–93. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 44: 1219–1228. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Lagerlund TD, Sharbrough FW, Brinkmann BH, Busacker NE, Cicora KM, O'Brien TJ (2000): Localization of the epileptic focus by low‐resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr 12: 273–282. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE Jr., Fallon JH, Keator D (1999): Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 156: 1149–1158. [DOI] [PubMed] [Google Scholar]


