Abstract
Memory impairment observed in patients with medial temporal lobe epilepsy (MTLE) is classically attributed to hippocampal atrophy. The contribution of extrahippocampal structures in shaping memory impairment in patients with MTLE is not yet completely understood, even though atrophy in MTLE extends beyond the hippocampus. We aimed to evaluate the neuropsychological profile of patients with MTLE focusing on memory, and to investigate whether gray matter concentration (GMC) distribution within and outside the medial portion of the temporal lobes would be associated with their neuropsychological performance. We performed a voxel based morphometry study of 36 consecutive patients with MTLE and unilateral hippocampal atrophy. We observed a significant simple regression between general and verbal memory performance based on Wechsler Memory Scale—Revised and the GMC of medial temporal and extratemporal structures in patients with left MTLE. We also performed a “regions of interest analysis” of the medial temporal lobe, and we observed that the GMC of the hippocampus, entorhinal, and perirhinal cortices were consistently associated with general and verbal memory performance in patients with MTLE. We also observed that the GMC of the cingulate and orbito‐frontal cortex are independently associated with verbal and general memory performances. Our results suggest that general and verbal memory impairments in patients with left MTLE are associated with atrophy of the hippocampus, the entorhinal, and the perirhinal cortex. We also suggest that atrophy and dysfunction of limbic and frontal structures such as the cingulate and the orbito‐frontal cortex contribute to memory impairment in MTLE. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: neuropsychological evaluation, medial temporal lobe epilepsy, magnetic resonance imaging
INTRODUCTION
There is evidence of association between medial temporal lobe epilepsy (MTLE) and progressive memory loss [Helmstaedter et al., 2003; Jokeit and Ebner, 1999]. In effect, more intense memory impairment is observed when the severity of epilepsy increases. Recognized factors contributing to a greater likelihood of higher memory impairment are an earlier age of seizure onset [Alessio et al., 2004; Dikmen et al., 1975; Dodrill, 1992; Lespinet et al., 2002; O'Leary et al., 1981, 1983], longer duration of epilepsy [Alessio et al., 2004; Delaney et al., 1980; Dikmen et al., 1975; Ladavas et al., 1979; Mirsky et al., 1960], higher seizure frequency [Aldenkamp et al., 1996; Alessio et al., 2004], and use of antiepileptic drugs (AEDs) [Alessio et al., 2004; Bennett, 1992; Smith, 1991]. Importantly, refractoriness to drug treatment is significantly associated to more severe memory loss, and the successful surgical treatment may stop or even reverse the memory loss [Helmstaedter et al., 2003].
The memory failure in patients with MTLE is related to the etiology of epilepsy. The presence of hippocampal atrophy (HA) and other signs of medial temporal sclerosis (MTS) detected by magnetic resonance imaging (MRI) are notably associated with the memory impairment frequently seen in patients with MTLE due to hippocampal sclerosis [Baxendale et al., 1998; Hermann et al., 1997; Meencke and Veith, 1991]. As predicted by the classical model of material‐specific memory, it has been hypothesized that MTS encompassing the left hippocampus would impair verbal memory [Meyer and Yate, 1955; Milner, 1958; Novelly et al., 1984], as opposed to MTS involving the right hippocampus resulting in visual memory deficits [Kimura, 1963; Milner et al., 1962]. However, evidence from studies involving MTLE patients have demonstrated that the relationship between side of hippocampal pathology and memory dysfunction is more evident in patients with left HA than in those with right HA on MRI [Baxendale, 1995; Hermann et al., 1997; Jones‐Gotman, 1996; Lencz et al., 1992; Rausch and Babb, 1993; Saling et al., 1993; Trenerry et al., 1995].
In a parallel line of research, it has been observed that the neuronal damage in patients with refractory MTLE is not restricted to the hippocampus, but involves extrahippocampal and extra‐temporal structures [Bonilha et al., 2003, 2004b]. Patients with MTLE may show atrophy in other regions anatomically and functionally linked to the hippocampus, such as the parahippocampal region, the temporal neocortex, the thalamus, and the medial portion of the frontal lobes [Bernasconi et al., 2000, 2003; Bonilha et al., 2003, 2004b, 2005]. Patients with MTLE possibly show a network of damage that encompasses different brain structures which are linked to the hippocampal system. Furthermore, the closer the structure is to the hippocampus, the more intense is its atrophy [Bonilha et al., 2003, 2004b].
Current research suggests that the whole hippocampal system is involved in different aspects of memory formation and consolidation. Specifically, while the hippocampus plays a critical role in the processes located between the initial formation of memories and their final repository in the neocortex [Eichenbaum, 2000], the parahippocampal region is involved in the intersection of perception and memory [Murray and Bussey, 1999], and mediates the extended persistence of cortical representations of memory [Eichenbaum, 2000]. Animal studies have shown that the parahippocampal region is particularly important for recognition memory [Brown and Aggleton, 2001; Eichenbaum, 2000], and that selective lesions to the parahippocampal cortex severely impair memory [Suzuki et al., 1993]. Moreover, the disconnection of the temporal lobe also results in significant memory deficits both in humans and in monkeys [Gaffan, 2005; Parker and Gaffan, 1998]. This is observed when white matter connecting frontal and temporal lobes is damaged, even when parahippocampal structures remain intact [Gaffan et al., 2001]. When the white matter underlying the temporal lobe or the fornix is damaged, the ensuing memory loss is termed dense amnesia and is thought to be the result of a broad disconnection of the ascending axon projections from the basal forebrain and brainstem that pass through the anterior medial temporal lobe on their path to widespread cortical targets [Easton et al., 2002; Gaffan et al., 2001]. The process of memory formation involves not only the medial portion of the temporal lobe but also other cortical areas, notably frontal areas, in special when sensory representations must be maintained in working memory. Both the medial temporal lobe and extra‐hippocampal regions are important for memory, and amnesia can result from damage to only some of these regions, suggesting that a large memory system is engaged or required for memory related tasks.
Patients with MTLE exhibit neuronal damage affecting brain structures that are probably crucial for the generation and maintenance of memory representations. Even though it is clear that the hippocampal damage in these patients play a significant role in structuring the memory deficit, it is not clear how the damage to extratemporal structures is relevant to define memory impairment in MTLE patients.
In this study, we used a voxel based automated neuroimaging technique in order to investigate the relationship between memory related neuropsychological tests and the distribution of gray matter concentration (GMC) in the brain of patients with MTLE. We aimed to find out which brain areas would exhibit a positive correlation between GMC and performance on the neuropsychological tests. We aimed to investigate the whole brain, and also to focus in anatomical regions of the medial temporal lobe. Moreover, we aimed to investigate if the atrophy from structures connected to the hippocampus would show a significant association with HA. Therefore, we also aimed to dissociate the influence of HA from extra‐HA on the memory impairment in patients with MTLE.
METHODS
Ascertainment of Subjects
We studied 36 consecutive patients with chronic refractory MTLE, who were referred from the out‐patient epilepsy clinic of our institution (UNICAMP hospital), where they were diagnosed based on a detailed neurological evaluation. The determination of the epileptic syndrome was based on ILAE criteria [Commission on classification and terminology of the International League Against Epilepsy, 1989]. Seizures were lateralized according to the medical history, a comprehensive neurological examination, interictal EEG, and prolonged video‐EEG monitoring. All patients were considered to have drug‐refractory MTLE [Engel, 1999], with unilateral seizure onset and unilateral HA on routine visual analysis of MRI diagnostic protocol [Kobayashi et al., 2003]. They signed a written informed consent approved by the Ethics Committee of our Institution, in accordance with the principles stated in the 1964 Declaration of Helsinki.
Neuropsychological Evaluation
Neuropsychological evaluation included: vocabulary and block design subtests of the Wechsler Adult Intelligence Scale—Revised (WAIS‐R) to estimate IQ; Edinburgh Handedness Inventory and Dichotic Listening Test Words to determine hemispheric dominance for language and, by inference, to lateralize verbal and visual memories (differences in the proportion equal or superior to 10% were considered suggestive of right or left ear advantage and, consequently, suggestive of left or right hemispheric dominance for language); Logical Memory and Verbal Pared Associates of the Wechsler Memory Scale—Revised (WMS‐R) to investigate verbal memory; and Figural Memory, Visual Reproduction and Visual Pared Associates of the WMS‐R to investigate visual memory.
To control for other cognitive functions that could influence memory tasks, we employed tests for language (Boston Naming Test/BNT), attention (Strub and Black Vigilance Test), and executive functions (Trail Making Test/TMT and Wisconsin Card Sorting Test/WCST) [Fromm‐Auch and Yeudall, 1983; Heaton et al., 1993; Kaplan et al., 1983; Kennedy, 1981; Oldfield, 1971; Strub and Black, 1993; Wechsler, 1981, 1987]. We did not use the same MRI control group for neuropsychological data. These tests were adapted for our population.
The results of each test were compared with results for normal controls matched for age and educational level. This normalization was performed by transforming the results from each patient's testing into Z scores (standardized scores defined by the number of standard deviations away from the mean of the respective control group).
Preprocessing of Neuroimaging Data
We applied a voxel based morphometry (VBM) protocol to assess the relationship between GMC and neuropsychological performance. VBM was performed on volumetric T1‐weighted images with either 1 mm isotropic voxels or with 1.5 × 0.97 × 0.97 mm3 voxels. All images were acquired on an Elscint Prestige 2 T scanner (TR = 22 ms, TE = 9 ms, flip angle = 35°, matrix = 256 × 220, field of view = 25 × 22 cm2, 1 mm sagittal slices). DICOM format images were transformed into ANALYZE format using MRIcro software (http://www.mricro.com) [Rorden and Brett, 2000]. The VBM analysis was performed using the software package SPM2 (Wellcome Department of Imaging Neuroscience, London, England; http://www.fil.ion.ucl.ac.uk) [Ashburner and Friston, 2000]. Images were normalized in order to match each individual brain's size and shape to the standard space. Normalization parameter estimation was employed using 12 linear and 7 × 8 × 7 nonlinear basis functions; in addition, a brain mask was used to ensure that the fit was based on the shape of the brain rather than the surrounding scalp. Spatially normalized images were resliced to an isotropic 1.5 mm voxel size. After normalization, images were submitted to tissue segmentation using SPM2's built‐in routines, which estimate the probability of GMC for each voxel. A pitfall of the “conventional” VBM is that during normalization brain areas that are atrophied can be artificially enlarged to match the standard space template, which is based on images from a neurologically healthy sample. As a result, GMC variations in the non‐healthy population can be washed out by normalization. To overcome this distortion and to keep the authenticity of the distribution of GMC, Good et al. have proposed a technique for modulating the estimated concentration of tissue in segmented images based on the spatial deformations selected during normalization [Good et al., 2001]. This technique compensates for the deformation of the brain tissue during the normalization process, preserving the quantity of gray matter while ensuring a good spatial alignment between patients and controls. We employed the modulation step developed by Good et al. As a final step, the images were convolved with a 10‐mm isotropic Gaussian kernel filter to minimize interindividual variability of sulci and gyri. This smoothing creates images that are more normally distributed and permits voxel‐wise analysis. The resulting images were regressed with the neuropsychological data in the search for a significant association between the neuropsychological scores and the probability of each voxel being gray matter.
Statistical Analysis
The results from the neuropsychological evaluation were transformed into Z scores (standardized scores defined by the number of standard deviations away from the mean of the respective control group). Group differences between left‐sided and right‐sided MTLE patients for neuropsychological performance were assessed in SPSS v12.0 using a multivariate analysis of variance (MANOVA). The scores of BNT and WMS‐R subtests were defined as dependent within subjects' variables and the side of MTLE as a fixed between subjects' factor.
We conducted a sequence of analyses of GMC. Initially, we investigated the correlation between the probability of each brain voxel being GMC and the scores of BNT and WMS‐R subtests. Each group of patients, i.e., left‐sided MTLE and right‐sided MTLE were investigated separately. This analysis was performed in SPM2 using simple regression. It did not include grand mean scaling, and used proportional threshold masking (set to 0.8), and brain masking.
Second, we aimed to investigate the association between the GMC of isolated medial temporal lobe structures and the performance on neuropsychological tests. We defined regions of interest (ROIs) within the medial aspect of the temporal lobe in a standard T1 MRI normal brain template (“colin27” matched to an average of 305 brains—the MNI30537) using a temporal lobe segmentation protocol [Bonilha et al., 2004a]. We defined ROIs corresponding to the hippocampus, the entorhinal cortex, and the perirhinal cortex. The GMC from each ROI was extracted using the software package MarsBar [Brett et al., 2002] and compared with the neuropsychological performance in SPSS v12.0 through a one‐tailed correlation analysis.
Third, we hypothesized that considerable associations between extratemporal GMC and neuropsychological performance could be an artifact of a double relation between the extratemporal GMC and the hippocampal GMC, and the hippocampal GMC and the neuropsychological performance. Therefore, we investigated the regions within the brain in which the probability of GMC would be significantly correlated with the GMC within the hippocampus. This analysis was also performed in SPM2 using simple regression. It did not include grand mean scaling, and used proportional threshold masking (set to 0.8), and brain masking.
Fourth, we employed a simultaneous multiple regression analysis with each neuropsychological test as dependent variables (or criterion) and the GMC for each brain voxel and the hippocampal GMC as predictors. Given that atrophy in the medial temporal lobe extends beyond the hippocampus, as well as the possible strong multicollinearity between the GMC scores, the multiple regression analysis would assess whether the medial temporal lobe structures can contribute over and above the hippocampus to predict the neuropsychological performance. The multiple linear regression applies a model in the form of y = β0 + β1 x 1 + β2 x 2 + β3 x 3 + …, where y is the criterion (in our case, each neuropsychological test), and x 1, x 2, … are the explanatory variables, so called predictors (the hippocampal, extrahippocampal, and extratemporal GMC). This analysis was performed in SPM2 using multiple regression. It did not include grand mean scaling, and used proportional threshold masking (set to 0.8), and brain masking.
However, we also hypothesized that the association between the extrahippocampal GMC and the neuropsychological tests would probably not survive the stringency imposed by the correction for multiple comparisons. Therefore, we also performed a multiple regression analysis with each neuropsychological test as a dependent variable, while the GMC extracted from ROIs defined by Anatomical Automatic Labeling (AAL) Freeware (http://www.cyceron.fr/freeware/) were used as predictors. ROIs comprised limbic structures and cortical areas within the temporal and frontal lobes. All ROIs were entered into the analyses. We decided to employ regions of interest defined from a template of the normal brain in order to avoid post‐hoc choosing of regions of interest based on the whole brain voxel‐wise results. Therefore, by using histologically defined anatomical regions, based on normal brain mapping, we avoided forcing artificial findings. While brains from patients with MTLE may exhibit gray matter atrophy, the employing of an anatomical mask to the MTLE brain would broadly encompass the specific anatomical area of interest. While analyzing an ROI, the mean GMC from the ROI is computed, thereby increasing the statistical power by reducing the number of multiple comparisons. We exported from SPM2 to MarsBar the design matrix obtained from the multiple regression set with neuropsychological test as a dependent variables and the GMC for each brain voxel and the hippocampal GMC as predictors. Subsequently, we extracted the data from this design matrix corresponding to ROIs within the limbic system. Finally, we calculated the significance of the regression of the mean probability of GMC within each ROI while portioning out the GMC within the hippocampus.
Whole brain VBM analyses were corrected for multiple comparisons through false discovery rate threshold of 5% with an extent threshold looking for clusters with at least 20 contiguous voxels [Genovese et al., 2002]. This technique controls the rate of statistical false alarms during multiple statistical comparisons. In the context of VBM, a false discovery rate of 0.05 implies that ∼5 of 100 voxels identified as statistically significant are actually a false alarm. We also employed an extent threshold looking for clusters with at least 20 contiguous voxels in order to focus the analysis on regions were the effects were more intense, thereby improving the conciseness of the results.
The determination and labeling of the brain regions determined as significant by the whole brain VBM analysis were performed by assessing their stereotaxic coordinates provided in the SPM output through the freeware Talairach Daemon client (http://ric.uthscsa.edu/projects/talairachdaemon.html).
RESULTS
Neuropsychological Profile of MTLE Patients
We studied 36 MTLE patients (23 women; 13 men) with mean age of 35 years (range: 17–54 years), mean educational level of 6 years (range: 1–16 years), and mean estimated IQ of 89 (range: 73–115). Nineteen patients had left MTLE and 17 had right MTLE.
Thirty‐four patients (94%) were right‐handed, 25 (70%) patients had left hemispheric dominance, and 8 (22%) had bilateral representation for language, three being patients with left MTLE. All except one patient with bilateral representation for language were right‐handed, indicating that their motor dominant hemisphere was the left one, and that the patient who had bilateral language representation and was left‐handed did have right‐sided MTLE.
The patients mean performances on the neuropsychological tests were BNT = −3.73 SD (range: 12.47/2.79); Vigilance Test = 0.38 errors (range: 0–2); TMTA = 61 s (range: 19/197) and 0.31 errors (range: 0–3); TMTB = 145 s (range: 49/281) and 1.41 errors (range: 0–10). Finally, on the WCST, 35 (97%) patients exhibited normal range of performance and only 1 patient exhibited impaired performance.
Overall, left and right MTLE patients combined performance on WMS‐R subtests can be summarized as follows: general memory – Z = −0.71 ± 1.12 (range from −2.79 to 2.64), verbal memory – Z = −0.59 ± 1.15 (range from −2.81 to 2.50), visual memory – Z = −0.52 ± 1.04 (range from −2.89 to 2.26), and delayed recall – Z = −0.86 ± 1.25 (range from −3.15 to 2.94).
Patients with left‐sided MTLE exhibited the following performance: general memory – Z = −0.83 ± 1.11 (range from −2.79 to 1.74), verbal memory – Z = −0.75 ± 1.08 (range from −2.81 to 1.46), visual memory – Z = −0.61 ± 1.03 (range from −2.89 to 1.17), and delayed recall – Z = −1.03 ± 1.01 (range from −3.15 to 0.6).
The performance of patients with right‐sided MTLE was the following: general memory – Z = −0.49 ± 1.18 (range from −2.15 to 2.64), verbal memory – Z = −0.32 ± 1.23 (range from −1.97 to 2.50), visual memory – Z = −0.40 ± 1.13 (range from −1.53 to 2.26), and delayed recall – Z = −0.52 ± 1.39 (range from −3.01 to 2.94).
Comparing patients with left and right MTLE, patients with left‐sided MTLE showed a significantly poorer performance on the BNT F(4,1) = 14.4, P = 0.002, and on the WMS‐R for general memory F(4,1) = 6.2, P = 0.019; verbal memory F(4,1) = 4.6, P = 0.047; and delayed recall F(4,1) = 6.4, P = 0.023. The performance on the WMS‐R for visual memory showed no significant difference between left‐ and right‐sided MTLE patients, F(4,1) = 3.6, P = 0.078. These findings are summarized in Figure 1.
Figure 1.
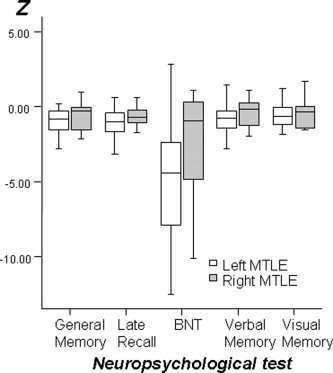
Box and whisker plots demonstrate the distribution of Z scores of the performance of patients with left‐ and right‐sided MTLE on memory related tests. Patients with left MTLE performed poorer on all tests except visual memory.
Whole Brain Voxel‐Wise Analysis: Correlation With Neuropsychological Data
Patients with left‐sided MTLE did exhibit a significant positive simple regression between GMC in selected brain regions and the scores in the WMS‐R for general and verbal memory (see Fig. 2). A decreased performance on general memory was significantly associated with a diminished GMC in limbic areas such as the parahippocampal cortex and cingulate gyri; in frontal areas, for instance, the superior and medial frontal gyri and orbital gyri; in cerebellar hemispheres; in the parietal lobe within the postcentral gyrus and inferior parietal lobule; in the occipital lobe, in the cuneus and precuneus; and in the superior temporal gyrus (Fig. 2 and Table I).
Figure 2.
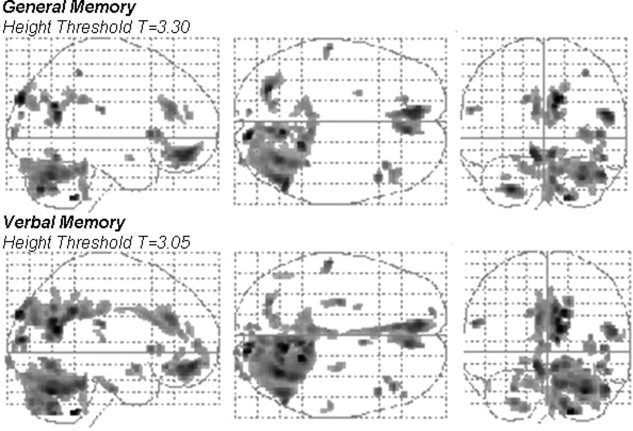
The parametric map of the t statistic depicts the location and the statistical significance of voxels with a significant correlation between density of gray matter and general memory (top row) and verbal memory (bottom row) in patients with left MTLE. The map is overlaid in a glass brain template. Images are shown in radiological convention, i.e., left side of images corresponds to the right brain hemisphere.
Table I.
Whole brain VBM results showing the voxel‐wise description of brain locations where there was a positive correlation between the GMC and the performance on general memory in patients with left MTLE
| Cluster size | Voxel wise | Spatial coordinates | Anatomical location | ||||
|---|---|---|---|---|---|---|---|
| T | equiv Z | X | Y | Z | Side | Location | |
| 272 | 7.41 | 4.89 | 12 | −89 | 32 | Right | Cuneus |
| 355 | 6.72 | 4.63 | 9 | −60 | 26 | Right | Precuneus |
| 179 | 6.33 | 4.48 | 51 | −59 | 20 | Right | Superior Temporal Gyrus |
| 273 | 6.3 | 4.47 | −26 | −72 | −41 | Left | Cerebellar Hemisphere |
| 36 | 6.28 | 4.46 | 20 | −45 | −48 | Right | Cerebellar Hemisphere |
| 832 | 6.14 | 4.4 | −9 | 48 | −12 | Left | Superior Frontal Gyrus |
| 3,818 | 5.93 | 4.31 | 47 | −65 | −30 | Right | Cerebellar Hemisphere |
| 471 | 5.27 | 4 | 12 | −63 | −41 | Right | Cerebellar Hemisphere |
| 64 | 5.13 | 3.93 | 45 | 17 | 3 | Right | Inferior Frontal Gyrus |
| 204 | 4.83 | 3.78 | −6 | 35 | 23 | Left | Anterior Cingulate |
| 44 | 4.76 | 3.75 | −60 | −24 | 23 | Left | Postcentral Gyrus |
| 20 | 4.69 | 3.71 | 35 | −41 | 53 | Right | Inferior Parietal Lobule |
| 69 | 4.67 | 3.69 | 35 | 23 | 3 | Right | Insula |
| 49 | 4.64 | 3.68 | 32 | −95 | 5 | Right | Middle Occipital Gyrus |
| 50 | 4.55 | 3.63 | −14 | −69 | 33 | Left | Precuneus |
| 20 | 4.52 | 3.61 | −9 | −89 | 20 | Left | Cuneus |
| 32 | 4.42 | 3.56 | −17 | 39 | −23 | Left | Orbital Gyrus |
| 86 | 4.37 | 3.53 | 42 | 29 | −12 | Right | Inferior Frontal Gyrus |
| 71 | 4.35 | 3.52 | −6 | −38 | 32 | Left | Cingulate Gyrus |
| 49 | 4.17 | 3.41 | 17 | −83 | 41 | Right | Precuneus |
| 22 | 4.09 | 3.36 | 3 | 48 | 18 | Right | Medial Frontal Gyrus |
| 22 | 4.01 | 3.32 | −23 | 2 | −17 | Left | Parahippocampal Gyrus |
| 70 | 3.86 | 3.23 | −32 | −65 | −33 | Left | Cerebellar Hemisphere |
| 55 | 3.76 | 3.16 | 14 | −65 | −12 | Right | Vermis |
| 24 | 3.69 | 3.12 | −6 | −50 | 38 | Left | Precuneus |
Brain sites where GMC correlated positively with the performance on the WMS‐R for General Memory, in patients with left MTLE. Results were corrected for multiple comparisons, Height threshold, T = 3.30; cluster ≥ 20 voxels; FDR corrected (P < 0.05).
The score in the WMS‐R for verbal memory was significantly correlated with the GMC in limbic regions, such as the ipsilateral and contralateral parahippocampal cortex and the cingulate gyri; in the temporal lobe and in the superior temporal gyrus; in frontal areas, the middle, medial, and inferior frontal gyri; in occipital areas, such as the cuneus, precuneus, and middle occipital gyrus; in the parietal lobe, in the postcentral gyrus; and in the cerebellar hemispheres (Fig. 2 and Table II).
Table II.
Whole brain VBM results showing the voxel‐wise description of brain locations where there was a positive correlation between the GMC and the performance on verbal memory in patients with left MTLE
| Cluster size | Voxel wise | Spatial coordinates | Anatomical location | ||||
|---|---|---|---|---|---|---|---|
| T | equiv Z | X | Y | Z | Side | Location | |
| 40 | 7.01 | 4.74 | 18 | −47 | −48 | Right | Cerebellar Hemisphere |
| 2504 | 6.77 | 4.65 | 11 | −60 | 18 | Right | Posterior Cingulate |
| 6115 | 6.35 | 4.49 | 32 | −65 | −32 | Right | Cerebellar Hemisphere |
| 62 | 6.08 | 4.37 | 8 | −89 | 11 | Right | Cuneus |
| 1021 | 5.85 | 4.27 | −9 | 51 | −11 | Left | Medial Frontal Gyrus |
| 1085 | 5.47 | 4.1 | 21 | −62 | −50 | Right | Cerebellar Hemisphere |
| 155 | 5.24 | 3.99 | 54 | −60 | 17 | Right | Superior Temporal Gyrus |
| 583 | 4.99 | 3.86 | −6 | 32 | 27 | Left | Cingulate Gyrus |
| 67 | 4.95 | 3.84 | −59 | −24 | 23 | Left | Postcentral Gyrus |
| 58 | 4.73 | 3.73 | 42 | 29 | −12 | Right | Inferior Frontal Gyrus |
| 171 | 4.62 | 3.67 | −26 | −72 | −41 | Left | Cerebellar Hemisphere |
| 75 | 4.61 | 3.66 | 17 | −83 | 41 | Right | Precuneus |
| 49 | 4.59 | 3.65 | 45 | 17 | 3 | Right | Inferior Frontal Gyrus |
| 38 | 4.56 | 3.63 | 48 | −60 | −15 | Right | Fusiform Gyrus |
| 97 | 4.51 | 3.61 | −27 | −15 | −30 | Left | Uncus |
| 27 | 4.36 | 3.52 | 5 | 5 | −12 | Right | Subcallosal Gyrus |
| 30 | 4.16 | 3.41 | −18 | 60 | −9 | Left | Superior Frontal Gyrus |
| 29 | 4.15 | 3.4 | 15 | −99 | −6 | Right | Lingual Gyrus |
| 24 | 4.15 | 3.4 | 26 | −98 | −8 | Right | Inferior Occipital Gyrus |
| 71 | 4.11 | 3.38 | −14 | −69 | 32 | Left | Cuneus |
| 63 | 3.99 | 3.3 | −8 | −89 | 18 | Left | Cuneus |
| 43 | 3.98 | 3.3 | 36 | 24 | 3 | Right | Inferior Frontal Gyrus |
| 56 | 3.97 | 3.3 | 36 | −92 | 12 | Right | Middle Occipital Gyrus |
| 22 | 3.93 | 3.27 | 0 | −87 | 5 | Left | Lingual Gyrus |
| 63 | 3.81 | 3.19 | 27 | −17 | −27 | Right | Parahippocampal Gyrus |
| 22 | 3.8 | 3.19 | 50 | 36 | −3 | Right | Middle Frontal Gyrus |
| 36 | 3.74 | 3.15 | 57 | −23 | 11 | Right | Superior Temporal Gyrus |
| 27 | 3.7 | 3.13 | −23 | 0 | −17 | Left | Parahippocampal Gyrus |
| 25 | 3.69 | 3.12 | 11 | −54 | −38 | Right | Cerebellar Hemisphere |
| 28 | 3.68 | 3.11 | 5 | 48 | 18 | Right | Medial Frontal Gyrus |
| 30 | 3.47 | 2.98 | −9 | −66 | 12 | Left | Posterior Cingulate |
| 64 | 3.28 | 2.85 | −50 | −59 | −24 | Left | Cerebellar Hemisphere |
Brain sites where GMC correlated positively with the performance on the WMS‐R for Verbal Memory, in patients with left MTLE. Results were corrected for multiple comparisons, Height threshold, T = 3.05; cluster ≥ 20 voxels; FDR corrected (P < 0.05).
We did not observe a significant association between the WMS‐R for visual memory and delayed recall and GMC while analyzing the whole brain of patients with left MTLE. Likewise, we did not observe a significant association between the GMC in patients with right‐sided MTLE and the performance on the BNT and on the WMS‐R for general memory, verbal memory, visual memory, and delayed recall.
Association of Neuropsychological Data With Regions of Interest in the Medial Temporal Lobe
When the GMC was extracted from the entorhinal cortex, the perirhinal cortex, and the hippocampus from patients with left and right MTLE, we observed that patients with left MTLE exhibited a significant correlation between (1) the performance on the WMS‐R for general memory and the GMC on the left perirhinal cortex (Pearson Correlation Coefficient = 0.47, P = 0.02), the GMC in the left hippocampus (Pearson = 0.42, P = 0.04), and a trend with the GMC in the entorhinal cortex (Pearson = 0.39, P = 0.05); (2) the performance on the WMS‐R for verbal memory and the GMC on the left perirhinal cortex (Pearson = 0.44, P = 0.03) and the GMC in the left hippocampus (Pearson = 0.42, P = 0.04); and (3) the performance on the WMS‐R for delayed recall and the GMC on the left perirhinal cortex (Pearson = 0.44, P = 0.03), the GMC in the left hippocampus (Pearson = 0.6, P = 0.002), and the GMC on the entorhinal cortex (Pearson = 0.45, P = 0.03) (Fig. 3).
Figure 3.
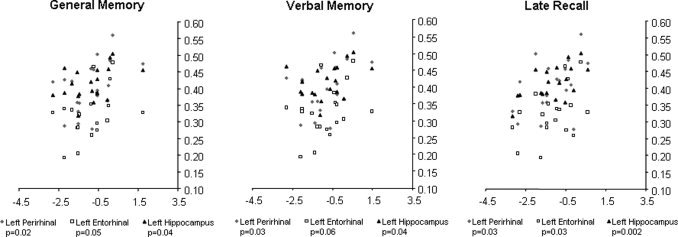
Scatter plots demonstrate the distribution of GMC extracted from ROIs defined in key structures of the left medial temporal lobe memory system (hippocampus, entorhinal, and perirhinal cortices) and the performance on general memory, verbal memory, and delayed recall in patients with left MTLE. The significance of the correlation between GMC and neuropsychological performance is shown below each graph for each ROI.
Association of Neuropsychological Data With Extra‐Hippocampal and Extra‐Temporal Regions of Interest
As a group, we observed that in patients with left MTLE the hippocampal GMC was correlated with GMC within limbic regions (parahippocampal gyri bilaterally and cingulate gyri), the parietal lobes (right inferior parietal lobule and postcentral gyri), the frontal lobes (superior and middle frontal gyrus), the temporal lobe (right superior temporal gyrus), and cerebellar hemispheres. The statistical map of the regions with GMC correlated with hippocampal GMC is shown in Figure 4, from which a listing of the details of the significant clusters is shown in Table III.
Figure 4.
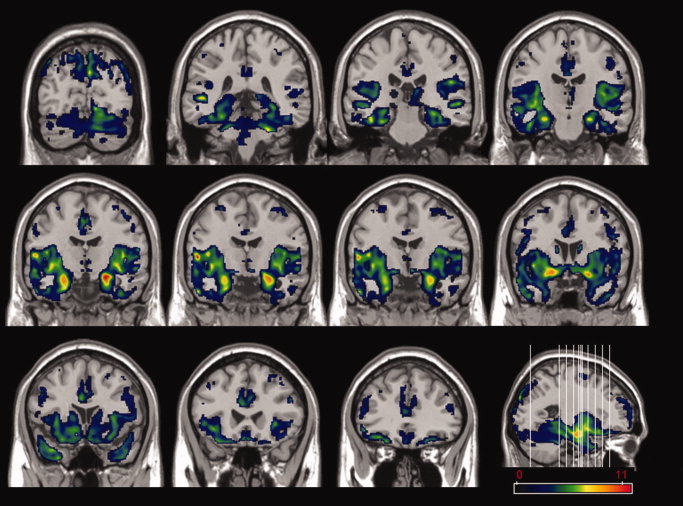
The parametric map of the t statistic depicts the location and the statistical significance of voxels with a significant correlation between densities of gray matter with the density of gray matter extracted from the left hippocampus. The map illustrates a multislice display of coronal images of a T1 template of a normal brain. A color bar indicating the Z score value is shown at the bottom right. A parasagittal slice of the T1 template is also displayed at the right, showing the location of the slices. Left side of images corresponds to the right brain hemisphere, and right side corresponds to the left brain hemisphere.
Table III.
Whole brain VBM results showing the voxel‐wise description of brain locations where there was a positive correlation between the GMC and the GMC extracted from the hippocampus, in patients with left MTLE
| Cluster size | Voxel wise | Spatial coordinates | Anatomical location | ||||
|---|---|---|---|---|---|---|---|
| T | equiv Z | X | Y | Z | Side | Location | |
| 116123 | 11.8 | 6.07 | 54 | −6 | 6 | Right | Precentral Gyrus |
| 11.79 | 6.07 | −24 | −9 | −17 | Left | Parahippocampal Gyrus | |
| 11.06 | 5.91 | 23 | −12 | −21 | Right | Parahippocampal Gyrus | |
| 225 | 6.28 | 4.46 | 42 | −50 | 54 | Right | Inferior Parietal Lobule |
| 4.25 | 3.46 | 33 | −42 | 59 | Right | Postcentral Gyrus | |
| 3.74 | 3.15 | 47 | −39 | 54 | Right | Inferior Parietal Lobule | |
| 209 | 5.51 | 4.12 | 27 | −8 | 60 | Right | Middle Frontal Gyrus |
| 3.91 | 3.26 | 33 | 2 | 57 | Right | Middle Frontal Gyrus | |
| 191 | 5.42 | 4.07 | −29 | 26 | 51 | Left | Superior Frontal Gyrus |
| 3.73 | 3.14 | −39 | 29 | 45 | Left | Middle Frontal Gyrus | |
| 3.47 | 2.98 | −26 | 12 | 53 | Left | Middle Frontal Gyrus | |
| 39 | 4.56 | 3.63 | −33 | −41 | 57 | Left | Postcentral Gyrus |
| 2.83 | 2.53 | −36 | −33 | 56 | Left | Postcentral Gyrus | |
| 57 | 4.13 | 3.39 | 38 | 30 | 41 | Right | Middle Frontal Gyrus |
| 4.09 | 3.37 | 44 | 23 | 42 | Right | Middle Frontal Gyrus | |
| 22 | 4.04 | 3.34 | 57 | −21 | 30 | Right | Postcentral Gyrus |
| 141 | 3.87 | 3.23 | 56 | −8 | 30 | Right | Precentral Gyrus |
| 3.48 | 2.98 | 56 | −11 | 44 | Right | Postcentral Gyrus | |
| 2.81 | 2.51 | 54 | −20 | 41 | Right | Postcentral Gyrus | |
| 82 | 3.61 | 3.06 | −23 | −42 | −48 | Left | Cerebellar Hemisphere |
| 52 | 3.57 | 3.04 | −57 | −17 | 30 | Left | Postcentral Gyrus |
| 55 | 3.43 | 2.95 | −6 | −18 | 68 | Left | Medial Frontal Gyrus |
| 47 | 3.41 | 2.94 | 21 | 47 | 41 | Right | Superior Frontal Gyrus |
| 3.11 | 2.73 | 23 | 39 | 36 | Right | Middle Frontal Gyrus | |
| 3.04 | 2.68 | 30 | 35 | 36 | Right | Middle Frontal Gyrus | |
| 102 | 2.85 | 2.54 | 51 | −56 | 18 | Right | Superior Temporal Gyrus |
| 33 | 2.67 | 2.41 | −51 | −27 | 51 | Left | Postcentral Gyrus |
| 27 | 2.4 | 2.2 | 27 | −72 | −36 | Right | Cerebellar Hemisphere |
Brain sites where the GMC correlated positively with the GMC in the left hippocampus, in patients with left MTLE. Results were corrected for multiple comparisons, Height threshold, T = 1.98; cluster ≥ 20 voxels; FDR corrected (P < 0.05).
As predicted, there was an extensive overlay between the structures that correlated with performance on general and verbal memory in patients with MTLE and the structures that correlated with the GMC within the hippocampus, what is exposed under the form of overlays of the statistical maps in Figure 5.
Figure 5.
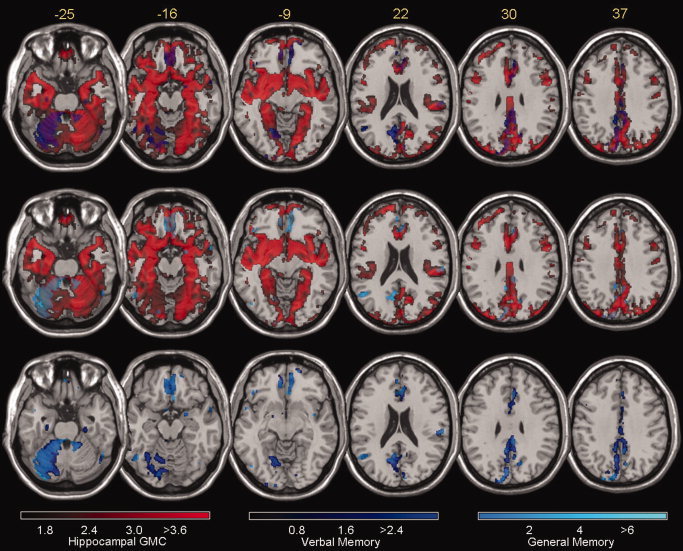
Overlay of parametric map of the t statistic shows the superimposition of areas where there is significant correlation between the GMC in each voxel to the GMC extracted from the hippocampus (in red, as per Fig. 4) to verbal memory (dark blue, as per Fig. 3) and general memory (light blue, as per Fig. 3). The figure shows a multislice display of axial images of a T1 template of a normal brain. Color bars indicating Z scores are shown at the bottom. Stereotaxic z values (rostrocaudal axis) are shown on the upper left of each slice. Top row displays in purple areas where the maps of correlation with hippocampal GMC and verbal memory coincide. Middle row displays in light gray the overlap between correlation with hippocampal GMC and general memory. Bottom row displays in light blue the overlap between general and verbal memory. Left side of images corresponds to the right brain hemisphere.
Regional Association With Memory Performance, Independent From the Hippocampus
Multiple regression analysis performed with the GMC from each brain voxel as a predictor and the performance on general and verbal memory as criterions, failed to demonstrate regions with association with the criterion while the hippocampal GMC was partialled out. However, when GMC from frontal and temporal ROIs were evaluated we observed that a subset of frontal regions was associated with performance on general and verbal memory independently from the hippocampus (Table IV, Fig. 6). The performance on the WMS‐R for general memory was positively correlated to the GMC within left anterior and posterior cingulate areas, right posterior cingulate, left medial and superior orbital areas, and right medial orbital areas. The performance on the WMS‐R for verbal memory was positively correlated to the GMC within left anterior, middle, and posterior cingulate areas, right posterior cingulate, left medial and superior orbital areas, and right medial orbital areas.
Table IV.
Regions of interest (ROI) where there is a positive correlation between the GMC within the ROI and performance on general and verbal memory tests, when the GMC extracted from the hippocampus is covaried out from the analysis
| Regions of interesta | Center of Mass | Volume (mm3) | Range (min/max) | Verbal Memory | General Memory | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | contrast | t | p | contrast | t | p | ||
| Left Anterior Cingulate 10(1876), 11(1917), 24(2870), 25(513), 32(3414) | −4.36 | 34.2 | 12.5 | 12,000 | −16/2 | −4/54 | −10/30 | 0.03 | 3.29 | 0.002 | 0.03 | 2.76 | 0.007 |
| Left Middle Cingulate 5(22), 6(1), 23(5048), 24(3361), 32(770) | −5.88 | −16.1 | 40.2 | 15,528 | −16/2 | −54/30 | 32/54 | 0.03 | 2.42 | 0.014 | — | — | — |
| Left Posterior Cingulate 23(1776), 26(632), 27(82), 29(860), 30(303) | −5.21 | −44.2 | 23.3 | 3,704 | −16/2 | −54/−32 | 6/32 | 0.03 | 3.07 | 0.004 | 0.03 | 2.28 | 0.018 |
| Right Posterior Cingulate 23 (885), 26(688), 27(56), 29(703), 30(144) | 7.18 | −43.1 | 20.5 | 2,680 | 2/16 | −58/−36 | 6/30 | 0.02 | 3 | 0.004 | 0.02 | 2.15 | 0.024 |
| Left Medial Orbital 10,(1604), 11(4095), 25(14) | −5.44 | 52.5 | −8.86 | 5,752 | −14/2 | 22/70 | −16/−2 | 0.03 | 2.99 | 0.004 | 0.03 | 2.87 | 0.006 |
| Right Medial Orbital 10 (2077), 11 (5446), 25(38) | 7.83 | 50.4 | −8.52 | 6,848 | 0/16 | 22/70 | −16/−2 | 0.02 | 2.48 | 0.012 | 0.02 | 2.09 | 0.027 |
| Left Superior Orbital 10 (41), 11(8932), 25(92), 47(32), 48(140) | −16.8 | 45.9 | −14.7 | 7,704 | −34/−6 | 10/70 | −26/−2 | 0.02 | 2.26 | 0.019 | 0.02 | 2.2 | 0.021 |
Values given are Brodmann Areas and values in parentheses are number of voxels.
Figure 6.
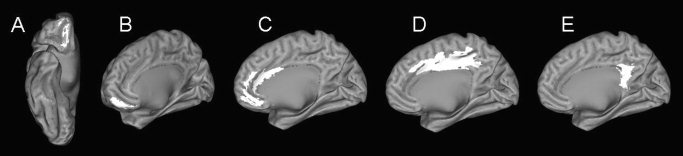
Regions of interest from which GMC was significantly correlated to performance on general and verbal memory (as per Table IV) are overlaid on an inflated cortical map from a normal subject [Van Essen et al., 2001]. (A) superior orbital, (B) medial orbital, (C) anterior cingulate, (D) middle cingulate, and (E) posterior cingulate.
DISCUSSION
We investigated MTLE patients with memory and language impairments but otherwise normal cognitive performance and we observed that patients with left MLTE exhibited a significantly poorer performance on general and verbal related memory tasks. When investigating the neural substrate of the memory impairment in these patients, this study produced three main findings: (1) left MTLE patients' performance on general and verbal memory was correlated with gray matter atrophy within the hippocampus, and also within cortical structures of the medial portion of the temporal lobe and limbic structures; (2) there is a significant correlation of atrophy of extra‐hippocampal and extra‐temporal structures with HA in left MTLE patients, what implies that the association between extra‐HA and memory performance should be taken carefully since it could represent the by‐product of a double association between HA and memory and between HA and extra‐temporal atrophy; (3) when the influence of HA is excluded, there is a significant association between frontal lobe areas and general and verbal memory deficits in patients with left MTLE.
At the present stage, it is still not possible to determine what guides extra‐hippocampal brain damage in patients with MTLE. Recent evidence suggest that, rather than just susceptibility to excitotoxic effects of seizures, atrophied structures within the brain of patients with MTLE follow a pattern of functional and anatomic connectivity to the hippocampus [Bernasconi et al., 2003; Bonilha et al., 2003, 2004b, 2005]. VBM studies addressing gray matter atrophy in these patients consistently demonstrated atrophy of limbic structures in addition to HA [Bonilha et al., 2004b, 2005; Keller et al., 2002a, 2002b]. Likewise, the thalamic atrophy in these patients is more intense in nuclei that are connected to the limbic system [Bonilha et al., 2005]. Altogether, it seems likely that the extra‐hippocampal damage in patients with MTLE results from deafferentation from loss of efferent neurons from the affected hippocampus, and therefore the predilection for distribution within limbic structures. Nonetheless, regardless of the mechanism underlying the neuronal loss in patients with MTLE, a striking feature concerning its physiopathology is the fact that gray matter atrophy is not restricted to the hippocampus but somewhat affects a vast extent of limbic structures, most of them part of the hippocampal memory system.
Atrophy to the hippocampus and extra‐hippocampal structures in patients with MTLE is potentially related to these patients' memory impairments. In fact, atrophy to medial temporal and other (limbic and cortical) regions is probably collective responsible for shaping memory deficits, since both the medial temporal and other cortical regions are known to be important for memory. Amnesia can result from damage to only some of these regions, suggesting that different elements of a large memory system are required and engaged for specific tasks or processes.
More specifically, declarative memory is established by reciprocal connections between the hippocampus and storage sites within the neocortex [Squire and Zola‐Morgan, 1991]. This form of memory (knowing that), in contrast with procedural memory (knowing how) [Squire, 2004] is essentially defined by the ability to bring to mind faces, places, lists, names, and other categorical data [Squire and Zola, 1996]. Because the connection between the hippocampus and the storage sites within the cortex is performed by medial temporal structures such as the entorhinal cortex and the perirhinal cortex [Squire and Zola‐Morgan, 1991], these structures altogether constitute the medial temporal lobe system which is essential for declarative memory [Squire et al., 2004]. The linkage between the neocortex and the medial portion of the temporal lobe, performed by the entorhinal, perirhinal, and parahippocampal cortices [Squire and Zola, 1996] is also the substrate for consolidation, which means that as time passes, stored information becomes less likely to be disturbed [Alvarez and Squire, 1994]. Consolidation occurs when the neural activity within the medial portion of the temporal lobe coactivates different regions of the neocortex that altogether enable the conscious recollection of an event. As an effect of repeated and simultaneous activation by the medial temporal lobe, the connection between neocortical structures strengthens and the storage of memory becomes independent of the medial temporal lobe [Alvarez and Squire, 1994]. This is why memory for remote events is spared after lesions to the medial temporal lobe.
Damage of the hippocampus and medial portion of the temporal lobe results in a more severe amnesia than lesions to the hippocampus alone, [Zola‐Morgan et al., 1994]. Besides the hippocampus, damage to the perirhinal cortex produces more severe memory impairment than damage to any other single component of the medial temporal lobe memory system [Squire and Zola, 1996]. Amnesia can also result from lesions to subcortical white matter pathways, particularly between the temporal and the frontal lobes, without lesion to the medial temporal cortical areas [Gaffan, 2002, 2005; Gaffan et al., 2001]. Lesions to the frontal lobes result in inability to assimilate novel tasks [Parker and Gaffan, 1998]. This is corroborated by functional neuroimaging studies that show widespread activity in the anterior prefrontal [Leung et al., 2004; Wiltgen et al., 2004] and cingulate cortices [Khader et al., 2005] during working memory related tasks. While the hippocampus and the medial portion of the temporal lobe play a role in orchestrating different neocortical areas in order to establish initial memory processes, the long‐term retrieval of consolidated memory is housed within the neocortex [Wiltgen et al., 2004]. More specifically, frontal lobe areas such as the dorsolateral prefrontal cortex, orbital cortex, and the cingulate possibly exert a top‐down control over other neocortical areas rendering possible the retrieval of consolidated information [Faw, 2003; Platel et al., 2003; Wiltgen et al., 2004].
Even though the memory deficit in patients with MTLE has been traditionally linked to HA [Baxendale et al., 1998; Helmstaedter et al., 2003; Hermann et al., 1997; Meencke and Veith, 1991], it is now recognized that these patients exhibit a more profound and diffuse brain lesion, most of the atrophied areas encompassing locations that are directly linked to memory establishment and consolidation (the medial portion of the temporal lobe) and consolidation and long‐term retrieval (the medial and orbital areas of the frontal lobes). As our study suggests, these areas contribute to shape the memory performance regarding general and verbal memory in patients with left MTLE. Moreover, cingulate and orbito‐frontal areas are associated to memory performance even when the hippocampal GMC is covaried out from the analysis. The results from our study indicate that the impaired memory performance in patients with MTLE is a result of the combined damage to memory related areas, specifically the hippocampal memory system and the frontal lobes.
We were not able to dissociate the effect on verbal and general Memory of medial temporal lobe structures such as the entorhinal and perirhinal cortices from the hippocampus. The medial temporal lobe works in concert to establish and consolidate declarative memory by its reciprocal connections to the temporal and extra‐temporal neocortex; however, it is likely that the type and intensity of memory impairment can be affected by the degree of the damage to the entorhinal and perirhinal cortices [Alessio et al., 2006; Brown and Aggleton, 2001]. We demonstrated that the GMC of the parahippocampal area is tightly correlated with the GMC within the hippocampus. As a consequence, it is difficult to dissociate the influence of the hippocampus and the remaining medial temporal lobe on memory performance. There is a close inter‐correlation between the GMC from these two sites, which is potentially stronger than the correlation between these sites and memory performance. Therefore, when the influence of the hippocampal GMC is covaried, the significant association of the medial temporal cortex to memory is washed away. Furthermore, smoothing the data, which improves voxel‐wise analyses by rendering the data more normally distributed, can increase the influence of atrophy to one structure to neighboring structures. VBM and conventional morphometry studies will face difficulties trying to dissociate the independent contribution of each region of the medial temporal lobe to memory impairment in patients with MTLE because of the high correlation of hippocampal and medial temporal lobe GMC. We believe that VBM can contribute to the understanding of the overall contribution of atrophy to extra‐hippocampal and extra‐temporal regions to memory. The study of the contribution of small structures of the medial temporal lobe to memory is probably more sensitive if performed using non‐smoothed data of anatomical resections of the temporal lobe, in patients surgically treated for epilepsy, compared to post‐operative memory performance. Therefore, we suggest that mapping temporal lobe lesions (or surgical resections) to neuropsychological memory impairment profile can be a promising way to address this question. Moreover, the investigation of other process‐constrained memory measures could also be helpful to distinguish the contribution of isolated brain regions to the formation of memory and the relative deficits in patients with MTLE.
In our study we have not addressed long‐term memory retrieval. Whether the orbito‐frontal cortex and cingulate areas are critical for the memory consolidation process in patients with MTLE could be addressed by studies aiming to dissociate short‐term declarative memory or long‐term retrieval performance in MTLE patients, with concurrent analysis of the corresponding brain morphometry.
Similarly, the main findings from this study are drawn from patients with left MTLE. Not observing significant effects from patients with right MTLE could be consequence of lack of statistical power due to the smaller sample size. However, both groups were similarly sized and composed by patients with similar profile, with comparable variability. In fact, three patients with left MTLE had bilateral representation for language, adding variability to the group, and reducing the power to detect significant effects. Nonetheless, the relationship between brain atrophy and memory and language in this group of patients with left MTLE was still detectable. Conversely, the same relationship was not demonstrated for patients with right MTLE, even though both groups were submitted to exactly the same analyses. This is possibly a reflection of the different biological nature of these two entities (left and right MTLE). This can be an indication of a salient frequently observed clinical feature of patients with left MTLE, who usually show more intense neuropsychological impairment than patients with right MTLE. It is possible that the association between regional atrophy and memory performance in patients with right MTLE is considerably smaller than that for patients with left MTE. Furthermore, it is also possible that the current standard neuropsychological evaluation is not sensitive to detect specific memory deficits of right MTLE patients. Thus, investigating other process constrained memory measures can help elucidate different contributions of different brain areas to the memory performance in patients with left and right MTLE.
In this study, we have aimed to demonstrate that there is an intense association between atrophy in extra‐hippocampal areas and general and verbal memory performance in patients with left MTLE. This is in agreement with the notion that memory formation results from an interaction between the hippocampus, the medial temporal lobe, and the extra‐temporal neocortex. As patients with MTLE do exhibit brain atrophy involving these areas, it is possible that the memory impairment observed in patients with MTLE is a consequence of not only hippocampal damage, but also medial temporal and frontal atrophy.
Acknowledgements
The authors thank all patients for agreeing in participating in this study.
REFERENCES
- Aldenkamp AP,Overweg J,Gutter T,Beun AM,Diepman L,Mulder OG ( 1996): Effect of epilepsy, seizures and epileptiform EEG discharges on cognitive function. Acta Neurol Scand 93: 253–259. [DOI] [PubMed] [Google Scholar]
- Alessio A,Damasceno BP,Camargo CH,Kobayashi E,Guerreiro CA,Cendes F ( 2004): Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav 5: 22–27. [DOI] [PubMed] [Google Scholar]
- Alessio A,Bonilha L,Rorden C,Kobayashi E,Min LL,Damasceno BP,Cendes F ( 2006): Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav 8: 593–600. [DOI] [PubMed] [Google Scholar]
- Alvarez P,Squire LR ( 1994): Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci USA 91: 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J,Friston KJ ( 2000): Voxel‐based morphometry–the methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Baxendale SA ( 1995): The hippocampus: functional and structural correlations. Seizure 4: 105–117. [DOI] [PubMed] [Google Scholar]
- Baxendale SA,Van Paesschen W,Thompson PJ,Duncan JS,Harkness WF,Shorvon SD ( 1998): Hippocampal cell loss and gliosis: Relationship to preoperative and postoperative memory function. Neuropsychiatry Neuropsychol Behav Neurol 11: 12–21. [PubMed] [Google Scholar]
- Bennett TL ( 1992): Cognitive effects of epilepsy and anticonvulsant medications In: Bennett TL,editor. Neuropsychology of Epilepsy. New York: Plenum; pp 73–95. [Google Scholar]
- Bernasconi N,Bernasconi A,Caramanos Z,Andermann F,Dubeau F,Arnold DL ( 2000): Morphometric MRI analysis of the parahippocampal region in temporal lobe epilepsy. Ann N Y Acad Sci 911: 495–500. [DOI] [PubMed] [Google Scholar]
- Bernasconi N,Bernasconi A,Caramanos Z,Antel SB,Andermann F,Arnold DL ( 2003): Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126: 462–469. [DOI] [PubMed] [Google Scholar]
- Bonilha L,Kobayashi E,Rorden C,Cendes F,Li LM ( 2003): Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 74: 1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L,Kobayashi E,Cendes F,Min LL ( 2004a): Protocol for volumetric segmentation of medial temporal structures using high‐resolution 3D magnetic resonance imaging. Hum Brain Mapp 22: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L,Rorden C,Castellano G,Pereira F,Rio PA,Cendes F,Li LM ( 2004b): Voxel‐based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 61: 1379–1384. [DOI] [PubMed] [Google Scholar]
- Bonilha L,Rorden C,Castellano G,Cendes F,Li LM ( 2005): Voxel‐based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage 25: 1016–1021. [DOI] [PubMed] [Google Scholar]
- Brett M,Anton JL,Valabregue R,Poline JB ( 2002): Region of interest analysis using an SPM toolbox (Abstract), Presented at the Eigth International Conferance on Functional Mapping of the Human Brain, Sendai, Japan.
- Brown MW,Aggleton JP ( 2001): Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51–61. [DOI] [PubMed] [Google Scholar]
- Commission on classification and terminology of the International League Against Epilepsy ( 1989): Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30: 389–399. [DOI] [PubMed] [Google Scholar]
- Delaney RC,Rosen AJ,Mattson RH,Novelly RA ( 1980): Memory function in focal epilepsy: A comparison of non‐surgical, unilateral temporal lobe and frontal lobe samples. Cortex 16: 103–117. [DOI] [PubMed] [Google Scholar]
- Dikmen S,Matthews CG,Harley JP ( 1975): The effect of early versus late onset of major motor epilepsy upon cognitive‐intellectual performance. Epilepsia 16: 73–81. [DOI] [PubMed] [Google Scholar]
- Dodrill CB ( 1992): Neuropsychology In: Laidlaw J,Richens A, Chadwick DW, editors. A Textbook of Epilepsy. Churchill Livingstone: London: pp 459–473. [Google Scholar]
- Easton A,Ridley RM,Baker HF,Gaffan D ( 2002): Unilateral lesions of the cholinergic basal forebrain and fornix in one hemisphere and inferior temporal cortex in the opposite hemisphere produce severe learning impairments in rhesus monkeys. Cereb Cortex 12: 729–736. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H ( 2000): A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Engel J Jr ( 1999): The timing of surgical intervention for mesial temporal lobe epilepsy: A plan for a randomized clinical trial. Arch Neurol 56: 1338–1341. [DOI] [PubMed] [Google Scholar]
- Faw B ( 2003): Pre‐frontal executive committee for perception, working memory, attention, long‐term memory, motor control, and thinking: A tutorial review. Conscious Cogn 12: 83–139. [DOI] [PubMed] [Google Scholar]
- Fromm‐Auch D,Yeudall LT ( 1983): Normative data for the Halstead‐Reitan neuropsychological tests. J Clin Neuropsychol 5: 221–238. [DOI] [PubMed] [Google Scholar]
- Gaffan D ( 2002): Against memory systems. Philos Trans R Soc Lond B Biol Sci 357: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D ( 2005): Widespread cortical networks underlie memory and attention. Science 309: 2172–2173. [DOI] [PubMed] [Google Scholar]
- Gaffan D,Parker A,Easton A ( 2001): Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia 39: 51–70. [DOI] [PubMed] [Google Scholar]
- Genovese CR,Lazar NA,Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Good CD,Johnsrude IS,Ashburner J,Henson RN,Friston KJ,Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Heaton RK,Chelune GJ,Talley JL,Kay GG,Curtiss G ( 1993): Wisconsin Card Sorting Test Manual—Revised and Expanded. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Helmstaedter C,Kurthen M,Lux S,Reuber M,Elger CE ( 2003): Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol 54: 425–432. [DOI] [PubMed] [Google Scholar]
- Hermann BP,Seidenberg M,Schoenfeld J,Davies K ( 1997): Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 54: 369–376. [DOI] [PubMed] [Google Scholar]
- Jokeit H,Ebner A ( 1999): Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. J Neurol Neurosurg Psychiatry 67: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones‐Gotman M ( 1996): Psychological evaluation for epilepsy surgery In: Shorvon S,Dreifuss F, Fish D, Thomas D, editors. The Treatment of Epilepsy. Oxford: Blackwell Science; pp 621–630. [Google Scholar]
- Kaplan EF,Goodglass H,Weintaub S ( 1983): The Boston Naming Test. Philadelphia: Lea & Febiger. [Google Scholar]
- Keller SS,Mackay CE,Barrick TR,Wieshmann UC,Howard MA,Roberts N ( 2002a): Voxel‐based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 16: 23–31. [DOI] [PubMed] [Google Scholar]
- Keller SS,Wieshmann UC,Mackay CE,Denby CE,Webb J,Roberts N ( 2002b): Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: Effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 73: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KJ ( 1981): Age effects on trail making test performance. Percept Mot Skills 52: 671–675. [DOI] [PubMed] [Google Scholar]
- Khader P,Burke M,Bien S,Ranganath C,Rosler F ( 2005): Content‐specific activation during associative long‐term memory retrieval. Neuroimage 27: 805–816. [DOI] [PubMed] [Google Scholar]
- Kimura D ( 1963): Right temporal‐lobe damage. Perception of unfamiliar stimuli after damage. Arch Neurol 8: 264–271. [DOI] [PubMed] [Google Scholar]
- Kobayashi E,Santos NF,Torres FR,Secolin R,Sardinha LA,Lopez‐Cendes I,Cendes F ( 2003): Magnetic resonance imaging abnormalities in familial temporal lobe epilepsy with auditory auras. Arch Neurol 60: 1546–1551. [DOI] [PubMed] [Google Scholar]
- Ladavas E,Umilta C,Provinciali L ( 1979): Hemisphere‐dependent cognitive performances in epileptic patients. Epilepsia 20: 493–502. [DOI] [PubMed] [Google Scholar]
- Lencz T,McCarthy G,Bronen RA,Scott TM,Inserni JA,Sass KJ,Novelly RA,Kim JH,Spencer DD ( 1992): Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 31: 629–637. [DOI] [PubMed] [Google Scholar]
- Lespinet V,Bresson C,N′Kaoua B,Rougier A,Claverie B ( 2002): Effect of age of onset of temporal lobe epilepsy on the severity and the nature of preoperative memory deficits. Neuropsychologia 40: 1591–1600. [DOI] [PubMed] [Google Scholar]
- Leung HC,Seelig D,Gore JC ( 2004): The effect of memory load on cortical activity in the spatial working memory circuit. Cogn Affect Behav Neurosci 4: 553–563. [DOI] [PubMed] [Google Scholar]
- Meencke HJ,Veith G ( 1991): Hippocampal sclerosis in epilepsy In: Lüders H,editor. Epilepsy Surgery. New York: Raven; pp 705–715. [Google Scholar]
- Meyer V,Yate AJ ( 1955): Intellectual changes following temporal lobectomy for psychomotor epilepsy. J Neurol Neurosurg Psychiatry 18: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B ( 1958): Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis 36: 244–257. [PubMed] [Google Scholar]
- Milner B,Branch C,Rasmussen T ( 1962): Study of short‐term memory after intracarotid injection of sodium amytal. Trans Am Neurol Assoc 87: 224–226. [Google Scholar]
- Mirsky AF,Primac DW,Marson CA ( 1960): A comparison of the psychological test performance of patients with focal and non‐focal epilepsy. Exp Neurol 2: 75–89. [DOI] [PubMed] [Google Scholar]
- Murray EA,Bussey TJ ( 1999): Perceptual‐mnemonic functions of the perirhinal cortex. Trends Cogn Sci 3: 142–151. [DOI] [PubMed] [Google Scholar]
- Novelly RA,Augustine EA,Mattson RH,Glaser GH,Williamson PD,Spencer DD,Spencer SS ( 1984): Selective memory improvement and impairment in temporal lobectomy for epilepsy. Ann Neurol 15: 64–67. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- O'Leary DS,Seidenberg M,Berent S,Boll TJ ( 1981): Effects of age of onset of tonic‐clonic seizures on neuropsychological performance in children. Epilepsia 22: 197–204. [DOI] [PubMed] [Google Scholar]
- O'Leary DS,Lovell MR,Sackellares JC,Berent S,Giordani B,Seidenberg M,Boll TJ ( 1983): Effects of age of onset of partial and generalized seizures on neuropsychological performance in children. J Nerv Ment Dis 171: 624–629. [DOI] [PubMed] [Google Scholar]
- Parker A,Gaffan D ( 1998): Memory after frontal/temporal disconnection in monkeys: conditional and non‐conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia 36: 259–271. [DOI] [PubMed] [Google Scholar]
- Platel H,Baron JC,Desgranges B,Bernard F,Eustache F ( 2003): Semantic and episodic memory of music are subserved by distinct neural networks. Neuroimage 20: 244–256. [DOI] [PubMed] [Google Scholar]
- Rausch R,Babb TL ( 1993): Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol 50: 812–817. [DOI] [PubMed] [Google Scholar]
- Rorden C,Brett M ( 2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Saling MM,Berkovic SF,O'Shea MF,Kalnins RM,Darby DG,Bladin PF ( 1993): Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task‐specific effects. J Clin Exp Neuropsychol 15: 608–618. [DOI] [PubMed] [Google Scholar]
- Smith DB ( 1991): Cognitive effects of antiepileptic drugs In: Smith DB,Treiman DM, Trimble MR, editors. Advances in Neurology. New York: Raven; pp 197–212. [PubMed] [Google Scholar]
- Squire LR ( 2004): Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem 82: 171–177. [DOI] [PubMed] [Google Scholar]
- Squire LR,Zola SM ( 1996): Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA 93: 13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR,Zola‐Morgan S ( 1991): The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Squire LR,Stark CE,Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Strub RL,Black FW ( 1993): Mental Status Examination in Neurology. Philadelphia: FA Davis. [Google Scholar]
- Suzuki WA,Zola‐Morgan S,Squire LR,Amaral DG ( 1993): Lesions of the perirhinal and parahippocampal cortices in the monkey produce long‐lasting memory impairment in the visual and tactual modalities. J Neurosci 13: 2430–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR,Westerveld M,Meador KJ ( 1995): MRI hippocampal volume and neuropsychology in epilepsy surgery. Magn Reson Imaging 13: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Van Essen DC,Drury HA,Dickson J,Harwell J,Hanlon D,Anderson CH ( 2001): An integrated software suite for surface‐based analyses of cerebral cortex. J Am Med Inform Assoc 8: 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D ( 1981): Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corporation. [Google Scholar]
- Wechsler D ( 1987): Wechsler Memory Scale—Revised Manual. San Diego, CA: Psychological Corporation/Harcourt Brace Jovanovich. [Google Scholar]
- Wiltgen BJ,Brown RA,Talton LE,Silva AJ ( 2004): New circuits for old memories: The role of the neocortex in consolidation. Neuron 44: 101–108. [DOI] [PubMed] [Google Scholar]
- Zola‐Morgan S,Squire LR,Ramus SJ ( 1994): Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4: 483–495. [DOI] [PubMed] [Google Scholar]


