Abstract
The dopaminergic system has been implicated in the pathogenesis and treatment of a variety of neuropsychiatric disorders, such as schizophrenia, depression, and addiction. (Dys)function of the dopaminergic system may be studied by combining [15O]H2O PET with a dopaminergic drug challenge. In this pilot study we investigated the suitability of the dopamine reuptake blocker methylphenidate (MP) as a dopaminergic probe. Measurements of regional cerebral blood flow (rCBF) were made at 10 and 30 min after placebo and MP (0.25 mg/kg) injection to seven healthy volunteers. During scanning the behavioral condition of the subjects was standardized using a continuous performance task. Growth hormone levels were assessed and subjective ratings were obtained. MP significantly elevated growth hormone levels. After receiving MP, the subjective experience varied from neutral to highly pleasurable. Ten minutes after MP administration, significant relative increases in rCBF were found in the rostral anterior cingulate (AC), temporal poles, and the supplementary motor area. Significant reductions were seen in the superior temporal gyri, right medial frontal gyrus, and right inferior parietal cortex. At 30 min after MP administration, increases were seen in the AC, temporal pole, and right cerebellum. No changes were observed in the striatum. The activation in the right rostral AC was significantly higher in the subjects with the highest euphoria scores compared to the subjects with minimal MP‐induced changes in euphoria. We suggest that the combined MP challenge with functional imaging, as described in our study, may be a useful tool to study the functional integrity of the dopaminergic system in psychiatric disorders. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: dopamine, emotions, regional cerebral blood flow, methylphenidate, humans
INTRODUCTION
The dopaminergic system has been implicated in the pathogeneses and treatment of a variety of neuropsychiatric disorders, such as schizophrenia, Parkinson's disease, depression, and addiction [Kapur and Mamo, 2003; Leenders, 2002; Naranjo et al., 2001; Volkow et al., 2002]. The involvement of dopamine (DA) in these disorders may be related to its function in the processing of rewarding or salient stimuli [Kapur, 2003; Kunig et al., 2000; Schmidt et al., 2001]. However, the exact role of DA in these processes is not completely known. The (dys)function of the dopaminergic system may be studied by combining functional neuroimaging with a dopaminergic drug challenge, such as the DA reuptake blocker methylphenidate (MP). With this approach, DA‐induced changes in regional neural activity can be assessed in living subjects.
The dopaminergic neurons are located in the substantia nigra (SN) and ventral tegmental area (VTA). The dopaminergic cells in the SN project primarily to the dorsal striatum, whereas the neurons of the VTA project most strongly to the limbic and cortical areas such as the nucleus accumbens, septum, amygdala, hippocampus, prefrontal, and cingulate cortex [Wise, 2004]. Administration of MP has been shown to induce substantial increases in DA levels in the projection areas of the dopaminergic system [During et al., 1992; Huff and Davies, 2002; Kuczenski and Segal, 1997] accompanied by strong subjective and behavioral effects [Wang et al., 1999].
Both positron emission tomography (PET) and functional MRI (fMRI) are increasingly used to measure drug‐induced changes in brain activity. These techniques provide in vivo measures of regional cerebral blood flow (rCBF), glucose utilization, or blood oxygenation, which serve as an index of neural activity [Herscovitch, 2001]. Previous studies that investigated the effects of dopaminergic drugs on neural activity have mainly used [18F]fluorodeoxyglucose PET (FDG) [Ernst et al., 1997; London et al., 1990a; Volkow et al., 1997, 1998; Vollenweider et al., 1998; Wolkin et al., 1987]. These studies showed that psychostimulants such as amphetamine, cocaine, or MP induce changes in cortical, subcortical, limbic, and cerebellar areas. Both increases and decreases have been reported, probably related to the route of administration, the dose administered [Vollenweider et al., 1998], the timing of the challenge, the use of single or repeated doses [Volkow et al., 1998], or whether changes in relative or absolute glucose metabolism were measured. Using FDG, neuronal glucose metabolism is established in a measurement over a period of ∼ 30 min. Therefore, this method it not suitable to detect short‐lasting changes in brain activation. In our study we were interested in the effect of MP at the time of the peak in subjective effects, which occurs ∼10 min after administration [Volkow et al., 1999; Wang et al., 1999]. In addition, we wished to investigate changes in neural activation at the time of the peak in DA concentration, ∼ 30 min after MP administration [Huff and Davies, 2002; Janowsky et al., 1978]. Compared to FDG, the temporal resolution of [15O]H2O PET and especially fMRI is much higher. The meth ods used to assess acute drug effects on the fMRI signal are currently under development and need further validation [Tracey, 2001]. Therefore, we decided to use [15O]H2O PET in our study. In a previous study using [15O]H2O PET, the effect of MP on absolute rCBF was investigated [Wang et al., 1994]. MP was found to induce a global decrease in blood flow, without any significant regional differences. In our study we were interested in the effect of MP on changes in relative regional CBF.
Previous studies have shown that the behavioral state of the subject may influence the MP‐induced effects on DA release or neuronal activity [Volkow et al., 1994, 1998]. Therefore, we tried to standardize the behavioral condition of the subjects during scanning using a continuous performance task [van Leeuwen et al., 1998]. The effect of MP on the behavioral state of the subjects was examined using verbal rating scales. We also assessed the effect of MP on growth hormone levels, as increases in the concentration of this hormone are assumed to reflect dopaminergic activity [Janowsky et al., 1978]. We used an MP dose of 0.25 mg/kg since previous studies in psychiatric patient groups have used comparable doses [Janowsky and Davis, 1976; Joyce et al., 1986]. In this pilot study we investigated the MP‐induced effects on rCBF in a small number of healthy volunteers. If MP induces changes in brain regions that are known to be involved in dopaminergic (dys)function, this probe may be used in studies of dopaminergic functional abnormalities in psychiatric patients.
SUBJECTS AND METHODS
Subjects
Seven healthy, right‐handed volunteers participated in the study (4 male, 3 female, mean age 22 years, range 19–26 years). All subjects gave written informed consent after written and verbal explanation of the study. Suitability to participate in the study was determined by an independent physician (psychiatrist) after a medical examination including an ECG and blood laboratory tests. Exclusion criteria were current or past psychiatric, neurological, cardiovascular, or other disease that could interfere with the study, dependence on any substance other than caffeine, and exposure to psychoactive drugs during the past 6 months, excluding alcohol and caffeinated products. The study was approved by the Medical Ethical Commission of the University Medical Center Groningen (UMCG).
General Design
The subjects were instructed to refrain from alcohol‐ and caffeine‐containing products for 24 h prior to each scan. On the day of the experiment, a total of four PET scans were made, two after placebo and two after MP injection. After placement in the scanner either placebo or MP, 0.25 mg/kg i.v. (intravenous), injected over 1 min, was administered and scans were made at 10 and 30 min after each injection. These time points were chosen on the basis of the expected peak in MP‐induced subjective effects (at 10 min) and DA levels (at 30 min). In order to avoid carry over the effects of MP, placebo injections were always administered first. The subjects were blind to the drug administered. Between the placebo and MP scans the subjects were allowed to leave the scanner for ∼ 30 min. Thereafter, they were repositioned in the scanner using marks on the face. During scanning the behavioral condition of the subjects was standardized using a continuous performance task (CPT). Immediately after each scan the subjective ratings were obtained and blood samples were taken for growth hormone levels. Blood pressure, heart rate, and ECG were monitored during the experiment.
Continuous Performance Task
The CPT in our study was based on a task used by van Leeuwen et al. [1998]. A sequence of digits (0–9) appeared on a computer screen and the subjects' task was to click a mouse button with the right index finger whenever the target sequence, a 3 followed by a 7, appeared. Stimuli were presented for 150 ms in the center of the screen, between two vertical lines that were permanently visible. The interstimulus interval was 1500 ms. The target digits and nontarget digits were pseudorandomly distributed with equal probability (10% for the target sequence “3–7” and nontarget sequence “3 non 7”). The task was initiated 1 min before bolus injection of [15O]H2O and continued throughout scan acquisition. The task was practiced before scanning.
Subjective Ratings
The behavioral effects were evaluated using a verbal analog rating scale. The subjects were asked to respond to the following descriptors, using a whole number between 0 (no effects) and 10 (maximal effects): euphoria, anxiety, happiness, sexual desire, desire for MP, alertness, annoyance, distrust, loss of control, restlessness, depression, and tiredness [Wang et al., 1997]. The subjects were also asked if they experienced the effects of the drug as pleasant or unpleasant.
Growth Hormone
Plasma growth hormone levels were assessed by radioimmunoassay.
PET Scans
For administration of the radioligand and MP or placebo, a venous cannula was placed in one of the veins in the right lower forearm. A second intravenous cannula was placed in the left arm for sampling of hormone levels. The subject was placed in the scanner and the head was fixed using a head restraint. The subjects were scanned in 3D acquisition mode using a Siemens ECAT Exact HR+ camera, giving 63 slices with a center‐to‐center distance of 2.425 mm. For each scan, 500 MBq of [15O]H2O in saline was injected and flushed with saline (total volume 32 mL) at a speed of 8 mL/s. After injection of the radioactive bolus, data were collected for a duration of 2 min in one frame [Poline et al., 1996].
Data and Image Analysis
Attenuation correction was performed by drawing ellipses on the brain images, assuming uniform attenuation. Statistical parametric mapping (SPM99) [Friston et al., 1995] was used for spatial transformation and statistical analysis. The origins were manually set at the anterior commissure. Changes in position due to repositioning of the subject could be adequately solved by the realignment routine of SPM. The images were normalized to the MNI (Montreal Neurological Institute) template and smoothed with a Gaussian kernel of 10 mm full‐width at half‐maximum (FWHM). The data were analyzed using multiple‐subjects conditions and covariates model with covariate 0. Proportional scaling was used to correct for MP‐induced changes in global blood flow. The images were scaled to a mean global activity of 50 mL/100 mL/min. The following contrasts were examined: differences between the two placebo scans (to assess the reproducibility of the scans), between the first placebo scan and first MP scan, and between the second placebo and second MP scan, using paired t‐tests. The initial T‐map threshold was set at 3.69 (P < 0.001), uncorrected for multiple comparisons. Results were considered significant at the 0.05 level, corrected for multiple comparisons. Clusters reaching this statistical threshold are included in the Discussion. The coordinates of maximum relative changes were converted from MNI space to Talairach space. To investigate the prediction that the anterior cingulate (AC) was differentially activated in subjects with either high or low euphoria scores, we conducted a (multigroup) region of interest (ROI) analysis using the MARSBAR toolbox [Brett et al., 2002] and ROI library [Tzourio‐Mazoyer et al., 2002] (Fig. 1). The two groups consisted of the three subjects with the highest and lowest scores for euphoria, respectively.
Figure 1.
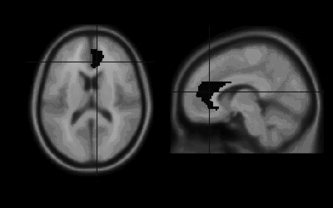
Region of interest (ROI) placement in the right anterior cingulate.
The effect of MP on subjective ratings and hormone levels was assessed with paired t‐tests.
Drugs
Methylphenidate was obtained from Fagron (The Netherlands) and infusions were prepared and provided by the pharmacy of the UMCG.
RESULTS
Growth Hormone
MP significantly elevated growth hormone levels at 10 min after administration (P < 0.01) and 30 min after administration (P < 0.01) (Fig. 2).
Figure 2.
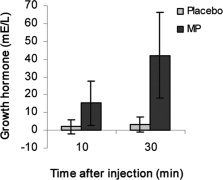
Growth hormone levels at 10 and 30 min after methylphenidate and placebo administration.
Subjective Effects
After receiving MP the subjective experience varied from neutral to highly pleasurable. Due to the variability in effects, the change in the different subjective ratings was not significant, except for happiness, 10 min after MP administration (P < 0.05).
Scan Results
Due to failure of the first MP scan of one of the (female) subjects, we only have data for six subjects at 10 min after MP administration. No differences were observed between the two placebo scans (P > 0.6), indicating reproducibility of the scans. Ten minutes after MP administration a significant relative increase in rCBF was found in the rostral AC, temporal poles, and the supplementary motor area. Significant relative reductions were seen in the superior temporal gyri, right medial frontal gyrus and right inferior parietal cortex. At 30 min after MP administration, increases were seen in the AC, temporal pole, and right cerebellum (Table I, Fig. 3). No changes were observed in the striatum, not even after lowering the initial significance threshold to P < 0.01 or after combining the two MP scans. At 10 min after MP administration the activation in the right rostral AC was significantly higher in the three subjects with the highest euphoria scores compared to the subjects with minimal MP‐induced changes in euphoria (T: 3.67, P < 0.01) (Fig. 4). At 30 min after MP administration, no significant differences were found in this region. No differences were observed in the left AC.
Table I.
Talairach coordinates of maximum relative changes in rCBF at 10 and 30 min after intravenous administration of methylphenidate (0.25 mg/kg)
| Brain region | Cluster | Peak voxel | Talairach coordinates | |||
|---|---|---|---|---|---|---|
| Size | P | T | x | y | z | |
| MP > placebo t = 10 min | ||||||
| Anterior cingulate | 1277 | < 0.001 | 9.09 | −4 | 37 | 2 |
| Right temporal pole* | 710 | < 0.001 | 7.94 | 52 | 0 | −39 |
| Left temporal pole* | 652 | < 0.001 | 8.11 | −57 | 6 | −29 |
| Supplementary motor area | 251 | 0.01 | 6.94 | −4 | 26 | 54 |
| MP > placebo t = 30 min | ||||||
| Anterior cingulate | 220 | 0.019 | 6.35 | 2 | 37 | 6 |
| Left temporal pole* | 280 | 0.006 | 6.18 | −57 | 6 | −29 |
| Right cerebellum | 321 | 0.003 | 6.68 | 40 | −71 | −22 |
| Placebo > MP t = 10 min | ||||||
| Right superiortemporal gyrus | 687 | < 0.001 | 7.14 | 40 | 1 | −19 |
| Left superior temporal gyrus | 202 | 0.028 | 5.51 | −40 | −8 | −8 |
| Right medial frontal gyrus | 338 | 0.002 | 5.33 | 30 | 66 | −10 |
| Right medial frontal gyrus | 183 | 0.041 | 5.03 | 32 | 50 | 20 |
| Right inferior parietal cortex | 209 | 0.024 | 4.90 | 55 | −32 | 24 |
Activation in the temporal poles extends outside the brain and is therefore questionable.
Figure 3.
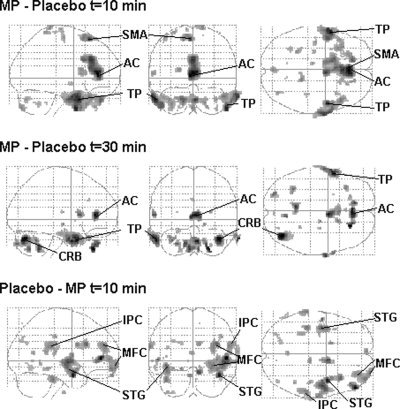
Glass brains (sagittal, coronal, and transverse projections) showing the location of significant relative increases (MP‐placebo) and decreases (Placebo‐MP) in rCBF at 10 and 30 min after MP administration (P < 0.001). AC: anterior cingulate, TP: temporal pole, SMA: supplementary motor area, CRB: cerebellum, STG: superior temporal gyrus, MFC: medial frontal gyrus, IPC: inferior parietal cortex.
Figure 4.
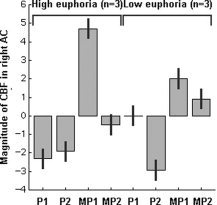
Relative rCBF changes in the right AC in the three subjects with the highest euphoria scores and the three subjects with low euphoria scores. P1: 10 min after placebo, P2: 30 min after placebo, MP1: 10 min after methylphenidate, MP2: 30 min after methylphenidate.
DISCUSSION
In our study we demonstrated a significant, MP‐induced relative increase in the rCBF in the rostral AC in healthy volunteers. At 10 min after MP administration, activation in the right rostral AC differed significantly between the subjects with the highest euphoria scores compared to the subjects with low euphoria scores, suggesting a relation between the magnitude of activation and the level of euphoria. Increases were also seen in the temporal poles, the supplementary motor area (SMA), and cerebellum. MP‐induced decreases were found in the superior temporal gyri, right medial frontal gyrus, and right inferior parietal cortex.
Changes in rCBF are coupled to changes in glucose consumption and assumed to be an index for changes in neuronal activation [Herscovitch, 2001]. Previous studies have shown dense dopaminergic innervation of the anterior cingulate and SMA [Berger et al., 1988; Gaspar et al., 1989], which could possibly explain the changes in activation in these areas in the current study. However, most changes in brain energy consumption are due to changes in (postsynaptic) glutamatergic signaling [Attwell and Laughlin, 2001]. Therefore, the findings in our study should also be understood in terms of anatomical circuits where dopaminergic stimulation induces changes in glutamate release in connected anatomical regions. Although previous studies have shown psychostimulant‐induced decreases in absolute global blood flow [Kahn et al., 1989; Wallace et al., 1996; Wang et al., 1994], it is unlikely that direct vasoactivity of DA is the dominant mechanism underlying the changes in relative rCBF [Schwarz et al., 2004]. In addition, no deviations in the relationship between local glucose utilization and local blood flow were found after administration of the dopamine agonist apomorphine [McCulloch et al., 1982].
The AC is part of the limbic system and includes numerous specialized subdivisions with a large range of cognitive, emotional, motor, nociceptive, and visuospatial functions. Two major subdivisions can be distinguished: the dorsal cognitive division and a rostral–ventral affective subdivision [Bush et al., 2000; Philips et al., 2003; Pizzagalli et al., 2001]. The (euphoria‐related) increase in rCBF in our study was located in the affective subdivision of the AC. This division is primarily involved in assessing the salience of emotional and motivational information and the regulation of emotional responses [Allman et al., 2001; Bush et al., 2000] and may be activated during efforts to cope with or control feelings [Posner and Rothbart, 1998]. This region has extensive connections with other limbic areas such as the striatum and amygdala [Devinsky et al., 1995; Kalivas and McFarland, 2003]. As stated above, the [15O]H2O PET signal can be understood in terms of changes in glutamate release. Therefore, the activation in the AC may be interpreted as a result of a change in the glutamatergic input from the amygdala [Jackson and Moghaddam, 2001].
Most studies in animals have reported psychostimulant‐induced activations in the AC [Cash et al., 2003; Chen et al., 1997; Marota et al., 2000; Pontieri et al., 1990; Stein et al., 1993]. Results from human studies show decreases or no effects of psychostimulants on absolute cerebral metabolism or blood flow [Ernst et al., 1997; Kahn et al., 1989; Wallace et al., 1996; Wang et al., 1994; Wolkin et al., 1987], except after relatively high doses [Vollenweider et al., 1998]. However, relative glucose metabolism or blood flow was found to be increased in the AC, comparable to the results from our study [Breiter et al., 1997; Vollm et al., 2004].
The AC activation in our study is probably not related to the experience of euphoria per se. In accordance with the role of the AC, we suggest that the activation is related to an increased need for emotional control during MP administration. This would agree with results from previous studies that reported increased activation of the (right) AC after administration of various psychoactive drugs [Gouzoulis et al., 1999; Vollm et al., 2004]. In these studies the subjects were also asked to perform a task that may have increased the need to control the drug‐induced emotional experiences. In addition, studies that specifically asked the subjects to attend to or control their emotions have found increased activation of the (right rostral) AC [Beauregard et al., 2001; Childress et al., 2004; Lane et al., 1997; Ochsner et al., 2004]. In a metaanalysis by Berthoz and Blair [2002] it was also concluded that the AC is a key structure in emotion regulation. The finding that the (right) rostral AC is activated during unpleasant emotions is also in agreement with the suggested role of the AC in emotional control [Boshuisen et al., 2002; Damasio et al., 2000; Zubieta et al., 2003]. In these studies the subjects experienced strong negative emotions. The experiments were performed in the PET scanner, thereby necessitating control of the experienced emotions.
Previous studies have shown that differences in AC activation may be related to baseline differences between the subjects. Cue‐induced activation in the rostral AC correlated to baseline striatal D2 occupancy in alcoholic subjects [Heinz et al., 2004]. In a study by Volkow et al. [1997], baseline striatal D2 correlated with MP‐induced changes in metabolism in the frontal cortex. Differences in AC activation may also be related to personality [Canli et al., 2001, Johnson et al., 1999; Sugiura et al., 2000]. Unfortunately, in our study we did not measure such parameters.
Results from previous studies indicated AC abnormalities in psychiatric patients. Several studies have found differences in (right rostral) AC function between depressed patients and healthy control subjects [Drevets, 1999; Ebert and Ebmeier, 1996; Kumari et al., 2003]. Moreover, the baseline activity in this region has been found to predict the response to antidepressant treatments [Mayberg et al., 1997; Pizzagalli et al., 2001; Wu et al., 1999]. The (right rostral) AC may also be involved in addiction [Bolla et al., 2004; Franklin et al., 2002; Goldstein and Volkow, 2002]. The AC abnormalities in these disorders may be related to the disturbances in emotion regulation and motivated behavior, probably due to a dysfunction of the dopaminergic system [Drevets, 1999; Ebert and Ebmeier, 1996; Volkow et al., 2002; Wu et al., 1999].
In our study, no changes were found in striatal activation. The striatum, and especially the ventral striatum, has been implicated in the processing of rewarding or reinforcing effects of dopaminergic drug stimuli [Ikemoto and Panksepp, 1999; Spanagel and Weiss, 1999; Ungless, 2004; Wise, 2004]. Therefore, we could have expected rCBF changes in this region. However, as stated previously, changes in rCBF are primarily caused by changes in (postsynaptic) glutamatergic transmission and changes in activation may therefore occur at sites that are downstream of the site of drug action [Eidelberg et al., 1997; Kadekaro et al., 1985; McCulloch, 1982; Schwartz et al., 1979]. A review of the literature shows that psychostimulant administration does not consistently affect striatal activation. Previous human and animal studies have reported activations [Chen at al., 1997; Dixon et al., 2005; Ernst et al., 1997; Marota et al., 2000; Schwarz et al., 2004], deactivations [Cash et al., 2003; Volkow et al., 1997], or no changes [Howell et al., 2002; Mehta et al., 2000; Vollm et al., 2004] in relative rCBF or brain metabolism. Drug administration also resulted in variable effects on absolute rCBF or metabolism. In most studies an increase in absolute brain activation was seen [Cash et al., 2003; Howell et al., 2002; Pontieri et al., 1990; Porrino and Lucignani, 1987; Vollenweider et al., 1998]; however, decreases have also been reported [London et al., 1990a; Volkow et al., 1998; Wang et al., 1994; Wolkin et al., 1987] and in other studies no significant changes were found [Cash et al., 2003; Ernst et al., 1997]. These differences may be due to differences in the pharmacological challenge [Pontieri et al., 1990], dose [Porrino and Lucignani, 1987; Vollenweider et al., 1998], use of anesthesia [Cash et al., 2003], timing of the challenge [Porrino, 1993; Stein and Fuller, 1993], route of administration [Porrino, 1993], the use of single or repeated doses [Volkow et al., 1998], immobilization [London et al., 1990b], and the state of cortical excitatory modulation [Tschanz et al., 1994]. It has been suggested that the effects of DA on brain activity may depend on the state of the dopaminergic system [Volkow et al., 1997, 1998]. The striatum receives glutamatergic projections from the cerebral cortex [Fonnum et al., 1981]. It has been shown that DA may both enhance or inhibit excitatory glutamate release in the striatum [Cepeda et al., 1993]. The DA‐induced glutamate response in the striatum is found to be dependent on the basal neuronal activity [Kiyatkin and Rebec, 1996]. It may be concluded that the nature of interaction between the cerebral cortex and striatum is dependent on the experimental conditions [Bamford et al., 2004, Morari et al., 1998].
A strong activation was seen in both temporal poles at 10 and 30 min after MP administration. Since this activation extends outside the brain, possible movement artifacts cannot be excluded. Nevertheless, the activation is in accordance with a role of the temporal poles in emotional processes [Dolan et al., 2000].
We have shown an MP‐induced activation of the (pre)supplementary motor area (pre‐SMA) (rostral part of BA 6). The pre‐SMA receives projections from the cingulate cortex [Luppino et al., 1993] and may play a role in motor control or response inhibition [Toma et al., 1999]. Activations in this region have been shown during response conflict or uncertainty [Hazeltine et al., 2000; Ullsperger and Cramon, 2003]. Possibly the activation in our study was due to the fact that subjects were required to lie still in the PET while being distracted by MP‐induced subjective experiences.
The cerebellar activation in our study is also difficult to interpret. Although the cerebellum is low in dopamine, there are dopaminergic projections to the cerebellum. Within the cerebellum, different glutamatergic projections have been described [Geurts et al., 2003]. These projections may be responsible for the observed changes in cerebellar activity. Evidence exists that the cerebellum is not only involved in motor functions, but also in cognitive and emotional processes [Rapoport et al., 2000; Schmahmann, 2004]. Increases in (right) cerebellar rCBF have also been reported in previous studies using psychoactive drugs [Gamma et al., 2000; Gouzoulis et al., 1999; Mehta et al., 2000; Volkow et al., 1997]. It has been hypothesized that dopamine affects reward‐dependent learning in the cerebellum [Schweighofer et al., 2004].
In our study (right‐sided) relative deactivations were found in the superior temporal gyri, medial frontal gyrus, and inferior parietal cortex. A right‐sided fronto‐parietal‐temporal network is most often associated with (sustained) attention, as assessed during performance of a CPT [Berman and Weinberger, 1990; Buchsbaum et al., 1990; Corbetta and Shulman, 2002; Hager et al., 1998; Pardo et al., 1991]. The deactivations in our study may related to a decrease in attention due to task habituation or allocation of attention to the MP‐induced emotional effects [Coull et al., 1998].
Limitations of the study include the fact that MP not only blocks DA uptake, but has also been shown to increase extracellular noradrenaline concentration [Kuczenski and Segal, 1997]. A contribution of this neurotransmitter to the observed effects can therefore not be excluded. Definitive conclusions on the involvement of a neurotransmitter system can only be drawn after administration of specific antagonists [McCulloch et al., 1980; Trugman and James, 1993]. However, previous studies have found strong correlations between DA increase and euphoria, the main behavioral effect in our study [Udo de Haes et al., 2005; Volkow et al., 1999]. Therefore, it can be assumed that the euphoria‐related activation in the AC in our study is indeed due to increased DA levels. Performance on the CPT was not quantified in our study and drug–task interactions therefore cannot be excluded. However, we deliberately choose a relatively simple task in order to avoid such effects and previous studies in healthy volunteers did not show significant effects of psychostimulants or changes in task load on CPT performance [Cattapan‐Ludewig et al., 2005; Ernst et al., 1997]. In our study we did not assess changes in absolute blood flow; the reported changes are relative to mean global blood flow. Since MP has been shown to reduce global blood flow, the regional increases in our study may in fact be due to a diminished reduction in blood flow in these areas [Black et al., 2002; Wang et al., 1994]. However, our study was set up to study local effects of MP and our data clearly show specific regional differences in activation. MP scans always followed the placebo scans but effects of order seem unlikely due to the fact that no differences in activation were observed between the two placebo scans. Finally, the small sample size is a limitation of our study and future studies should be performed to confirm our results.
In conclusion, the most important finding in our study was the euphoria‐related relative increase in right rostral AC activation after administration of MP. We suggest that the activation is related to the role of this region in emotional control. This finding may be relevant with respect to previous studies that have shown the involvement of the (right) rostral AC in the pathogenesis of psychiatric disorders such as depression [Drevets, 1999; Ebert and Ebmeier, 1996; Kumari et al., 2003; Mayberg et al., 1997; Pizzagalli et al., 2001; Wu et al., 1999] or addiction [Bolla et al., 2004; Franklin et al., 2002; Goldstein and Volkow, 2002]. As stated before, previous studies have also indicated a role of the dopaminergic system in the AC‐related disturbances in emotion regulation and motivated behavior [Drevets, 1999; Ebert and Ebmeier, 1996; Volkow et al., 2002; Wu et al., 1999]. Therefore, we suggest that the combined MP challenge with functional imaging, as described in our study, may be a useful tool to study the functional integrity of the dopaminergic system in psychiatric disorders.
Acknowledgements
We thank Sascha Russo for contributions to the medical screening of the volunteers, Thomas Gladwin for programming the continuous performance task, and Simone Reinders for SPM advice.
REFERENCES
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P ( 2001): The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci 935: 107–117. [PubMed] [Google Scholar]
- Attwell D, Laughlin SB ( 2001): An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D ( 2004): Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42: 653–663. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P ( 2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C ( 1988): Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol 273: 99–119. [DOI] [PubMed] [Google Scholar]
- Berman KF, Weinberger DR ( 1990): Lateralisation of cortical function during cognitive tasks: regional cerebral blood flow studies of normal individuals and patients with schizophrenia. J Neurol Neurosurg Psychiatry 53: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz S, Blair RJR ( 2002): Emotions: from neuropsychology to functional imaging. Int J Psychol 37: 193–203. [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS ( 2002): A possible substrate for dopamine‐related changes in mood and behavior: prefrontal and limbic effects of a D3‐preferring dopamine agonist. Proc Natl Acad Sci U S A 99: 17113–17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E ( 2004): Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci 16: 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen ML, Ter‐Horst GJ, Paans AM, Reinders AA, den‐Boer JA ( 2002): rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry 52: 126–135. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB ( 2002): Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Neuroimage 16: 497. [Google Scholar]
- Buchsbaum MS, Nuechterlein KH, Haier RJ, Wu J, Sicotte N, Hazlett E, Asarnow R, Potkin S, Guich S ( 1990): Glucose metabolic rate in normals and schizophrenics during the Continuous Performance Test assessed by positron emission tomography. Br J Psychiatry 156: 216–227. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD ( 2001): An fMRI study of personality influences on brain reactivity to emotional stimuli. Behav Neurosci 115: 33–42. [DOI] [PubMed] [Google Scholar]
- Cash D, Read SJ, Lythgoe D, Williams SCR, Roberts TJ, Ireland MD, Smart SC, Hunter AJ ( 2003): Autoradiographic and functional MRI assessment of rat brain response to amphetamine under halothane and alpha‐chloralose anaesthesia. J Cereb Blood Flow Metab 23: 31. [Google Scholar]
- Cattapan‐Ludewig K, Hilti CC, Ludewig S, Vollenweider FX, Feldon J ( 2005): Rapid visual information processing in schizophrenic patients: the impact of cognitive load and duration of stimulus presentation. A pilot study. Neuropsychobiology 52: 130–134. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS ( 1993): Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A 90: 9576–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG ( 1997): Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 38: 389–398. [DOI] [PubMed] [Google Scholar]
- Childress AR, Wang Z, Sciortino N, Detre J, Germain A, Hole A, MacDougall M, Vietri J, O'Brien CP ( 2004): Brain substrates of “attempted” vs. “successful” inhibition of cue‐induced craving for cocaine vs. sex. Soc Neurosci Abstr 464.6. [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RS, Frith CD ( 1998): Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia 36: 1325–1334. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD ( 2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA ( 1995): Contributions of anterior cingulate cortex to behaviour. Brain 118(Pt 1): 279–306. [DOI] [PubMed] [Google Scholar]
- Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AM ( 2005): Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology 48: 236–245. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P ( 2000): Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage 11: 203–209. [DOI] [PubMed] [Google Scholar]
- Drevets WC ( 1999): Prefrontal cortical‐amygdalar metabolism in major depression. Ann N Y Acad Sci 877: 614–637. [DOI] [PubMed] [Google Scholar]
- During MJ, Bean AJ, Roth RH ( 1992): Effects of CNS stimulants on the in vivo release of the colocalized transmitters, dopamine and neurotensin, from rat prefrontal cortex. Neurosci Lett 140: 129–133. [DOI] [PubMed] [Google Scholar]
- Ebert D, Ebmeier KP ( 1996): The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry 39: 1044–1050. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, Spetsieris P, Alterman R, Kelly PJ, Dogali M, Fazzini E, Beric A ( 1997): Metabolic correlates of pallidal neuronal activity in Parkinson's disease. Brain 120: 1315– 1324. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik J, Schmidt M, Jons PH, Liebenauer LL, Hardy KK, Cohen RM ( 1997): Intravenous dextroamphetamine and brain glucose metabolism. Neuropsychopharmacology 17: 391–401. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Storm‐Mathisen J, Divac I ( 1981): Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience 6: 863–873. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR ( 2002): Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51: 134–142. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ ( 1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX ( 2000): 3,4‐Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H(2](15)O)‐PET in healthy humans. Neuropsychopharmacology 23: 388–395. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP ( 1989): Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine‐beta‐hydroxylase. J Comp Neurol 279: 249–271. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De‐Schutter E, Dieudonne S ( 2003): Unraveling the cerebellar cortex: cytology and cellular physiology of large‐sized interneurons in the granular layer. Cerebellum 2: 290–299. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND ( 2002): Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis‐Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Bull U, Sass H ( 1999): Neurometabolic effects of psilocybin, 3,4‐methylenedioxyethylamphetamine (MDE) and d‐methamphetamine in healthy volunteers. A double‐blind, placebo‐controlled PET study with [18F]FDG. Neuropsychopharmacology 20: 565–581. [DOI] [PubMed] [Google Scholar]
- Hager F, Volz HP, Gaser C, Mentzel HJ, Kaiser WA, Sauer H ( 1998): Challenging the anterior attentional system with a continuous performance task: a functional magnetic resonance imaging approach. Eur Arch Psychiatry Clin Neurosci 248: 161–170. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD ( 2000): Neural activation during response competition. J Cogn Neurosci 12( Suppl 2): 118–129. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser‐Sinopoli SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P ( 2004): Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161: 1783–1789. [DOI] [PubMed] [Google Scholar]
- Herscovitch P ( 2001): Can [15O] water be used to evaluate drugs? J Clin Pharmacol Suppl: 11S–20S. [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP ( 2002): Cocaine‐induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 159: 154–160. [DOI] [PubMed] [Google Scholar]
- Huff JK, Davies MI ( 2002): Microdialysis monitoring of methylphenidate in blood and brain correlated with changes in dopamine and rat activity. J Pharm Biomed Anal 29: 767–777. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J ( 1999): The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward‐seeking. Brain Res Brain Res Rev 31: 6–41. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B ( 2001): Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci 21: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, Davis JM ( 1976): Methylphenidate, dextroamphetamine, and levamfetamine. Effects on schizophrenic symptoms. Arch Gen Psychiatry 33: 304–308. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Leichner P, Parker D, Judd L, Huey L, Clopton P ( 1978): The effect of methylphenidate on serum growth hormone: influence of antipsychotic drugs and diagnosis. Arch Gen Psychiatry 35: 1384–1389. [DOI] [PubMed] [Google Scholar]
- Johnson DL, Wiebe JS, Gold SM, Andreasen NC, Hichwa RD, Watkins GL, Boles‐Ponto LL ( 1999): Cerebral blood flow and personality: a positron emission tomography study. Am J Psychiatry 156: 252–257. [DOI] [PubMed] [Google Scholar]
- Joyce PR, Donald RA, Nicholls MG, Livesey JH, Abbott RM ( 1986): Endocrine and behavioural responses to methylphenidate in depression. Psychol Med 16: 531–540. [DOI] [PubMed] [Google Scholar]
- Kadekaro M, Crane AM, Sokoloff L ( 1985): Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A 82: 6010–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DA, Prohovnik I, Lucas LR, Sackeim HA ( 1989): Dissociated effects of amphetamine on arousal and cortical blood flow in humans. Biol Psychiatry 25: 755–767. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K ( 2003): Brain circuitry and the reinstatement of cocaine‐seeking behavior. Psychopharmacology (Berl) 168: 44–56. [DOI] [PubMed] [Google Scholar]
- Kapur S ( 2003): Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160: 13–23. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D ( 2003): Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV ( 1996): Dopaminergic modulation of glutamate‐induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol 75: 142–153. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS ( 1997): Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 68: 2032–2037. [DOI] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, Brammer MJ, Poon L, Simmons A, Williams SC, Checkley SA, Sharma T ( 2003): Neural abnormalities during cognitive generation of affect in treatment‐resistant depression. Biol Psychiatry 54: 777–791. [DOI] [PubMed] [Google Scholar]
- Kunig G, Leenders KL, Martin‐Solch C, Missimer J, Magyar S, Schultz W ( 2000): Reduced reward processing in the brains of Parkinsonian patients. Neuroreport 11: 3681–3687. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ ( 1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Leenders KL ( 2002): Disease process and drug treatments in Parkinson's disease. Eur Neuropsychopharmacol 12: 575–580. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN Jr ( 1990a): Cocaine‐induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]‐fluorodeoxyglucose. Arch Gen Psychiatry 47: 567–574. [DOI] [PubMed] [Google Scholar]
- London ED, Wilkerson G, Ori C, Kimes AS ( 1990b): Central action of psychomotor stimulants on glucose utilization in extrapyramidal motor areas of the rat brain. Brain Res 512: 155–158. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G ( 1993): Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. J Comp Neurol 338: 114–140. [DOI] [PubMed] [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE ( 2000): Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in rat. Neuroimage 11: 13–23. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT ( 1997): Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8: 1057–1061. [DOI] [PubMed] [Google Scholar]
- McCulloch J ( 1982): Mapping functional alterations in the CNS with [14C]deoxyglucose In: Iverson LL, Iverson SD, Snyder SH, editors. Handbook of psychopharmacology: new techniques in psychopharmacology 15. New York: Plenum; p 321–410. [Google Scholar]
- McCulloch J, Savaki HE, Sokoloff L ( 1980): Influence of dopaminergic systems on the lateral habenular nucleus of the rat. Brain Res 194: 117–124. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Kelly PA, Ford I ( 1982): Effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow (with an appendix on its statistical analysis). J Cereb Blood Flow Metab 2: 487–499. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW ( 2000): Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20: RC65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari M, Marti M, Sbrenna S, Fuxe K, Bianchi C, Beani L ( 1998): Reciprocal dopamine‐glutamate modulation of release in the basal ganglia. Neurochem Int 33: 383–397. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Tremblay LK, Busto UE ( 2001): The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 25: 781–823. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004): For better or for worse: neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME ( 1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R ( 2003): Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual‐Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ ( 2001): Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 158: 405–415. [DOI] [PubMed] [Google Scholar]
- Poline JB, Vandenberghe R, Holmes AP, Friston KJ, Frackowiak RS ( 1996): Reproducibility of PET activation studies: lessons from a multi‐center European experiment. EU concerted action on functional imaging. Neuroimage 4: 34–54. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Crane AM, Seiden LS, Kleven MS, Porrino LJ ( 1990): Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology (Berl) 102: 175–182. [DOI] [PubMed] [Google Scholar]
- Porrino LJ ( 1993): Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology (Berl) 112: 343–351. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lucignani G ( 1987): Different patterns of local brain energy metabolism associated with high and low doses of methylphenidate. Relevance to its action in hyperactive children. Biol Psychiatry 22: 126–138. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK ( 1998): Attention, self‐regulation and consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, van‐Reekum R, Mayberg H ( 2000): The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 12: 193–198. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD ( 2004): Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16: 367–378. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte‐Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A ( 2001): Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry 34: 66–72. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Smith CB, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink DJ, Gainer H ( 1979): Metabolic mapping of functional activity in the hypothalamo‐neurohypophysial system of the rat. Science 205: 723–725. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Zocchi A, Reese T, Gozzi A, Garzotti M, Varnier G, Curcuruto O, Sartori I, Girlanda E, Biscaro B, Crestan V, Bertani S, Heidbreder C, Bifone A ( 2004): Concurrent pharmacological MRI and in situ microdialysis of cocaine reveal a complex relationship between the central hemodynamic response and local dopamine concentration. Neuroimage 23: 296–304. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Doya K, Kuroda S ( 2004): Cerebellar aminergic neuromodulation: towards a functional understanding. Brain Res Brain Res Rev 44: 103–116. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F ( 1999): The dopamine hypothesis of reward: past and current status. Trends Neurosci 22: 521–527. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA ( 1993): Cocaine's time action profile on regional cerebral blood flow in the rat. Brain Res 626: 117–126. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, Sato K, Ono S, Schormann T, Zilles K, Fukuda H ( 2000): Correlation between human personality and neural activity in cerebral cortex. Neuroimage 11(5 Pt 1): 541–546. [DOI] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H ( 1999): Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event‐related fMRI study. J Neurosci 19: 3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I ( 2001): Prospects for human pharmacological functional magnetic resonance imaging (phMRI). J Clin Pharmacol Suppl: 21S–28S. [PubMed] [Google Scholar]
- Trugman JM, James CL ( 1993): D1 dopamine agonist and antagonist effects on regional cerebral glucose utilization in rats with intact dopaminergic innervation. Brain Res 607: 270–274. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Griffith KE, Haracz JL, Rebec GV ( 1994): Cortical lesions attenuate the opposing effects of amphetamine and haloperidol on neostriatal neurons in freely moving rats. Eur J Pharmacol 257: 161–167. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Kortekaas R, van Waarde A, Maguire RP, Pruim J, den Boer JA ( 2005): Assessment of methylphenidate‐induced changes in binding of continuously infused [(11]C)‐raclopride in healthy human subjects: correlation with subjective effects. Psychopharmacology 183: 322–330. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von‐Cramon DY ( 2003): Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci 23: 4308–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA ( 2004): Dopamine: the salient issue. Trends Neurosci 27: 702–706. [DOI] [PubMed] [Google Scholar]
- Van‐Leeuwen TH, Steinhausen HC, Overtoom CC, Pascual‐Marqui RD, van't‐Klooster B, Rothenberger A, Sergeant JA, Brandeis D ( 1998): The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav Brain Res 94: 97–110. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, Burr G, Cooper T, Wolf A ( 1994): Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16: 255–262. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N ( 1997): Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry 154: 50–55. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Gatley J, Ding YS, Wong C, Pappas N ( 1998): Differences in regional brain metabolic responses between single and repeated doses of methylphenidate. Psychiatry Res 83: 29–36. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR ( 1999): Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291: 409–415. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ ( 2002): Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem 78: 610–624. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Maguire RP, Leenders KL, Mathys K, Angst J ( 1998): Effects of high amphetamine dose on mood and cerebral glucose metabolism in normal volunteers using positron emission tomography (PET). Psychiatry Res 83: 149–162. [DOI] [PubMed] [Google Scholar]
- Vollm BA, de‐Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM ( 2004): Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology 29: 1715–1722. [DOI] [PubMed] [Google Scholar]
- Wallace EA, Wisniewski G, Zubal G, vanDyck CH, Pfau SE, Smith EO, Rosen MI, Sullivan MC, Woods SW, Kosten TR ( 1996): Acute cocaine effects on absolute cerebral blood flow. Psychopharmacology (Berl) 128: 17–20. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Ferrieri R, Schlyer DJ, Alexoff D, Pappas N, Lieberman J, King P, Warner D, Wong C, Hitzemann RJ, Wolf AP ( 1994): Methylphenidate decreases regional cerebral blood flow in normal human subjects. Life Sci 54: PL143–146. [DOI] [PubMed] [Google Scholar]
- Wang G‐J, Volkow ND, Hitzemann RJ, Wong C, Angrist B, Burr G, Pascani K, Pappas N, Lu A, Cooper T, Lieberman JA ( 1997): Behavioral and cardiovascular effects of intravenous methylphenidate in normal subjects and cocaine abusers. Eur Addict Res 3: 9–54. [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Pappas NR, Wong CT, Hitzemann RJ, Netusil N ( 1999): Reproducibility of repeated measures of endogenous dopamine competition with [11C]raclopride in the human brain in response to methylphenidate. J Nucl Med 40: 1285–1291. [PubMed] [Google Scholar]
- Wise RA ( 2004): Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494. [DOI] [PubMed] [Google Scholar]
- Wolkin A, Angrist B, Wolf A, Brodie J, Wolkin B, Jaeger J, Cancro R, Rotrosen J ( 1987): Effects of amphetamine on local cerebral metabolism in normal and schizophrenic subjects as determined by positron emission tomography. Psychopharmacology (Berl) 92: 241–246. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE Jr, Fallon JH, Keator D ( 1999): Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 156: 1149–1158. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA ( 2003): Regulation of human affective responses by anterior cingulate and limbic mu‐opioid neurotransmission. Arch Gen Psychiatry 60: 1145–1153. [DOI] [PubMed] [Google Scholar]


