Abstract
Native English speakers with no knowledge of Chinese were trained on 60 Chinese characters according to one of three mapping conditions: orthography to pronunciation and meaning (P + M), orthography to pronunciation (P), and orthography to meaning (M). Following the training, fMRI scans taken during passive viewing of Chinese characters showed activation in brain regions that partially overlap the regions found in studies of skilled Chinese readers, but typically not found in alphabetic readers. Areas include bilateral middle frontal (BA 9), right occipital (BA 18/19), and fusiform (BA 37) regions. The activation pattern of Chinese characters was similar across the three groups. However, peak location was different in the left middle frontal region between groups. Direct contrasts between the groups also revealed stronger activation of left middle frontal in the P + M group. The results suggest that learners acquired skill in reading Chinese characters using a brain network similar to that used by Chinese native speakers. The results are consistent with the system accommodation hypothesis: The brain's reading network accommodates to features of an acquired writing system. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: Chinese, reading, learning, fMRI
INTRODUCTION
In word reading, graphic forms (visual symbols) are mapped to pronunciations and meanings that are part of the reader's spoken language. These mappings from graphic form to language are different across writing systems. In an alphabetic system, the mapping is from a graphic unit (a letter) to a phoneme. In Chinese, a logographic or morphosyllabic system, the mapping is from a graphic unit (a character) to a syllable/morpheme. Combined with differences in the visual appearance of the writing, these differences in mapping can lead to differences in the details of reading processes, despite the existence of universals in reading across writing systems [Perfetti et al., 2005]. They can also lead to differences in how reading is implemented in the brain.
Written Chinese maps square‐shaped characters onto syllables, which are often morphemes. Although about 85% of characters consist of radicals that can provide moderately reliable clues to pronunciation or meaning, there is no principle of compositionality analogous to that of alphabetically written words. The character does not contain graphic elements that correspond to subsyllabic units in the way that an alphabetic word contains letters that correspond to phonemes. Thus, the ultimate determiner of the character's meaning and pronunciation is the whole character itself, as uniquely specified by the set of radicals and their spatial configuration within the character. In addition to these differences in the principles of mapping, the two‐dimensional square layout of the character provides another factor confronted by the learner, contrasting with the linear layout of most alphabetic writing. Both types of differences could matter for how the brain implements reading.
The nature of any brain differences, however, is not obvious if they only follow the descriptions of alphabetic and Chinese writing systems. Despite the profound differences in the two writing systems at the principle level, behavioral research shows universal processes of phonology in reading. The phonology (pronunciation) of words is a demonstrated part of Chinese silent reading just as it is in alphabetic reading [Chua, 1999; Perfetti and Tan, 1998, 1999; Perfetti and Zhang, 1995; Tan and Perfetti, 1997; Xu et al., 1999]. Thus, the activation of phonology is universal in word reading, but the specific details of how this occurs depend on the writing system [Perfetti et al., 2005; Tan et al., 1995]. Chinese phonology is retrieved only after orthographic identification of the character (threshold style), whereas English phonology can be assembled in cascade style with the processing of orthographic information. This, along with several related facts that derive from the differences between the writing systems, produces systematic reading differences between Chinese and alphabetic reading [Perfetti et al., 2005]. These facts, in addition to descriptions of the writing systems, lead to the expectation of differences in the functional neuroanatomy for reading.
Reviews of research on alphabetic writing have consistently identified a functional network of brain areas for skilled reading, although there remains considerable uncertainty about the exact functions of each area and relationships between areas within this network [Bolger et al., 2005; Fiez and Petersen, 1998; Mechelli et al., 2003; Price, 2000; Tan et al., 2005]. This network includes a left ventral occipitotemporal pathway that supports orthographic processing, left superior temporal and inferior parietal regions for phonological processing, and the left inferior frontal gyrus for semantic and phonological processing [McCandliss et al., 2003].
Recently, neuroimaging studies of Chinese reading have developed a picture of the functional neuroanatomy of skilled reading that is partly convergent and partly divergent with the results of alphabetic studies [reviewed by Bolger et al., 2005; Tan et al., 2005]. An example of convergence is that the left fusiform gyrus is activated in Chinese reading as it is in alphabetic reading [Chee et al., 1999, 2000; Tan et al., 2000, 2001]. However, Chinese readers show additional right hemisphere activation in occipital and fusiform regions [Tan et al., 2000, 2001, 2005], a result also found using source localization of a high‐density event related potential (ERP) data set [Liu and Perfetti, 2003]. Divergence between Chinese and English reading is also observed in the frontal regions. Chinese readers shows less activation in the left inferior frontal gyrus (BA 45/44) and more activation in the left middle frontal gyrus (BA 9), near the junction of superior frontal and precentral sulci [Siok et al., 2003, 2004; Tan et al., 2000, 2001, 2003]. Chinese readers also show more activation in the left inferior parietal cortex and less activation in the left superior temporal cortex. Differences are seen also in dyslexia, where reduced activation is observed for dyslexic versus normal readers in left temporal regions for English participants [Shaywitz et al., 2002], but in the left middle frontal gyrus (LMFG) for Chinese participants [Siok et al., 2004].
The question we pose is whether the differences observed in skilled reading across writing systems are also seen when readers of English learn Chinese. If the writing system imposes constraints on processing, the brain may need to accommodate to these constraints. For example, if Chinese characters require extra spatial analysis of the subcharacter components (radicals) that is not needed for linear alphabetic reading, a larger role in processing for right hemisphere visual areas could be expected at an early stage in character learning. And if the activation in the LMFG reflects a necessary integration of form, pronunciation, and meaning representation for Chinese character processing, as is implied by the lexical constituency theory's analysis of Chinese reading [Perfetti et al., 2005], then the learners too should show this activation (LMFG). On the other hand, if the activation pattern shown by skilled Chinese readers is a function of skill that must be acquired through extensive experience, then learners with limited Chinese experience might not show this pattern.
Thus, our primary goal was to investigate whether beginning learners of Chinese show evidence of accommodation to the new writing system. The system accommodation hypothesis predicts that learning to read in a new writing system requires recruitment of neural resources that can specifically support the features of the new writing system. In other words, the reading network changes to accommodate the new system. The alternative hypothesis is that readers assimilate the new system into the procedures used for the old writing system. Although the assimilation hypothesis allows neural networks to reflect differences in skill, it does not predict that learners will show patterns of activation that correspond more to those of skilled readers of the system they are learning than to their native language system, at least at the early stage of learning.
A second goal of our study was to examine phonology and semantics as separable constituents of a word. In most cases, for either first or second language, orthography, phonology, and meaning are interconnected parts of a word and all are involved in word reading. Finding areas that specifically respond to semantic and phonological processing has relied on contrasts between tasks, such as homophone versus semantic decision tasks [Tan et al., 2001]. A controlled learning study provides an opportunity to access semantic and phonological processing separately by controlling what participants learn [Sandak et al., 2004].
To control the lexical constituents acquired by the learner, we taught one group of learners only the character's connection to meaning. We provided an English meaning equivalent, but no Chinese pronunciation. For another group, we taught the character's connection to its Chinese pronunciation, but provided no meaning. In a third, we taught both pronunciation and meaning, providing the full set of lexical constituents. This training‐based separation of phonology and meaning could be informative, especially if learners show activation in the LMFG, whose role in Chinese reading is not yet determined.
MATERIALS AND METHODS
Subjects
Twenty‐nine English native speakers, all right handed, were trained to read a set of 60 Chinese characters. Among them, six participants performed poorly on the behavioral test and did not undertake the fMRI scan. Among the 23 fMRI participants, 7 were trained on both the pronunciations and meanings (P+M), 8 were trained on the pronunciations only (P), and 8 were trained on the meanings only (M).
Materials
The 60 Chinese characters and their English translations were all commonly used nouns in both Chinese and English (Appendix Table 1). In order to facilitate the learning of meanings, we selected characters that belong to three broad semantic categories: animal, man‐made objects, and natural objects, for example,  (/chong/2, WORM),
(/chong/2, WORM),  (/che/1, CAR), and
(/che/1, CAR), and  (/bing/1, ICE). Among these 60 characters, 16 were simple characters and the rest were compound characters comprising 2–5 radicals. The average number of radicals was 2.27; the average number of strokes was 8.55. There were some shared radicals among characters that belonged to the same semantic category. The average frequency of their English translations was 80/million.
(/bing/1, ICE). Among these 60 characters, 16 were simple characters and the rest were compound characters comprising 2–5 radicals. The average number of radicals was 2.27; the average number of strokes was 8.55. There were some shared radicals among characters that belonged to the same semantic category. The average frequency of their English translations was 80/million.
Novel Chinese characters, English words, and English pseudowords were also used as stimuli in the fMRI scan. The novel characters were real Chinese characters not used in the training session. So their visual forms were square‐shaped legal characters but neither pronunciation nor meaning was available to the participants. Their average number of radicals was 2.25, and their average number of strokes was 8.53. The English words were four to six letters long (mean = 4.4) with a Kuçera and Francis [ 1967] written frequency greater than 100/million (mean = 293), and included a broad range of categories. Familiarity ratings [Coltheart, 1981] were greater than 550 (mean = 594.9) on a scale from 100 to 700. The English pseudowords were in consonant‐vowel‐consonant (CVC) form, and did not form words in Chinese when pronounced.
Learning Session Procedure
The training procedure was automated, individualized, and criterion‐based. The training materials were presented on three compact disks (CD) that had a Hyper Text Marker Language (HTML) based interface. Each CD contained 20 characters. Each participant received the first CD on the first day, then carried out the training on his or her home computer, provided it had audio and video capabilities, or on a laboratory computer. On the second day, the participant returned to the laboratory, where he or she was required to pass a test on the first set of characters before receiving a CD for the second set of characters. The test was a computerized naming or translation task that did not require speed, but only accuracy. This procedure was repeated through the third day, when the participant received the third set of characters after passing a test on the second set.
The HTML interface had a framed structure and was composed by a top and a bottom frame. When browsing the CDs with a web browser, learners could view a table containing 20 characters that was shown in the top frame. By clicking on a given character in the table, the participant brought the character into a large font display in the left side of the bottom frame. In the center of the bottom frame, there was a video of a native speaker producing the corresponding Chinese pronunciation and/or the English translation according to the training condition. The Chinese pronunciation was provided by a Chinese speaker, and the English meaning equivalent was produced by an English speaker. Participants who learned both pronunciation and meaning saw the native Chinese speaker for pronunciation and the native English speaker for English translation in order to help direct attention to each of the two connections to be learned.
Participants were randomly assigned to one of the three groups. All participants were instructed to spend 2 h on each day's materials. Study times for each day were self‐reported and logged. Most participants were able to complete the 3‐day training in a total of 6 h. However, if a participant did not pass the test on any of the 3 days, he or she received an extra hour to study the 20 characters for that day and then took the test again. Participants who were not able to pass the test with the extra hour of studying were dropped from the experiment and received compensation for the total training time. Six participants failed to complete the training; 23 participants reached the criterion to continue the study.
Behavioral Session Procedure
After the training was completed, each participant underwent computerized testing on all 60 characters. Participants in the P+M condition performed both a naming task and a category judgment task. Those in the P condition performed the naming task and those in the M condition performed the category judgment task. Speed and accuracy of responses were measured on these behavioral tasks, but only accuracy was used as a criterion for continuing with the fMRI scans.
In the naming task, a Chinese character was shown in the middle of the screen following a 500‐ms fixation (“+”). The character remained on the screen until the participant read it aloud. A microphone detected the response and recorded the reaction time, and then the next character was shown. An experimenter hand coded the response accuracy.
In the category judgment task, a character was shown in the center of the screen following a 500‐ms fixation. The character remained on the screen until the participant pressed one of three keys to indicate its semantic category membership (animal, man‐made objects, or natural objects). Reaction times and accuracies were recorded by the computer.
fMRI Session Procedure
The fMRI session involved four types of stimuli: the 60 Chinese characters learned during training, 60 novel Chinese characters not previously seen, 60 commonly used English words not included in the training, and 60 English pseudowords (pronounceable letter strings). Participants performed a passive viewing task, in which stimuli occurred in blocks with fixation as the baseline. For each stimulus type, there were three experimental blocks with 20 stimuli in each block. A fixation block followed each experimental block, creating a total of 12 experimental blocks and 12 fixation blocks that were randomized and counterbalanced across participants. Each stimulus was presented for 500 ms, followed by a 500‐ms blank screen, a total of 20 s for each block and a total of 480 s to complete the scan.
Image Acquisition
Scannings were performed on a 3T Siemens Allegro MRI scanner in the Brain Imaging Research Center at University of Pittsburgh. Visual stimuli were projected to a screen that was viewed via a mirror placed in front of the participants' eyes. Anatomical images were acquired using a T1 weighted high resolution (MPRAGE) localizer scan. These images contained 160 axial slices with 1 mm thickness, 0 mm gap, and 256 × 256 imaging matrix. The TI (inversion time) was 800 ms, repetition time (TR) was 1,800 ms, and echo time (TE) was 3.49 ms with an 8° flip angle and 256‐mm field of view.
A T2* gradient‐echo echo planar imaging sequence was used to collect functional MRI images. The functional scans contained 35 axial slices with 3.3 mm thickness, 0 mm gap, and 64 × 64 imaging matrix. The TR was 2 s, and the TE was 35 ms, with a 70° flip angle and 210‐mm field of view. Two hundred and forty three‐dimensional (3D) functional volumes were collected for each participant.
Data Analysis Procedure
The AFNI software package (http://afni.nimh.nih.gov/afni) was used to analyze the fMRI data. First, data were preprocessed with 3D movement correction, spatial filtering (FWHM = 6 mm), and normalization. Then a deconvolution model was applied to each participant's data to calculate the activation coefficient of each experimental condition relative to the fixation baseline. As a result, there was one 3D activation coefficient volume for each experimental condition from each participant. Using participants as a random factor, voxelwise F‐tests and two‐tailed t‐tests tested whether the activation coefficients of various experimental conditions were different from 0 and from each other, resulting in 3D F and t maps. These maps were thresholded using an α level of P < 0.005. The statistical maps were registered to Talairach coordinates, and only clusters of active voxels larger than 170 mm3 (Monte Carlo simulation with 1,000 iterations and α < 0.001) are reported as significant foci of change.
RESULTS
Behavioral Results
The mean reaction times and accuracies of naming and category judgment tasks are shown in Table I. Although the pronunciation and meaning group (P+M) tended to have longer reaction times and lower accuracies than did participants who learned only pronunciation (P) or meaning (M), there were no significant differences between training groups (all P values > 0.1).
Table I.
The mean of reaction times and accuracies of naming and category judgment tasks
| Naming | Category judgment | |||
|---|---|---|---|---|
| Mean RT(ms) | Mean accuracy(%) | Mean RT (ms) | Mean accuracy (%) | |
| Pronunciation + meaning group | 2856 (314)a | 91.7 (2.4) | 2348 (344) | 91.9 (2.5) |
| Pronunciation group | 2397 (393) | 95 (1.2) | — | — |
| Meaning group | — | — | 2287 (210) | 95.4 (1.3) |
Values in parentheses are standard error.
fMRI Results
We report two sets of results, one for the overall training effects for the full group of 23 participants, and a second for the effects of specific training conditions.
Overall results for all participants
Because all three groups learned the orthographic forms of a single set of Chinese characters, they comprise a meaningful sample of 23 orthographic learners. This total sample allows maximal statistical power in identifying brain regions activated by orthographic processing. Accordingly, English real words, English pseudowords, learned Chinese characters, and novel Chinese characters were subtracted from the fixation baseline condition to provide a general picture of cortical regions involved in reading for all 23 learners (Table II).
Table II.
Peak activations of the three groups combined (x, y, z corrdinates in Talairach space and t value)
| Broad‐man area | Gyral location | English words > fixation | English pseudowords > fixation | Chinese learned characters > fixation | Chinese novel characters > fixation | Chinese learned > Englishwords | English words > Chinese learned |
|---|---|---|---|---|---|---|---|
| 6 | Left precentral | −52,−3,46(5.81) | −49,3,33(7.62) | −50,−5,46(8.17) | −50,2,46(4.40) | −52,1,36(6.05) | |
| −32,−9,60(4.11) | |||||||
| Right precentral | 54,−1,43(6.32) | 40,−5,50(4.26) | 50,2,43(4.97) | 40,−2,30(6.30) | |||
| Left superior medial frontal | −7,4,49(5.13) | −4,7,48(10.96) | −7,5,48(5.51) | −2,8,47(7.38) | −17,23,54(4.97) | ||
| −14,46,44(4.95) | |||||||
| Right superior medial frontal | 10,50,30(4.96) | 3,9,49(4.32) | 2,9,48(9.43) | 1,9,48(7.22) | 2,9,46(6.18) | 8,52,30(5.60) | |
| 6,38,48(4.22) | |||||||
| 6/9/46 | Left middle frontal | −47,5,34(5.34) | −45,25,20(6.12) | −49,12,33(6.36) | −54,21,29(5.34) | −43,22,24(3.82) | |
| −34,46,18(4.26) | |||||||
| Right middle frontal | 50,20,29(5.48) | 44,14,33(4.41) | 46,21,26(9.10) | 39,27,31(3.70) | |||
| 44,20,25(4.54) | |||||||
| Left insula | −37,13,9(6.03) | −31,21,7(6.03) | −29,21,8(4.44) | ||||
| Right insula | 31,16,11(8.45) | 30,20,6(7.09) | 29,18,10(5.34) | ||||
| 45/46/47 | Left inferior frontal | −50,25,20(4.58) | −42,35,10(4.738) | −44,25,16(3.90) | −49,34,−5(4.45) | ||
| Right inferior frontal | 52,24,2(3.94) | 43,28,14(4.52) | 42,24,−7(3.81) | ||||
| 21/22 | Left middle/ superior temporal | −60,−33,4(4.72) | −59,−32,8(5.373) | −56,−59,23(5.96) | |||
| −62,−46,5(5.15) | |||||||
| Right middle/ superior temporal | 58,−27,2(4.06) | 51,−62,21(6.01) | |||||
| 53,−45,9(4.44) | |||||||
| 38 | Left superior temporal | −38,16,−26(4.16) | −46,13,−27(3.60) | ||||
| Right superior temporal | 33,4,−28(4.00) | 33,23,−25(3.90) | |||||
| 7 | Left superior parietal | −33,−62,45(9.54) | −30,−64,46(7.56) | −22,−66,56(7.05) | |||
| Right superior parietal | 28,−62,48(10.14) | 25,−63,43(10.47) | 26,−60,51(7.23) | ||||
| 40 | Left inferior parietal | −56,−44,24(5.40) | −36,−52,41(4.98) | −35,−54,39(9.06) | −33,−54,39(7.83) | ||
| Right inferior parietal | 32,−54,41(4.83) | 30,−56,41(7.25) | 35,−55,40(4.57) | ||||
| 17/18/19 | Left middle/ inferior occipital | −32,−86,−7(12.59) | −31,−86,−8(12.939) | −31,−88,−4(19.13) | −31,−86,−8(12.94) | −42,−77,−3(5.33) | |
| −32,−87,−1(4.37) | |||||||
| Right middle/ inferior occipital | 30,−88,−7(9.93) | 32,82,−5(7.67) | 29,−87,−6(14.38) | 30,−89,−5(8.28) | 39,−80,10(5.27) | ||
| 37 | Left fusiform | −39,−44,−16(5.69) | −45,−51,−13(8.119) | −46,−64,−13(10.48) | −46,−67,−11(11.01) | −48,−56,−11(4.05) | |
| 20/37 | Right fusiform | 36,−39,−16(5.73) | 42,−62,−10(5.557) | 42,−63,−14(8.84) | 43,−59,−10(6.04) | 46,−61,−11(6.14) | |
| 18 | Left cuneus | −9,−89,21(5.25) | |||||
| 18 | Right cuneus | 4,−90,21(4.34) |
For English real words, the activated brain areas (relative to fixation) included bilateral inferior (BA 45) and medial frontal (BA 6; SMA), left middle frontal (BA 9), bilateral superior and middle temporal (BA 22), bilateral occipital (BA 17/18/19), and bilateral fusiform (BA 37) regions. The occipital and fusiform activations were much stronger in the left hemisphere than in the right (Fig. 1a). A nearly identical set of regions was found for English pseudowords relative to fixation (see Table II).
Figure 1.
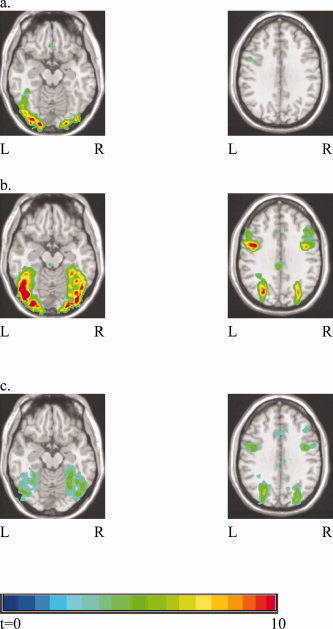
Subtraction maps based on 23 participants from all three groups. T‐statistics map in the left shows occipital and fusiform gyri (z = −12). Map in the right shows middle frontal gyrus (z = 31). (a) English words > fixation; (b) learned Chinese characters > fixation; (c) learned Chinese characters > English words. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For learned Chinese characters, the activated brain regions (relative to fixation) were symmetrical and overlapped only partly with regions observed for English words and pseudowords. Activation localized to bilateral medial frontal (BA 6; SMA), precentral (BA 6), middle frontal (BA 9), insular, occipital (BA 17/18/19), and fusiform (BA 37) regions (Fig. 1b and Table II). The novel Chinese characters had a similar pattern of activation. Significant differences between learned and novel characters emerged in contrasts between training conditions and will be shown for individual groups later.
To specifically examine the differences across languages, activation during the learned Chinese character blocks was contrasted with the real English word blocks using a two‐tailed t‐test (Table II). There was significantly more activation for Chinese characters in bilateral dorsal precentral (BA 6), superior frontal (BA 6), middle frontal (6/9/46), insular, occipital (BA 17/18/19), fusiform (BA 37), and superior parietal (BA 7) regions (Fig. 1c). There was significantly more activation for English words in bilateral superior frontal (BA 8), inferior frontal (BA 47), middle/superior temporal, (BA 21/22), and cuneate regions.
On the basis of our subtraction maps and the literature, four ROIs were selected to calculate the percentage of signal change (Fig. 2): left fusiform, right fusiform, left middle frontal, and right middle frontal. Two 3‐factor ANOVAs were carried out respectively on the fusiforms and middle frontal areas. The factors were hemisphere (left vs. right), language (Chinese vs. English), and familiarity (known vs. unknown).
Figure 2.
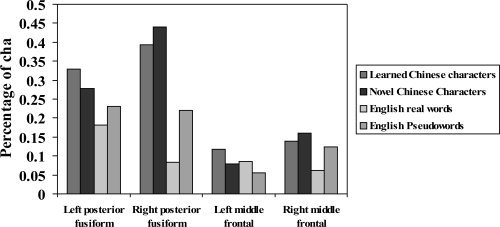
Percentage of change for four types of stimuli at four ROIs (left posterior fusiform: x = −43, y = −60, z = −12; right posterior fusiform: x = 43, y = −60, z = −12; left middle frontal: x = −45, y = 14, z = 31; right middle frontal: x = 45, y = 14, z = 31).
The fusiforms ANOVA showed a significant language effect (F(1,22) = 27.469, MSE = 0.055, P < 0.001) and a hemisphere by language interaction (F(1,22) = 15.89, MSE = 0.02, P = 0.001). Chinese characters produced more activation than English words at bilateral fusiforms, but the difference was larger in the right fusiform than in the left.
The middle frontal ANOVA showed a significant hemisphere by familiarity interaction (F(1,22) = 9.44, MSE = 0.007, P = 0.006). For both Chinese and English, in left middle frontal, the known stimuli (learned Chinese characters and English real words) produced more activation than did unknown stimuli (novel Chinese characters and English pseudowords). However, in right middle frontal, the unknown stimuli produced more activation than known.
Results for specific training
Multiple comparisons were carried out separately for the three training groups. For each group, the voxelwise difference values were calculated by subtracting the activation coefficients for the fixation condition from those for the learned Chinese characters and then testing the significance of these difference values using two‐tailed t‐tests against 0. In addition, the activation coefficients of learned characters for the P+M group were tested against those of the other two groups to obtain phonological and semantic learning effects.
The regions of significant activation, relative to fixation, are listed for each training condition in Table III. A generally similar pattern of results was found across all three groups. Of particular interest was the activation observed across all groups in the bilateral middle frontal gyrus (LMFG), near the junction of the inferior frontal and precentral sulci (BA 6/9/44). Activation of this region is consistent with results from Chinese native speakers [Tan et al., 2005]. One point to note is that the coordinates of the peak activated voxel in LMFG varied across training groups. Specifically, while the peak voxels of the P+M group and the M group were separated by 8 mm, they were about 19 mm distant from the peak voxel of the P group (Table III and Fig. 3a,b).
Table III.
Peak activations of the three groups separated (x, y, z corrdinates in Talairach space and t value)
| Broadman area | Gyral location | Chinese learned characters > fixation | P+M > P | P+M > M | ||
|---|---|---|---|---|---|---|
| P+M group | P group | M group | ||||
| 6 | Left precentral | −47,−7,56(7.26) | −48,−3,50(5.53) | −45,0,32(9.20) | ||
| Right precentral | 51,0,36(7.30) | 44,−4,49(6.99) | 40,−1,29(5.62) | 48,−15,36(4.50) | ||
| Left superior medial frontal | −6,4,48(7.23) | −6,1,55(6.25) | −6,12,53(8.33) | |||
| Right superior medial frontal | 2,10,47(5.30) | 4,2,55(4.37) | 2,10,51(5.42) | |||
| 8 | Left superior frontal | −22,31,45(3.88) | −18,26,46(5.26) | |||
| 6/9/45/46 | Left middle frontal | −45,21,24(4.64) | −44,4,33(12.09) | −45,28,20(5.52) | −40,0,39(5.35) | −35,13,41(5.04) |
| −48,11,31(4.11) | −38,12,44(4.47) | −40,4,43(4.22) | ||||
| Right middle frontal | 34,24,24(10.48) | 47,5,33(8.07) | 45,21,27(7.17) | |||
| Left insula | −30,18,11(7.45) | −29,24,8(8.87) | −34,20,10(5.03) | |||
| Right insula | 28,19,10(5.04) | 32,18,8(8.65) | 31,17,14(4.77) | |||
| 22/39 | Right middle/superior temporal | 55,−46,4(4.70) | ||||
| 54,−58,25(5.06) | ||||||
| 45,−53,31(4.99) | ||||||
| 7 | Left superior parietal | −30,−63,46(7.53) | −28,−70,49(8.08) | −30,−59,46(8.53) | ||
| Right superior parietal | 29,−70,43(5.73) | 29,−62,48(8.36) | 28,−60,51(8.10) | |||
| Left and right precuneus | 2,−54,37(6.03) | |||||
| 40 | Left inferior parietal | −30,−54,39(9.78) | −38,−47,40(10.21) | −30,−46,43(5.53) | ||
| Right inferior parietal | 36,−53,43(4.17) | 34,−50,42(7.13) | 31,−39,39(5.70) | |||
| 17/18/19 | Left middle/inferior occipital | −33,−84,−7(13.42) | −39,−75,−10(14.74) | −31,−90,−14(11.68) | ||
| Right middle/inferior occipital | 35,−83,−7(8.20) | 29,−85,4(15.32) | 34,−85,2(10.42) | |||
| 37 | Left fusiform | −42,−71,−14(10.97) | −37,−44,−15(10.27) | −45,−67,−12(7.50) | ||
| 20/37 | Right fusiform | 37,−71,−13(9.44) | 44,−65,−13(8.62) | 31,−43,−13(11.25) | ||
Figure 3.
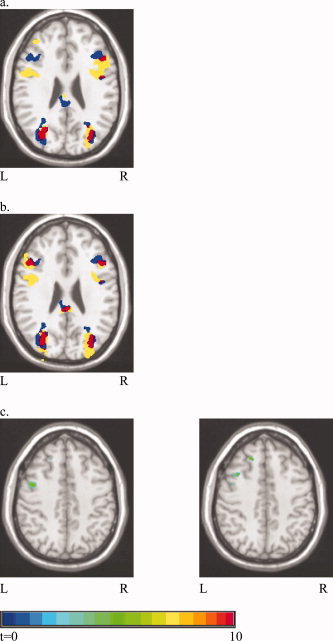
Overlapping maps of two of the three training groups. (a) P+M and P groups at z = 25: blue represents P+M only, yellow represents P only, and red represents P+M and P overlap; (b) P+M and M groups at z = 25: blue represents P+M only, yellow represents M only, and red represents P+M and M overlap; (c) P+M > P (left one) and P+M > M (right one) maps at z = 40. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The between‐groups comparison found that the LMFG was activated significantly more in the P+M group than in either the P group or the M group (Fig. 3c). Relative to the M group, the P+M group also showed more activation in the right middle/superior temporal gyrus and precuneus bilaterally.
DISCUSSION
A striking result from this study is the extent to which the brain activation pattern of English speakers viewing Chinese resembled that of Chinese native speakers. This pattern of similarity resulted after learning only 60 characters over a 3–4‐day period (total, 6–9 h). A second important result concerns the identification of neural correlates of the three lexical constituents—orthographic, phonological, and semantic constituents—that together specify the identity of a printed word. We discuss each of these in turn, beginning with a discussion of orthographic processing within occipital and fusiform cortex.
Studies of English speakers that have contrasted different types of orthographic stimuli have helped to identify a putative visual word form area (VWFA) located within the left fusiform gyrus [Cohen et al., 2002; Dehaene et al., 2001; Nobre et al., 1998]. Whether the left fusiform gyrus really contains a word form area is a matter of contention, [Price and Devlin, 2003]. However, because it is activated by the presentation of word‐like stimuli, the VWFA has been argued to support orthographic processing through “extensive visual experience with a class of stimuli [that] drives enhancement of perceptual mechanisms and changes in the supporting functional architecture in the left fusiform gyrus” [McCandliss et al., 2003: 296]. The question of what kind of experience might be necessary to alter the functional architecture of the left fusiform gyrus remains open for learning to read in a native language. Booth et al. [ 2001] found that adults showed fusiform activation when reading words, while children instead showed activation in posterior superior temporal regions (Wernicke's area), suggesting that extended experience is needed for the fusiform to be involved in reading English.
For adults, the degree of fusiform activation may decrease with word familiarity. Thus, pseudowords not previously viewed may activate the fusiform gyrus even more than real words do [Fiez and Petersen, 1998]. Furthermore, after training native speakers on English pseudowords, Sandak et al. [ 2004] found that phonologically‐based training produced decreased activation in the fusiform, perhaps reflecting increased efficiency of orthographic processing that could result from learning letter–phoneme connections.
Because English and Chinese stimuli both activated the left fusiform (Fig. 3), our results confirm that this region can respond to word forms in general, not just to linear alphabetic strings. Although the left fusiform has previously been shown to be active for Chinese native reading, our results further suggest that this region responds to “Chinese character like” stimuli after only a modest level of exposure to characters. Stronger activation of the left fusiform during Chinese reading might be attributed to the learned but less familiar visual properties of characters. This possibility seems consistent with the result that pseudowords, which are less familiar than real words, produced slightly stronger activation than did English words in both the present study and other studies [Brunswick et al., 1999; Dehaene et al., 2002; Fiez et al., 1999]. We conclude that the left fusiform quickly responds to learning a new writing system that uses very different visual forms.
In the present study, English words produced much larger and stronger activation in the left occipital and fusiform regions than in the right occipital and fusiform (Figs. 1a and 3). This result is in line with other results from studies of alphabetic readers [Cohen et al., 2000; Dehaene et al., 2001, 2002]. However, when the same 23 participants were viewing Chinese characters (Fig. 1b), the occipital and fusiform activation was equivalent across hemispheres. Furthermore, the direct contrast between Chinese characters and English words showed that Chinese characters produced stronger activation in both left and right fusiform cortex, although the difference of signal change in the right fusiform was significantly larger than in the left fusiform. These findings essentially replicate, in learners, multiple studies of native Chinese reading that have consistently found stronger activation in the right occipital and fusiform regions than is found in English [Liu and Perfetti, 2003; Siok et al., 2003, 2004; Tan et al., 2000, 2001, 2003].
The question becomes what is there about Chinese characters that produces this right hemisphere involvement. The timing of the right occipital activation is early, at 100–150 ms, according to ERP source analysis of Chinese character naming by Liu and Perfetti [ 2003]. Furthermore, Peng et al. [ 2003] found that, at a very brief exposure (51 ms), high frequency characters produced more right fusiform activity than did low frequency characters.
Liu and Perfetti [ 2003] suggested that the complex visual form of Chinese characters requires spatial processing that may be supported by the right hemisphere. In particular, while the stroke composition of a radical creates a stimulus of high spatial frequencies, the spatial relations between the radicals (right–left, top–bottom, inside– outside) add a low spatial frequency component to the visual analysis. Right hemisphere occipital cortex may be well suited for this task, based on claims that it is specialized for global and low spatial frequency information analysis [Hellige, 1995; Kitterle et al., 1992]. In Chinese reading, the right ventral occipital‐to‐fusiform pathway might play an important role in analyzing the spatial relations of radicals, although a more direct test of this hypothesis is needed. The fact that learners as well as skilled Chinese readers show this right hemisphere activation is consistent with the assumption that specific stimulus characteristics of Chinese characters are responsible.
A second region that distinguishes Chinese from English word reading is an area in the posterior portion of the LMFG, near the junction of the inferior prefrontal and precentral sulci (BA9/44/6). This LMFG region is more activated in reading Chinese compared either with a nonreading baseline or with English [Tan et al., 2000, 2001, 2003]. The function of this region in reading Chinese is not completely clear. In the research on Chinese native speakers, the function of the LMFG region has been linked to semantic access [Tan et al., 2000] and to addressed phonology [Tan et al., 2005].
A possibility we raise here is that the LMFG region functions as a working memory component that provides essential support for reading a Chinese character. This speculation is consistent with evidence linking LMFG more generally to working memory and executive functions. For example, Courtney et al. [ 1998] found that the LMFG was involved in spatial working memory (BA 46) and working memory for faces (BA 9). Verbal working memory [Petrides et al., 1993] and central executive functions [D'Esposito et al., 1995] have also be linked to regions within the LMFG. In reading Chinese, these memory and executive functions may be especially important because there is no cascade‐style processing in which each segment can be added to an unfolding phonological representation, as in English. Instead, phonology and meaning are both retrieved from memory based on the character as a whole (with assistance from phonetic and semantic radicals) as an orthographic threshold is reached [Perfetti et al., 2005]. This may make it necessary to retain the character form until both meaning and pronunciation are retrieved, and the LMFG region identified in Chinese word reading may support this memory process.
This hypothesis is supported by the fact that all three of our Chinese learner groups showed greater LMFG activation for characters relative to fixation and to English words, regardless of their learning condition. Though differences in the spatial location of the peak activation across the groups raises the possibility of some differentiation across groups, overall the results generally suggest that associating a learned character with either a Chinese pronunciation or a meaning was sufficient to activate the LMFG region. Thus, the results are consistent with the hypothesis that the LMFG helps to support a visual memory for the character so that a constituent associated with the character can be retrieved.
An alternative hypothesis also merits consideration. Specifically, the fact that phonological and semantic information appears to be accessed in Chinese only after a threshold for orthographic activation has been reached may lead to the recruitment of additional executive resources necessary to support a three‐way integration of graphic form, phonological form, and meaning. Support for this “executive control” hypothesis comes from the fact that dual association of pronunciation and meaning with orthography is associated with increased activation within the LMFG; that is, the P+M learners showed an increase in a superior region of LMFG (z = 39–44) that was not observed for the other two groups (P+M > P and P+M > M columns in Table III and Fig. 3c).
Another interesting finding is that the right middle frontal gyrus showed more activation for unknown stimuli. This result is consistent with existing evidence suggesting that the right hemisphere and especially right frontal systems are organized principally to process novel challenges [Goldberg et al., 1994].
In addition to the fusiform and middle frontal areas we have discussed, three other regions showed stronger activation for Chinese than for English: premotor cortex (BA 6), insula, and superior parietal (BA 7). In native Chinese speakers, Tan et al. [ 2005] found activation in the premotor cortex during character reading, which they attributed to the experience of repeated character copying during the learning to read Chinese. However, both the left premotor cortex and insula have been found to produce greater activation in a speaker's second language (French) than first language (English) during an auditory word repetition task [Klein et al., 2006]. Thus it is unclear whether these three regions are Chinese specific or L2 general. But there is no doubt that the network observed in learning a second language is composed by language specific and L2 general brain regions.
Finally, we return to the system accommodation hypothesis as a generalization concerning the brain's response to learning to read in a new writing system. This hypothesis assumes the possibility of universals in reading. At the neural level, the left fusiform gyrus may be universally involved in skilled visual word recognition. Phonology is universal at a different level, clearly implicated in word reading across writing systems by substantial behavioral research [Perfetti and Tan, 1998; Perfetti et al., 2005], without necessarily having a universal neural implementation. The system accommodation hypothesis adds a general brain–behavior relation to this universal picture, proposing that the reading network acquired by the brain through alphabetic reading cannot simply assimilate a logographic writing system into its procedures for reading. Accommodation to the structural properties of the new writing system occurs. This accommodation includes the recruitment of visual areas that can support the visual layout and composition of characters and of a left middle frontal area that supports lexical‐level character processing, perhaps reinstating the orthographic form of the character.
An interesting corollary to the accommodation hypothesis is that it may not have a symmetrical scope. That is, it may be that an alphabetic brain needs more accommodation for reading Chinese than a Chinese brain needs for alphabetic reading. Chinese cannot be read alphabetically, whereas English words (or other alphabetic words), in principle, could be read in a quasi‐Chinese style, this is, holistically or at least with little decomposition and minimal reference to sublexical phonology [Wang et al., 2003]. The Dual Route Theory of pronouncing written words [Coltheart et al., 2001] allows this possibility explicitly. (Note that the use of word shape cues is not what is at stake here; although such cues can be helpful, reading words in English or in Chinese includes identification of constituents, i.e. letters or radicals.) The implication of this asymmetry is that Chinese–English bilinguals, when they read English, could show a pattern more similar to the pattern they show for Chinese. Some evidence for this comes from a study comparing English and Chinese [Nelson et al., unpublished work]. If this turns out to be correct, we would conclude that the Chinese style of reading is actually more universal, whereas the alphabetic style is more specialized.
Table I.
Learning materials
| Chi‐nese | Pronun‐ciation | Meaning | Chi‐nese | Pronun‐ciation | Meaning | Chi‐nese | Pronun‐ciation | Meaning |
|---|---|---|---|---|---|---|---|---|

|
/chong/2 | WORM |

|
/che/1 | CAR |

|
/bing/1 | ICE |

|
/die/2 | BUTTERFLY |

|
/chuang/2 | BED |

|
/chun/1 | SPRING |

|
/e/2 | GOOSE |

|
/ding/1 | NAIL |

|
/dao/3 | ISLAND |

|
/gui/1 | TURTLE |

|
/fang/2 | HOUSE |

|
/di/4 | EARTH |

|
/hou/2 | MONKEY |

|
/gong/1 | BOW |

|
/dong/1 | WINTER |

|
/hu/3 | TIGER |

|
/jia/1 | HOME |

|
/feng/1 | WIND |

|
/lv/2 | DONKEY |

|
/jian/4 | SWORD |

|
/gu/3 | VALLEY |

|
/ma/3 | HORSE |

|
/jiu/3 | WINE |

|
/he/2 | RIVER |

|
/mao/1 | CAT |

|
/lu/2 | STOVE |

|
/hong/2 | RAINBOW |

|
/niao/3 | BIRD |

|
/men/2 | DOOR |

|
/huo/3 | FIRE |

|
/she/2 | SNAKE |

|
/qi/2 | CHESS |

|
/pu/4 | WATERFALL |

|
/shi/1 | LION |

|
/qiang/1 | GUN |

|
/qiu/1 | AUTUMN |

|
/wa/1 | FROG |

|
/qiao/2 | BRIDGE |

|
/ri/4 | SUN |

|
/xia/1 | SHRIMP |

|
/sheng/2 | ROPE |

|
/sha/1 | SAND |

|
/xiang/4 | ELEPHANT |

|
/wan/3 | BOWL |

|
/san/1 | MOUNTAIN |

|
/xiong/2 | BEAR |

|
/xie/2 | SHOE |

|
/shui/3 | WATER |

|
/ya/1 | DUCK |

|
/yi/3 | CHAIR |

|
/tian/1 | SKY |

|
/ying/1 | EAGLE |

|
/zhen/1 | NEEDLE |

|
/wu/4 | FOG |

|
/yv/2 | FISH |

|
/zhi/3 | PAPER |

|
/xing/1 | STAR |

|
/zhu/1 | PIG |

|
/zuo/1 | DESK |

|
/yue/4 | MOON |
Contributor Information
Ying Liu, Email: liuying@pitt.edu.
Charles Perfetti, Email: perfetti@pitt.edu.
REFERENCES
- Bolger DJ,Perfetti CA,Schneider W ( 2005): Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp 25: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR,Burman DD,Van Santen FW,Harasaki Y,Gitelman DR,Parrish TB,Mesulam MM ( 2001): The development of specialized brain systems in reading and oral‐language. Child Neuropsychol 7: 119. [DOI] [PubMed] [Google Scholar]
- Brunswick N,McCrory E,Price CJ,Frith CD,Frith U ( 1999): Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke's Wortschatz? Brain 122: 1901–1917. [DOI] [PubMed] [Google Scholar]
- Chee MW,Tan EWL,Thiel T ( 1999): Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci 19: 3050–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW,Weekes B,Lee KM,Soon CS,Schreiber A,Hoon JJ,Chee M ( 2000): Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: Evidence from fMRI. Neuroimage 12: 392–403. [DOI] [PubMed] [Google Scholar]
- Chua FK ( 1999): Phonological recoding in Chinese logograph recognition. J Exp Psychology Learn Mem Cogn 25: 876–891. [Google Scholar]
- Cohen L,Dehaene S,Naccache L,Lehéricy S,Dehaene‐Lambertz G,Hénaff MA,Michel F ( 2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123: 291. [DOI] [PubMed] [Google Scholar]
- Cohen L,Lehéricy SP,Chochon F,Lemer C,Rivaud S,Dehaene S ( 2002): Language‐specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125: 1054. [DOI] [PubMed] [Google Scholar]
- Coltheart M ( 1981): The MRC psycholinguistic database. Q J Exp Psychol A 33: 497–505. [Google Scholar]
- Coltheart M,Rastle K,Perry C,Langdon R,Ziegler J ( 2001): DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev 108: 204–256. [DOI] [PubMed] [Google Scholar]
- Courtney SM,Petit L,Maisog JM,Ungerleider LG,Haxby JV ( 1998): An area specialized for spatial working memory in human frontal cortex. Science 279: 1347. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Detre JA,Alsop DC,Shin RK,Atlas S,Grossman M ( 1995): The neural basis of the central executive system of working memory. Nature 378: 279. [DOI] [PubMed] [Google Scholar]
- Dehaene S,Naccache L,Cohen L,Le Bihan D,Mangin JF,Poline JB,Rivière D ( 2001): Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4: 752. [DOI] [PubMed] [Google Scholar]
- Dehaene S,Le Clec'H G,Poline JB,Le Bihan D,Cohen L ( 2002): The visual word form area: A prelexical representation of visual words in the fusiform gyrus. Neuroreport 13: 321. [DOI] [PubMed] [Google Scholar]
- Fiez JA,Petersen SE ( 1998): Neuroimaging studies of word reading. Proc Natl Acad Sci USA 95: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA,Balota DA,Raichle ME,Petersen SE ( 1999): Effects of lexicality, frequency, and spelling‐to‐sound consistency on the functional anatomy of reading. Neuron 24: 205–218. [DOI] [PubMed] [Google Scholar]
- Goldberg E,Podell K,Lovell M ( 1994): Lateralization of frontal lobe functions and cognitive novelty. J Neuropsychiatry Clin Neurosci 6: 371–378. [DOI] [PubMed] [Google Scholar]
- Hellige JB ( 1995): Hemispheric asymmetry for components of visual information processing In: Davidson RJ,Hugdahl K, editors. Brain Asymmetry. Cambridge, MA: MIT Press; pp 99–121. [Google Scholar]
- Kitterle FL,Hellige JB,Christman S ( 1992): Visual hemispheric asymmetries depend on which spatial frequencies are task relevant. Brain Cogn 20: 308–314. [DOI] [PubMed] [Google Scholar]
- Klein D,Watkins KE,Zatorre RJ,Milner B ( 2006): Word and nonword repetition in bilingual subjects: A PET study. Hum Brain Mapp 27: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuçera H,Francis WN ( 1967): Computational Analysis of Present‐Day American English. Providence, RI: Brown. [Google Scholar]
- Liu Y,Perfetti CA ( 2003): The time course of brain activity in reading English and Chinese: An ERP study of Chinese bilinguals. Hum Brain Mapp 18: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B,Cohen L,Dehaene S ( 2003): The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci 7: 293–299. [DOI] [PubMed] [Google Scholar]
- Mechelli A,Gorno‐Tempini ML,Price CJ ( 2003): Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. J Cogn Neurosci 15: 260. [DOI] [PubMed] [Google Scholar]
- Nobre AC,Allison T,McCarthy G ( 1998): Modulation of human extrastriate visual processing by selective attention to colours and words. Brain 121: 1357. [DOI] [PubMed] [Google Scholar]
- Peng DL,Xu D,Jin Z,Luo Q,Ding GS,Perry C,Zhang L,Liu Y ( 2003): Neural basis of the non‐attentional processing of briefly presented words. Hum Brain Mapp 18: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA,Zhang S ( 1995): Very early phonological activation in Chinese reading. J Exp Psychol Learn Mem Cogn 21: 24–33. [Google Scholar]
- Perfetti CA,Tan LH ( 1998): The time course of graphic, phonological, and semantic activation in Chinese character identification. J Exp Psychol Learn Mem Cogn 24: 101–118. [DOI] [PubMed] [Google Scholar]
- Perfetti CA,Tan LH ( 1999): The constituency model of Chinese word identification In: Wang J, editor. Reading Chinese Script: A Cognitive Analysis. Mahwah, NJ: Erlbaum; pp 115–134. [Google Scholar]
- Perfetti CA,Liu Y,Tan LH ( 2005): The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112: 43–59. [DOI] [PubMed] [Google Scholar]
- Petrides M,Alivisatos B,Meyer E,Evans AC ( 1993): Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 90: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ ( 2000): The anatomy of language: Contributions from functional neuroimaging. J Anat 197: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ,Devlin JT ( 2003): The myth of the visual word form area. Neuroimage 19: 473–481. [DOI] [PubMed] [Google Scholar]
- Sandak R,Mencl WE,Frost SJ,Rueckl JG,Katz L,Moore DL,Mason SA,Fulbright RK,Constable RT,Pugh KR ( 2004): The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cogn Affect Behav Neurosci 4: 67. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA,Shaywitz SE,Pugh KR,Mencl WE,Fulbright RK,Skudlarski P,Constable RT,Marchione KE,Fletcher JM,Lyon GR,Gore JC ( 2002): Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 52: 101. [DOI] [PubMed] [Google Scholar]
- Siok WT,Jin Z,Fletcher P,Tan LH ( 2003): Distinct brain regions associated with syllable and phoneme. Hum Brain Mapp 18: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT,Perfetti CA,Jin Z,Tan LH ( 2004): Biological abnormality of impaired reading is constrained by culture. Nature 431: 71. [DOI] [PubMed] [Google Scholar]
- Tan LH,Perfetti CA ( 1997): Visual Chinese character recognition: Does phonological information mediate access to meaning? J Mem Lang 37: 41–57. [Google Scholar]
- Tan LH,Hoosain R,Peng DL ( 1995): Role of early presemantic phonological code in Chinese character identification. J Exp Psychol Learn Mem Cogn 21: 43–54. [Google Scholar]
- Tan LH,Spinks JA,Gao JH,Liu HL,Perfetti CA,Xiong JH,Stofer KA,Pu YL,Liu YJ,Fox PT ( 2000): Brain activation in the processing of Chinese characters and words: A functional MRI study. Hum Brain Mapp 10: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH,Liu HL,Perfetti CA,Spinks JA,Fox PT,Gao JH ( 2001): The neural system underlying Chinese logograph reading. Neuroimage 13: 836–846. [DOI] [PubMed] [Google Scholar]
- Tan LH,Spinks JA,Feng CM,Siok WT,Perfetti CA,Xiong JH,Fox PT,Gao JH ( 2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH,Laird A,Li K,Fox PT ( 2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M,Koda K,Perfetti CA ( 2003): Alphabetic and nonalphabetic L1 effects in English word identification: A comparison of Korean and Chinese English L2 learners. Cognition 87: 129. [DOI] [PubMed] [Google Scholar]
- Xu Y,Pollatsek A,Potter MC ( 1999): The activation of phonology during silent Chinese word reading. J Exp Psychol Learn Mem Cogn 25: 838–857. [DOI] [PubMed] [Google Scholar]


