Abstract
We used emotional expectancy to study attentional modulation in the processing of emotional stimuli. During functional magnetic resonance imaging (fMRI), volunteers saw emotional and neutral expectancy cues signaling the subsequent presentation of corresponding emotional or neutral pictorial stimuli. As a control, emotional and neutral pictures were presented without preceding expectancy cue, resulting in a 2 × 2 factorial design with the factors “expectancy” and “emotion.” Statistical analysis revealed a significant positive interaction effect between these factors in the medial prefrontal cortex (MPFC, Brodmann area [BA] 9/10), amygdala, and dorsal midbrain. In all these regions, expectancy augmented the neural response to emotional but not to neutral pictures. Time course analysis of raw data suggests that this augmented activation was not preceded by baseline increases in MPFC and amygdala during the period of emotional expectancy. In a post‐scanning session, the paradigm was presented for a second time to allow emotional intensity rating. Again, a significant interaction between expectancy and emotion was observed, with intensity ratings specifically enhanced in emotional photographs preceded by expectancy. There was a positive correlation between intensity ratings and blood oxygenation level‐dependent (BOLD) signals in the left amygdala. We conclude that specific components of the emotion network show enhanced activation in response to emotional stimuli when these are preceded by expectancy. This enhancement effect is not present in neutral pictures and might parallel accentuated subjective feeling states. Hum. Brain Mapp 2005. © 2005 Wiley‐Liss, Inc.
Keywords: emotion, attention, functional magnetic resonance imaging, medial prefrontal cortex, amygdala
INTRODUCTION
Daily life shows that attention influences our emotional experience. When we direct our attention to an emotion (e.g., sadness), we tend to experience this emotion more intensely. Conversely, if we turn our attention away from the emotion and direct it to a more demanding cognitive task, the emotion tends to fade. This influence of attention on emotional experience is also found in psychiatric patients. Patients with major depressive disorder, for example, show an attention bias toward negative contents and deficits in shifting the focus of their attention to positive stimuli [Murphy et al., 1999]. This seems to contribute to the intensity and perpetuation of depressed mood and anhedonia in this disorder. Accordingly, a therapeutic premise behind cognitive behavioral therapy (CBT) is to modify maladaptive attentional processes to alleviate emotional symptoms.
Functional imaging has been used to study the neural correlates of attentional control in humans. Whereas relatively few studies have focused on attentional modulation in the emotion domain, a large body of work has examined attention effects in extrastriate visual areas. These studies have generally adopted one of two approaches to examine attentional modulation. The classic approach is to compare different tasks in relation to the same set of visual stimuli [Buchel et al., 1998a; Corbetta et al., 1990; Kastner et al., 1998; O'Craven et al., 1997; Tootell et al., 1998]. The premise is that each task will require subjects to attend to a different object feature, e.g., color or velocity. Because such paradigms allow the study of the attention effect that appears during the period of stimulus presentation, we shall refer to them here (for convenience) as “simultaneous attention” paradigms. In an alternative approach, a number of studies have incorporated a period of expectancy before the presentation of the actual stimulus [Posner et al., 1980]. In such paradigms, an expectancy cue is presented to direct the subject's attention to a specific visual feature of the subsequent stimulus such as color [Chawla et al., 1999], motion [Chawla et al., 1999; Shulman et al., 1999], or spatial location [Hopfinger et al., 2000; Kastner et al., 1999]. We shall refer to these paradigms here as “preceding attention” tasks. Although both approaches have been used to demonstrate enhanced activation in extrastriate visual areas during attended versus unattended stimulus processing, the preceding attention approach also allows the study of attention‐related baseline activity increases occurring before stimulus onset.
In contrast to the visual domain, relatively few imaging studies have investigated attentional modulation in emotional stimulus processing. Most of these studies have used simultaneous attention paradigms. Within these paradigms, two variants can be distinguished. The first variant is based on the comparison between explicit and implicit tasks in relation to emotional photographs [Fichtenholtz et al., 2004; Keightley et al., 2003; Lane et al., 1997, 2001; Lange et al., 2003; Liberzon et al., 2000; Northoff et al., 2004; Teasdale et al., 1999; Winston et al., 2003]. The underlying assumption is that explicit tasks (e.g., pleasant vs. unpleasant) involve stronger emotional attention through selective attention to emotional aspects of the photograph (attended condition). In contrast, implicit tasks (e.g., indoors vs. outdoors) direct the attentional focus to nonemotional features of the stimulus (unattended condition). It is noteworthy that these studies found consistent activation in the medial prefrontal cortex (MPFC; including the anterior cingulate) during attended compared to unattended emotional picture viewing. The second variant uses distraction tasks to modulate attentional processes during emotional stimulus presentation [Lane et al., 1999; Pessoa et al., 2002; Vuilleumier et al., 2001]. The premise is that demanding distraction tasks suppress emotional attention because they compete for attentional resources with the processing of emotional stimuli. Two of these studies [Lane et al., 1999; Pessoa et al., 2002] also observed activation in the MPFC during low compared to high distraction conditions. In addition, studies of both variants reported activation of a variety of other regions such as the amygdala, midbrain, posterior cingulate, insula, lateral prefrontal cortex, and superior temporal gyrus. With regard to these regions, however, findings varied considerably across studies.
Several studies have employed expectancy paradigms to investigate preceding emotional attention. This includes the expectancy of emotional photographs [Ueda et al., 2003], painful stimuli [Buchel et al., 1998a, 1999; Jensen et al., 2003; LaBar et al., 1998; Ploghaus et al., 2003], monetary rewards [Breiter et al., 2001; Critchley et al., 2001; Knutson et al., 2001], and aversive or appetitive odor [Gottfried et al., 2002]. Whereas these studies concentrated on the period of expectancy preceding the actual emotional stimulus, our present study focused on the interaction effect between expectancy and emotion observed during the period of stimulus presentation. We intermittently presented an expectancy cue before emotional picture presentation (Fig. 1). Signaling an emotional picture, this expectancy cue induced preceding emotional attention to the subsequent picture. Some emotional pictures were not preceded by the expectancy cue. As a further control, neutral pictures were presented with and without neutral expectancy cues, resulting in a 2 × 2 factorial design with the factors “expectancy” and “emotion.” The interaction term between these factors allowed the identification of the expectancy effect specific for the emotional condition. By employing such an expectancy paradigm, we were able to modulate emotional attention without varying the task demands in relation to the stimuli. In our paradigm, attended (with expectancy) and unattended (without expectancy) conditions only differed in the period preceding the stimulus presentation. The period of stimulus presentation itself did not vary between conditions. In this way, we were able to avoid potentially confounding task‐related activation. Analogous to studies on feature‐selective attention in the visual domain, we hypothesized that emotional expectancy would enhance neural responses to emotional stimuli in key regions of the emotion network, such as the amygdala and MPFC. We anticipated that this expectancy effect would be specific for the emotional condition. Consistent with our hypothesis, we found larger activation in the MPFC (Brodmann area [BA] 9/10), amygdala, and dorsal midbrain during emotional stimulus processing when it was preceded by emotional expectancy. This augmentation of neural response was specific for the emotional condition (interaction effect) and was paralleled by a behavioral interaction effect, with intensity ratings specifically enhanced in emotional photographs preceded by expectancy.
Figure 1.
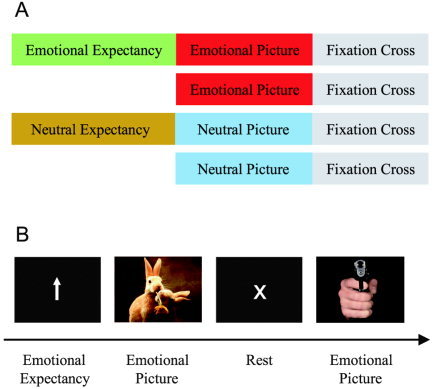
Paradigm for fMRI. A: Experimental conditions. Participants viewed emotional and neutral photographs. In half of the trials these were preceded by an expectancy period indicating whether the subsequent picture was emotional or neutral. Each picture was followed by a fixation cross. B: Example trials. The trials “emotional picture with preceding expectancy period” and “emotional picture without preceding expectancy period” are illustrated. Stimuli are not drawn to scale.
SUBJECTS AND METHODS
Subjects
Seventeen healthy volunteers (9 women, 8 men; age range, 21–37 years) with no history of neurological or psychiatric illness (based on a physician's examination and interview) participated in this study after giving written informed consent. The institutional review board of the Beth Israel Deaconess Medical Center approved this study.
Experimental Design
The paradigm distinguished between emotional (50% positive/pleasant, 50% negative/unpleasant) and neutral pictures (Fig. 1). Pictures were selected from the International Affective Picture System [IAPS, Lang et al., 1999] in a manner so that their standard valence scores were: (1) clearly positive, negative, or neutral; and (2) approximately balanced between positive and negative pictures relative to the neutral pictures. The mean for positive, negative, and neutral pictures was 7.68 (± 0.27, standard deviation [SD]), 2.00 (± 0.32), and 5.10 (± 0.34), respectively. Each picture presentation (5 s) was followed by a rest period (8.5 s) that allowed subjects to emotionally recover from the picture [Garrett and Maddock, 2001]. Before half of the photographs, attention‐directing cues were presented (expectancy period, 5 s; Fig. 1). An upwards‐pointing arrow indicated that an emotional photograph would follow (emotional expectancy). A horizontal arrow signaled a neutral picture (neutral expectancy). Half of the photographs were not preceded by an arrow (pictures without expectancy). The instruction for upwards‐pointing arrows was to build up attention for subsequent emotional picture presentation. During neutral expectancy, subjects had to build up attention for subsequent neutral picture presentation.
The paradigm distinguished between emotional and neutral conditions as well as between conditions with and without expectancy (Fig. 1), resulting in a 2 × 2 factorial design with the factors expectancy and emotion. Sixty‐four trials per condition were presented over eight runs. The four conditions were pseudorandomized and counterbalanced within and across runs. Between emotional conditions (with and without expectancy) as well as between neutral conditions, pictures were matched for valence, arousal, and dominance based on the normative data of the IAPS [Lang et al., 1999], thus ruling out confounding activation in the comparison between expected and nonexpected conditions. Because valence and arousal are correlated strongly in pictorial emotional stimuli [Anderson et al., 2003b; Lang et al., 1999], we were not able to match emotional and neutral pictures according to arousal. This mismatch, however, did not affect our main findings, which concern the term (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy), reflecting the positive interaction between the factors expectancy and emotion.
The emotional stimuli presented were taken from a standardized picture set [IAPS; Lang et al., 1999] that is used widely to study the comparison between positive (i.e., pleasant), negative (i.e., unpleasant), and neutral stimulus processing. Pictures from this set are matched largely between valences with regard to color, luminance, complexity, and semantic content [Lang et al., 1999]. For instance, a negative picture drawn from this set shows a suicidal man with a gun, whereas the corresponding neutral picture shows the same man in the same position holding a hairdryer. We acknowledge that we cannot rule out slight differences in visual properties between single pictures of different conditions. Given that there were 64 trials (pictures) for each condition, however, we assume that most of the remaining variability was canceled out over the course of the experiment. The nonpictorial stimuli (arrows, fixation cross) were of equal size, color, and luminance and were centered on a black background.
Before the experiment, subjects were familiarized with the paradigm by completing a test run with 20 trials. Subjects were instructed to promptly press a button whenever they saw a photograph. This button press allowed the monitoring of the attentiveness of the subject. The button press did not require a specific judgment task that could have modulated the emotional response [Taylor et al., 2003]. This simple task design thus allowed the functional magnetic resonance imaging (fMRI) investigation of emotional stimulus processing and its attentional modulation unaffected of interfering cognitive task demands. Due to technical difficulties, reaction times were not recorded in three subjects.
Picture judgments were conducted outside the scanner 1 day after the fMRI session. The paradigm (including expectancy periods) was presented to the subjects for a second round, this time, each picture was followed by a task period that consisted of emotional intensity rating, valence rating, and surprise recognition test. All three responses were given using a visual analogue scale. Emotional intensity rating scores ranged from low (1) through medium (5) to high (9). Similarly, valence assessment ranged from very negative (1) through neutral (5) to very positive (9). For picture recognition, choices ranged from “definitely not seen during fMRI” (1) through “maybe” (5) to “definitely seen” (9). For the purpose of the surprise recognition task, 24 new photographs were included in the slide show. The surprise recognition task was not tested in one subject due to technical difficulties. We were aware that emotional responses might attenuate when pictures are seen for a second time [Ishai et al., 2004]; however, this repetition effect applies equally to all picture conditions and was not expected to affect the differences between conditions.
Functional MRI Data Acquisition
MR images were acquired on a 3 Tesla GE VH/1 (Milwaukee, WI) whole‐body scanner equipped with echo planar imaging (EPI) capabilities using the standard head coil for radiofrequency transmission and signal reception. A 3D T1‐weighted structural image (1 mm3 voxel size) was acquired for each subject for anatomical reference. For functional imaging, a gradient‐echo EPI sequence was used with a repetition time (TR) of 3.016 s, an echo time (TE) of 20 ms, and a matrix of 64 × 64. Using a midsagittal scout image, 36 contiguous axial slices were acquired parallel to the bicommissural plane covering the entire brain in less than 3 s (flip angle = 90 degrees, field of view [FOV] = 24 cm, 3 mm slices, skip 1 mm). In total, 196 T2*‐weighted functional images were acquired per run. The first four acquisitions of each run were discarded due to T1‐saturation effects. Blood oxygenation level‐dependent (BOLD) images were reconstructed to yield isotropic voxels, 4 mm on edge.
Because the gradient‐echo echoplanar images used for fMRI can be degraded in the presence of nonuniform magnetic fields, we paid special attention to the image quality in the anterior medial temporal lobes and orbitofrontal brain regions. First, pilot echoplanar images were obtained and were visually inspected before fMRI acquisition to ensure good image quality in the brain regions of interest. Second, the images were corrected for residual geometric distortion [Jezzard and Balaban, 1995] based on a magnetic field map acquired with a 1‐min reference scan [Alsop, 1995]. This correction realigns the echoplanar images with the higher quality T1 images used for determining the transformation to the standard atlas.
Functional MRI Analysis
Image processing and statistical analysis were carried out using SPM99 (Wellcome Department of Imaging Neuroscience, London, UK). Each set of functional volumes was realigned to the first volume, spatially normalized [Friston et al., 1995a] to a standard SPM99 template based upon the Montreal Neurological Institute (MNI) reference brain [Evans et al., 1993], and finally smoothed using an 8‐mm full‐width half‐maximum (FWHM) Gaussian kernel. The effect of global differences in scan intensity was removed by scaling each scan in proportion to its global intensity. Low‐frequency drifts were removed using a temporal high‐pass filter with a frequency of 1/200 Hz. High‐frequency drifts were removed by applying a low‐pass filter convolving our data with the hemodynamic response function (HRF). Before statistical analysis, a whole‐brain mask was created and was specified explicitly based on each subject's normalized in‐plane anatomical image. This was done to ensure that statistics were carried out in all brain regions, including those where signals may be low in some subjects due to susceptibility artifacts (K. Christoff, http://www-psych.stanford.edu/~kalina/SPM99/Tools/glm_specmask.html).
Condition and subject effects were estimated using general linear model approach [Friston et al., 1995b]. We modeled six regressors of interest, convolved with the canonical HRF as implemented in SPM99 [Friston et al., 1998]. Besides the four experimental conditions (emotional and neutral pictures with and without preceding expectancy; Fig. 1), we also modeled the emotional and neutral expectancy periods preceding the picture presentation. Although not involved in the comparisons of this study, these two conditions were modeled to reduce the possible confound of picture period by preceding expectancy‐related BOLD responses. Main comparisons involving the periods of emotional and neutral expectancy will be analyzed in a separate study. In a first‐level analysis, parameter estimates of stimulus‐related activity were obtained at each voxel for each regressor and each subject [Friston et al., 1995b]. Contrast images were constructed, whereby the size of a given effect at each voxel constitutes the image. For second‐level random‐effects analysis [Friston et al., 1999], these single‐subject contrasts were entered into one‐sample t‐tests across the 17 subjects. Corresponding to the 2 × 2 factorial design of our paradigm with the factors expectancy and emotion, statistical parametric maps were estimated for the main effects of expectancy and emotion as well as for the positive and negative interaction between both factors. The positive interaction effect was determined using the serial subtraction (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy). Analogously, the negative interaction term was (emotional picture without expectancy > emotional picture with expectancy) > (neutral picture without expectancy > neutral picture with expectancy). The positive interaction term was then decomposed by studying the expectancy effect for emotional and neutral conditions separately (emotional picture with expectancy > emotional picture without expectancy and neutral picture with expectancy > neutral picture without expectancy). Finally, a valence‐oriented analysis was carried out distinguishing between positive (i.e., pleasant) and negative (i.e., unpleasant) picture conditions. For this analysis, we separately modeled positive and negative picture periods (with and without preceding expectancy, respectively) as regressors of interest. The individual interaction contrasts (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy) were entered into paired t‐tests across the 17 subjects comparing the interaction effect between positive and negative picture conditions.
A global height threshold was set at P < 0.001 (uncorrected) corresponding to a t score > 3.69. We indicate where results survive corrections for multiple comparisons at P < 0.05 [false discovery rate (FDR); Genovese et al., 2002]. In relation to the ongoing debate on potential attentional modulation of amygdala activity [Pessoa et al., 2002; Vuilleumier et al., 2001], small volume correction (SVC; P < 0.05) was carried out for this a priori region of interest [Worsley et al., 1996]. The SVC was based on a sphere centered on the peak amygdala activation revealed by the contrast emotional vs. neutral pictures (6‐mm radius, corresponding to the approximate size of the amygdala; x/y/z = 24, −4, −24 and x/y/z = −24, −8, −20). Given the small size and the shape of the amygdala, as well as the voxel size of 4 mm and the smoothing kernel of 8 mm, it is acknowledged that our procedure of SVC does not allow the complete isolation of amygdala activation from activation in adjacent structures, e.g., the hippocampus and parahippocampal gyrus.
For correlation analysis, individual interaction effects in brain activation and in emotional intensity rating were correlated with each other. This analysis was carried out for the peak voxels in MPFC, midbrain, left and right amygdala as identified by the positive interaction contrast (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy; cf. Table I). The individual interaction effect on brain activation was extracted from the statistical parametric t map created for this contrast. The individual interaction score in intensity rating was determined by subtracting the expectancy effect in neutral pictures from the effect in emotional pictures for each subject.
Table I.
Maximum t values and peak voxel coordinates for regions activated in main and interaction contrasts
| Region | Pictures | Expectancy* emotion interaction | ||
|---|---|---|---|---|
| Expected > nonexpecteda | Emotional > neutrala | Positive | Negative | |
| L superior/medial frontal gyrus (BA9, 10) | — | 3.29 (−4, 56, 20) | 4.66 (−8, 64, 24) | — |
| R superior/medial frontal gyrus (BA9, 10) | 5.30 (12, 64, 20) | — | — | — |
| L middle/inferior frontal gyrus (BA8) | 4.76 (−44, 20, 52) | — | — | — |
| R middle/inferior frontal gyrus (BA9, 44, 45, 46) | — | 7.49 (48, 8, 20) | — | — |
| R middle/inferior frontal gyrus (BA10) | — | — | — | 6.28 (40, 56, 8) |
| L precentral gyrus (BA6) | — | — | — | 5.02 (−64, 0, 20) |
| L amygdala | — | 5.27 (−24, −8, −20) | 3.16 (−20, −8, −20)b | — |
| R amygdala | — | 5.26 (24, −4, −24) | 3.11 (28, −4, −28)b | — |
| L hippocampus | 4.95 (−28, −28, −8) | 5.42 (−28, −12, −20) | — | — |
| R hippocampus | 5.24 (32, −16, −20) | 4.40 (28, −12, −20) | — | — |
| L putamen | 4.63 (−28, −20, 0) | — | — | — |
| R putamen | — | 4.14 (16, −4, 8) | — | — |
| L thalamus | 4.67 (−20, −28, −4) | — | — | — |
| R thalamus | 5.40 (16, −4, 12) | — | — | — |
| Dorsal midbrain | — | 7.09 (−8, −28, −12) | 5.69 (−4, −28, −12) | — |
| R insula | — | 4.99 (44, 12, 12) | — | — |
| L superior temporal gyrus (BA42) | — | — | — | 4.43 (−68, −28, 16) |
| L temporal pole (BA38) | 5.84 (−52, 20, −16) | — | — | — |
| L middle/inferior temporal gyrus (BA37, 39) | 5.17 (−56, −56, −20) | 7.51 (−48, −80, 12) | — | — |
| R superior temporal gyrus (BA22) | — | — | — | 6.39 (60, −28, 0) |
| R temporal pole (BA38) | 4.57 (52, 16, –20) | — | — | — |
| R middle/inferior temporal gyrus (BA37, 39) | 5.91 (40, −52, −24) | 10.17 (56, −64, 0) | — | — |
| L fusiform gyrus (BA37) | 5.07 (−40, −60, −20) | 8.33 (−52, −56, −24) | — | — |
| R fusiform gyrus (BA37) | 5.91 (40, −52, −24) | 8.42 (44, −48, −24) | — | — |
| L inferior parietal lobule (BA40) | 4.99 (−60, −60, 36) | — | — | 5.26 (−48, −40, 36) |
| R inferior parietal lobule (BA40) | — | — | — | 6.02 (48, −40, 36) |
| Posterior cingulate gyrus (BA23, 31) | — | — | — | 5.71 (0, −28, 36) |
| L cerebellum | 4.89 (−40, −60, −24) | 8.55 (−52, −60, −28) | — | — |
| R cerebellum | 5.90 (12, −76, −20) | 9.42 (44, −56, −28) | — | — |
| L occipital and lingual gyri (BA17, 18, 19) | 7.68 (−8, −88, −4) | 7.12 (−4, −92, 4) | — | — |
| R occipital and lingual gyri (BA17, 18, 19) | 4.76 (16, −84, −4) | 9.73 (4, −68, −4) | — | — |
P < 0.001 uncorrected at a voxel‐level unless otherwise noted. L, left; R, right.
Also significant at P < 0.05 false discovery rate‐corrected at a voxel level.
P < 0.05 corrected for multiple comparisons across a small volume of interest.
For regions revealed by the positive interaction contrast (MPFC, amygdala, and midbrain), we explored the time course of activation for the expectancy period. For this purpose, we resampled the time series of the BOLD signals in 2‐s time bins [cf. Sakai and Passingham, 2003]. The resampling was based on jitter decomposition. Parameter estimates were extracted from the time bins and plotted against time. The size of effect within each bin was averaged across the experiment for the 17 subjects, separately for each of the four conditions and each peak voxel. Time bins covered the time period from baseline (three time points) to seven time points after the onset of picture presentation. To standardize the baselines between conditions, we normalized the curves by defining the average of the three baseline time points as zero for each condition. Data were not convolved with the canonical HRF for this time course analysis.
RESULTS
Behavioral Performance
The expectancy paradigm was presented to the subjects twice, during fMRI and in a post‐scanning session. The second session allowed the investigation of the effect of preceding expectancy on subjective picture rating and recognition.
Emotional intensity ratings (Fig. 2) showed significant effects for the factors emotion (repeated‐measures analysis of variance [ANOVA]; F [1,16] = 120.5, P < 0.001) and expectancy (F [1,16] = 10.5, P = 0.005), and the interaction term between expectancy and emotion (F [1,16] = 9.1, P = 0.008). Emotional photographs received higher intensity scores than neutral photographs did (mean difference, 2.91 ± 0.27 standard error of the mean [SEM]). At the same time, photographs were rated as more intense after an expectancy cue, especially in the emotional condition where the mean expectancy effect was 0.24 (± 0.07). Even though the expectancy effect was smaller than the emotion effect was, it was consistent across the subjects, which explains the significant differential effect in the repeated‐measures analysis.
Figure 2.
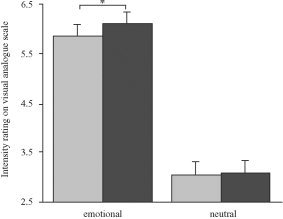
Intensity rating. Emotional intensity of pictures was assessed using a nine‐point visual analogue scale. Larger values on the ordinate reflect higher intensity scores. Emotional pictures were rated significantly more intense when they were preceded by expectancy. Error bars show the standard error of the mean (SEM). *P < 0.05.
Valence ratings revealed that pictures classified as emotional and neutral in the paradigm were experienced as such. The mean valence scores for positive, negative, and neutral pictures were 7.26 (± 0.18), 5.14 (± 0.07), and 1.81 (± 0.13), respectively.
Recognition performance, represented by d′ [e.g., Green and Swets 1966], showed a significant effect for the factor emotion (repeated‐measures ANOVA; F [1,15] = 8.9, P = 0.009) with better performance in the emotional condition. There was no significant effect for the factor expectancy (F [1,15] = 2.8, P = 0.12) and the interaction term between expectancy and emotion (F [1,15] = 0.4, P = 0.53).
The on‐line button press served to assess the attentiveness of the subjects during the fMRI session. No judgment was required. Reaction times showed a significant effect of expectancy in the repeated‐measures ANOVA (F [1,13] = 39.6, P < 0.001) with faster responses in the conditions with preceding expectancy. By contrast, the effect of emotion (F [1,13] = 0.08, P = 0.79) and the interaction between expectancy and emotion (F [1,13] = 0.57, P = 0.47) did not reach significance.
Functional MRI Data
Main effect of expectancy
To investigate the effect of expectancy on pictorial stimulus processing, brain activity during the presentation of pictures was compared between conditions with and without preceding expectancy (Table I). Because emotional and neutral pictures were collapsed in this contrast, this analysis revealed activation related to expectancy independent of emotion. The comparison revealed activation in the medial and lateral prefrontal cortex, hippocampus, putamen, thalamus, cerebellum, inferior parietal lobule, temporal pole, and occipitotemporal visual regions. These signal increases were significant at P < 0.05 FDR‐corrected. No significant expectancy effect was found in the amygdala at P < 0.05 SVC.
Main Effect of Emotion
To identify the effect of emotion in our study group, we contrasted brain activity during emotional versus neutral pictures (Table I). Because pictures with and without expectancy were not distinguished in this contrast, this comparison revealed activation related to emotional stimulus processing independent of expectancy. The main effect of emotion involved a number of regions that had also shown an expectancy effect. These common regions include the MPFC, lateral prefrontal cortex, hippocampus, putamen, cerebellum, and occipitotemporal visual regions. In addition, the effect of emotion also revealed regions that had not appeared in the above expectancy contrast, such as the amygdala, dorsal midbrain, and insula.
Interaction Between Expectancy and Emotion
To identify the expectancy effect specific for the emotional condition, we tested the positive interaction between expectancy and emotion (Fig. 3, Table I). This analysis was critical to the experiment to cancel out general expectancy effects such as picture expectancy. The positive interaction contrast (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy) revealed a specific effect of emotional expectancy in the MPFC and dorsal midbrain (P < 0.001 uncorrected). Figure 3C indicates that the MPFC showed this effect more pronounced during positive (i.e., pleasant) picture conditions and the midbrain during negative (i.e., unpleasant) picture conditions. A significant positive interaction effect was also observed in the amygdala after SVC (P < 0.05 SVC). The negative interaction contrast (emotional picture without expectancy > emotional picture with expectancy) > (neutral picture without expectancy > neutral picture with expectancy), identifying the effect of “unexpectedness” specific for the emotional condition, showed activations in the lateral prefrontal cortex, superior temporal gyrus, inferior parietal lobule, and posterior cingulate gyrus (P < 0.001 uncorrected; Table I).
Figure 3.
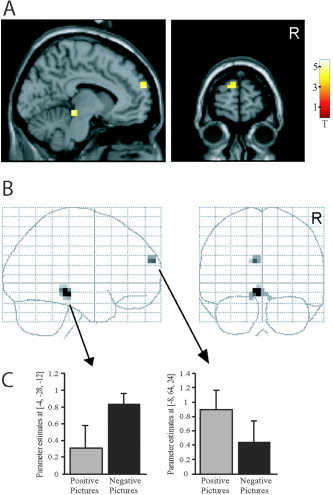
Positive interaction effect. Regions showing activation in the contrast (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy), i.e., positive interaction between emotion and expectancy, presented in section A (through x = −8, y = 64, z = 24) and B glass‐brain views. P < 0.001 uncorrected. The bar diagram (C) separately indicates the contribution of positive (i.e., pleasant) and negative (i.e., unpleasant) picture conditions to the effects observed in midbrain and medial prefrontal cortex.
In the next step, we decomposed the positive interaction term in a post‐hoc analysis (Table II). To determine the effect of expectancy in the emotional condition, brain activity during the presentation of emotional pictures was compared between conditions with and without preceding expectancy (Fig. 4, Table II). This contrast revealed activation in the medial and lateral prefrontal cortex, hippocampus, putamen, thalamus, dorsal midbrain, temporal pole, cerebellum, and occipitotemporal visual regions. These signal increases were significant at P < 0.05 FDR‐corrected. A significant expectancy effect was also found in the amygdala after SVC (P < 0.05 SVC). To assess the effect of expectancy on picture processing independent of emotional processes, we compared brain activity during the presentation of neutral pictures between conditions with and without expectancy (Table II). Like the expectancy effect in the emotional condition, this contrast revealed activation in the lateral prefrontal cortex, putamen, thalamus, and occipitotemporal visual regions. The expectancy effect in the neutral condition did not involve the MPFC, amygdala, and dorsal midbrain.
Table II.
Maximum t values and peak voxel coordinates for regions activated in post‐hoc contrasts
| Region | Expected > nonexpected | |
|---|---|---|
| Emotional pictures* | Neutral pictures | |
| L superior/medial frontal gyrus (BA9,10) | 6.22 (−8,60,20) | — |
| L middle/inferior frontal gyrus (BA10,47) | 4.26 (−56,32,−8) | 4.39 (−48,52,−8) |
| R middle/inferior frontal gyrus (BA10,47) | — | 4.36 (48,52,−4) |
| R amygdala | 4.38 (24,0,−28)a | — |
| L hippocampus | 4.44 (−28,−32,−8) | — |
| R hippocampus | 5.24 (32,−16,−20) | — |
| L putamen | 4.64 (−28,−16,−8) | 4.44 (−28,−24,−4) |
| L thalamus | 4.05 (−16,−12,8) | — |
| R thalamus | 4.57 (24,−28,0) | 4.21 (16,−4,12) |
| Dorsal midbrain | 3.90 (8,−36,−20) | — |
| L temporal pole (BA38) | 5.54 (−52,16,−16) | — |
| R temporal pole (BA38) | 4.93 (52,16,−16) | — |
| L fusiform gyrus (BA37) | 4.70 (−40,−56,−24) | 5.14 (−40,−60,−20) |
| R fusiform gyrus (BA37) | 4.16 (40,−52,−24) | 5.05 (52,−52,−20) |
| L inferior parietal lobule (BA40) | — | 4.53 (−60,−60,40) |
| R inferior parietal lobule (BA40) | — | 5.06 (56,−60,44) |
| L cerebellum | 4.44 (−36,−72,−24) | — |
| R cerebellum | 8.86 (12,−72,−20) | — |
| L occipital and lingual gyri (BA17,18,19) | 7.66 (−8,−76,−12) | 7.52 (−4,−84,16) |
| R occipital and lingual gyri (BA17,18,19) | 5.83 (16,−84,−4) | 4.39 (4,−88,0) |
P < 0.001 uncorrected at a voxel‐level unless otherwise noted.
Also significant at P < 0.05 false discovery rate (FDR)‐corrected at a voxel‐level.
P < 0.05 corrected for multiple comparisons across a small volume of interest.
Figure 4.
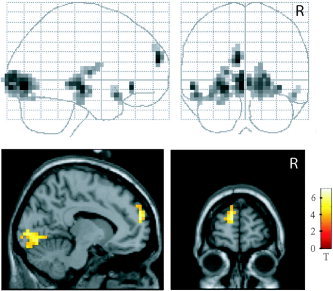
Emotional expectancy effect. Comparison between emotional conditions with and without preceding expectancy, presented in glass‐brain and section (through x = −8, y = 60, z = 20) views. The section views do not display all regions activated in this contrast (cf. Table II). P < 0.05 false discovery rate (FDR)‐corrected.
Finally, we compared the interaction effect (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy) between positive (pleasant) and negative (unpleasant) picture conditions. We found larger interaction effects in the left (x = −40, y = −4, z = −4) and right (x = 40, y = 0, z = −8) insula during negative (unpleasant) compared to positive (pleasant) picture conditions (P < 0.001 uncorrected). Positive picture conditions showed larger interaction effects in the left (x = −56, y = −40, z = −8) and right (x = 48, y = −32, z = −12) middle temporal gyrus (BA 21) as well as in the cuneus (x = 0, y = −92, z = 16) relative to negative conditions (P < 0.001 uncorrected). No significant valence differences were observed in the MPFC and dorsal midbrain at P < 0.001 uncorrected and in the amygdala at P < 0.001 uncorrected and P < 0.05 SVC.
Correlation Between fMRI and Behavioral Data
Because the positive interaction term (emotional picture with expectancy > emotional picture without expectancy) > (neutral picture with expectancy > neutral picture without expectancy) best reflects attentional modulation specific for emotional stimulus processing, our correlation analysis focused on this effect. In fMRI, interaction between expectancy and emotion was present in the MPFC, midbrain, and amygdala, whereas at the behavioral level, a significant interaction was found in intensity rating. We therefore tested for correlation between the interaction effects in fMRI activation (peak voxels in MPFC, midbrain, and left and right amygdala; cf. Table I, third column) and intensity rating. This analysis revealed a significant correlation in the left amygdala (Fig. 5, Pearson r = 0.58, P = 0.014). The more that expectancy enhanced intensity rating scores specifically in emotional pictures, the larger was the interaction effect observed in the left amygdala.
Figure 5.
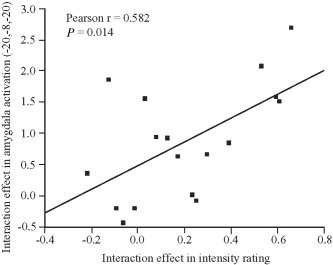
Correlation of interaction effects. Correlation between positive interaction effects in fMRI activation (left amygdala) and intensity ratings. The interaction effect on left amygdala activation was extracted from the peak voxel as identified by the positive interaction contrast (x = −20, y = −8, z = −20, cf. Table I). The interaction effect in intensity rating was determined by subtracting the expectancy effect in neutral pictures from the effect in emotional pictures.
Time Course of Activation
To illustrate the time course of the hemodynamic response in the MPFC, amygdala, and midbrain, the time series of BOLD signals was resampled in 2‐s time bins (Fig. 6). The emotional picture conditions with and without preceding expectancy both produced signal increases in the MPFC (Fig. 6A, cf. Table I). In contrast, no meaningful signal increases were found in the two neutral conditions. In good agreement with the predicted delay of the hemodynamic response, the signal peak was seen approximately 6 s after the onset of the emotional picture and decayed over the course of the subsequent rest period. Larger signal increases were observed during emotional pictures when they were preceded by emotional expectancy; this observation reflects the above‐reported significant signal increases in the statistical parametric mapping contrast emotional picture with expectancy > emotional picture without expectancy (Fig. 4, Table II). Similar time courses were observed in the right amygdala (Fig. 6B) and dorsal midbrain (Fig. 6B) during the picture period. As with the MPFC, emotional pictures produced larger signal intensities than neutral pictures did, and greater signal increases were seen when emotional pictures were preceded by expectancy (cf. Fig. 4, Table II). In the MPFC and amygdala, signal increases relative to rest were only observed 6–10 s after the onset of the expectancy cue (i.e., 1–5 s after picture onset). The left amygdala showed time courses similar to the right amygdala. During the expectancy period, we observed no signal increases in the MPFC and amygdala, whereas slight signal increases were seen in the midbrain.
Figure 6.
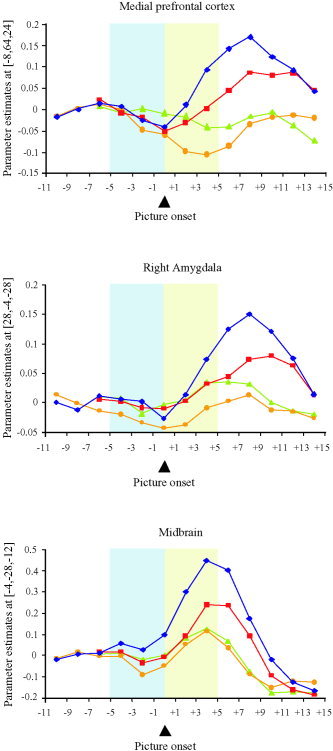
Time histogram. Size of effect in the medial prefrontal cortex (MPFC), right amygdala, and dorsal midbrain over time. The curves represent the four conditions, emotional pictures with expectancy (rhomb), emotional pictures without expectancy (squares), neutral pictures with expectancy (circles), and neutral pictures without expectancy (triangles). The picture period (shaded area after picture onset) is preceded by the expectancy period (shaded area before picture onset) in the conditions with expectancy. In the conditions without expectancy, the picture presentation is preceded by the rest period. Values refer to the peak voxels as identified by the positive interaction contrast (cf. Table I): MPFC: x = −8, y = 64, z = 24. Right amygdala: x = 28, y = −4, z = −28. Dorsal midbrain: x = −4, y = −28, z = −12. The peak voxel in the left amygdala (x = −20, y = −8, z = −20) showed a time course similar to the right amygdala. Comparable findings were obtained when the analysis referred to clusters of activation (cf. Fig. 3A,B) instead of peak voxels.
DISCUSSION
The aim of the present experiment was to investigate how expectancy affects the processing of emotional compared to neutral stimuli. In fMRI, we observed a significant positive interaction between the factors emotion and expectancy in the medial prefrontal cortex (MPFC, BA 9/10), amygdala, and dorsal midbrain. At the behavioral level, we also found a significant interaction effect with higher intensity ratings to emotional photographs when they were preceded by expectancy. These findings suggest that expectancy specifically augments the neural response to emotional stimuli in specific components of the emotion network. This enhancement of brain activity might reflect accentuated subjective feeling states.
Interaction Between Expectancy and Emotion
Our expectancy paradigm refers to studies on feature‐selective attention in the visual domain [Chawla et al., 1999; Hopfinger et al., 2000; Kastner et al., 1999; Shulman et al., 1999]. In these studies, the expectancy of a specific stimulus feature (e.g., color, motion) has been shown to induce increased activation in appropriately specific visual areas during subsequent stimulus presentation. Analogous to these findings, the present study hypothesized that emotional expectancy would enhance the neural response to emotional stimuli in specific regions of the emotion network. To identify brain regions showing such enhanced activation, we contrasted emotional picture conditions with versus without preceding expectancy. To control for nonspecific aspects of expectancy (e.g., picture expectancy), we subtracted the expectancy effect in the neutral condition from the above contrast. The resulting positive interaction term revealed activation in the MPFC (BA 9/10), amygdala, and midbrain. These three regions were also identified as part of the emotion network with the contrast “emotional versus neutral pictures.” We conclude that these three regions represent the part of the emotion network whose response to emotional stimuli is particularly modulated by emotional expectancy.
The fMRI findings were paralleled by a corresponding interaction effect in subjective picture rating. Emotional pictures received higher emotional intensity rating scores when they were preceded by emotional expectancy. This expectancy effect was specific for emotional pictures (as opposed to neutral pictures) and for the affective rating (emotional intensity) as opposed to the cognitive control task (surprise recognition test). Taken together, the behavioral data show that increased selective attention to an emotional stimulus induced by emotional expectancy is associated with accentuated subjective feeling states.
Simultaneous and Preceding Attention
Our data show that expectancy enhances the neural response to emotional but not neutral stimuli in the MPFC, amygdala, and midbrain. Because emotional expectancy can be considered a form of preceding attention, one may interpret our findings as attentional modulation of emotional stimulus processing. This is one of the first studies to examine attentional modulation using emotional expectancy. Previous studies have addressed this issue by adopting simultaneous attention paradigms [Fichtenholtz et al., 2004; Keightley et al., 2003; Lane et al., 1997, 1999, 2001; Liberzon et al., 2000; Northoff et al., 2004; Pessoa et al., 2002; Winston et al., 2003]. Besides the MPFC, amygdala, and dorsal midbrain, these studies also observed attentional modulation in a variety of other brain regions such as the posterior cingulate gyrus, insula, lateral prefrontal cortex, and superior temporal gyrus. Differences in findings may well be related to paradigm differences. In contrast to our expectancy paradigm, simultaneous attention paradigms vary task demands in relation to emotional stimuli to modulate attention. Across conditions, tasks might not differ in the attention factor exclusively but also in other aspects, e.g., cognitive processes involved or simply task difficulty [Fichtenholtz et al., 2004]. Given the mutual suppression between cognitive and emotional processes [Northoff et al., 2004], it cannot be ruled out that such task differences affect emotional stimulus processing and its modulation by attention. These differences are difficult to control for and might have contributed to activations inconsistently observed across studies. Because our paradigm did not vary the task demands present during picture presentation, we can exclude confounding task differences in our findings.
Medial Prefrontal Cortex
The positive interaction effect observed in the present study concerns the MPFC, amygdala, and midbrain. The dorsal part of the MPFC (BA 9/10) activated in our study has been implicated in a number of cognitive aspects of emotion processing [Blood et al., 1999; Blood and Zatorre, 2001; Drevets and Raichle, 1998; Fossati et al., 2003, 2004; Frith and Frith, 1999; Gusnard et al., 2001; Hornak et al., 1996; Northoff et al., 2004; Taylor et al., 2003]. Consistent with our results, several studies have suggested a role for the MPFC in emotion regulation [Beauregard et al., 2001; Ochsner et al., 2002, 2004; Phillips et al., 2003; Price, 1999] and more specifically in attentional modulation of emotion processing [Fichtenholtz et al., 2004; Keightley et al., 2003; Lane et al., 1997, 1999, 2001; Liberzon et al., 2000; Northoff et al., 2004; Pessoa et al., 2002; Winston et al., 2003]. These attention studies used simultaneous attention paradigms to study attentional modulation in emotional stimulus processing. They observed higher medial prefrontal activity when tasks required subjects to direct their attention to the emotional content of stimuli compared to the activity observed for implicit or distraction tasks.
In some of these simultaneous attention studies, the activation focus extended into the anterior cingulate (BA 24/32) [Fichtenholtz et al., 2004; Lane et al., 1997; Winston et al., 2003]. In our study, the main site of MPFC activation (as identified by the positive interaction term) was confined to the superior frontal gyrus at the border of BA 9 and BA 10. This activation did not extend into the anterior cingulate, even when the threshold was lowered to P < 0.05 uncorrected. We suggest that this discrepancy exists because in contrast to our paradigm, the simultaneous attention studies involved explicit cognitive tasks. This interpretation is supported by a recent meta‐analysis [Phan et al., 2002] showing that the anterior cingulate is specifically engaged in emotional paradigms that involve explicit cognitive tasks. In contrast, emotional conditions activate the more anterior part of the MPFC regardless of the presence or absence of explicit cognitive demand. Phan et al. [2002] therefore concluded that the MPFC is engaged in implicit cognitive aspects of emotion processing that are common across emotional tasks. Our results suggest that this implicit cognitive function is implicated with attentional modulation of emotional stimulus processing.
Our findings are also consistent with a recent positron emission tomography (PET) study on treatment‐specific effects of CBT in patients with major depression [Goldapple et al., 2004]. Before treatment, these patients demonstrated hyperactivity in the MPFC; after successful treatment with CBT, a significant signal decrease was observed in this region. Such decrease was not seen, however, with paroxetine‐facilitated clinical recovery. In the context of our present findings, one could speculate that the decrease of MPFC activity may reflect a CBT‐conditioned reduction of the attention bias toward negative contents in recovered patients, which might contribute to improvement of the emotional state.
Amygdala
Positive interaction between expectancy and emotion was also observed in the amygdala; however, this finding was less robust than was that in the MPFC and midbrain. This could be due in part to increased susceptibility‐induced signal losses and geometric distortions complicating echoplanar imaging in this region [Merboldt et al., 2001]. Alternatively, the amygdala might in fact be characterized by weaker expectancy‐related modulation. This alternative is in accordance with previous studies suggesting that the amygdala shows a considerable degree of automaticity in responding to emotional stimuli [Anderson et al., 2003a; Davidson et al., 2004; Dolan and Vuilleumier, 2003]. For example, the amygdala was activated not only during explicit but also during implicit emotional tasks, with implicit tasks involving less emotional attention [Winston et al., 2002, 2003]. Similarly, Vuilleumier et al. [2001] found that activation of the amygdala to emotional faces was relatively unaffected by a distraction task, whereas strong modulation was observed in the fusiform gyrus. Finally, studies using backward‐masked faces showed that the amygdala can even be activated during preattentive processing of emotional stimuli [Hendler et al., 2003; Morris et al., 1998; Whalen et al., 1998]. Nevertheless, there are also indications that amygdala activity is not entirely independent of attentional processes. Using a high demand distraction task, Pessoa et al. [2002] were able to eliminate differential responses to emotional faces in the amygdala. They concluded that the distraction task had exhausted attentional resources and that the processing of emotional faces in the amygdala required some degree of attention. Our study confirms this finding using a different paradigm to modulate attention. Whereas Pessoa et al. [2002] distracted subjects from emotional stimuli to eliminate neural responses, our study focused the subjects' attention to the stimuli to enhance the responses.
The interaction effect observed in our study showed a positive correlation between emotional intensity ratings and BOLD signals in the left amygdala. Subjects who showed particularly high expectancy effects in intensity ratings of emotional pictures revealed stronger interaction effects in the left amygdala. This finding suggests that attentional modulation of amygdala activity is particularly relevant for how intensely an emotional stimulus is subjectively experienced. This conclusion is in line with recent functional imaging studies that used olfactory [Anderson et al., 2003b], gustatory [Small et al., 2003], and visual [Phan et al., 2004; Rotshtein et al., 2001] stimuli to demonstrate a role of the amygdala in emotional intensity processing.
Recent neuroimaging studies have also implicated the amygdala in the detection of novel stimuli [Dubois et al., 1999; Schwartz et al., 2003; Whalen et al., 2001; Wright et al., 2003]. For instance, greater amygdala activation was observed during passive viewing of novel compared to that during viewing of familiar neutral faces [Schwartz et al., 2003]. In the present study, we observed greater activation in the amygdala when emotional stimuli were preceded by an expectancy cue. This effect was specific for the emotional condition (positive interaction between expectancy and emotion), and the reverse contrast (negative interaction term) did not reveal activation in any other section of the amygdala. One may therefore conclude that modulation of novelty did not play a relevant role in our paradigm. Apparently, the emotional expectancy cue employed here did not considerably reduce the extent of novelty associated with the subsequent emotional stimulus. This seems plausible, because all pictures (expected as well as nonexpected) were novel and the emotional expectancy cue did not specify what type of stimulus (pleasant or unpleasant) would follow.
Previous studies have observed reciprocal activation between amygdala and MPFC [Beauregard et al., 2001; Drevets and Raichle, 1998; Hariri et al., 2003; Keightley et al., 2003; Kim et al., 2003; Lange et al., 2003; Phan et al., 2005; Shin et al., 2004; Taylor et al., 2003]. Based on this pattern of activation and on the dense anatomical connections between these two structures [Amaral et al., 1992; Perez‐Jaranay and Vives, 1991], it has been suggested that the MPFC serves as a top‐down modulator of the emotional response in the amygdala [Davidson et al., 2000; Drevets, 1999; Morgan and LeDoux, 1995; Phillips et al., 2003]. At first glance, our results seem to contradict the above studies, given that we observed parallel rather than reciprocal activation (Fig. 6). Our result may be reconciled with these previously reported results, however, when the cognitive components involved are considered. It seems that the studies mentioned above observed activation in the MPFC during cognitive tasks that interfered by some means or another with emotional stimulus processing (e.g., distraction). Medial prefrontal activity might thus have suppressed the amygdala response in these studies. In the present paradigm, the cognitive component (expectancy) did not involve an explicit task demand, but rather served to direct the subject's attention to the emotional content of the picture. The medial prefrontal activation observed in the expected emotional condition might thus have enhanced the response in the amygdala. This interpretation is supported by our participants' own subjective ratings. Extending to previous work, our findings therefore suggest that the modulatory role of the MPFC might not only involve suppression but also enhancement of emotional responses in the amygdala.
In addition to top‐down modulation, one may also consider bottom‐up processes to interpret our results, suggesting that MPFC activity may arise as a function of amygdala activity, leading to apparently positively related time courses. The present data do not allow determination of which mechanism is predominant in our paradigm.
Midbrain
The third region activated in the positive interaction contrast was the dorsal midbrain. Given the small size of the anatomical structures located in the dorsal midbrain and the relatively low resolution of functional imaging data, it is difficult to assign this signal to a distinct anatomical region. The signal might derive from the periaqueductal grey, reticular nuclei, or raphe nuclei, all of which are implicated in the generation of autonomic responses associated with emotions. These regions possess dense anatomical connections with the MPFC and amygdala [Amaral et al., 1992; Price, 1999] that represent higher brain centers modulating emotional responses [LeDoux, 2000; Neafsey, 1990]. The midbrain signal observed in our study might also be associated with the inferior or superior colliculus. The superior colliculus is part of the extrageniculostriate visual pathway, which provides rapid but coarse emotion‐related signals to the amygdala. Coactivation of superior colliculus and amygdala was observed in previous neuroimaging studies on emotional responses [Morris et al., 2001; Vuilleumier et al., 2003].
Baseline Shifts and Gain Modulation
In the visual domain, gain modulation and baseline shifts have been proposed as mechanisms underlying the attentional regulation of the neural response to stimulus features [Kanwisher and Wojciulik, 2000]. Both (not mutually exclusive) concepts are aimed at explaining the enhanced activation in specialized regions during attended versus unattended stimulus processing. Gain modulation describes a situation where attention acts as a multiplier of the neural response [Kanwisher and Wojciulik, 2000]. In gain modulation, the difference between attended and unattended conditions results from an interactive (stimulus‐evoked) component of attention [Rees and Frith, 1998]. In contrast, baseline shifts represent an attention‐related additive elevation of the baseline firing rate in the attentionally modulated brain regions [Kanwisher and Wojciulik, 2000; Rees and Frith, 1998]. Because such a tonic component of attention is independent of stimulus‐evoked activity, it can also be observed during the expectancy of a specific stimulus feature. Expectancy paradigms therefore represent a useful tool to study baseline shifts. In these paradigms, baseline shifts become visible as expectancy‐related signal increases occurring before the onset of the actual stimulus. Such baseline increases have been found in specialized visual areas during the expectancy of color [Chawla et al., 1999], motion [Chawla et al., 1999; Shulman et al., 1999], and spatial location [Hopfinger et al., 2000; Kastner et al., 1999; Luck et al., 1997].
Employing an emotional expectancy paradigm, the present study allowed us to address the question whether baseline shifts might also be present in the emotion domain. To determine whether baseline shifts were preceding the enhanced activation observed in the MPFC, amygdala, and midbrain, the time course of the raw BOLD signals was plotted for these regions (Fig. 6). This time histogram did not reveal expectancy‐induced baseline increases in the MPFC and amygdala before the onset of emotional picture presentation. Based on the delay of the hemodynamic response observed in previous studies [e.g., Liao et al., 2002], one would hypothesize the expectancy‐evoked response to occur approximately 4–6 s after the onset of the expectancy cue. Parameter estimates extracted from this time bin, however, showed no signal increases compared to the previous time bins in the MPFC and the amygdala. In this region, signal increases relative to rest only began 8–10 s after the onset of the expectancy cue (i.e., 3–5 s after picture onset). Our findings therefore argue against baseline increases in the MPFC and amygdala before picture onset. One might speculate that in these regions, gain modulation rather than baseline shifts represents the mechanism predominantly underlying the enhanced neural response to emotional stimuli after expectancy. In contrast, in the dorsal midbrain the time histogram seems to reveal a slight signal increase during emotional expectancy relative to the preceding rest period. This could potentially reflect a positive baseline shift in this region during the expectancy of emotional stimuli. Our study, however, was not designed optimally to evaluate the issue of baseline shifts. Our data do not allow the exclusion of a late onset of expectancy‐related, stimulus‐independent signal increases. Such late baseline shifts could potentially be buried in the peak of activation observed during subsequent picture presentation. Nevertheless, this alternative seems unlikely for the MPFC and amygdala, because the baseline increases would not begin until 6–10 s (depending on the region) after the onset of the expectancy cue. To address this issue better, a future paradigm will also have to incorporate longer or unpaired expectancy periods, the latter representing expectancy conditions without subsequent picture presentation.
Besides unpaired cues, future expectancy studies might also consider the investigation of ambiguous and misleading expectancy cues. Ambiguous expectancy cues signal that an emotional or neutral picture is to come, whereas misleading cues provide false information. Both ambiguous and misleading cues could be useful to control for nonspecific expectancy effects (such as picture expectancy) present in contrasts between expected and nonexpected picture conditions. These more sophisticated expectancy conditions, however, will bring about considerable experimental challenges. Specifically, unpaired, ambiguous, and misleading expectancy conditions all involve further psychological factors, such as “uncertainty” and “distrust.” These factors are difficult to control for and are likely to compromise the psychological effect of expectancy in the main condition (congruent expectancy). Given the paucity of work on this issue, the present study focused on congruent expectancy conditions. To control for nonspecific expectancy effects also in absence of unpaired, ambiguous, and misleading conditions, the present study made use of the interaction contrast.
Another interesting variant of expectancy cue concerns valence‐selective expectancy [Buchel et al., 1998b, 1999; Gottfried et al., 2002; LaBar et al., 1998; Ueda et al., 2003]. In contrast to the emotional expectancy cue used in our study, valence‐selective cues provide information as to whether a pleasant or unpleasant stimulus will follow. In the present paradigm, we refrained from valence‐selective cues to prevent additional conditioning effects. Although any expectancy naturally depends on previous learning, valence‐selective expectancy cues stronger involve appetitive and aversive conditioning, both of which are associated with amygdala function [Bechara et al., 1995; Buchel et al., 1998b, 1999; Gallagher et al., 1990; Gottfried et al., 2002; LaBar et al., 1998; Parkinson et al., 2000]. Because our emotional expectancy cue was followed in equal proportions by pleasant and unpleasant pictures, the potentially confounding effect of appetitive or aversive conditioning should be minimal in our study. This assumption is supported by the time histogram, which does not show signal increases in the amygdala until 8–10 s after the onset of the emotional expectancy cue (i.e., 3–5 s after picture onset).
Emotional Attention and Arousal
As our paradigm involved emotional and neutral, as well as expected and nonexpected conditions, it is important to disentangle nonspecific effects such as arousal. Confounding arousal effects could in principle be due to differences between pictorial stimuli or to nonspecific effects of expectancy. In our experimental design, these issues were addressed as follows: pictorial stimuli were matched with regard to arousal, valence, and dominance between conditions with and without expectancy. The unavoidable mismatch between emotional and neutral pictures with regard to arousal does not affect our main findings, which did not concern the comparison between emotional and neutral conditions but rather the interaction term. To exclude nonspecific effects of expectancy, we incorporated neutral control conditions. The comparison of expectancy effects between emotional and neutral conditions (positive interaction contrast) allowed the identification of the expectancy effect specific for the emotional condition. Expectancy‐related nonspecific arousal effects were eliminated in this interaction contrast.
These methodological considerations aside, our findings further argue against a confounding arousal effect. First, arousal might be regarded as a nonspecific state of increased neural excitability. One may therefore expect that a general arousal effect would influence all parts of the emotion network in a similar (nonspecific) manner. Contrary to this hypothesis, the positive interaction contrast from our study identified a selective subset of emotional regions, namely the MPFC, amygdala, and midbrain. Second, if preceding emotional attention caused a nonspecific state of increased neural excitability accounting for the interaction effect observed, one would expect a general baseline increase in the MPFC and amygdala during the period of emotional expectancy. We observed no such baseline shift in these regions. Third, brain regions activated by the contrasts “all expected versus all non‐expected pictures” and “expected versus nonexpected neutral pictures” may be associated with an enhanced neural readiness to process stimuli (i.e., arousal). In our study, these regions were dissociable from the areas associated with the positive interaction between emotion and expectancy.
Despite all of these considerations, one could argue that the positive interaction effect involves an arousal component specific for the expected emotional condition. We cannot exclude such a specific arousal effect. It is acknowledged that this issue could have been addressed more appropriately by using objective psychophysiological measures (e.g., skin conductance).
CONCLUSIONS
Similar to studies on feature‐selective attention in the visual domain, we adopted expectancy (preceding attention) to study attentional modulation in the emotion domain. We observed that expectancy augmented the neural response to emotional stimuli in the amygdala, medial prefrontal cortex, and dorsal midbrain. This augmentation effect was specific for the emotional condition (significant positive interaction effect) and was paralleled by corresponding behavioral findings. When preceded by expectancy, emotional pictures received higher rating scores for emotional intensity. We conclude that preceding emotional attention enhances the response to emotional stimuli at behavioral and neuronal level.
Acknowledgements
This work was carried out at the Center for Non‐Invasive Brain Stimulation, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, and was supported by the Postdoc Programme of the German Academic Exchange Service (DAAD; D/02/46858 to F.B.), the National Institutes of Health (NCRR; K24 RR018875 to A.P.‐L.), and the German Research Foundation (DFG; Heisenberg 304/4‐1 to G.N.).
REFERENCES
- Alsop D (1995): Correction of ghost artifacts and distortion in echo‐planar MR imaging with an iterative image reconstruction technique. Radiology 197: 388. [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. 1992. Anatomical organisation of the primate amygdaloid complex In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. New York: Wiley‐Liss; p 1–66. [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD (2003a): Neural correlates of the automatic processing of threat facial signals. J Neurosci 23: 5627–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N (2003b): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P (2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR (1995): Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ (2001): Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA 98: 11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC (1999): Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci 2: 382–387. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P (2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 619–639. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ (1999): Amygdala‐hippocampal involvement in human aversive trace conditioning revealed through event‐related functional magnetic resonance imaging. J Neurosci 19: 10869–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ (1998a): The functional anatomy of attention to visual motion. A functional MRI study. Brain 121: 1281–1294. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ (1998b): Brain systems mediating aversive conditioning: an event‐related fMRI study. Neuron 20: 947–957. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ (1999): The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1990): Attentional modulation of neural processing of shape, color, and velocity in humans. Science 248: 1556–1559. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001): Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29: 537–545. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH (2000): Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull 126: 890–909. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Maxwell JS, Shackman AJ (2004): The privileged status of emotion in the brain. Proc Natl Acad Sci USA 101: 11915–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P (2003): Amygdala automaticity in emotional processing. Ann N Y Acad Sci 985: 348–355. [DOI] [PubMed] [Google Scholar]
- Drevets WC (1999): Prefrontal cortical‐amygdalar metabolism in major depression. Ann N Y Acad Sci 877: 614–637. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME (1998): Reciprocal suppression of rCBF during emotional versus higher cognitive processes. Cogn Emot 12: 353–385. [Google Scholar]
- Dubois S, Rossion B, Schiltz C, Bodart JM, Michel C, Bruyer R, Crommelinck M (1999): Effect of familiarity on the processing of human faces. Neuroimage 9: 278–289. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993): 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE Nuclear Science Symposium and Medical Imaging 3: 1813–1817. [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, McCarthy G, LaBar KS (2004): Emotion‐attention network interactions during a visual oddball task. Brain Res Cogn Brain Res 20: 67–80. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H (2003): In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H (2004): Distributed self in episodic memory: neural correlates of successful retrieval of self‐encoded positive and negative personality traits. Neuroimage 22: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998): Event‐related fMRI: characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley K, Poline J, Frith CD, Frackowiak RS (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ (1999): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U (1999): Interacting minds—a biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC (1990): The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci 10: 1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Maddock RJ (2001): Time course of the subjective emotional response to aversive pictures: relevance to fMRI studies. Psychiatry Res 108: 39–48. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H (2004): Modulation of cortical‐limbic pathways in major depression: treatment‐specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61: 34–41. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2002): Appetitive and aversive olfactory learning in humans studied using event‐related functional magnetic resonance imaging. J Neurosci 22: 10829–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. 1966. Signal detection theory and psychophysics. New York: Wiley; 455 p. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003): Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53: 494–501. [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben‐Bashat D, Malach R, Bleich A (2003): Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage 19: 587–600. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3: 284–291. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D (1996): Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 34: 247–261. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG (2004): Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci USA 101: 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S (2003): Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40: 1251–1257. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS (1995): Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 34: 65–73. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E (2000): Visual attention: insights from brain imaging. Nat Rev Neurosci 1: 91–100. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG (1998): Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 282 108–111. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL (2003): An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia 41: 585–596. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ (2003): Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14: 2317–2322. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998): Human amygdala activation during conditioned fear acquisition and extinction: a mixed‐trial fMRI study. Neuron 20: 937–945. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ (1999): Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 37: 989–997. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ (1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fort C, Johnson S, Ryan L, Trouard T (2001): Dissociable representations of emotional state in dorsal and ventral medial prefrontal cortex. Neuroimage 13: 437. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. 1999. International Affective Picture System (IAPS). Instruction manual and affective ratings (Rep. No. A‐4). Gainesville, Florida: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, Gray JA, Phillips ML (2003): Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry 53: 226–232. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Liao CH, Worsley KJ, Poline JB, Aston JA, Duncan GH, Evans AC (2002): Estimating the delay of the fMRI response. Neuroimage 16: 593–606. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S (2000): Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology 23: 508–516. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R (1997): Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Fransson P, Bruhn H, Frahm J (2001): Functional MRI of the human amygdala? Neuroimage 14: 253–257. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE (1995): Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681–688. [DOI] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ (2001): Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124: 1241–1252. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES (1999): Emotional bias and inhibitory control processes in mania and depression. Psychol Med 29: 1307–1321. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ (1990): Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res 85: 147–165; discussion 165–166. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual‐Leone A, Schlaug G (2004): Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional‐cognitive interaction. Hum Brain Mapp 21: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002): Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ (2004): For better or for worse: neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL (1997): Voluntary attention modulates fMRI activity in human MT‐MST. Neuron 18: 591–598. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ (2000): Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci 12: 405–413. [DOI] [PubMed] [Google Scholar]
- Perez‐Jaranay JM, Vives F (1991): Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res 564: 97–101. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME (2005): Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry 57: 210–219. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I (2004): Neural correlates of individual ratings of emotional salience: a trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003): Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D (2003): Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci 7: 197–200. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ (1980): Attention and the detection of signals. J Exp Psychol 109: 160–174. [PubMed] [Google Scholar]
- Price JL (1999): Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 877: 383–396. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD (1998): How do we select perceptions and actions? Human brain imaging studies. Philos Trans R Soc Lond B Biol Sci 353: 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshtein P, Malach R, Hadar U, Graif M, Hendler T (2001): Feeling or features: different sensitivity to emotion in high‐order visual cortex and amygdala. Neuron 32: 747–757. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE (2003): Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL (2003): Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol Psychiatry 53: 854–862. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK (2004): Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61: 168–176. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M (1999): Areas involved in encoding and applying directional expectations to moving objects. J Neurosci 19: 9480–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T (2003): Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39: 701–711. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I (2003): Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18: 650–659. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SC, Checkley SA (1999): Functional MRI study of the cognitive generation of affect. Am J Psychiatry 156: 209–215. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM (1998): The retinotopy of visual spatial attention. Neuron 21: 1409–1422. [DOI] [PubMed] [Google Scholar]
- Ueda K, Okamoto Y, Okada G, Yamashita H, Hori T, Yamawaki S (2003): Brain activity during expectancy of emotional stimuli: an fMRI study. Neuroreport 14: 51–55. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2001): Effects of attention and emotion on face processing in the human brain: an event‐related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2003): Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6: 624–631. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL (2001): A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1: 70–83. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ (2003): Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage 20: 84–97. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ (2002): Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 5: 277–283. [DOI] [PubMed] [Google Scholar]
- Worsley K, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, Rauch SL (2003): Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage 18: 660–669. [DOI] [PubMed] [Google Scholar]


