Abstract
Recent data suggest that chronic tinnitus is a “phantom auditory perception” caused by maladaptive neuroplasticity and subsequent hyperactivity in an extended neuronal network including the primary auditory cortex, higher‐order association areas, and parts of the limbic system. It was suggested that attenuation of this tinnitus‐associated hyperactivity may offer a rational option for lasting tinnitus reduction. Here, we tested the hypothesis that tinnitus loudness can be attenuated by low‐frequency repetitive transcranial magnetic stimulation (rTMS) individually navigated to cortical areas with excessive tinnitus‐related activity as assessed by [15O]H2O positron‐emission tomography (PET). Nine patients with chronic tinnitus underwent this combined functional imaging and rTMS‐study. Group analysis of the PET data showed tinnitus‐related increases of regional cerebral blood flow in the left middle and inferior temporal as well as right temporoparietal cortex and posterior cingulum. Repetitive TMS was performed at 1 Hz and 120% of the motor threshold for 5, 15, and 30 min, navigated to the individual maximum of tinnitus‐related cortical hyperactivity. A noncortical stimulation site with the same distance to the ear served as sham control. Tinnitus loudness was reduced after temporoparietal, PET‐guided low‐frequency rTMS. This reduction, lasting up to 30 min, was dependent on the number of stimuli applied, differed from sham stimulation, and was negatively correlated with the length of the medical history of tinnitus in our patients. These data show the feasibility and effectiveness of rTMS guided by individual functional imaging to induce a lasting, dose‐dependent attenuation of tinnitus. Of note, these effects were related to stimulation of cortical association areas, not primary auditory cortex, emphasizing the crucial role of higher‐order sensory processing in the pathophysiology of chronic tinnitus. Hum Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: positron emission tomography, auditory cortex, auditory pathways, neuronal plasticity, neuronavigation, parietal lobe, temporal lobe, cerebral cortex
INTRODUCTION
Tinnitus, i.e., the perception of elementary sounds or noise in the absence of auditory stimuli, is a frequent and often severely disabling symptom of different disorders of the auditory system [see Lockwood et al.,2002, for review]. Although specific training strategies [Flor et al.,2004; Jastreboff and Jastreboff,2000] and the use of antidepressants [Folmer and Shi,2004] as well as benzodiazepines [Gananca et al.,2002; Shulman et al.,2002] may offer relief to some patients, there are currently no effective treatments based on neurophysiological evidence. Up to now, efforts to develop rational therapies have been constrained by fragmentary pathophysiological knowledge.
However, recent evidence points to a pivotal role of the central nervous system and particularly cortical functions in the pathophysiology of tinnitus [Norena and Eggermont,2003]. In analogy to disorders like phantom limb pain [Flor et al.,1995] or focal dystonia [Bara‐Jimenez et al.,1998], chronic tinnitus may result from maladaptive neuroplastic changes of the central nervous system [Moller,2000; Muhlnickel et al.,1998]. In this pathophysiological model, “auditory phantom perception” is the consequence of deafferentation‐induced disinhibition in the central auditory system, reflected in irregular hyperactivity of neuronal networks integrated in the processing of auditory information [Eggermont and Roberts,2004; Rauschecker,1999]. Accordingly, recent imaging studies have focused on the role of the cerebral cortex for the development and perpetuation of tinnitus. Functional imaging data from [15O]H2O positron emission tomography (PET) in patients who could alter tinnitus loudness by voluntary movements of mouth or face [Lockwood et al.,1998,2001] or eye movements [Giraud et al.,1999] have pointed toward an association of tinnitus with increased regional cerebral blood flow (rCBF), predominantly in temporoparietal areas of the cerebral cortex. In line with these results, PET studies using transient reduction of tinnitus by lidocaine also revealed significantly increased rCBF in temporoparietal cortical activity during tinnitus perception [Andersson et al.,2000; Mirz et al.,1999; Reyes et al.,2002]. The data on the laterality of these changes vary between different studies with trials using lidocaine as the method for tinnitus suppression showing preferentially right hemispheric changes [Mirz et al.,1999; Reyes et al.,2002] and the other studies, a left hemispheric predominance [Giraud et al.,1999; Lockwood et al.,2001]. Studies on tinnitus using functional MRI (fMRI) are scarce. In patients with lateralized tinnitus, Melcher et al. [2000] showed a reduced blood oxygenation level‐dependent (BOLD) signal change in the contralateral inferior colliculus in response to auditory stimulation, suggesting subcortical abnormalities in chronic tinnitus. To some extent, the functional relevance of these activations for actual tinnitus perception remained unclear, because, for principal reasons, correlative rCBF‐ or BOLD signal‐based neuroimaging data are not suited to demonstrate causal relations between “activation” and behavior or symptom [Cohen et al.,1997]. Functional relevance of temporoparietal cortical areas for tinnitus perception was first verified by a short‐term suppression of tinnitus immediately after interference with the activity in these areas by high‐frequency (10 Hz, 120% of the motor threshold (MT), 3 s) transcranial magnetic stimulation (rTMS) [Plewnia et al.,2003a]. This initial finding was replicated and extended by studies demonstrating the tinnitus‐suppressing effects of rTMS of different frequencies [De Ridder et al.,2004,2005] and of high‐frequency rTMS as well as anodal transcranial direct current stimulation (tDCS) [Fregni et al.,2006], a technique to transiently modulate the excitability of cortical areas by transcranial application of a weak direct current (DC). In a large study of 114 patients with unilateral tinnitus, De Ridder et al. [2005] replicated, extended, and significantly strengthened the result that tinnitus can be transiently suppressed by rTMS (90% MT, 1–20 Hz, 200 pulses each) to an area near the auditory cortex on the contralateral side of the tinnitus in ∼53% of the patients. Most interestingly, the effectiveness of rTMS for tinnitus reduction decreased with the length of the medical history of tinnitus in these patients. Tinnitus duration correlated negatively with the stimulation frequency inducing maximal tinnitus suppression. In a recent study, Fregni et al. [2006] provided evidence that anodal tDCS of the left temporoparietal area may induce a similar transient tinnitus reduction, as does high‐frequency rTMS.
Going beyond the very short‐lasting “virtual lesion” effects of high‐frequency rTMS, low‐frequency rTMS (∼1 Hz) can be used for the induction of longer‐lasting reductions (up to 30 min) of focal cortical excitability [Chen et al.,1997; Plewnia et al.,2003b] and activity [Lee et al.,2003]. These long‐term depression (LTD)‐like changes can lead to relevant behavioral modifications [Hoffman et al.,2003; Siebner et al.,1999] and offer an interesting method for probing and possibly treating brain hyperexcitability syndromes [Hoffman and Cavus,2002].
Recently, analogous procedures have also been applied in tinnitus. In a first case series of three subjects, five daily sessions of low‐frequency rTMS were applied to a location near the primary auditory cortex [Eichhammer et al.,2003]. The study was performed in a crossover design with a sham‐coil mimicking the noise of rTMS. A specific benefit of rTMS could be demonstrated in one out of three patients. The other two patients showed a reduction of tinnitus in both the true rTMS and the sham condition. A 4‐week rTMS treatment of the one responding subject did not yield a further amplification of this effect [Langguth et al.,2003]. In another study by the same group [Eichhammer et al.,2003; Kleinjung et al.,2005; Langguth et al.,2003], 1 Hz rTMS was applied on 5 days to a temporal stimulation site. This intervention showed a discrete but lasting reduction of the subjective burden of tinnitus as quantified by a tinnitus questionnaire. No significant change was found after treatment with the placebo coil. The mean change in tinnitus score after stimulation was ∼8% (reduction). The general concept that cortical brain stimulation could ultimately provide an effective treatment to selected patients with otherwise untreatable tinnitus is further supported by a case report on the implantation of extradural electrodes over the auditory cortex of one subject resulting in permanent suppression of tinnitus [De Ridder et al.,2004]. However, the immediate effects of low‐frequency rTMS on tinnitus loudness, linking the tinnitus suppression with the excitability‐modulating properties of rTMS, have not been explored yet. Further evidence for the effectiveness of low‐frequency rTMS in chronic tinnitus is needed and can be gained by applying a technologically optimized approach with PET‐guided individual neuronavigation and testing for significant dose–effect relations. To this end, we used functional information from [15O]H2O PET to navigate low‐frequency rTMS of different durations to the area of highest tinnitus‐associated increase of rCBF in the temporoparietal cortex. A noncortical control stimulation (lower occiput) within the same distance to the ear served as comparably loud and uncomfortable control stimulation. Based on the present knowledge on the functional neuroanatomy of tinnitus and the neurophysiological effects of low‐frequency rTMS, we predicted that this strategy will lead to a reduction of tinnitus loudness that depends on the dose of rTMS (=number of stimuli) and outlasts the time of stimulation in the order of the known LTD‐like effects of rTMS (i.e., 30 min or more). Thus, we set out to provide further evidence for the significance of focal cortical hyperactivity as a relevant neurophysiological basis of tinnitus and a potential pathophysiology‐based target for novel treatment approaches.
MATERIALS AND METHODS
Study Design
In the present study, individuals with chronic tinnitus were (1) screened on the effect of an intravenous (i.v.) bolus of lidocaine on tinnitus loudness. In subjects who reported a transient reduction of tinnitus this intervention was used to obtain (2) individual and (3) general patterns of tinnitus‐related neuronal activity by [15O]H2O PET. Then, (4) in a randomized, controlled, crossover design low‐frequency rTMS was navigated to the maximum of individual tinnitus‐related activity and to a noncortical (sham) position. Intensity and duration of changes in tinnitus loudness were assessed after different numbers of rTMS stimuli. This randomized, placebo‐controlled, crossover trial was approved by the local ethics committee and was in accordance with the Declaration of Helsinki.
Screening Procedure
Sixteen patients were recruited from a general community sample, gave informed consent, and were screened for tinnitus suppression in response to a bolus injection of lidocaine in order to use this suppression as a control condition in PET perfusion‐imaging [Reyes et al.,2002]. Lidocaine (1.5 mg/kg) was injected i.v. over 1 min under ECG monitoring by a cardiologist because of the risk of lidocaine‐induced cardiac arrhythmia. Only patients with unequivocal tinnitus reduction were included in the further study.
Study Participants
Nine subjects (2 women, 7 men, 49–68 years old) with chronic (>1 year) compensated subjective tinnitus were included in the further study at the local PET center and the Hertie‐Institute for Clinical Brain Research. Detailed histories, otolaryngologic examinations, and standard audiometric measures were obtained by an otolaryngologist (Table I). Heart disease, previous history of seizures or brain lesions, metal implants, cardiac pacemaker, and current use of psychotherapeutic drugs were exclusion criteria.
Table I.
Patient data
| Subject | Age/sex | Duration (years) | Laterality | Audiometric measures* | Stimulation site | rTMS intensity |
|---|---|---|---|---|---|---|
| 1a | 59/m | 10 | Bilateral | 69 dB HL, pan | Right BA 39 | 91% |
| 2 | 59/m | 10 | Bilateral | 55 dB HL, pan | Left BA 39 | 74% |
| 3 | 58/m | 2 | Bilateral | 7 dB HL, hf | Right BA 39 | 58% |
| 4 | 49/m | 4 | Bilateral | 12 dB HL, hf | Left BA 22 | 82% |
| 5 | 57/f | 9 | Bilateral | 15 dB HL, hf | Left BA 39 | 96% |
| 6 | 68/m | 4 | Bilateral | 6 dB HL, hf | Right BA 22 | 55% |
| 7a | 68/m | 15 | Right | 6 dB HL, hf | Left BA 39 | 60% |
| 8 | 53/f | 4 | Bilateral | 15 dB HL, hf | Left BA 39 | 77% |
| 9b | 59/m | 10 | Bilateral | 21 dB HL, pan | Right BA 39 | 67% |
Hearing level (pure tone average = hearing loss at [0.5+1+2+3 kHz]/4); hf: high‐frequency hearing loss; pan: pancochlear hearing loss.
Nonresponder.
Excluded due to tinnitus increase after rTMS.
[15O]H2O PET
Neuronal activity, reflected in changes of rCBF associated with tinnitus perception, was assessed with [15O]H2O PET. Since it is a quiet method, it is especially suited for the investigation of central auditory functions, particularly tinnitus. All PET scans were acquired on 2 days with two conditions each: first scan at rest (“tinnitus‐ON”), then immediately after i.v. injection of lidocaine (“tinnitus‐OFF”). We followed this order in all subjects to avoid the contamination of the control condition by residuals of the drug effect. Earplugs were inserted and subjects were instructed to fixate on a red LED. After bolus injection of 1.8 GB [15O]H2O, dynamic data were acquired with a GE (Milwaukee, WI) Advance PET scanner in 3D mode. As an index for tissue perfusion, we calculated the sum of counts (no decay correction) from injection to 100 s after the turning point of the whole brain time–activity curve. Attenuation‐corrected images were calculated with the standard software of the scanner (FORE rebinning, 2D filtered backprojection). For individual patient analysis, all images were realigned with the software SPM2 (Wellcome Department of Cognitive Neurology, London, UK) and intensity normalized. A mean image (four scans) was calculated for anatomical orientation, difference images (tinnitus‐ON minus tinnitus‐OFF) were calculated after smoothing (10 mm Gauss) and thresholded at 5% of mean cortical activity. These thresholded individual difference images were used for rTMS navigation as described below.
Group effects in rCBF measured with PET were analyzed with the software SPM2. After realignment, all scans were transformed into standard space using the standard perfusion template and smoothed (12 mm Gaussian). For statistical analysis, each scan was assigned to one of two conditions (tinnitus‐ON or tinnitus‐OFF) resulting in 11 parameters (two conditions, nine subjects) that were estimated within the general linear model after proportional scaling. A voxel‐level t‐threshold of 3.41 was selected, corresponding with an uncorrected P < 0.001. For correction for multiple testing, we used SPM's built‐in method that compares cluster size with estimated image “smoothness.” For illustration purposes, we created maximum intensity projections (“glass brain”) with an extent threshold large enough to separate significant and not significant clusters (400 voxels, Fig. 1). To quantify the intensity of individual tinnitus‐specific patterns of neuronal activation, we calculated the mean ΔrCBF in a mask comprising all voxels of the three significant SPM‐clusters.
Figure 1.
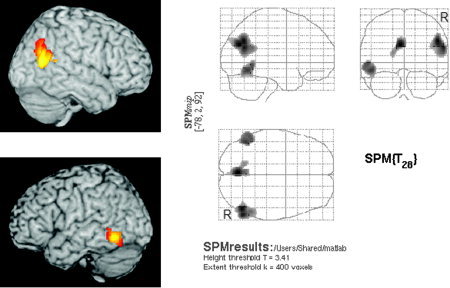
SPM2 analysis (n = 9) of tinnitus‐related hyperactivity. Significant tinnitus‐related activity was found in the right gyrus angularis (BA 39, corrected P = 0.001, T max = 5.29, MNIx,y,z [52/−66/32]), in the left lower temporal cortex (BA 37/21, corrected P = 0.015, T max = 4.51, MNIx,y,z [−54/−52/−4]) and in the posterior cingulum (BA 31, corrected P = 0.01, T max = 5.41, MNIx,y,z [−4/−72/28]).
Navigated rTMS
PET images (mean and difference) were coregistered with the individual structural MRI (Magnetom Vision, Siemens, Germany) and fed into the neuronavigation device (Brainsight, Magstim, Whitland, UK). The target area of rTMS was selected on the basis of the individual thresholded difference images. Stimulation was navigated to the cluster with maximal tinnitus‐related rCBF increase in both temporoparietal cortices. Repetitive TMS was carried out continuously with 1 Hz at 120% of the individual motor threshold (n = 9) defined as the minimal intensity necessary to evoke motor evoked potentials (MEP) of >50 μV in a small hand muscle (m. abd. poll. brevis) in at least 5 of 10 stimuli. In two sessions (different days) and in randomized order, stimulation was navigated either to the individual activation maximum in the temporoparietal cortex or the control spot (sham stimulation) at the lower occiput. In order to obtain a reliable sham condition, the localization of control stimulation was individually matched according to the distance to the ear resulting in comparable noise (between 60 and 75 dB) and aversive sensations (pricking, muscle twitches). Due to the limited penetration of magnetic fields produced by standard TMS coils as used here, a functionally relevant stimulation of cerebral cortex by the sham intervention can be ruled out. Subjects were naive with respect to the treatment conditions. Three stimulation trains (5, 15, 30 min, corresponding to 300, 900, 1800 stimuli) were applied during one session with intertrain intervals of 30 min.
Outcome Measures and Statistical Analysis
Modifications of tinnitus loudness by rTMS were assessed every minute after each train of stimulation by a discrete visual analog scale (VAS) consisting of 11 steps (primary endpoint). Subjects were instructed to remember tinnitus loudness before each intervention. The numbers (−5 to +5) were associated with adjectives describing changes in the loudness of tinnitus compared to the tinnitus loudness before each stimulation train. Zero represented no change, +1 to +5 an increase, and −1 to −5 a decrease in tinnitus loudness. The extent of tinnitus reduction was quantified by the sum scores over 30 min after each train (max = −150/+150) and the sum scores after each intervention (3 × 30 min, max = −450/+450).
The rTMS‐specific effect was determined using a Wilcoxon nonparametric test for paired samples on the VAS sum scores obtained after each stimulation condition (temporoparietal vs. sham). Statistical testing for dose dependency (effect of stimulation time) was performed by Friedman analysis of variance on the sum of VAS scores after each intervention (5, 15, 30 min TMS) for verum and sham stimulation separately. Correlation between rTMS effects (VAS sum score) and duration of tinnitus was computed by Spearman's nonparametric correlation analysis.
Based on the voxel‐wise statistical group analysis, we generated a post‐hoc ROI comprising all voxels of significant SPM clusters to extract an individual index of tinnitus‐related activation and to exploratively compare this index with rTMS outcome.
RESULTS
Lidocaine Effects
In 12 of 16 subjects, lidocaine injection resulted in a transient reduction of tinnitus. The duration of this reduction varied between 5 min and several hours. Two subjects reported no or only minimal (VAS ≥ −1) attenuation. In two patients lidocaine induced a temporary increase of tinnitus loudness. As substantial tinnitus reduction was the target of the study, these subjects (n = 4) were excluded. Three of the 12 responders withdrew from the study before the PET scans (claustrophobia in the MRI‐scanner, objections to the injection of [15O]H2O, no‐show).
Imaging Data
In all subjects, at least one temporoparietal cluster with a ΔrCBF greater than 5% was detected and used as stimulation target (Table I). Statistical group analysis (n = 9) revealed three areas of increased rCBF associated with tinnitus perception: right gyrus angularis (Brodmann area (BA) 39, corrected P = 0.001, T max = 5.29, MNIx,y,z [52/−66/32]), left middle and inferior temporal cortex (BA 37/21, corrected P = 0.015, T max = 4.51, MNIx,y,z [−54/−52/−4]) and posterior cingulate cortex (BA 31, corrected P = 0.01, T max = 5.41, MNIx,y,z [−4/−72/28]).
Adverse Reactions
All subjects (n = 16) reported mild to moderate adverse reactions including vertigo, dizziness, and numbness or tingling of mouth and tongue immediately after the injection of lidocaine lasting up to 10 min. No cardiovascular complications were observed. In one patient of the nine studied with rTMS the protocol was discontinued because tinnitus loudness increased after 5 min of rTMS to the area of tinnitus‐related activity. It returned to baseline after ∼30 min.
Navigated rTMS
Low‐frequency rTMS navigated to the individual area of maximal tinnitus‐related temporoparietal cortical activation reduced tinnitus perception up to 30 min in 6 of 8 subjects (Table I). Group analysis (n = 8) indicated a dose‐dependent reduction of tinnitus intensity after rTMS (Friedman analysis of variance [ANOVA], χ2 = 7.0, P = 0.03), an effect that was not found after sham stimulation (Fig. 2). Direct comparison of rTMS and sham stimulation revealed that rTMS to the tinnitus‐related maximum of activation resulted in a greater reduction of tinnitus loudness (Wilcoxon, Z = −2.2, P = 0.028).
Figure 2.
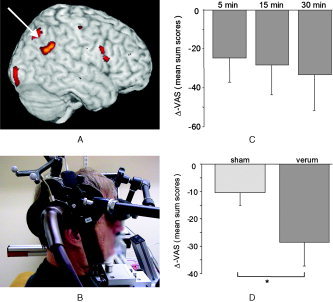
A: Exemplary single‐subject image (white arrow points to the area stimulated). B: Neuronavigated rTMS. C: Mean (n = 8) reduction of tinnitus (averaged sum scores of VAS ratings) after rTMS to the tinnitus‐related area of maximal activity after 5, 15, and 30 min. D: Compared with sham stimulation.
There was a negative correlation between the amount of tinnitus reduction (VAS sum score) after verum TMS and the duration of the illness (Spearman = −0.62; P = 0.05; one‐sided).
Two subjects reported no change of tinnitus. These patients showed excessively higher tinnitus‐related ΔrCBF than the patients reacting to rTMS (Fig. 3). On visual inspection of individual difference images this hyperactivity was mainly located in the posterior cingulum.
Figure 3.
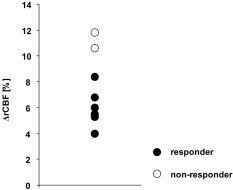
Individual tinnitus‐related ΔrCBF (tinnitus‐ON minus tinnitus‐OFF, average over all significant clusters). The two subjects showing no reduction of tinnitus after rTMS were those with the highest tinnitus‐related ΔrCBF.
DISCUSSION
The present study strengthens the conceptual link of chronic tinnitus with pathologically enhanced cortical activation, provides further evidence for a causative relation between cortical hyperactivity and disease, and verifies the hypothesis that low‐frequency rTMS guided to hyperactive temporoparietal areas beyond the classically defined auditory pathways can suppress tinnitus in a dose‐dependent manner. Finally, the longer the patients had been suffering from tinnitus the less likely neurostimulation was to reduce tinnitus loudness.II
Table II.
Attenuation of tinnitus loudness after rTMS
| Subject | Σ−VAS* (max. reduction),** response duration (VAS < 0) | |||||
|---|---|---|---|---|---|---|
| Verum | Sham | |||||
| 5 min rTMS | 15 min rTMs | 30 min rTMS | 5 min rTMS | 15 min rTMS | 30 min rTMs | |
| 1a | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | −14 (−3), 7 min | −16 (−3), 8 min | 0 | 0 | 0 |
| 3 | −60 (−2), 30 min | −75 (−5), 19 min | n.o. | 0 | 0 | n.o. |
| 4 | 0 | 0 | −3 (−1), 3 min | 0 | 0 | 0 |
| 5 | −96 (−5), 27 min | −114 (−5), 24 min | −132 (−5), 30 min | −36 (−3), 14 min | −81 (−5), 30 min | −62 (−5), 24 min |
| 6 | −17 (−5), 7 min | −24 (−5), 6 min | −59 (−5), 20 min | 0 | −6 (−5), 2 min | −27 (−5), 9 min |
| 7a | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | −24 (−2), 12 min | 0 | −24 (−2), 12 min | 0 | −19 (−1), 19 min | −7 (−1), 24 min |
Sum scores of VAS after rTMS.
Maximal tinnitus reduction (VAS).
Nonresponder.
n.o., not obtained.
Rather than using imaging of baseline metabolism (e.g., Fluorodeoxyglucose‐PET) [Eichhammer et al.,2003] or applying rTMS based on standardized coordinates on the head surface [De Ridder et al.,2005; Plewnia et al.,2003a], we directly combined functional imaging with [15O]H2O PET and stereotactic neuronavigation to optimize the effectiveness of focal modulation of cortical hyperactivity by rTMS at the individual patient level. This approach was chosen because of an assumed high between‐subject variability of focal tinnitus‐related cortical hyperactivity. Of note, by using the individual ΔrCBF images for targeting, stimulation was focused predominantly to areas of higher‐order processing (BA 39, 22). The tinnitus‐suppressing effects of this intervention indicate that these structures represent critical elements of a neuronal network subserving tinnitus perception. However, acknowledging the inherently limited signal‐to‐noise ratio in individual rCBF images, some uncertainty with respect to the exact Brodmann areas must remain. The group data analysis, however, confirms the pivotal role of sensory association cortex rather than primary auditory areas.
Until now, we are aware of six studies using [15O]H2O PET for the functional imaging of tinnitus [Andersson et al.,2000; Giraud et al.,1999; Lockwood et al.,1998,2001; Mirz et al.,1999; Reyes et al.,2002]. Two of them induced the tinnitus‐OFF condition by lateral gaze [Giraud et al.,1999; Lockwood et al.,2001], one by orofacial movements [Lockwood et al.,1998], and three by lidocaine [Andersson et al.,2000; Mirz et al.,1999; Reyes et al.,2002]. Concordantly, these imaging studies on tinnitus indicate enhanced activation in cortical areas involved in different levels of processing and emotional appraisal of auditory stimuli (BA 41, 42, 21, 22, 39) in both hemispheres. In line with these data, the brain regions activated in association with tinnitus perception in this study extended from the middle temporal gyrus (BA 21), a heteromodal brain region participating in auditory processing [Goycoolea et al.,2005], to cortical areas involved in the integration (BA 39, gyrus angularis; BA 37, fusiform gyrus [Booth et al.,2003]) and emotional validation (BA 31, posterior cingulate cortex [Gundel et al.,2003; Vogt et al.,1992]) of sensory stimuli. These data complement clinical observations that tinnitus perception is tightly linked with cognition and emotional processes [Folmer et al.,2001; Newman et al.,1997; Zenner and Zalaman,2004]. Like prior imaging studies, our group data showed no significant tinnitus‐related hyperactivation of the primary auditory cortex [Giraud et al.,1999; Reyes et al.,2002]. These findings support the notion that tinnitus‐related neuroplastic changes as documented also in the primary auditory cortex by electrophysiological methods [Diesch et al.,2004; Muhlnickel et al.,1998] are not necessarily associated with enhanced perfusion or metabolism. This is in agreement with a recent study in animals showing that an acute noise trauma (supposed to induce tinnitus) is not immediately followed by an increase in firing rates [Norena and Eggermont,2003].
The present data, demonstrating suppression of tinnitus by low‐frequency rTMS navigated to areas of tinnitus‐related cortical activity, add further evidence to the hypothesis that cortical hyperactivity is functionally relevant and in a causative relation with the perception of tinnitus. In comparison to the short‐term effects induced by high‐frequency rTMS (the “virtual‐lesion paradigm,” [Plewnia et al.,2003a]), we were now able to prolong tinnitus suppression substantially and in a dose‐dependent manner with low‐frequency rTMS. Low‐frequency rTMS is a well‐known technique to reduce cortical excitability up to 30 min, as demonstrated in studies on the primary motor cortex [Chen et al.,1997; Muellbacher et al.,2000]. Sharing central properties such as frequency and dose dependence, spreading to functionally linked cortical regions and continuation after stimulation, the effects of low‐frequency rTMS on neuronal excitability are attributed to similar mechanisms as the known cellular phenomenon “long‐term depression” (LTD) [Hoffman and Cavus,2002; Wang et al.,1996]. Although the immediate excitability‐decreasing properties of low‐frequency rTMS are well documented by means of electrophysiological studies, data on concurrent behavioral effects with comparable temporal dynamics as demonstrated here are still scarce. It has been shown that low‐frequency rTMS can have transient beneficial effects on symptoms of focal dystonia [Siebner et al.,1999] and Parkinson's disease [Lefaucheur et al.,2004; Siebner et al.,2000; Sommer et al.,2002]. In healthy subjects, low‐frequency rTMS modulated finger motor performance [Kobayashi et al.,2004], and low‐frequency rTMS to the somatosensory cortex can reduce proprioceptive acuity [Balslev et al.,2004]. It is conceivable that the tinnitus‐attenuating effect of rTMS is mediated through similar mechanisms, i.e., the reduction of neuronal excitability in an area of tinnitus‐related hypermetabolism. This is in line with studies on the effects of rTMS on the excitability of the motor cortex showing that the number of stimuli influenced the duration of after effects [Peinemann et al.,2004; Touge et al.,2001]. At the behavioral level, our results demonstrate a dependence of rTMS‐induced tinnitus reduction on the number of stimuli applied (“dose dependence”).
It has been hypothesized that repeated applications of rTMS may induce long‐term effects on behavioral parameters. Accordingly, studies with multiple sessions of rTMS over several days or weeks have demonstrated beneficial effects on different neuropsychiatric disorders and symptoms presumably associated with a disturbance of cortical activity, e.g., major depression [Fitzgerald et al.,2003] or auditory hallucinations [Hoffman et al.,2005; Poulet et al.,2005]. It must be mentioned, however, that these findings remain controversial [Hausmann et al.,2004; Schonfeldt‐Lecuona et al.,2004] and that the consequences of repeated rTMS on the physiological activity of cortical neurons are still to be determined.
Different from previous studies applying low‐frequency rTMS to the area above the primary auditory cortex [Eichhammer et al.,2003; Kleinjung et al.,2005], our functional PET‐guided stimulation was focused to the inferior parietal lobule (BA 39) in six subjects and to the secondary auditory cortex (BA 22) in two subjects. In line with our previous results [Plewnia et al.,2003a] and recent PET studies [Andersson et al.,2000; Giraud et al.,1999; Lockwood et al.,2001; Mirz et al.,1999; Reyes et al.,2002], the present data provide compelling evidence for the notion that a broad neuronal network extending beyond the primary auditory pathways is involved in the generation and perpetuation of tinnitus [Cacace,2003; Moller,2003] and integrative auditory cortical areas represent essential components subserving tinnitus perception at least in a subgroup of patients with chronic tinnitus. Since most of the participating subjects had bilateral tinnitus, aspects of tinnitus laterality were not addressed in this study. From our data, one might also express the concern that an increasing chronification of tinnitus lowers the chances to successfully interfere with pathologically enhanced cortical activity by neurostimulation. A similar observation has been made by De Ridder et al. [2005] with the “virtual lesion paradigm.” Of note, the two subjects with no response to rTMS were those with excessive tinnitus‐related ΔrCBF and very long histories of tinnitus. It seems possible that in these cases chronified extensive hypermetabolism exceeds the efficacy of rTMS and/or the tinnitus‐associated network is more distributed (e.g., with prominent hyperactivity in the posterior cingulum) and thus less accessible by rTMS. With rTMS the risk of a transient increase of tinnitus loudness has to be taken into account, which may be related to interindividual differences in the susceptibility to low‐frequency rTMS as demonstrated by increased motor cortex excitability in a subgroup of subjects after 1 Hz rTMS [Touge et al.,2001]. With respect to the tinnitus‐suppressing effect of the sham stimulation in three subjects, it is likely that the noise of rTMS (∼70 dB) induces a transient reduction of tinnitus in terms of residual inhibition.
In conclusion, rTMS navigated to the individual maximum of tinnitus‐related cortical hyperactivity can reduce tinnitus not only during stimulation or for seconds thereafter, but for up to 30 min. Most important, there is a clear dose–effect relationship between TMS and tinnitus reduction. Cumulative effects from the shorter to the longer durations of rTMS are unlikely, but cannot be completely ruled out. Even if this were true, however, the conclusion would be justified that an increasing amount of rTMS causes a more pronounced suppression of tinnitus. These findings emphasize the effectiveness of rTMS as an interventional tool and support the pathophysiological concept of maladaptive plasticity reflected in focal cortical hyperactivity in chronic tinnitus. Together with previous data, our results make rTMS an interesting candidate for neurophysiology‐based new treatment strategies. Repeated application possibly furthered by priming with high‐frequency rTMS [Iyer et al.,2003] or tDCS [Fregni et al.,2006], or combined with specific behavioral or pharmacological interventions, may stabilize the effects of rTMS and must be the next step.
REFERENCES
- Andersson G, Lyttkens L, Hirvela C, Furmark T, Tillfors M, Fredrikson M (2000): Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol 120: 967–972. [DOI] [PubMed] [Google Scholar]
- Balslev D, Christensen LO, Lee JH, Law I, Paulson OB, Miall RC (2004): Enhanced accuracy in novel mirror drawing after repetitive transcranial magnetic stimulation‐induced proprioceptive deafferentation. J Neurosci 24: 9698–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara‐Jimenez W, Catalan MJ, Hallett M, Gerloff C (1998): Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol 44: 828–831. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2003): Relation between brain activation and lexical performance. Hum Brain Mapp 19: 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AT (2003): Expanding the biological basis of tinnitus: crossmodal origins and the role of neuroplasticity. Hear Res 175: 112–132. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997): Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology 48: 1398–1403. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual‐Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD (1997): Functional relevance of cross‐modal plasticity in blind humans. Nature 389: 180–183. [DOI] [PubMed] [Google Scholar]
- De Ridder D, De Mulder G, Walsh V, Muggleton N, Sunaert S, Moller A (2004): Magnetic and electrical stimulation of the auditory cortex for intractable tinnitus. Case report. J Neurosurg 100: 560–564. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Verstraeten E, Van der Kelen K, De Mulder G, Sunaert S, Verlooy J, Van de Heyning P, Moller A (2005): Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otol Neurotol 26: 616–619. [DOI] [PubMed] [Google Scholar]
- Diesch E, Struve M, Rupp A, Ritter S, Hulse M, Flor H (2004): Enhancement of steady‐state auditory evoked magnetic fields in tinnitus. Eur J Neurosci 19: 1093–1104. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE (2004): The neuroscience of tinnitus. Trends Neurosci 27: 676–682. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Langguth B, Marienhagen J, Kleinjung T, Hajak G (2003): Neuronavigated repetitive transcranial magnetic stimulation in patients with tinnitus: a short case series. Biol Psychiatry 54: 862–865. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J (2003): Transcranial magnetic stimulation in the treatment of depression: a double‐blind, placebo‐controlled trial. Arch Gen Psychiatry 60: 1002–1008. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995): Phantom‐limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375: 482–484. [DOI] [PubMed] [Google Scholar]
- Flor H, Hoffmann D, Struve M, Diesch E (2004): Auditory discrimination training for the treatment of tinnitus. Appl Psychophysiol Biofeedback 29: 113–120. [DOI] [PubMed] [Google Scholar]
- Folmer RL, Shi YB (2004): SSRI use by tinnitus patients: interactions between depression and tinnitus severity. Ear Nose Throat J 83: 107–108, 110, 112 passim. [PubMed] [Google Scholar]
- Folmer RL, Griest SE, Martin WH (2001): Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg 124: 394–400. [DOI] [PubMed] [Google Scholar]
- Fregni F, Marcondes R, Boggio P, Marcolin MA, P RS, G ST, Nitsche M, Pascual‐Leone A (2006): Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol (in press). [DOI] [PubMed] [Google Scholar]
- Gananca MM, Caovilla HH, Gananca FF, Gananca CF, Munhoz MS, da Silva ML, Serafini F (2002): Clonazepam in the pharmacological treatment of vertigo and tinnitus. Int Tinnitus J 8: 50–53. [PubMed] [Google Scholar]
- Giraud AL, Chery‐Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, Lavenne F, Collet L (1999): A selective imaging of tinnitus. Neuroreport 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Goycoolea M, Mena I, Neubauer S (2005): Functional studies of the human auditory pathway after monaural stimulation with pure tones. Establishing a normal database. Acta Otolaryngol 125: 513–519. [DOI] [PubMed] [Google Scholar]
- Gundel H, O'Connor MF, Littrell L, Fort C, Lane RD (2003): Functional neuroanatomy of grief: an FMRI study. Am J Psychiatry 160: 1946–1953. [DOI] [PubMed] [Google Scholar]
- Hausmann A, Kemmler G, Walpoth M, Mechtcheriakov S, Kramer‐Reinstadler K, Lechner T, Walch T, Deisenhammer EA, Kofler M, Rupp CI (2004): No benefit derived from repetitive transcranial magnetic stimulation in depression: a prospective, single centre, randomised, double blind, sham controlled “add on” trial. J Neurol Neurosurg Psychiatry 75: 320–322. [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Cavus I (2002): Slow transcranial magnetic stimulation, long‐term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry 159: 1093–1102. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH (2003): Transcranial magnetic stimulation of left temporoparietal cortex and medication‐resistant auditory hallucinations. Arch Gen Psychiatry 60: 49–56. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu YT, Carroll K, Krystal JH (2005): Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry 58: 97–104. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM (2003): Priming stimulation enhances the depressant effect of low‐frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 10867–10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM (2000): Tinnitus retraining therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol 11: 162–177. [PubMed] [Google Scholar]
- Kleinjung T, Eichhammer P, Langguth B, Jacob P, Marienhagen J, Hajak G, Wolf SR, Strutz J (2005): Long‐term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg 132: 566–569. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual‐Leone A (2004): Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology 62: 91–98. [DOI] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Wiegand R, Marienhegen J, Maenner P, Jacob P, Hajak G (2003): Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. Neuroreport 14: 977–980. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ (2003): Acute remapping within the motor system induced by low‐frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 5308–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Menard‐Lefaucheur I, Cesaro P, Nguyen JP (2004): Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson's disease. Clin Neurophysiol 115: 2530–2541. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW (1998): The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50: 114–120. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Wack DS, Burkard RF, Coad ML, Reyes SA, Arnold SA, Salvi RJ (2001): The functional anatomy of gaze‐evoked tinnitus and sustained lateral gaze. Neurology 56: 472–480. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF (2002): Tinnitus. N Engl J Med 347: 904–910. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ Jr, Levine RA (2000): Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83: 1058–1072. [DOI] [PubMed] [Google Scholar]
- Mirz F, Pedersen B, Ishizu K, Johannsen P, Ovesen T, Stodkilde‐Jorgensen H, Gjedde A (1999): Positron emission tomography of cortical centers of tinnitus. Hear Res 134: 133–144. [DOI] [PubMed] [Google Scholar]
- Moller AR (2000): Similarities between severe tinnitus and chronic pain. J Am Acad Audiol 11: 115–124. [PubMed] [Google Scholar]
- Moller AR (2003): Pathophysiology of tinnitus. Otolaryngol Clin North Am 36: 249–266, v–vi. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M (2000): Effects of low‐frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111: 1002–1007. [DOI] [PubMed] [Google Scholar]
- Muhlnickel W, Elbert T, Taub E, Flor H (1998): Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95: 10340–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CW, Wharton JA, Jacobson GP (1997): Self‐focused and somatic attention in patients with tinnitus. J Am Acad Audiol 8: 143–149. [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ (2003): Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183: 137–153. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR (2004): Long‐lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 115: 1519–1526. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Bartels M, Gerloff C (2003a): Transient suppression of tinnitus by transcranial magnetic stimulation. Ann Neurol 53: 263–266. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Lotze M, Gerloff C (2003b): Disinhibition of the contralateral motor cortex by low‐frequency rTMS. Neuroreport 14: 609–612. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, d'Amato T, Saoud M (2005): Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry 57: 188–191. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP (1999): Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci 22: 74–80. [DOI] [PubMed] [Google Scholar]
- Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH (2002): Brain imaging of the effects of lidocaine on tinnitus. Hear Res 171: 43–50. [DOI] [PubMed] [Google Scholar]
- Schonfeldt‐Lecuona C, Gron G, Walter H, Buchler N, Wunderlich A, Spitzer M, Herwig U (2004): Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport 15: 1669–1673. [DOI] [PubMed] [Google Scholar]
- Shulman A, Strashun AM, Goldstein BA (2002): GABAA‐benzodiazepine‐chloride receptor‐targeted therapy for tinnitus control: preliminary report. Int Tinnitus J 8: 30–36. [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos‐Baumann AO, Auer C, Catala MD, Conrad B, Pascual‐Leone A (1999): Low‐frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology 52: 529–537. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B (2000): Short‐term motor improvement after sub‐threshold 5‐Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson's disease. J Neurol Sci 178: 91–94. [DOI] [PubMed] [Google Scholar]
- Sommer M, Kamm T, Tergau F, Ulm G, Paulus W (2002): Repetitive paired‐pulse transcranial magnetic stimulation affects corticospinal excitability and finger tapping in Parkinson's disease. Clin Neurophysiol 113: 944–950. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC (2001): Are the after‐effects of low‐frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol 112: 2138–2145. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang X, Scheich H (1996): LTD and LTP induced by transcranial magnetic stimulation in auditory cortex. Neuroreport 7: 521–525. [DOI] [PubMed] [Google Scholar]
- Zenner HP, Zalaman IM (2004): Cognitive tinnitus sensitization: behavioral and neurophysiological aspects of tinnitus centralization. Acta Otolaryngol 124: 436–439. [DOI] [PubMed] [Google Scholar]


