INTRODUCTION
The last two decades have seen tremendous advances in our understanding of human brain structure and function, particularly at the level of systems neuroscience where neuroimaging methods have led to better delineation of brain networks. Even more striking advances have been reported in molecular genetics research where the Human Genome Project has provided a rough draft of the human genome including the total number of genes and their chromosomal locations. Yet, despite significant progress in molecular genetics and in neuroimaging‐based research, there has been relatively little integration of the two fields. Indeed, fewer than 50 peer‐reviewed “imaging genomics” articles—manuscripts where genetic information was used to inform neuroimaging experiments—were published by December 2006. However, as shown in Figure 1, the annual publication rates for human functional and anatomic imaging studies including genetic data either for sample selection or for grouping has increased each year since the completion of the Human Genome Project in 2003. Since the assimilation of genetics information into neuroimaging methods promises to significantly improve our understanding of normal and pathologic brain function, this Special Issue of Human Brain Mapping is devoted to imaging genomics. We hope that the 11 articles in this Special Issue spur additional research by providing several novel findings and by reviewing our current state of understanding in imaging genomics research.
Figure 1.
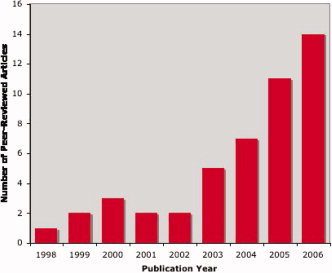
Imaging Genomics Literature. Annual publication rates for all peer‐reviewed human functional and anatomic brain imaging studies where molecular genetic data were used either for sample selection or grouping, excluding case reports or reports of single small families. Publication rates increased dramatically after the completion of the Human Genome Project.
In order for a trait to be a useful phenotype for studies in imaging genomics, it must be influenced by genetic factors (i.e., heritable). Several articles in this Special Issue document the heritability of brain anatomy, as indexed by structural MRI. Giedd and colleagues review their ongoing work applying structural equation modeling to pediatric brain morphometric data collected from 150 monozygotic and dizygotic healthy twin pairs [Giedd et al.,2007]. These data demonstrate that most brain morphometric measures are highly heritable and have minimal shared environmental effects in childhood. To complement the article by Giedd and colleagues, Kahn and coworkers review work documenting genetic influences on neuroanatomic measures in adults [Kahn et al., 2007]. Adult twin studies indicate that genetic effects vary across brain regions, with the highest heritabilities reported for frontal lobe volumes (90–95%), moderate heritability for hippocampal volume (40–69%), and environmental factors influencing medial brain areas. Together, these manuscripts leave little doubt that gross human neuroanatomy is strongly influenced by genetics across the life span, particularly in the neocortex. However, it is also quite likely that environmental factors are also important determinants of brain anatomy, particularly during key developmental periods. The Saguenay Youth Study examines the impact of one such environmental factor, maternal cigarette smoking during pregnancy, on variation in brain structure. Pausova, Paus and their colleagues present on overview of this study, carried out in a geographically isolated population to facilitate the search for genes interacting with this and other environmental influences [Pausova et al.,2007]. The heritability of the prefrontal contributions to emotional processing, as assessed by functional MRI, were estimated by Perusse and colleagues [Perusse et al., 2007]. Finally, Glahn, Thompson, and Blangero review the current state of genetic investigations in anatomic and functional MRI, focusing on methods to discover novel genes influencing specific traits in psychiatric and neurological illnesses [Glahn et al.,2007].
Bearden and colleagues review anatomic and functional neuroimaging studies of individuals with, and genetic risk for, schizophrenia [Bearden et al.,2007]. They argue that neuroimaging methods offer a powerful way to bridge the neurobiology of genes and psychotic behaviors. Specifically, the effects of DISC1, a putative risk gene for schizophrenia, on neuroanatomic variation is described, as are complementary studies of individuals with chromosomal abnormalities associated with schizophrenia (22q11 deletion syndrome). Gothelf and colleagues directly extend this work by presenting novel functional MRI data comparing children with 22q11 deletion syndrome to children with other developmental delays and typically developing children [Gothelf et al.,2007]. Although groups did not differ on task performance, the 22q11 group showed greater left parietal activation when compared to either control group. To examine response inhibition in Fragile X syndrome, a disorder with known genetic etiology, Hoeft and colleagues employed a similar experimental design. In this manuscript, age, gender, and IQ‐matched comparison subjects showed increased right ventrolateral prefrontal cortex activation and increased right caudate activity when compared to children with Fragile X syndrome [Hoeft et al.,2007]. By contrast, the Fragile X group showed increased activation in left ventrolateral prefrontal cortex, compared to healthy children, suggesting that right fronto‐striatal dysfunction may be linked to the genetic abnormalities found in Fragile X syndrome and that increased left ventrolateral prefrontal activation may be linked to compensatory processes in this group.
The final three manuscripts in this Special Issue use animal models to inform imaging genomics. Henkelman and colleagues present a meta‐analysis of studies involving anatomic imaging of behavioral mouse mutants [Henkelman et al., 2007]. Of the 15 different mutant genotypes evaluated, 13 had abnormal neuroimaging findings suggesting that genetic processes affecting behavior generally alter neuroanatomy. Pitiot and colleagues outline computational approaches to derive quantitative neuroanatomic phenotypes from rodents, and the use of these phenotypes in genetic studies of complex traits [Pitiot et al., 2007]. Rogers and colleagues present heritability estimates for neuroanatomic traits obtained from baboon colonies [Rogers et al.,2007]. These estimates are strikingly similar to those reported by Giedd et al., and Kahn et al., and support the value of cross‐primate imaging genomics studies.
REFERENCES
- Bearden CE,van Erp TG,Thompson PM,Toga AW,Cannon TD ( 2007). Cortical mapping of genotype‐phenotype relationships in schizophrenia. Hum Brain Mapp. Apr 16; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote C,Beauregard M,Girard A,Mensour B,Mancini‐Marie A,Perusse D ( 2007). Individual variation in neural correlates of sadness in children: A twin fMRI study. Hum Brain Mapp. Apr 16; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN,Schmitt JE,Neale MC ( 2007). Structural brain magnetic resonance imaging of pediatric twins. Hum Brain Mapp. Apr 16; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC,Thompson PM,Blangero J ( 2007). Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Hum Brain Mapp. Apr 17; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D,Hoeft F,Hinard C,Hallmayer JF,Van Dover Stoecker J,Antonarakis SE,Morris MA,Reiss AL ( 2007). Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp. Apr 10; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F,Hernandez A,Parthasarathy S,Watson CL,Hall SS,Reiss AL ( 2007). Fronto‐striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. Apr 16; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausova Z,Paus T,Abrahamowicz M,Almerigi J,Arbour N,Bernard M,Gaudet D,Hanzalek P,Hamet P,Evans AC,Kramer M,Laberge L,Leal S,Leonard G,Lerner J,Lerner RM,Mathieu J,Perron M,Pike B,Pitiot A,Richer L,Séguin JR,Syme C,Toro R,Tremblay RE,Veillette S,Watkins K ( 2007). Genes, maternal smoking and the offspring brain and body during adolescence: Design of the Saguenay Youth Study. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS,Brouwer RM,Boomsma DI,Kahn RS,Hulshoff Pol HE ( 2007). Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp. Apr 5; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J,Kochunov P,Lancaster J,Shelledy W,Glahn D,Blangero J,Fox P (2007).Heritability of brain volume, surface area and shape: An MRI study in an extended pedigree of baboons.Hum Brain Mapp. Apr 16; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


