Abstract
The prefrontal cortex (PFC) has been implicated in the ability to apply semantic organizational strategies during verbal encoding and episodic learning. However, there has been no direct evidence demonstrating which specific areas in the PFC are engaged after cognitive training using semantic organizational strategies in healthy adult human subjects. In this study, we investigated the effects of semantic strategic training on brain activity and changes in behavioral performance, after cognitive training, using functional MRI (fMRI). There was a significant activation in bilateral dorsolateral prefrontal (DLPF) and orbitofrontal (OFC) areas after cognitive training. These results demonstrate the engagement of bilateral DLPF and OFC cortex during strategic memory processes, particularly when mobilization and effort of effective use of strategies are required. The functional adaptations observed here may also shed light on some of the processes underlying recovery with cognitive rehabilitation in patient populations with brain injury. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: fMRI, prefrontal, strategy, cognitive training
INTRODUCTION
The involvement of prefrontal cortex (PFC) in episodic memory and learning has been demonstrated in a number of studies [Fletcher et al.,1998; Fletcher and Henson, 2001; Savage et al.,2001; Schacter and Tulving,1994; Shallice et al.,1994; Shimamura et al., 1991; Tulving,1962]. The term “episodic memory” refers to a system responsible for the encoding, storing, and retrieving of personally experienced and highly temporally specific events or episodes [Tulving,1983].
It has been proposed that prefrontal activation during episodic memory tasks reflects the applications of executive functioning processes that support and enhance different components of learning and memory [Buckner et al.,1999]. In this context, strategic processes such as semantic organizational strategies, which play an important role in learning and memory, are thought to be controlled by distinct regions of the PFC. Semantic processing is supported by regions in the left inferior prefrontal cortex (IPFC), whereas updating, manipulating, and monitoring operations are mediated by the dorsolateral prefrontal cortex (DLPFC) [Owen et al.,1996]. A recent study found, in addition to the above areas, increased activation in the orbital frontal cortex (OFC) during verbal encoding in a paradigm that manipulated semantic organization using word lists [Savage et al.,2001]. Furthermore, activation predicted which subjects would initiate the use of effective strategies during free recall. The authors were able to demonstrate the important role of the OFC in strategic memory by initiating the early mobilization of effective behavioral strategies in novel situations.
In spite of the numerous lines of evidence indicating the importance of the PFC for episodic memory, it is largely unknown to what extent the functioning of the neural systems that underlie the strategic semantic processes may be altered by cognitive training. Recent studies with healthy adult human subjects have indicated that memory systems are affected by training. For instance, one study investigated the effects of working memory training over a period of 5 weeks on cortical activity in healthy humans with functional MRI (fMRI) before, during, and after training [Olesen et al.,2004]. They found increased activation in the middle frontal gyrus and superior and inferior parietal cortices after training. Increased activation was related to improved performance in working memory capacity. The improvement in performance also generalized to include nontrained working memory and reasoning tasks. The authors suggested that cortical plasticity might have explained these signal changes. Nevertheless, the paradigm used in this study was based on a visuospatial working memory task and the aim was not to investigate the strategic processes associated with cortical activity after training. Changes in functional brain activity in adulthood and aging related to training‐induced memory improvement have also been demonstrated by another study [Nyberg et al.,2003]. In this PET study, younger healthy adults but not older adults showed increased activity during memory encoding of word lists in left DLPFC and left occipitoparietal cortex after training using a classical mnemonic‐strategy, the method of loci, to memorize and retrieve word lists. This method involves learning to visualize a series of mental landmarks. The whole experiment, including the encoding and loci training, was carried out while subjects were in the scanner. The authors' main objective was to study whether age‐related activity changes were associated with the size of training‐related gains using this particular memory learning method.
To our knowledge, there has been no investigation of the specific areas in the PFC that are engaged after cognitive training using semantic organizational strategies in healthy adult human subjects. In the present study, our aim was to investigate the effects of semantic strategic training on brain activity in healthy subjects and the neural correlates associated with changes in behavioral performance after cognitive training using a verbal memory‐encoding paradigm. These findings may be of interest in trying to understand which strategies may be useful for people with acquired memory impairments.
SUBJECTS AND METHODS
Subjects
Fifteen healthy, right‐handed adult subjects were included (nine males and six females) with a mean age of 38.8 years (SD 12.92) and mean education of 7.93 years (SD 3.49). Their mean estimated Full Scale IQ assessed on the WASI was in the average range (96.87, SD 6.63). Memory performance assessed on standardized tests before the experimental study was in the average range for episodic recognition (Warrington Recognition Memory, Words = 24.07, SD 0.70, and Faces = 23.27, SD 0.96) and immediate memory/attentional span (Digit Span WAIS‐III = 10.3, SD 0.83). The participants were free of psychiatric or neurological illness and all gave informed consent. They had no previous experience with semantic organizational tests and had never undergone memory training. The study was approved by the Ethics Committee of the Department of Neurology, University of São Paulo, Brazil.
Experimental Paradigm
The paradigm used in the current investigation was based on the California Verbal Learning Test (CVLT) [Knoke et al., 1998], which is a clinical measure of strategic verbal memory. Subjects were scanned while they encoded word lists in Portuguese under three different conditions: 16 unrelated words (UR), 16 related‐nonstructured words (RNS), and 16 related‐structured words (RS) and a fixation baseline (+; see Fig. 1).
Figure 1.
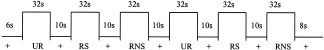
Experimental paradigm. Experimental conditions: fixation baseline (+); unrelated words (UR); related‐structured words (RS); related‐non‐structured words (RNS). There were three runs, each run with 48 words and total number of words pre‐training or post‐training = 144 words. FMRI parameter: three runs (all with all novel words). Each run 256 s (4 min, 16 s). One image per slice every 2 s = 128 data points each run; 16 axial slices; slice thickness = 8 mm (7, skip 1).
This unique paradigm allows for greater or lesser use of semantic organization according to the condition. For the UR condition, the words presented were not related in any sense, therefore making it difficult to apply semantic organizational strategies. For the RNS condition the words were related in terms of categories; however, they were presented so that no two words from the same category occurred consecutively within the list. For the RS condition the words were related in terms of categories and presented grouped together in categories (see Table I for sample word lists).
Table I.
Three sample word lists for the RNS and RS conditions (fuzzy categories in parentheses)
| Category | List RNS | Category | List RS |
|---|---|---|---|
| Body parts | Eye | Toiletries | Toothpaste |
| (Land animals) | Penguin | (Fruits) | Razor |
| Cleaning supplies | Dustpan | Toys | Shampoo |
| (Water animals) | Oyster | (Vegetables) | Comb |
| Detergent | Lemon | ||
| Squid | Peach | ||
| Beaver | Grapes | ||
| Finger | Cherry | ||
| Cod | Doll | ||
| Sponge | Blocks | ||
| Deer | Crayons | ||
| Ankle | Swing | ||
| Bucket | Lettuce | ||
| Salmon | Spinach | ||
| Nose | Radish | ||
| Wolf | Celery |
Scanning was carried out twice: before and after semantic organizational strategy training on the same day. The word lists were generated from 32 categories of words with four words in each category. The paradigm was tested extensively in pilot studies in order to develop word lists that prompted significant differences in semantic clustering. In addition, the inclusion of categories that shared “fuzzy” semantic relationships (e.g., land animals and water animals) made the task more challenging and minimized ceiling effects for clustering in normal subjects [Dellis et al.,1987]. At the start of the study, subjects were instructed to look at words presented on the screen and try to remember as many words as they could. Following each scan participants were instructed to produce as many words as they could remember in any order. There was no specific instruction to apply any strategy when subjects were scanned before training.
All stimuli were visually presented on a screen and synchronized with the scanner via an optic relay triggered by the radiofrequency pulse. Functional scanning included the verbal encoding paradigm with three conditions and a control fixation baseline alternating in a block design. The presentation order of the conditions was randomized. There were three runs with novel words. After each run the volunteers were tested for word list recall.
Semantic Organizational Strategy Training
After the subjects were scanned they were taken to a different room and given a 30‐min period of cognitive training. The training consisted of teaching them to apply semantic organizational strategies to a set of different word lists. Subjects were instructed to organize the words into categories and to then memorize and retrieve the words according to each category using novel word lists. After training they were taken to the scanner and instructed to look at words presented on the screen and apply the semantic organizational strategies. It was explained to them that the use of this strategy could help them improve recall. The post‐training paradigm used novel words.
Image Acquisition and Analysis
Gradient echo planar imaging (EPI) data were acquired on a GE Signa 1.5 T system (General Electric, Milwaukee WI). Tape was gently applied to the forehead in order to provide feedback to the subjects and to minimize head movement. In addition, the head was kept in a tight position within a quadrature birdcage headcoil with cushions. In total, 100 T 2*‐weighted images depicting blood oxygen level‐dependent (BOLD) contrast were acquired over 5 min (for each task) at each of 15 axial noncontiguous 7‐mm thick planes (interslice gap 0.7 mm, in‐plane resolution 3.125 × 3.125 mm) parallel to the intercommissural (AC–PC) line: TE 40 ms, TR 2 s. This EPI dataset provided almost complete brain coverage.
Individual Analysis
The data were first realigned [Bullmore et al.,1999a,b] to minimize motion‐related artifacts and were smoothed using a Gaussian filter (FWHM 7.2 mm). Responses to the experimental paradigms were then detected by time‐series analysis using Gamma variant functions (peak responses at 4 and 8 s) to model the BOLD response. The analysis was implemented as follows: first, each experimental condition was convolved separately with the 4‐ and 8‐s Poisson functions to yield two models of the expected hemodynamic response to that condition. The weighted sum of these two convolutions that gave the best fit to the time series at each voxel was then computed. This weighted sum effectively allows voxel‐wise variability in time to peak hemodynamic response. In order to constrain the possible range of fits physiologically plausible BOLD responses, the constrained fitting procedure was adopted [Friman et al.,2003]. Following this fitting operation, a goodness of fit statistic was computed at each voxel. This was the ratio of the sum of squares of deviations from the mean intensity value due to the model (fitted time series) divided by the sum of 7 squares due to the residuals (original time series minus model time series). This statistic is called the SSQratio. The percentage BOLD signal change at each voxel was also calculated. This was [(fitmax – fitmin)/mean signal intensity] × 100, where fitmax and fitmin were the maximum and minimum values of the fitted response for the time series in question. In order to sample the distribution of SSQratio under the null hypothesis that observed values of SSQratio were not determined by experimental design (with minimal assumptions), the time series at each voxel was permuted using a wavelet‐based resampling method [Brammer et al.,2004; Breakspear et al.,2004]. This process was repeated 10 times at each voxel and the data combined over all voxels, resulting in 10 permuted parametric maps of SSQratio at each plane for each subject. The same permutation strategy was applied at each voxel to preserve spatial correlational structure in the data during randomization. Combining the randomized data over all voxels yields the distribution of SSQratio under the null hypothesis. A test that any given voxel is activated at any required type I error can then be carried out by obtaining the appropriate critical value of SSQratio from the null distribution. For example, SSQratio values in the observed data lying above the 99th percentile of the null distribution have a probability under the null hypothesis of ≤0.01. We have shown that this permutation method gives very good type I error control with minimal distributional assumptions [Bullmore et al.,2001].
Group Mapping
In order to extend inference to the group level, the observed and randomized SSQratio maps were transformed into standard by a two‐stage process involving first a rigid body transformation of the fMRI data into a high‐resolution inversion recovery image of the same subject followed by an affined transformation onto a Talairach template [Brammer et al.,1997]. By applying the two spatial transformations computed above for each subject to the statistic maps obtained by analyzing the observed and wavelet‐randomized data, a generic brain activation map (GBAM) could be produced for each experimental condition. The median observed SSQratio over all subjects at each voxel (median values were used to minimize outlier effects) could then be tested at each intracerebral voxel in standard space against a critical value of the permutation distribution for median SSQratio ascertained from the spatially transformed wavelet‐permuted data [Brammer et al.,1997]. In order to increase sensitivity and reduce the multiple comparison problem encountered in fMRI, hypothesis testing was carried out at the cluster level [Bullmore et al.,1999a,b], initially for structural image analysis, and it was subsequently shown to give excellent cluster‐wise type I error control in both structural and fMRI analysis. When applied to fMRI data, this method estimates the probability of occurrence of clusters under the null hypothesis using the eight distribution of median SSQratios computed from spatially transformed data obtained from wavelet permutation of the time series at each voxel (see above). Image‐wise expectation of the number of false‐positive clusters under the null hypothesis is set for each analysis at <1.
Differences Between Conditions in the Group
Analysis of variance (ANOVA) was carried out on the SSQratio maps in standard space by first computing the difference in median SSQratio between condition A (before training) and B (after training) at each voxel. Subsequent inference of the probability of this difference under the null hypothesis was made by reference to the null distribution obtained by repeated random permutation of conditions assignment (before and after training) and recomputation of the difference in median SSQratios between the two condition “assignment groups” obtained from the resampling process. Cluster‐level maps were then obtained as described above.
RESULTS
Behavioral Data
Free recall
Fifteen healthy adult subjects were scanned, before and after cognitive training, during encoding of word lists under three different conditions: 16 unrelated words (UR), 16 related‐nonstructured words (RNS), and 16 related‐structured words (RS). After each scan participants were instructed to produce as many words as they could remember in any order and tested for word list recall. Results were first analyzed in terms of the total number of words recalled pre‐ and post‐training. A paired t‐test analysis showed a significant difference with an increased number of words remembered after training (pre‐training mean words: 44.07 (8.46 SD) and post‐training mean words: 49.07 (7.74 SD); t = –4.82, df =14, P < 0.01).
Semantic organizational strategy
In order to analyze whether subjects were able to apply the semantic organizational strategy after training, we used a measure of semantic clustering scores before and after training. Semantic clustering scores were defined as a consecutive recall of two words from the same category. Clustering scores reflected the proportion of clustered responses out of the total possible clusters for that condition defined as [clusters/(words recalled‐categories recalled)]. This allows for measuring the subject's tendencies to group words together actively according to their shared semantic features. Results indicated that subjects used semantic strategies significantly more after training (pre‐training mean clustering: 0.34 (0.08 SD) and post‐training mean clustering: 0.43 (0.05 SD); t = –4.52, df = 14, P < 0.01).
fMRI Data
Cluster‐based ANOVA tests were used to investigate the brain system's underlying semantic organization after training during verbal learning encoding. A series of comparisons were carried out before and after training with the three conditions (UR, RNS, and RS). A comparison between the RNS post‐training and RNS pre‐training conditions (see Table II) showed significant difference in activation in bilateral DLPFC (middle frontal gyrus) corresponding to BA 9/46; bilateral IPFC, BA 45 (inferior frontal gyrus); right OFC, BA 11/47 (orbitary gyrus); bilateral cerebellum and occipital cortex and precuneus (P < 0.01 cluster level). Activation was greater during the RNS post‐training condition. Figure 2 shows the areas of increased brain activity after training with the RNS posttraining > RNS pre‐training conditions.
Table II.
Brain regions showing significant signal changes after strategic semantic organizational training during the RNS condition
| Brain region | Hemisphere | Talairach coordinates (x, y, z) | P |
|---|---|---|---|
| Middle frontal gyrus (9/46) | L | −45, 19, 21 | <0.01 |
| Inferior frontal gyrus (45/47) | L | −44, 18, 0 | <0.01 |
| Middle frontal gyrus (9/46) | L | −44, 7, 37 | <0.01 |
| Middle frontal gyrus (9/46) | R | 58, 9, 24 | <0.01 |
| Inferior frontal gyrus (45/47) | R | 58, 19, 18 | <0.01 |
| Precuneos (7) | L | −2, −67, 52 | <0.01 |
| Middle occipital gyrus (18) | L | −25, −91, 18 | <0.01 |
| Fusiform gyrus (19) | L | −50, −64, −12 | <0.01 |
| Cerebellum | L | −46, −63, −30 | <0.01 |
Figure 2.
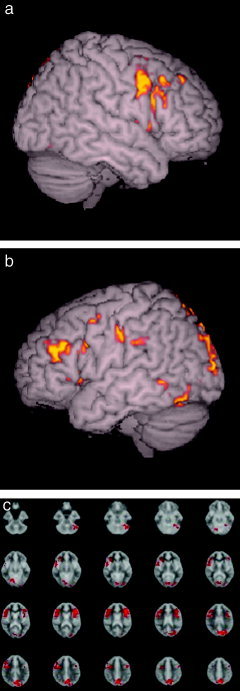
fMRI statistical map from the ANOVA test between the RNS post‐training > RNS pre‐training conditions (P < 0.01 cluster level). a: Right hemisphere showing increased activity in the middle frontal gyrus (9/46) (x, y, z; 58, 9, 24), inferior frontal gyrus (45/47) (58, 19, 18). b: Left hemisphere showing the increase in the middle frontal gyrus (9/46) (−45, 19, 21), inferior frontal gyrus (45/47) (−44, 18, 0), middle frontal gyrus (9/46) (−44, 7, 37), middle occipital gyrus (18) (−25, − 91, 18), fusiform gyrus (19) (−50, −64, −12). c: Axial slices showing, in addition to the above areas, increase in the left precuneus (7) (−2, −67, 52) and left cerebellum (−46, − 63, − 30).
The comparison between the UR post‐training and UR pre‐training conditions (see Table III, Fig. 3) indicated significant differences in activation in the bilateral DLPC, BA 9/45; bilateral IPFC, BA 45; left OFC, BA 11/47; bilateral cerebellum, occipital cortex and precuneus (P < 0.01, cluster level), with greater activation during the UR post‐training condition. There was no difference in PFC activation when comparing the RS post‐training and > RS pre‐training conditions nor a decrease in activation during post‐training in any of the conditions.
Table III.
Brain regions showing significant signal changes after strategic semantic organizational training during the UR condition
| Brain region | Hemisphere | Talairach coordinates (x, y, z) | P |
|---|---|---|---|
| Middle frontal gyrus (9/46) | L | −47, 29, 24 | <0.01 |
| Medical frontal gyrus (10) | L | −31, 54, 12 | <0.01 |
| Inferior frontal gyrus (45/47) | L | −46, 21, 0 | <0.01 |
| Middle frontal gyrus (9/46) | R | 58, 9, 24 | <0.01 |
| Inferior frontal gyrus (45/47) | R | 43, 6, 41 | <0.01 |
| Precuneos (7) | R | 17, −68, 25 | <0.01 |
| Lingual gyrus (18) | R | 17, −78, −2 | <0.01 |
| Lingual gyrus (18) | L | −20, −75, −2 | <0.01 |
Figure 3.
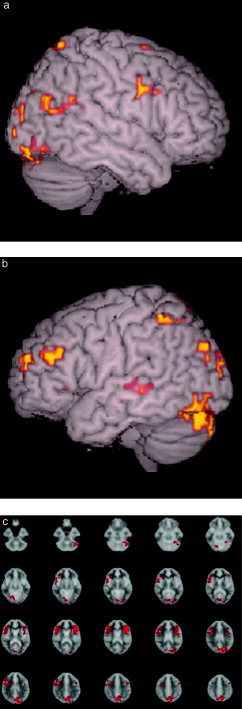
fMRI statistical map from the ANOVA test between the UR post‐training > UR pre‐training conditions (P < 0.01 cluster level). a: Right hemisphere demonstrating increase in activity in the middle frontal gyrus (9/46) (x, y, z; 58, 9, 24), inferior frontal gyrus (45/47) (43, 6, 41), lingual gyrus (18) (17, −78, −2). b: Left hemisphere showing increase in the middle frontal gyrus (9/46) (−47, 29, 24), medial frontal gyrus (10) (−31, 54, 12), inferior frontal gyrus (45/47) (−46, 21, 0), lingual gyrus (18) (−20, −75, −2). c: Axial slices showing, in addition to the above, increase in the right precuneus (7) (17, −68, 25).
Subsequently, a comparison between the conditions considering only the trials before training was carried out for the subjects. When comparing the UR > RNS conditions, there was a significant activation in the bilateral cerebellum and occipital cortex; the RNS > UR, in the left OFC, left cerebellum and left parietal cortex; the UR > RS, in the bilateral cerebellum and occipital cortex; RS > UR, no activation; RNS > RS, in the left cerebellum; RS > RNS, no activation.
DISCUSSION
The present study investigated the neural correlates associated with semantic organizational strategy application after cognitive training and changes in behavioral performance using a verbal encoding paradigm. An important aspect of this paradigm was the ability to manipulate the level of use of semantic organizational strategies through the design of the experimental conditions. All subjects were scanned before and after a period of cognitive training with semantic organizational strategies. The primary comparisons between conditions pre‐ and post‐training identified increased activity in bilateral DLPFC (BA 9/46), IPFC (BA 45), and right OFC (BA 11/47) regions after training for the RNS condition and bilateral DLPC, IPFC, and left OFC regions for the UR condition. For the RS, there was no significant change in the level of activation after training in the PFC area. Changes in behavioral performance were also demonstrated after training with a significant improvement in word list recall in addition to increased use of semantic organizational strategies.
Previous studies have shown the engagement of left IPFC and DLPFC during semantic processing and updating in verbal encoding paradigms [D'Esposito et al.,1998; Demb et al.,1995; Owen et al.,1996]. In the current study, there was, in addition to these areas, an unexpected increase in right DLPFC and IPFC activation after training. A number of authors have put forward different hypotheses for the involvement of the right PFC in episodic memory [Buckner and Peterson,1996; Fletcher et al.,1997; Henson et al.,1999; Rugg et al;1996; Schacter et al.,1996; Tulving,1983], including the adoption of a retrieval mode whenever one refers back in time to past experiences [Tulving,1983] and degree of retrieval effort [Schacter et al.,1996]. An alternative hypotheses suggests that it reflects processes operating after retrieval, including monitoring of suitability of information to direct and guide one's behavioral processes [Fletcher et al.,1997; Henson et al.,1999; Rugg et al.,1996; Shallice et al.,1994]. A possible explanation for the current right PFC engagement could be the greater demand on attentional and monitoring processes required in the effort to apply accurate semantic strategies. It is of note that the right PFC engagement in the present study occurred during the RNS and UR conditions but not during the RS condition. In the RNS the words belonging to the same category were randomly presented, and therefore subjects would have to apply greater attention to each one and monitor the order of their presentation to organize them into categories. In the UR, we speculate that subjects may have been attempting to apply the strategy, even though this would prove to be difficult given that the words were unrelated. In the RS the words were presented in an organized fashion according to their categories, minimizing the organizational demands, and the subjects only had to remember the categories to facilitate retrieval.
The finding of increased activation in the OFC before training in the RNS condition is similar to the finding of a previous study by Savage et al. [2001], who demonstrated engagement of the OFC during a verbal encoding paradigm without cognitive training associated with early mobilization of behavioral strategies. Human lesion and neuroimaging studies indicated that the OFC may mediate the early inhibition of automatic behavior favoring a more well planned and organized one, particularly in novel situations [Bechara et al.,1996; Damasio,1996; Nobre et al.,1999]. The OFC is anatomically positioned as a convergence zone for emotional and cognitive information processing with reciprocal connections with limbic structures, anterior cingulate, and association cortex. It is possible that in the current study the engagement of the OFC was associated before training with some of the subjects' identifications of a situation in which behavioral strategies could be applied.
The bilateral cerebellum activation found in this study was also unexpected, since the paradigm used the same type of stimuli before and after training. Recently, a study suggested that the cerebellum is in fact active during a wide range of activities not directly related to movement [Bower and Parsons,2003]. They suggested a role for it as a support structure for the rest of the brain involving monitoring incoming sensory information and making continuous adjustments in how that information is acquired. In a lesion study mentioned by the authors, subjects with atrophy in the cerebellum had difficulties with the planning of the steps to solve the Tower of Hanoi task. Other neuroimaging studies also suggested that the cerebellum participates in working memory, attention, and impulse control [Bower and Parsons,2003]. We speculate that the current bilateral cerebellum activation was associated only after training with the increased requirement to monitor the sensory information being received—in this case the visually presented words— supporting the other brain structures involved in the application of the semantic strategies.
Another finding was an association between higher levels of PFC activity and higher strategic memory capacity after training. This is consistent with previous studies showing a positive correlation between cortical prefrontal activity and working memory capacity [Klingberg et al.,2002; Olesen et al.,2004; Poldrack and Gabrieli,2001]. Since in the current study we trained the subjects for a period of 30 min, it is difficult to argue for an explanation of cortical plasticity, as suggested in previous studies. However, one study using an 88‐min short‐term language comprehension training during the interval between 12 PET scans in patients with aphasia found improved language performance correlated with increased brain activity in the right temporal gyrus and left precuneus [Musso et al.,1999]. The authors suggested that this short‐term training was capable of inducing functional brain reorganization. The findings of the current study could indicate that the effort in applying cognitive strategy involved recruitment of regions of PFC required in the operation of this strategy, probably supporting greater levels of working memory functioning.
In summary, our study demonstrated that training‐induced changes in strategic episodic memory performance were related to increased bilateral prefrontal cortical activation in healthy adult subjects. The behavioral and signal changes observed in this study as a consequence of cognitive training may also reflect the recruitment of a network of areas, each area playing a specialized role in one or more aspects of strategic episodic memory while depending on the participation and support from the others in order to produce an efficient response. The functional adaptations observed here may shed light on some of the processes underlying recovery with cognitive rehabilitation in patient populations with brain injury. However, it is also possible that recovery of function, particularly that supported by rehabilitation, might be related to the compensatory use of strategies to support learning. In addition, other factors different from the effects of short‐term practice with the use of a clustering strategy are likely to be at least as important in supporting recovery of function. Future studies are needed in brain‐injured populations.
REFERENCES
- Bechara A, Tranel D, Damásio H, Damásio AR (1996): Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 6: 215–225. [DOI] [PubMed] [Google Scholar]
- Bower JM, Parsons L (2003): Rethinking the lesser brain. Sci Am 289: 51–57. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SCR, Grasby PM, Howard RJ, Woodruff PWR, Rabe‐Hesketh S (1997): Generic brain activation mapping in fMRI: a non‐parametric approach. Magn Reson Imaging 15: 763–770. [DOI] [PubMed] [Google Scholar]
- Breakspear M, Brammer MJ, Bullmore ET, Das P, Williams LM (2004): Spatiotemporal wavelet resampling for functional neuroimaging data. Hum Brain Mapp 23: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Peterson SE (1996): What does neuroimaging tell us about the role of prefrontal cortex in memory retrieval? Semin Neurosci 8: 47–55. [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE (1999): Frontal cortex contributes to human memory formation. Nat Neurosci 2: 311–314. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Brammer MJ, Rabe‐Hesketh S, Curtis V, Morris RG, Williams SCR, Sharma T, McGuire PK (1999a): Methods for diagnosis and treatment of stimulus‐correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 7: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ (1999b): Global, voxel and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Long C, Suckling J, Fadili J, Calvert GA, Zelaya F, Carpenter TA, Brammer MJ (2001): Colored noise and computational inference in neurophysiological fMRI time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 12: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR (1996): The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Dellis DC, Kramer JH, Kaplan E, Ober BA (1987): California verbal learning test: manual. San Antonio, TX: Psychological. [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD (1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn 7: 1–13. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Rugg MD (1997): The functional neuroanatomy of episodic memory. Trends Neurosci 20: 213–218. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith RS, Frackowiak RSJ, Dolan RJ (1998): The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain 121: 1249–1256. [DOI] [PubMed] [Google Scholar]
- Friman O, Borga M, Lunderberg GP, Knutsson H (2003): Adaptive analysis of FMRI data. Neuroimage 19: 837–845. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ (1999): Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain 122: 1367–1381. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H (2002): Increased brain activity in frontal and parietal cortex underlies the development of visuo‐spatial working memory capacity during childhood. J Cogn Neurosci 14: 1–10. [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijnties M (1999): Training‐induced brain plasticity in aphasia. Brain 122: 1781–1790. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM (1999): Orbitofrontal cortex is activated by breaches of expectation in tasks of visual attention. Nat Neurosci 2: 11–12. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Stigsdotter A, Petersson KM, Ingvar M, Backman L (2003): Neural correlates of training‐related memory improvement in adulthood and aging. Proc Natl Acad Sci U S A 100: 13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T (2004): Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7: 75–79. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M (1996): Evidence for a two‐stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 6: 352–354. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD (2001): Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain 124: 67–82. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ (1996): Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain 119: 2073–2083. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AL, Rauch SL (2001): Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain 124: 219–231. [DOI] [PubMed] [Google Scholar]
- Schacter D, Tulving E (1994): Memory systems. Cambridge, MA: MIT Press. [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS (1996): Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A 93: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ (1994): Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368: 633–635. [DOI] [PubMed] [Google Scholar]
- Tulving E (1962): Subjective organization in free recall of 'unrelated' words. Psychol Rev 69: 344–254. [DOI] [PubMed] [Google Scholar]
- Tulving E (1983): Elements of episodic memory. Oxford: Clarendon Press. [Google Scholar]
- Tulving E, Kapur S, Craik FI, Habib R, Houle S (1994): Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


