Abstract
Carpal tunnel syndrome (CTS) is a common entrapment neuropathy of the median nerve characterized by paresthesias and pain in the first through fourth digits. We hypothesize that aberrant afferent input from CTS will lead to maladaptive cortical plasticity, which may be corrected by appropriate therapy. Functional MRI (fMRI) scanning and clinical testing was performed on CTS patients at baseline and after 5 weeks of acupuncture treatment. As a control, healthy adults were also tested 5 weeks apart. During fMRI, sensory stimulation was performed for median nerve innervated digit 2 (D2) and digit 3 (D3), and ulnar nerve innervated digit 5 (D5). Surface‐based and region of interest (ROI)‐based analyses demonstrated that while the extent of fMRI activity in contralateral Brodmann Area 1 (BA 1) and BA 4 was increased in CTS compared to healthy adults, after acupuncture there was a significant decrease in contralateral BA 1 (P < 0.005) and BA 4 (P < 0.05) activity during D3 sensory stimulation. Healthy adults demonstrated no significant test–retest differences for any digit tested. While D3/D2 separation was contracted or blurred in CTS patients compared to healthy adults, the D2 SI representation shifted laterally after acupuncture treatment, leading to increased D3/D2 separation. Increasing D3/D2 separation correlated with decreasing paresthesias in CTS patients (P < 0.05). As CTS‐induced paresthesias constitute diffuse, synchronized, multidigit symptomatology, our results for maladaptive change and correction are consistent with Hebbian plasticity mechanisms. Acupuncture, a somatosensory conditioning stimulus, shows promise in inducing beneficial cortical plasticity manifested by more focused digital representations. Hum Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: alternative medicine, somatotopy, neuropathy, Hebbian plasticity, nerve entrapment
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy, with a prevalence of 3.72% in the United States. CTS etiology is characterized by compression of the distal median nerve and an elevated pressure in the carpal tunnel. Ischemic injury to the median nerve induces a range of symptoms primarily in the first through fourth digit, including paresthesias, pain, and weakness. Paresthesias represent the most common symptom [Nora et al.,2005] and result from ectopic impulse activity generated by ischemic nerve damage [Mogyoros et al.,2000; Ochoa and Torebjork,1980].
Surgery is considered the most definitive treatment, with as high as 70–90% of subjects reporting freedom from nocturnal pain after surgery [Katz et al.,1998]. Nevertheless, surgery drives up costs and there have been long‐term follow‐up studies suggesting that patients who have undergone carpal tunnel release do no better that those who have been managed with conservative care [Nathan et al.,2001]. Conservative treatments vary from injection of corticosteroids to neutral wrist splinting [Wilson and Sevier,2003]. Injection of corticosteroids has been found to improve symptoms in more than 75% of patients, with short‐term improvement in median‐nerve conduction. However, symptoms generally recur within 1 year. In addition, a recent review by O'Connor et al. [2003] found that current evidence suggests that short‐term benefit can be derived from oral steroids, splinting, ultrasound, yoga, and carpal bone mobilization, but the durability of these treatments is not known.
Acupuncture for the treatment of CTS has been evaluated in several studies. Naeser et al. [2002] found a significant reduction in McGill Pain Questionnaire scores and median nerve sensory latency after a mixed laser acupuncture/micro‐transcutaneous electrical nerve stimulation (MicroTENS) treatment protocol compared to sham stimulation in a crossover design trial. Chen [1990] reported almost uniform improvement (97.2% success rate by questionnaire) using conventional manual and electrical acupuncture. However, the mechanism by which acupuncture affects CTS is not well understood. Furthermore, it is not clear how acupuncture might impact the central manifestations of CTS, a peripheral neuropathic condition.
CTS leads to altered afferent processing throughout the somatosensory system (e.g., peripheral and central nervous systems), as measured by somatosensory evoked potentials (SEPs) in the spinal cord, brainstem, and primary sensorimotor cortex (SMC) [Tinazzi et al.,1998]. The digits occupy a significant portion of the human somatotopic map in the primary somatosensory cortex and are represented in consecutive order along the postcentral gyrus, with digit 1 (D1) most ventrolateral and D5 most dorsomedial [Penfield and Boldrey,1937]. Using magnetoencephalography (MEG), Tecchio et al. [2002] demonstrated that the cortical representation distance between D1 and D5 in the contralateral primary somatosensory cortex (SI) depended on the patients' qualitative symptomatology—expansion when paresthesias prevailed and contraction when pain prevailed. Further, compared to D5 (ulnar nerve innervated), the cortical response for D3 (median nerve innervated) was amplified. We have also reported differences in central somatosensory processing between CTS patients and healthy adults using functional MRI (fMRI) [Napadow et al.,2006]. We found that CTS patients experienced cortical hyperactivation to nonnoxious somatosensory stimulation of affected digits and expanded cortical representations. We also found that the primary somatosensory representations for D2 and D3 were closer together (blurred) than in healthy adults, with the separation distance correlating negatively with a degree of pathology as measured by delay in the sensory nerve conduction latency.
It is widely known that altered afferent input patterns drive neuroplasticity in the cerebral cortex [Merzenich et al.,1983, 1984]. In fact, aberrant somatotopy has been described for other chronic pain states, such as complex regional pain syndrome (CRPS, type I, see Maihofner et al. [2003]), and evidence exists that as these conditions resolve, so does the maladaptive central plasticity [Maihofner et al.,2004]. Additionally, aberrant somatotopy also results from synchronized afferent input in a condition known as syndactyly, where adjacent digits on the hand are fused together. Electrophysiology and MEG studies have demonstrated that syndactyly produces blurred cortical representations of the affected digits [Allard et al.,1991], while surgical separation of these digits induces normalized cortical reorganization [Mogilner et al.,1993]. This suggests that peripherally induced maladaptive cortical plasticity can in fact be corrected by appropriate therapy.
To study central reorganization associated with acupuncture‐treated CTS, we used fMRI, an imaging modality that can noninvasively map digit somatotopy [Gelnar et al.,1998; Kurth et al.,1998; McGlone et al.,2002]. The aim of the present study was to assess cortical reorganization for CTS patients postacupuncture and to correlate these changes with the degree of clinical improvement. As CTS is associated with cortical hyperactivation and more blurred digit representations in contralateral SI, we hypothesize that successful acupuncture treatment will diminish the extent of somatosensory cortical hyperactivation and separate the cortical representations of median nerve innervated digits. Furthermore, as some subjects respond better to acupuncture than others, we hypothesize that improvement in somatotopy will correlate with improvement in clinical variables. This study in CTS patients represents the first investigation of longitudinal effects in the brain following therapeutic intervention and its outcome may impact future rehabilitation strategies for CTS patients.
SUBJECTS AND METHODS
Subject Recruitment and Evaluation
All participants in the study provided written informed consent in accordance with the Human Research Committee of the Massachusetts General Hospital. A total of 25 subjects were enrolled in this study: 13 adults affected by CTS and 12 age‐ and sex‐matched healthy adults. Of the 13 CTS patients initially studied, one was removed for excessive motion during fMRI scanning and two were removed because they dropped out of the study immediately after the initial clinical and fMRI evaluation. Of the 12 healthy adults recruited, 9 completed the test–retest clinical and fMRI evaluations (see below). Clinical evaluation was completed for both groups by an experienced physician (J.A.) at the Spaulding Rehabilitation Hospital and included medical history, nerve conduction studies (Cadwell Sierra EMG/NCS Device, Kennewick, WA), grip strength (BTE Work Simulator, Hanover, MD), sensory threshold testing using Semmes Weinstein monofilaments, and testing for Phalen's and Tinel's sign. Nerve conduction studies (NCSs) using the method described by Ma and Liveson [1983] were performed on both the median and ulnar nerves of the most symptomatic hand (based on subject history). Both groups completed the Boston Carpal Tunnel Syndrome Questionnaire (BCTSQ) [Levine et al.,1993]. Subjects were screened and excluded if they were pregnant or had a history of diabetes mellitus, rheumatoid arthritis, thyroid dysfunction, wrist fracture or direct trauma to median nerve, atrophy of the thenar eminence, carpal tunnel surgery, current use of prescriptive opioid pain medications, psychiatric and neurological disorders, head trauma with loss of consciousness, or other serious cardiovascular, respiratory, or renal illness. Subjects were also excluded if they had any contraindication for undergoing MRI (e.g., pacemaker), or any contraindication for acupuncture (e.g., anticoagulation therapy). CTS patients were included if they had experienced pain and/or paresthesias for greater than 3 months in the median nerve distribution of the affected hand: D1, D2, D3, and the radial aspect of D4. Further, NCS findings needed to be consistent with mild to moderate CTS. Mild CTS was defined by delayed distal latency of median sensory nerve conduction across the wrist (>3.7 ms and/or >0.5 ms compared to ulnar sensory nerve conduction) with normal motor nerve conduction [Stevens,1997; You et al.,1999]. Moderate CTS was defined to be the same as mild CTS plus delayed distal latency of median motor nerve conduction across the wrist (>4.2 ms), but with normal motor amplitudes. Subjects with severe CTS, defined by prolonged median sensory and motor latencies with either absent sensory nerve action potentials and/or reduced (50%) median motor amplitudes, were excluded. Patients were also excluded if they demonstrated any evidence on NCS of generalized peripheral neuropathy or localized ulnar nerve entrapment. This study was set up as an experimental neuroimaging evaluation, and not as a randomized placebo‐controlled clinical trial to test for the specific effects of acupuncture.
Acupuncture Treatment
Acupuncture was performed by experienced practitioners on CTS patients over a 5‐week period, after baseline clinical and fMRI evaluation. Treatments were provided three times per week for 3 weeks and two times per week for the remaining 2 weeks. A semi‐individualized approach was used wherein every subject was treated for 10 min with 2 Hz electro‐acupuncture at common acupoints (Fig. 1): unilateral TW‐5 (triple‐warmer 5, dorsal aspect of forearm) to PC‐7 (pericardium 7, first wrist crease). This was followed by manual needling at acupoints chosen by the practitioner based on the individual symptoms of the presenting patient. Three points were chosen out of the following six: HT‐3 (heart 3, medial aspect of elbow), PC‐3 (pericardium 3, medial aspect of elbow crease), SI‐4 (small intestine 4, ulnar aspect of wrist), LI‐5 (large intestine 5, radial aspect of wrist), LI‐10 (large intestine 10, lateral aspect of forearm), LU‐5 (lung 5, lateral aspect of elbow crease). These points were stimulated with a manual even needle technique where a deqi response was obtained.
Figure 1.
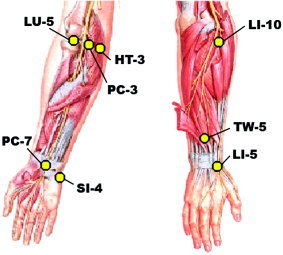
Acupoints utilized in our protocol for treatment of CTS. Figure adapted from F.H. Netter and J.T. Hansen, Atlas of Human Anatomy, ICON Learning Systems, 3rd Ed., 2002. Netter illustration used with permission of Elsevier Inc. All rights reserved.
fMRI Image Acquisition and Stimulation Protocol
fMRI test–retest scanning was completed on 10 CTS patients (six female; mean age, 51.1, range, 31–60) before (baseline) and following 5 weeks of acupuncture, with an interval of 40.6 ± 4.4 days (mean ± standard deviation, SD) between scan sessions. fMRI scanning was also completed on nine age‐ and sex‐matched healthy adults (six female; mean age, 46.9, range, 32–59) spaced at least 5 weeks apart, with an interval of 44.0 ± 7.7 days between scan sessions. Five patients presented with CTS symptoms in both hands, while five presented with only unilateral symptomatology. For patients with bilateral CTS symptoms, testing was done on the more affected hand (self report). In all cases, the more affected hand was also the patient's dominant hand. The chronicity of symptoms (self reported) ranged from 4 months to 10 years, with 8 of 10 patients having symptoms for longer than 1 year. While patients reported both pain and dysesthesias, a pain/dysesthesia ratio was calculated from subjective responses to individual questions of the BCTSQ (pain: Q1–Q5, dysesthesias: Q6–Q10).
Functional scans were acquired using a 3.0 T Siemens (Erlangen, Germany) Allegra MRI System equipped for echo planar imaging with quadrature headcoil. The subject lay supine in the scanner with the head immobilized using cushioned supports. Two sets of structural images were collected using a T1‐weighted MPRAGE sequence (TR/TE = 2.73/3.19 ms, flip angle = 7°, field of view (FOV) = 256 × 256 mm; slice thickness = 1.33 mm). Blood oxygenation level‐dependent (BOLD) functional imaging was performed using a gradient echo T2*‐weighted pulse sequence (TR/TE = 3000/30 ms, flip angle = 90°, FOV = 200 × 200 mm, 38 sagittal slices, slice thickness = 3.0 mm with 0.6 mm interslice gap, matrix = 64 × 64, 90 time points). Image collection was preceded by four dummy scans to allow for equilibration of the MRI signal.
During an fMRI session, three digits were individually stimulated in a pseudorandomized manner for three scan runs each. Digits were chosen from the more affected hand of CTS patients (right hand: n = 8) and on the dominant hand of healthy adult volunteers (right hand: n = 8). The stimulated digits included D2, D3, and D5, thereby testing the somatosensory response for affected (median n., D2, D3) and nonaffected (ulnar n., D5) digits in CTS patients. A single fMRI scan run consisted of a block design protocol with four 30‐s blocks of stimulation (ON‐block), alternating with five 30‐s blocks of no stimulation (OFF‐block). Stimulation was delivered via pad electrodes attached to the volar aspect (glabrous skin) of the middle and distal phalanx of D2, D3, and D5. The stimulus consisted of 100 Hz constant current electrostimulation (HANS LH202H, Neuroscience Research Center, Peking University, Beijing, China). In order to avoid habituation, the peak amplitude of the 100 Hz electrostimulation was modulated ±25% by a slow varying sinusoidal envelope (0.2 Hz) throughout each 30‐s ON‐block. The electrical current intensity during each scan was percept‐matched for each individual to be strong but nonpainful (just below the pain threshold by 0.2 mA) in order to normalize for differences in pain and somatosensory sensitivity, particularly because CTS is reported to involve abnormal somatosensory threshold levels [Nishimura et al.,2003]. Current intensity was determined immediately prior to each scan to ensure that the stimulus was not painful. Three scans were completed for each digit. Subjects were instructed to keep their eyes closed and to pay close attention to the sensations felt at the stimulated digit.
Single‐Subject fMRI Data Analysis
Analysis was carried out using a combination of software including FEAT (FMRI Expert Analysis Tool) v. 5.1, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl), surface mapping (SUMA) in AFNI [Cox,1996], and Freesurfer [Dale et al.,1999]. Data collected from left‐hand dominant healthy adults (one subject) or predominant left‐handed CTS patients (two subjects) were mirror‐reversed across the mid‐sagittal plane. The following prestatistics processing was applied: motion correction using MCFLIRT [Jenkinson et al.,2002]; nonbrain removal using Brain Extraction Tool (BET) [Smith,2002]; spatial smoothing using a Gaussian kernel of full‐width at half‐maximum (FWHM) 5 mm; mean‐based intensity normalization; and highpass temporal filtering (sigma = 30.0 s). Time‐series statistical analysis was carried out using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction [Woolrich et al.,2001]. The hemodynamic response function utilized in the general linear model (GLM) analysis was defined by the block design paradigm convolved with a prescribed gamma function (SD = 3 s, mean lag = 6 s).
Structural T1‐weighted MPRAGE images from each subject were averaged and the Freesurfer software package was used to generate a model of the cortical surface through intensity normalization, skull‐stripping, segmentation, and tessellation of the gray/white matter interface surface [Dale et al.,1999]. This reconstructed cortical surface was inflated using spring force and metric‐preserving terms. The tessellated surface was also projected onto a unit sphere (spherical space), using algorithms that minimize metric distortions, thereby parameterizing the surface into a spherical‐based coordinate system [Fischl et al.,1999a]. The cortical surface of each subject brain was normalized to an average brain template by maximizing alignment of the folding patterns in a spherical representation [Fischl et al.,1999b], a process that provides accurate group‐averaged statistical analysis over surface elements from topologically homologous regions in the brains of different subjects. Surface‐based analyses for somatosensory mapping in SI have been used in the past [Moore et al.,2000]. These postprocessing techniques improve both anatomical region attribution and functional data visualization by respecting the topological curvature inherent in the sulcal and gyral structure found around the central sulcus. Hence, these techniques are especially suited for studies of somatotopy, as described below.
Surface‐Based Group fMRI Data Analysis
Functional images from each subject were coregistered with their averaged T1‐weighted MPRAGE structural volume using an automated procedure that included particular focus on central sulcus coregistration. Separate scan runs for each stimulated digit were assessed for excessive motion artifact (removed if >3 mm on any axis) and averaged under a fixed effects model. The percent signal change was then calculated and the mean value taken along a normal vector through the cortical gray matter thickness was also calculated and normalized to a standard mesh surface (defined by nodes and mesh elements). Group maps for each stimulated digit and activation difference maps (CTS patients: post‐ vs. preacupuncture; healthy adults: at 5 weeks vs. baseline) were calculated using a fixed effects statistical model, which limits false negatives but applies only to the subjects in the model and cannot be extended to the population. Resultant statistical parametric maps were corrected for multiple comparisons at a false discovery rate (FDR) of 0.05 [Genovese et al.,2002] and clustered at an area equivalent to the cross‐section of three image voxels (29.3 mm2).
ROI‐Based fMRI Data Analysis
In order to set up a random effects analysis that takes into account intersubject variability, the extent of SMC activation and somatotopy were quantified with the aid of user‐defined anatomical ROIs, outlined on the smoothed white matter (gray/white boundary) surface. The ROIs included putative BA 1, defined as the crest of the postcentral gyrus, and putative BA 4, defined as the anterior bank of the central sulcus [Gelnar et al.,1998; Moore et al.,2000]. The superior margin of the ROIs was taken to be the superior edge of the hemisphere, thereby avoiding potential contamination from dorsal interhemispheric vein artifacts. The inferior edge of the ROIs was defined by a posterior extension of the inferior frontal sulcus, thereby avoiding the parietal operculum. These ROIs were also used and visualized in a previous publication [Napadow et al.,2006], which was a cross‐sectional analysis of the same subjects as in this longitudinal study. The extent of activation for each individual during test and retest was defined as the surface area of statistically significant fMRI signal increase. The mean extent of activation in BA 1 and BA 4 ROIs for CTS patients and healthy adults was compared with a Student's paired t‐test (significant for α < 0.05). Hence, this analysis employed a random effects model that accounted for intersubject variance.
Somatotopy was assessed by finding the standard mesh surface‐based location of the percent signal change center‐of‐mass of the largest hand area cluster in BA 1 contralateral to digit stimulation. The hand area in SI was defined broadly as the postcentral gyrus involution adjacent to the omega shaped fold in the central sulcus around a knob in the precentral gyrus [Yousry et al.,1997]. The center‐of‐mass location for each individual was also found in a group‐averaged surface to ensure intergroup comparability. The location was quantified on the group‐averaged surface by the surface‐based distance from a common convenient landmark—the lateral edge of the parietal operculum on the crest of the postcentral gyrus. Center‐of‐mass digit representations in BA 1 and D2/D3 separation distance were compared between test/retest conditions for CTS patients and healthy adults with a Student's paired t‐test (significant for α < 0.05). Through increased sensitivity due to intersubject averaging and center‐of‐mass calculations, spatial differences in somatotopy for both patients and controls could be derived that approached the single‐subject voxel resolution. Furthermore, Spearman's rank correlation and Pearson's correlation tests were used to determine if the change in D2/D3 separation distance correlated with pertinent discrete (BCTSQ, change in paresthesias) or continuous (nerve conduction latency) clinical testing variables, respectively.
RESULTS
In our cohort of patients, acupuncture produced improvement in both subjective (BCTSQ questionnaire) and objective (nerve sensory latencies, grip strength) outcome measures (details provided below). Imaging results demonstrated test–retest cortical plasticity in the CTS cohort treated by acupuncture.
Neurophysiological and Clinical Results
While a more complete treatment of the neurophysiological and clinical data has been presented elsewhere (Audette et al., in review), the following results were found for the cohort of patients included in this neuroimaging study. In our cohort of patients, dysesthesias were more dominant than pain in 7/10 patients, as derived from self‐report in the BCTSQ. The remaining 3/10 patients had pain/dysesthesia ratios less than 1.1 (i.e., close to an equal amount of pain and dysesthesias). The clinical results for our CTS patients demonstrated that dysesthesia levels from the BCTSQ (Q6–10, 1–5 scale) decreased (improved) from 2.48 ± 0.22 (mean ± SEM) to 1.86 ± 0.23 (paired t‐test, P < 0.001) after 5 weeks of acupuncture. Specifically, paresthesia levels improved from 2.7 ± 0.6 to 1.3 ± 0.5 (paired t‐test, P < 0.005). However, overt pain levels (Q1–5) did not improve significantly by BCTSQ (1.80 ± 0.27 to 1.66 ± 0.29, P > 0.1). The average median nerve sensory latency to D2 and D3 was 3.48 ± 0.05 ms (mean ± SEM) and 3.80 ± 0.07 ms, respectively, and improved to 3.16 ± 0.05 ms (paired t‐test, P < 0.05) and 3.33 ± 0.06 ms (P < 0.005), respectively. Furthermore, mean improvement in sensory latency was 0.14 ± 0.02 ms greater for D3 than for D2 (paired t‐test, P < 0.05). In healthy adults, no statistically significant change in median nerve sensory latencies was found for either D2 or D3 (P > 0.1) in our 5‐week test–retest evaluation. Further, there was no significant change in the ulnar sensory latency to D5 in either CTS patients (baseline: 2.09 ± 0.04 ms; post: 2.00 ± 0.03 ms, P > 0.1) or healthy adults. After acupuncture, percent change in grip strength in the more affected hand for CTS patients was significantly improved (20.3 ± 3.6%, P < 0.05), while healthy adults did not show a significant change (0.55 ± 3.44%, P > 0.1).
fMRI Surface‐Based Group Comparisons
Stimulation of digits 2, 3, and 5 produced fMRI activation in precentral and postcentral (SI) gyri, and the secondary somatosensory cortex, or SII (Table I). After acupuncture treatment the CTS group activation maps for median nerve innervated D3 demonstrated decreased extent of activation in the contralateral precentral and postcentral gyrus, as well as the dorsolateral prefrontal and inferior parietal cortices (group mean maps, Fig. 2; statistical difference maps, Fig. 3). No statistically significant difference in the extent of precentral or postcentral activity was found for D2 or D5 stimulation in CTS patients. No differences were found in precentral or postcentral gyrus for test–retest results in healthy adults for D2, D3, and D5 (Fig. 3). Visualizing the SI cluster center‐of‐mass (from Table I) showed that the D2 and D3 representations in SI were in close proximity to one another at baseline and were more separated after acupuncture treatment (Fig. 4). Representation of the three digits in SI for healthy control subjects showed similar test–retest localization (Fig. 4).
Table I.
Summary of carpal tunnel syndrome (CTS) baseline and postacupuncture group map sensorimotor cortical activations
| Group, digit and region | Talairach x, y, z (mm) | P value (10−x) | Cluster size (mm2) |
|---|---|---|---|
| CTS baseline | |||
| 2 | |||
| SI | −51, −15, 50 | 8.40 | 322.95 |
| PrCG | −41, −15, 58 | 7.21 | 166.22 |
| SII | −51, −12, 22 | 8.75 | 550.02 |
| 3 | |||
| SI | −50, −16, 52 | 8.83 | 520.59 |
| PrCG | −39, −17, 62 | 6.88 | 140.08 |
| SII | −52, −13, 22 | 9.39 | 702.72 |
| 5 | |||
| SI | −45, −20, 54 | 7.94 | 146.57 |
| PrCG | −39, −19, 62 | 7.55 | 28.03 |
| SII | −50, −13, 22 | 8.62 | 411.17 |
| CTS post acup | |||
| 2 | |||
| SI | −47, −13, 42 | 9.05 | 471.86 |
| PrCG | −38, −15, 62 | 9.60 | 60.42 |
| SII | −51, −15, 23 | 9.12 | 749.33 |
| 3 | |||
| SI | −51, −16, 51 | 8.13 | 127.45 |
| PrCG | −38, −18, 63 | 5.39 | 9.57 |
| SII | −51, −14, 23 | 6.79 | 72.61 |
| 5 | |||
| SI | −46, −18, 55 | 8.44 | 192.74 |
| PrCG | −38, −16, 63 | 9.70 | 58.28 |
| SII | −51, −13, 22 | 8.09 | 205.97 |
| Healthy baseline | |||
| 2 | |||
| SI | −47, −15, 46 | 8.73 | 98.78 |
| PrCG | −39, −12, 54 | 7.17 | 126.54 |
| SII | −52, −13, 22 | 10.05 | 474.95 |
| 3 | |||
| SI | −51, −17, 51 | 8.44 | 175.97 |
| PrCG | −38, −10, 58 | 6.71 | 38.54 |
| SII | −54, −12, 22 | 10.15 | 330.45 |
| 5 | |||
| SI | −50, −20, 53 | 6.43 | 53.65 |
| PrCG | —, —, — | — | — |
| SII | −55, −13, 21 | 7.36 | 224.12 |
| Healthy Week 5 | |||
| 2 | |||
| SI | −47, −14, 43 | 7.82 | 174.48 |
| PrCG | −39, −11, 53 | 5.83 | 24.36 |
| SII | −52, −12, 22 | 8.52 | 307.71 |
| 3 | |||
| SI | −51, −17, 51 | 6.84 | 100.81 |
| PrCG | −41, −18, 57 | 6.37 | 13.48 |
| SII | −48, −11, 20 | 6.03 | 38.97 |
| 5 | |||
| SI | −49, −20, 54 | 7.89 | 84.25 |
| PrCG | —, —, — | — | — |
| SII | −52, −12, 22 | 6.82 | 216.85 |
Cluster center‐of‐mass (COM) Talairach coordinates, significance, and extent for SI, precentral gyrus, and SII. SI, primary somatosensory cortex; PrCG, precentral gyrus; SII, secondary somatosensory cortex; P value, mean P value for cluster, expressed as 10−x; cluster size, size of cluster on the surface, expressed as mm2).
Figure 2.
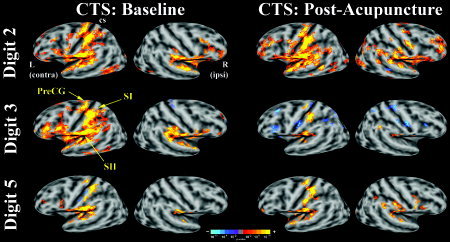
Group maps of CTS patients at baseline and postacupuncture for D2, D3, and D5 nonnoxious electrostimulation. Activation (color‐coded P‐value) was overlaid onto group‐averaged inflated brains with gray‐scale defined curvature (sulci dark, gyri light). Both right (ipsilateral) and left (contralateral) hemispheres are shown. Hyperactivity in contralateral sensorimotor cortex seen for median nerve innervated D3 diminished after acupuncture treatment. Differences for D2 and ulnar nerve innervated D5 were less profound (cs: central sulcus).
Figure 3.
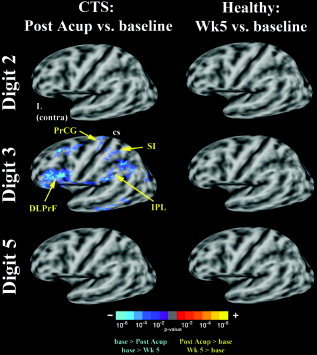
Difference maps for CTS patients (baseline vs. postacupuncture) and healthy adults (baseline vs. 5 weeks later) for D2, D3, and D5 nonnoxious electrostimulation. Decreased fMRI activation was seen for D3 in CTS patients in contralateral SI, precentral gyrus, as well as the dorsolateral prefrontal and inferior parietal cortices. Differences for D2 and ulnar nerve innervated D5, as well as all three digits in healthy adults were less profound (cs: central sulcus).
Figure 4.
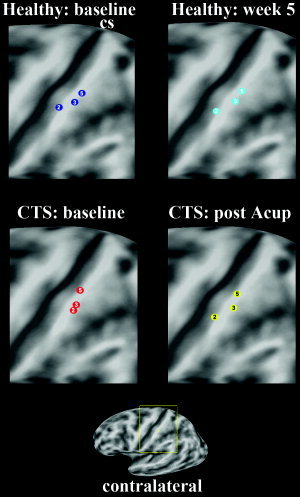
Visualization of the group map contralateral SI representations for digits 2, 3, and 5 was done by localizing the cluster center‐of‐mass. While healthy adults demonstrated similar test–retest localization for all three digits, in CTS patients the D2 and D3 representations, which were closely grouped at baseline, were further apart after acupuncture treatment.
fMRI ROI‐Based Comparisons: Extent and Somatotopy
With innocuous D2 and D3 stimulation the extent of fMRI activity in contralateral BA 1 ROI was increased in CTS patients compared to healthy adults [Napadow et al.,2006]. After acupuncture, there was a significant decrease in contralateral BA 1 activity for D3 stimulation from 162.5 ± 32.2 mm2 (mean ± SEM) to 66.6 ± 22.6 mm2 (paired t‐test, P < 0.005, Fig. 5A). There was no significant change in BA 1 activity after acupuncture (P > 0.1) for stimulation of either D2 (pre: 151.0 ± 32.1 mm2; post: 107.7 ± 25.0 mm2) or D5 (pre: 88.7 ± 33.2 mm2; post: 116.6 ± 28.0 mm2). Furthermore, in healthy adults no significant differences were seen in the extent of BA 1 activity for D2, D3, or D5 (P > 0.1). Activity in BA 4 was also increased for D2 and D3 in CTS patients compared to healthy adults. After acupuncture the extent of activity for D3 diminished significantly in BA 4 from 144.7 ± 45.3 mm to 45.4 ± 21.3 mm (P = 0.014, Fig. 5B). There was no significant change in BA 4 activity after acupuncture (P > 0.1) for stimulation of either D2 (pre: 115.3 ± 38.4 mm2; post: 88.6 ± 16.6 mm2) or D5 (pre: 63.3 ± 26.3 mm2; post: 59.7 ± 19.0 mm2). In healthy adults, no significant differences were seen in BA 4 activity for test–retest fMRI with D2, D3, or D5 (P > 0.1).
Figure 5.
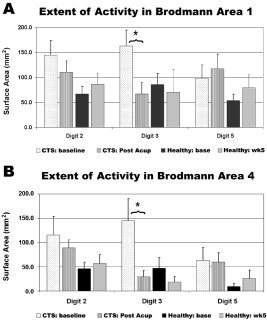
ROI analysis. During D3 stimulation, a significant decrease in activity was seen in both (A) BA 1 and (B) BA 4 for CTS patients after acupuncture treatment (*P < 0.005). No significant difference was seen for D2 or D5 in CTS patients or for any digit in control healthy adults.
In our cross‐sectional study, analysis of somatotopy demonstrated that CTS patients had diminished D2/D3 separation distance compared to healthy adults due to a medial shift of the D2 primary sensory representation [Napadow et al.,2006]. After acupuncture therapy we found that the D2 representation shifted laterally away from the D3 representation (paired t‐test, P = 0.029) by an average of 2.17 ± 0.79 mm (mean ± SEM; Fig. 6). This shift in D2 for CTS patients after acupuncture resulted in a trend toward greater D2/D3 separation distance (pre: 3.81 ± 1.05 mm; post: 5.55 ± 0.79 mm; P = 0.058). No significant shifts were seen for either D3 or D5 (P > 0.1). Moreover, no significant shifts were seen in healthy adults for D2, D3, or D5 (P > 0.1).
Figure 6.
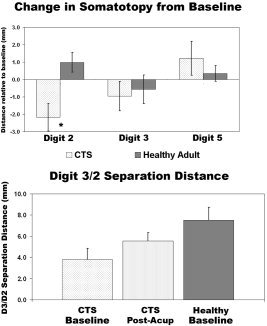
Somatotopy for CTS patients and healthy adults was defined by the distance of fMRI center‐of‐mass activation from the lateral edge of the parietal operculum. After acupuncture, there was a significant lateral shift in the cortical representation for D2 (P = 0.029). This shift produced an increase in the D3/D2 separation distance for CTS patients (P = 0.058), bringing it closer to that found for healthy adults.
Correlation of fMRI Results with Neurophysiological/Clinical Variables
Improvement in median sensory nerve conduction latencies for D3 varied among CTS patients after receiving acupuncture therapy (see below). We found that the change in conduction latency for D3 was negatively correlated with the change in D2/D3 separation distance (r = −0.72, P < 0.05), i.e., the more the conduction latency decreased (improved), the greater the expansion in D2 and D3 separation distance (Fig. 7). In addition, as we hypothesized that somatosensory cortical plasticity in our cohort of CTS patients was driven by paresthesias, we explored changes in self‐reported paresthesias (BCTSQ 8) after acupuncture treatment and found that 7/10 patients reported improvement (see below). Furthermore, those patients who did not report improvement did not have a significant expansion of the D2/D3 separation distance, which correlated negatively with the change in paresthesias, i.e., the more paresthesias improved, the more the D2/D3 separation distance increased (Spearman's rank correlation test, r = −0.73, P < 0.05, Fig. 8). No significant correlations were found between the change in D2/D3 separation distance and changes in other clinical measures such as neuropathic pain severity, sensory thresholds, or grip strength. Finally, we found a predictive positive correlation (r = 0.72, P < 0.05) between CTS patients' baseline value of D2 median sensory latency and the eventual change in D2/D3 separation distance, i.e., worse conduction block predicts greater separation in abnormally overlapping digits after acupuncture treatment.
Figure 7.
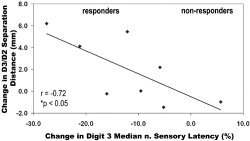
Correlation analysis demonstrated that improvement in D3 conduction latency correlated negatively with the change in D2/D3 separation distance (r = −0.72, P < 0.05), i.e., the more improvement in peripheral nerve dysfunction, the more improvement in cortical somatotopy.
Figure 8.
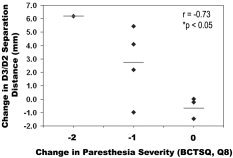
The change in D2/D3 separation distance correlated negatively with the change in paresthesia score (Spearman r = −0.73, P < 0.05). The more improvement in paresthesias, the wider the change in separation.
Comparison of fMRI Stimulus Requirement
Although the stimulation intensity varied for different individuals, no significant difference was found between CTS patients (D2 = 6.4 ± 0.6 mA, D3 = 6.4 ± 0.6 mA, D5 = 5.1 ± 0.5 mA) and healthy adults (D2 = 6.0 ± 0.6 mA, D3 = 5.9 ± 0.6 mA, D5 = 4.6 ± 0.8 mA) for any digit tested, either at baseline or after acupuncture treatment (after a period of at least 5 weeks for healthy adults), suggesting comparability of fMRI findings. As we set fMRI stimulus current intensity relative to subjects' pain threshold, it was important to evaluate whether differences in stimulus strength may have contributed to differences in fMRI results. For both groups, D5 required significantly less current than D2 or D3 (Student's t‐test, P < 0.05).
DISCUSSION
Our results demonstrate adaptive neuroplasticity in CTS patients treated with acupuncture. Specifically, the cortical hyperactivation to nonnoxious stimuli in CTS patients was diminished for D3 after treatment. We also observed that the separation between D2 and D3 cortical representations, which was found to be contracted in CTS patients [Napadow et al.,2006], increased after acupuncture therapy. In fact, the increase in D3/D2 separation correlated with the change in both median nerve sensory conduction latency and reported paresthesias. These data suggest a beneficial cortical plasticity associated with improved CTS symptomatology.
In a cross‐sectional study of CTS patients, we attributed the cortical hyperactivation in response to stimulation of CTS affected digits to Hebbian plasticity mechanisms [Napadow et al.,2006]. CTS patients' persistent paresthesias and pain, which represent increased afferent input for the affected digits, drive use‐dependent enlargement of cortical representation fields in SI. Similar models have been demonstrated by electrophysiological mapping and by fMRI with increased innocuous tactile stimulation in primates [Jenkins et al.,1990] and humans [Hodzic et al.,2004], respectively. While short‐term mechanisms for increased extent in activation have been attributed to GABAergic “disinhibition” [Calford and Tweedale,1988], and the release of existing “latent” subthreshold thalamocortical and corticocortical excitatory inputs from inhibitory control [Dykes,1997; Jones,1993; Levy et al.,2002], longer‐term changes may be reinforced via synaptic strengthening from voltage‐gated, N‐methyl‐D‐aspartate (NMDA) receptor‐mediated long‐term potentiation (LTP) [Brown et al.,1988; Buonomano and Merzenich,1998]. This process applies to temporally correlated synaptic activity and is known as Hebbian plasticity [Hebb,1949; Rauschecker,1991]. Similar mechanisms in the cortex may also apply for nonphysiological pain states [Rygh et al.,2005; Zhang et al.,2003], and increased SI response has been noted for other chronic pain states such as CRPS I [Juottonen et al.,2002; Maihofner et al.,2003]. Under this model, if CTS‐associated pain and paresthesias were to abate, normal temporal patterns of afferent input could then drive neuroplasticity back toward normal cortical function. We propose that diminished paresthesias, reported by our cohort of CTS patients after acupuncture treatment, contributed to the reduction of cortical hyperactivation seen for D3 stimulation.
In CTS, paresthesias are typically spread diffusely over median nerve innervated digits and produce greater synchrony in afferent signaling from adjacent digits than is typically seen in normal signal transmission. This temporal coherence may also drive synaptic strengthening through LTP and Hebbian plasticity mechanisms, thereby blurring cortical representations for CTS affected digits [Napadow et al.,2006]. Similar blurring of representations has been noted in rat [Godde et al.,1996], monkey [Wang et al.,1995], and human [Pilz et al.,2004] studies of multidigit synchronous coactivation, where digits with synchronous stimulation showed increased multidigit receptive fields and closer SI cortical representations.
If CTS‐associated paresthesias could be reduced, normal temporal patterns of afferent input could then drive neuroplasticity back toward normalized somatotopy. In our cohort of CTS patients the D2/D3 separation distance was found to increase (on average) after acupuncture treatment. This increase was due mostly to a lateral shift in the D2 cortical representation, away from the more medially located D3 cortical representation. Furthermore, the increase in D2/D3 separation was correlated with the change in paresthesia severity—the greater decrease in paresthesias, the greater the increase in D2/D3 separation. Similar neuroplastic changes can be inferred from a magnetoencephalography study of surgically repaired congenital syndactyly in humans [Mogilner et al.,1993]. In syndactyly, digital webbing leads to the transmission of more temporally coherent afferent impulses for adjacent digits (similar to paresthesias), and this is reflected in cortical somatotopy. Presurgery, the authors noted that syndactyly was associated with overlap in adjacent digit representations. After surgical separation of the webbed digits, independent digital function resulted in greater separation in cortical representations. These results were also consistent with Hebbian plasticity mechanisms governing changes in cortical representation.
While beneficial cortical plasticity (manifested as more focused contralateral SI representation) was observed for D3 somatosensory stimulation, we did not see appreciable plasticity with regard to the extent of activity for either D2 or D5 cortical representation. In the case of D2, this was an interesting finding, given that sensory conduction latencies improved for both D3 and D2. Also, D2 was shown to move laterally away from D3 in an investigation of somatotopy. However, we did note a statistically greater effect in the improvement of D3 conduction latency as compared to the improvement in D2 conduction latency. This greater degree of improvement in D3 conduction latency may have reflected more normalized afferent input coming from this digit, thereby driving a more profound change in cortical representation. Another possibility is that changes in somatotopy may precede changes in the extent of cortical representation. In other words, a beneficial shift in the center‐of‐mass of the cortical digit representation may precede the normalization of inhibitory control of the cortical area surrounding that representation. The existence of a “permissive state” favoring disinhibition and plasticity [Dykes,1997] may persist in the cortex in order to facilitate even further shifting of the D2 cortical representation, while more normalized lateral inhibition around the D3 representation keeps its representation more focused. Future studies should address this possibility by imaging digit somatotopy at a later time in the healing process.
Furthermore, our acupuncture protocol may have contributed to the greater improvement in D3 (compared with D2). Nerve tracing studies in the peripheral median nerves of primates have demonstrated that different digit territories are significantly segregated at the wrist level to distinct fascicles within the nerve cross‐section [Brushart,1991]. A peripheral digital somatotopy in median nerve fascicles has also been suggested via microneurography studies in humans [Hallin,1990]. Thus, it is conceivable that our acupuncture protocol, which included electro‐acupuncture at acupoint PC‐7 over the midpoint of the wrist crease, was more effective in treating the region of the nerve cross‐section containing fascicle afferents from D3 more so than D2. This differential effect could then lead to more notable improvement in D3 than D2 sensory latencies, while a more normalized afferent input stream could in turn shape the cortical representation pattern for the more improved digit, D3.
Hence, in the specific case of acupuncture treatment at the site of injury, the evoked needle sensation may function as a sensory conditioning stimulus. In other words, acupuncture needle stimulation, either by hand or by electricity, generates evoked sensations (and perhaps fascicular involvement) distinct from the pain and paresthesias typically perceived by the brain from that injured region and works to erode the pain memory coded by the chronic pain condition. This mechanism has been previously suggested by Flor [2002] in the treatment of phantom limb pain, which has been ascribed to pathological cortical plasticity driven by random peripheral input. To test this hypothesis, Lotze et al. [1999] used a myoelectric prosthesis to provide normalized sensory, motor, and visual feedback to phantom limb patients and demonstrated decreased pain and cortical reorganization compared to a cosmetic prosthesis alone. The myoelectric prosthesis provided relevant and correlated sensory input, thus driving beneficial plasticity in the brain—a model that we posit to be similar to acupuncture in the treatment of CTS.
If acupuncture acts as a sensory conditioning stimulus, its evoked afferent signaling should be transmitted by nonnociceptive peripheral fibers. Several studies have investigated the nerve fiber type associated with acupuncture stimulation, and evidence from human studies suggests that acupuncture stimuli are carried by A‐β, A‐γ, and A‐δ fibers [Lu,1983; Wang et al.,1985]. This would apply to nonnoxious acupuncture, where practitioners attempt to stimulate needles without eliciting sharp pain. In addition to providing sensory conditioning stimuli, acupuncture may also work peripherally to address the ischemic damage in the carpal tunnel through the stimulated release of vasodilatory peptides such as calcitonin gene‐regulated peptide (CGRP) [Andersson and Lundeberg,1995; Lundeberg,1993; Sato et al.,2000]. Peripherally addressing the ischemic insult could then modify the maladaptive neuroplasticity seen in CTS patients. However, acupuncture may also drive neuroplasticity in the sensorimotor cortex directly via modulation in subcortical [e.g., limbic, see Hui et al.,1997, 2000; Napadow et al.,2005; Wu et al.,1997] and/or cortico‐cortical circuits to relieve any centrally sustained pain. Acupuncture may modulate the CNS directly through classic spinal‐thalamic [Kandel et al.,2000] or indirectly through spino‐limbic‐cortical pathways [Bernard et al.,1992; Willis and Westlund,1997]. In fact, SI receives robust projections from limbic regions including the amygdala [Amaral et al.,1992; Zald,2003], and amygdaloid projections to primary sensory cortices are more widespread than corticoamygdaloid projections [Parent,1996]. Moreover, cortical sensory regions demonstrate enhanced response during exposure to emotionally valenced sensory stimuli [Lang et al.,1998; Quirk et al.,1997]. Acupuncture has been shown to downregulate amygdala activity in human fMRI studies [Hui et al.,1997, 2000; Napadow et al.,2005; Wu et al., 1999; Zhang et al.,2003] and in a study using chronically implanted recording electrodes in monkey [Jacobs et al.,1977]. Thus, acupuncture may also modulate neuroplasticity in SI via limbic‐cortical projections. However, the most likely scenario is that acupuncture modulates cerebral activity both directly, through central processing of acupuncture stimuli, and indirectly, through sensory conditioning of the peripheral median nerve.
Conservative treatment options for CTS are in demand. Current options vary from injection of corticosteroids to neutral wrist splinting [Wilson and Sevier,2003] and have been found to be effective, although symptom recurrence is common. While carpal tunnel release surgery is still considered the most definitive treatment [Katz et al.,1998], the surgical option is expensive and long‐term follow‐up studies suggest that outcomes are no better than with conservative care [Nathan et al.,2001]. If acupuncture is to have a place in the armamentarium of conservative treatment options, it would be beneficial to find a predictive neurophysiological variable for screening patients at baseline. In this study, we found that the D2 median nerve sensory latency at baseline correlates positively with the eventual change in D2/D3 separation distance. If the latter is to be interpreted as beneficial plasticity, future evaluations of acupuncture for CTS may have reason to explore baseline D2 sensory latency as predictive of posttreatment improvement.
The current study should be viewed in light of some limitations. Even though the changes in symptomatology and nerve function were trending toward normality, the duration of the acupuncture treatment may not have been sufficiently long to allow as full a recovery as possible in all individuals. Further, our clinical and imaging assessments should have incorporated a long‐term follow‐up to investigate if the beneficial effects of acupuncture are maintained or even increased after cessation of treatment. While our study design type was mainly experimental and not a placebo controlled clinical trial, an ideal design would also include a natural history and control stimulation group in order to evaluate the specific effects of acupuncture and placebo response. However, it should be noted that exact design of a placebo group has been controversial in the acupuncture research field [Paterson and Dieppe,2005]. Furthermore, studies on the natural history of CTS without intervention have found that 81% to 88% of patients with mild CTS and 45% to 71% with moderate CTS on nerve conduction studies either remained the same or worsened [Padua et al.,1998, 2001]. Thus, spontaneous improvement in our cohort of relatively chronic mild to moderate CTS patients over a 5‐week period seems unlikely. However, as we did not directly test for specific effects, our neuroplasticity results can be more strongly attributed to improved CTS symptomatology than to the specific effects of acupuncture. Future studies will need to incorporate a placebo control group and long‐term follow‐up in the analysis of cortical plasticity. Furthermore, as carpal tunnel release surgery is considered to be the most effective course of treatment (especially for severe CTS), future studies should assess neuroplasticity after surgical release for comparison with the results of more conservative treatment options.
In summary, this study details the changes in digit cortical representations in CTS patients following acupuncture treatment. Cortical hyperactivation was diminished for D3 stimulation and blurred digit representations became differentiated as peripheral nerve function and symptomatology improved. The reduction of paresthesias may have driven beneficial plasticity in CTS patients. Furthermore, acupuncture may act as a correlated somatosensory conditioning stimulus to aid the evolution of this beneficial plasticity. Future studies should aim to resolve the optimal duration of acupuncture treatment and the durability of the changes in cortical somatotopy during and after acupuncture treatment of CTS.
Acknowledgements
We thank Drs. Aimee J. Nelson and Judith D. Schaechter, who contributed to data analysis and provided feedback on a draft of the article, and Ping Yao, who contributed to the clinical acupuncture protocol.
REFERENCES
- Allard T, Clark SA, Jenkins WM, Merzenich MM (1991): Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol 66: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Amaral D, Price J, Pitkanen A, Carmichael S (1992): Anatomical organization of the primate amygdaloid complex In: Aggleton J, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley‐Liss; p 1–66. [Google Scholar]
- Andersson S, Lundeberg T (1995): Acupuncture — from empiricism to science: functional background to acupuncture effects in pain and disease. Med Hypotheses 45: 271–281. [DOI] [PubMed] [Google Scholar]
- Bernard J, Huang GF, Besson JM (1992): Nucleus centralis of the amygdala and the globus pallida ventralis. Electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68: 551–569. [DOI] [PubMed] [Google Scholar]
- Brown TH, Chapman PF, Kairiss EW, Keenan CL (1988): Long‐term synaptic potentiation. Science 242: 724–728. [DOI] [PubMed] [Google Scholar]
- Brushart TM (1991): Central course of digital axons within the median nerve of Macaca mulatta. J Comp Neurol 311: 197–209. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM (1998): Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R (1988): Immediate and chronic changes in responses of somatosensory cortex in adult flying‐fox after digit amputation. Nature 332: 446–448. [DOI] [PubMed] [Google Scholar]
- Chen G (1990): The effect of acupuncture treatment on carpal tunnel syndrome. Am J Acupunct 19: 5–9. [Google Scholar]
- Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Dykes RW (1997): Mechanisms controlling neuronal plasticity in somatosensory cortex. Can J Physiol Pharmacol 75: 535–545. [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H (2002): Phantom‐limb pain: characteristics, causes, and treatment. Lancet Neurol 1: 182–189. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV (1998): Fingertip representation in the human somatosensory cortex: an fMRI study. Neuroimage 7: 261–283. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR (1996): Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport 8: 281–285. [DOI] [PubMed] [Google Scholar]
- Hallin RG (1990): Microneurography in relation to intraneural topography: somatotopic organisation of median nerve fascicles in humans. J Neurol Neurosurg Psychiatry 53: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D (1949): The organization of behavior. New York: John Wiley & Sons. [Google Scholar]
- Hodzic A, Veit R, Karim AA, Erb M, Godde B (2004): Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. J Neurosci 24: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K, Liu J, Rosen B, Kwong K (1997): Effects of acupuncture on human limbic system and basal ganglia measured by fMRI. Neuroimage 5: s226. [Google Scholar]
- Hui KK, Liu J, Makris N, Gollub RL, Chen AJ, Moore CI, Kennedy DN, Rosen BR, Kwong KK (2000): Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Hum Brain Mapp 9: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Anderson G, Bailey S, Ottaviano J, McCarthy V (1977): Neurophysiological correlates of acupuncture: limbic and thalamic responses to analgesic studies in non‐human primates. TIT J Life Sci 7: 37–42. [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic‐Robles E (1990): Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Jones EG (1993): GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 3: 361–372. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Gockel M, Silen T, Hurri H, Hari R, Forss N (2002): Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 98: 315–323. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T (2000): Principles of neural science. New York: McGraw Hill. [Google Scholar]
- Katz JN, Keller RB, Simmons BP, Rogers WD, Bessette L, Fossel AH, Mooney NA (1998): Maine Carpal Tunnel Study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community‐based cohort. J Hand Surg Am 23: 697–710. [DOI] [PubMed] [Google Scholar]
- Kurth R, Villringer K, Mackert BM, Schwiemann J, Braun J, Curio G, Villringer A, Wolf KJ (1998): fMRI assessment of somatotopy in human Brodmann area 3b by electrical finger stimulation. Neuroreport 9: 207–212. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V (1998): Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology 35: 199–210. [PubMed] [Google Scholar]
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN (1993): A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am 75: 1585–1592. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG (2002): Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol 52: 755–761. [DOI] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H (1999): Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci 2: 501–502. [DOI] [PubMed] [Google Scholar]
- Lu GW (1983): Characteristics of afferent fiber innervation on acupuncture points zusanli. Am J Physiol 245: R606–612. [DOI] [PubMed] [Google Scholar]
- Lundeberg T (1993): Peripheral effects of sensory nerve stimulation (acupuncture) in inflammation and ischemia. Scand J Rehabil Med Suppl 29: 61–86. [PubMed] [Google Scholar]
- Ma D, Liveson J (1983): Nerve conduction handbook. Philadelphia: FA Davis. [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F (2003): Patterns of cortical reorganization in complex regional pain syndrome. Neurology 61: 1707–1715. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F (2004): Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63: 693–701. [DOI] [PubMed] [Google Scholar]
- McGlone F, Kelly EF, Trulsson M, Francis ST, Westling G, Bowtell R (2002): Functional neuroimaging studies of human somatosensory cortex. Behav Brain Res 135: 147–158. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D (1983): Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8: 33–55. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM (1984): Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol 224: 591–605. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Grossman JA, Ribary U, Joliot M, Volkmann J, Rapaport D, Beasley RW, Llinas RR (1993): Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci U S A 90: 3593–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyoros I, Bostock H, Burke D (2000): Mechanisms of paresthesias arising from healthy axons. Muscle Nerve 23: 310–320. [DOI] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM (2000): Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 84: 558–569. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Hahn KA, Lieberman BE, Branco KF (2002): Carpal tunnel syndrome pain treated with low‐level laser and microamperes transcutaneous electric nerve stimulation: A controlled study. Arch Phys Med Rehabil 83: 978–988. [DOI] [PubMed] [Google Scholar]
- Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK (2005): Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 24: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Kettner NW, Ryan A, Kwong KK, Audette J, Hui KK (2006): Somatosensory cortical plasticity in carpal tunnel syndrome — a cross‐sectional fMRI evaluation. Neuroimage (in press). [DOI] [PubMed] [Google Scholar]
- Nathan PA, Wilcox A, Emerick PS, Meadows KD, McCormack AL (2001): Effects of an aerobic exercise program on median nerve conduction and symptoms associated with carpal tunnel syndrome. J Occup Environ Med 43: 840–843. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Ogura T, Hase H, Makinodan A, Hojo T, Katsumi Y, Yagi K, Mikami Y, Kubo T (2003): Objective evaluation of sensory function in patients with carpal tunnel syndrome using the current perception threshold. J Orthop Sci 8: 625–628. [DOI] [PubMed] [Google Scholar]
- Nora DB, Becker J, Ehlers JA, Gomes I ( 2005): What symptoms are truly caused by median nerve compression in carpal tunnel syndrome? Clin Neurophysiol 116: 275–183. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Torebjork HE (1980): Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain 103: 835–853. [DOI] [PubMed] [Google Scholar]
- O'Connor D, Marshall S, Massy‐Westropp N (2003): Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua L, Padua R, Lo Monaco M, Aprile I, Paciello N, Nazzaro M, Tonali P (1998): Natural history of carpal tunnel syndrome according to the neurophysiological classification. Ital J Neurol Sci 19: 357–361. [DOI] [PubMed] [Google Scholar]
- Padua L, Padua R, Aprile I, Pasqualetti P, Tonali P (2001): Multiperspective follow‐up of untreated carpal tunnel syndrome: a multicenter study. Neurology 56: 1459–1466. [DOI] [PubMed] [Google Scholar]
- Parent A (1996): Carpenter's human neuroanatomy. Baltimore: Williams & Wilkins. [Google Scholar]
- Paterson C, Dieppe P (2005): Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. Br Med J 330: 1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Pilz K, Veit R, Braun C, Godde B (2004): Effects of co‐activation on cortical organization and discrimination performance. Neuroreport 15: 2669–2672. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE (1997): Fear conditioning enhances different temporal components of tone‐evoked spike trains in auditory cortex and lateral amygdala. Neuron 19: 613–624. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP (1991): Mechanisms of visual plasticity: Hebb synapses, NMDA receptors, and beyond. Physiol Rev 71: 587–615. [DOI] [PubMed] [Google Scholar]
- Rygh LJ, Svendsen F, Fiska A, Haugan F, Hole K, Tjolsen A (2005): Long‐term potentiation in spinal nociceptive systems—how acute pain may become chronic. Psychoneuroendocrinology 30: 959–964. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Shimura M, Uchida S (2000): Calcitonin gene‐related peptide produces skeletal muscle vasodilation following antidromic stimulation of unmyelinated afferents in the dorsal root in rats. Neurosci Lett 283: 137–140. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JC (1997): AAEM minimonograph 26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine . Muscle Nerve 20: 1477–1486. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Padua L, Aprile I, Rossini PM (2002): Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp 17: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G, Volpato D, Testoni R, Bonato C, Manganotti P, Miniussi C, Fiaschi A (1998): Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain 121: 1785–1794. [DOI] [PubMed] [Google Scholar]
- Wang KM, Yao SM, Xian YL, Hou ZL (1985): A study on the receptive field of acupoints and the relationship between characteristics of needling sensation and groups of afferent fibres. Sci Sin [B] 28: 963–971. [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM (1995): Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN (1997): Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Sevier TL (2003): A review of treatment for carpal tunnel syndrome. Disabil Rehabil 25: 113–119. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Wu MT, Hsien JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK (1997): Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain–preliminary experience. Radiology 212: 133–141. [DOI] [PubMed] [Google Scholar]
- You H, Simmons Z, Freivalds A, Kothari MJ, Naidu SH (1999): Relationships between clinical symptom severity scales and nerve conduction measures in carpal tunnel syndrome. Muscle Nerve 22: 497–501. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]
- Zald DH (2003): The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]
- Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, Zeng YW, Luo F, Chen AC, Han JS (2003): Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res 982: 168–178. [DOI] [PubMed] [Google Scholar]


