Abstract
The inter‐related cognitive constructs of working memory (WM) and processing speed are fundamental components to general intellectual functioning in humans. Importantly, both WM and processing speed are highly susceptible to disruption in cases of brain injury, neurologic illness, and even in normal aging. A goal of this article is to summarize and critique the functional imaging studies of speeded working memory in neurologically impaired populations. This review focuses specifically on the role of the lateral prefrontal cortex in mediating WM performance and integrates the relevant WM literature in healthy adults with the current findings in the clinical literature. One important finding emerging from a summary of this literature is the dissociable contributions made by ventrolateral and dorsolateral prefrontal cortex (VLPFC and DLPFC) in guiding performance on tasks of WM. Throughout this review, it is shown that when cerebral resources are challenged, it is DLPFC, and often right DLPFC specifically, that plays a critical role in modulating WM functioning. In addition, this article will examine the relationship between task performance and brain activation across studies to clarify the role of increased DLPFC activity in clinical samples. Finally, explanations are offered for the observed increased DLPFC activation and the potentially unique role of right DLPFC in mediating WM performance during periods of cerebral challenge. Hum. Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: functional imaging, prefrontal cortex, working memory, TBI, MS, brain injury
INTRODUCTION
The primary purpose of this review is to summarize and critique functional imaging studies of working memory (WM) in neurologically impaired populations. This review will specifically focus on the role of prefrontal cortex (PFC) in modulating task performance during variable WM demands. In doing so, first the importance of examining WM functioning will be discussed followed by a brief overview of the roles of dorsolateral (DLPFC) and ventrolateral (VLPFC) prefrontal cortex in WM functioning established previously in healthy adults. In examining how PFC mediates WM performance, several forms of “cerebral challenge” will be considered. For the purposes of this article, the term cerebral challenge in a given neural network can be defined as: a transient or permanent increase in the requirements of the basal neural network resulting in adjustment in that network to meet demands. The forms of cerebral challenge discussed herein may be temporary (e.g., sleep deprivation), task related (e.g., in response to fluctuations in task demand), due to changes in the brain over the course of a lifespan (e.g., aging), or due to injury or disease (e.g., trauma, multiple sclerosis). One important observation emerging from the literature is that the contributions made by VLPFC and DLPFC cortex in guiding performance on tasks of working memory are dissociable. Throughout this critique, it will be shown that when cerebral resources are challenged, it is DLPFC, and often right DLPFC specifically, that plays a critical role in modulating WM functioning. In addition, an important aim of this article is to examine the relationship between task performance and brain activation across studies to increase the interpretability of altered brain activation in clinical samples. In the final section of this review, explanations for the increased brain activation observed during cerebral challenge and the potentially unique role of right DLPFC in mediating WM performance are presented.
Working Memory and Processing Speed as Basic Cognitive Components
By the nature of the cognitive tasks used to examine WM in functional imaging experiments, the inter‐related cognitive constructs of WM and processing speed are often examined simultaneously. WM is a fundamental cognitive ability and was posited by Dr. Goldman‐Rakic to be at the foundation of all higher cognitive functioning [Courtney,2004]. Separately, processing speed has also been thought to be a fundamental component to general intellectual functioning in humans [Salthouse,1996a; Salthouse and Coon,1993]. There is evidence that information processing efficiency in human cognition is influenced by the interaction between processing speed and its influence on the size and flexibility of the WM buffer [DeLuca et al.,2004a; Demaree et al.,1999; Salthouse,1996b; Salthouse and Coon,1993]. In this literature, WM has been defined as the ability to both maintain and manipulate a limited amount of information over a brief period of time [Baddeley,1992] and processing speed has been defined as either the amount of time it takes to process a predetermined amount of information, or the volume of information that can be processed within a prescribed amount of time [Deluca et al.,2004b]. Processing speed and WM are integral to a variety of cognitive functions and there is mounting evidence that decrements in these two areas occur almost universally in neurologically impaired populations. For example, both WM and processing speed deficits have been noted after brain trauma (TBI) [McDowell et al.,1997; Stuss et al.,1985] multiple sclerosis [Demaree et al.,1999; Mostofsky et al.,2003; Rao et al.,1989a; Rao et al.,1989b], schizophrenia [Cohen et al.,1997; Saykin et al.,1991,1994], dementia [Bradley et al.,1989; Collette et al.,1999; Morris and Baddeley,1988] and normal aging [Salthouse,1992,1996a, 1996b; Salthouse and Coon,1993]. Moreover, processing speed has been shown to account for significant variance in the other cognitive deficits observed in clinical populations [Archibald and Fisk,2000; DeLuca et al.,2004a, 2004b; Demaree et al.,1999; Kail,1998; Li et al.,2004; Litvan et al.,1988]. In sum, WM and processing speed are: (1) fundamental cognitive components; (2) critical to a variety of cognitive functions; and (3) highly susceptible to disruption in cases of brain injury, neurologic illness, and even in normal aging. Early examination of deficits in neurologically impaired populations using functional imaging techniques have focused on tasks measuring WM and associated processing speed. The current review will examine how PFC modulates performance on tasks of WM and processing speed and how this modulation is neurally represented.
Role of PFC in WM
There is a large corpus of literature in the neurosciences aimed at better understanding the role of PFC in memorial functioning. More specifically, the use of functional imaging techniques, such as blood oxygen level dependent functional MRI (fMRI) and positron emission tomography (PET), have aided greatly in clarifying the roles of VLPFC and DLPFC during WM. For much of the work discussed here, DLPFC is composed primarily of middle and superior frontal gyri, or areas that translate loosely to Brodmann areas (BA) 46 and 9 (and occasionally parts of 8 and 10) and VLPFC is composed primarily of inferior frontal gyrus, or areas translating roughly to BA 44, 45, and 47. Over the past decade, competing explanations have emerged describing the dissociable roles of DLPFC and VLPFC in WM. From one perspective, investigators supporting a material‐specific, or domain‐dependent, hypothesis maintained that DLPFC and VLPFC are organized similarly to posterior regions of the brain with dorsal regions processing primarily information about spatial localization and ventral regions processing information about the object recognition. Thus, according to the original proposal of the domain dependent hypothesis, the roles of these two frontal regions are dissociable based upon the types of material to be processed [Courtney et al.,1996; Haxby et al.,2000; McCarthy et al.,1996]. Separately, a process‐specific hypothesis has proposed that the roles of the DLPFC and VLPFC are differentiated primarily based on the type of information processing to occur. More specifically, a process‐specific hypothesis maintains that, regardless of the type of material being processed, VLPFC is more generally dedicated to the rehearsal and maintenance of internal representations and DLPFC facilitates “executive” functions such as updating, monitoring, and manipulating new information [Owen,1997; Petrides,2000; Petrides et al.,1993; Rypma and D'Esposito,1999; Rypma et al.,1999]. Understanding the roles of DLPFC and VLPFC in WM is complicated further by evidence in animal models providing support for both of these hypotheses. More recently, investigators have provided some evidence that both process specific and domain specific hypotheses may coexist [Johnson et al.,2003] and a summary by Courtney [2004] integrates these distinct perspectives and offers clarification for the types of information indicated by “domain‐dependent,” including information about context, task instruction, and even motivation. Although work remains to be done to characterize fully how DLPFC and VLPFC are differentially involved in WM information processing, there is sufficient evidence suggesting that their primary roles are dissociable. This dissociation in humans has been corroborated in investigations of non‐human primates revealing important cytoarchitectural differences between VLPFC and DLPFC [Owen,1997; Petrides,1996; Petrides and Pandya,1999]. There is thus much work distinguishing VLPFC and DLPFC in WM functioning in healthy adults, and in the current article it will be shown that these two prefrontal cortical regions are differentially allocated during periods of cerebral challenge.
During tasks of WM, suprathreshold demands require the recruitment of additional cerebral resources (i.e., cognitive control mechanisms) to increase the proficiency or capacity of information processing as task load increases. For the purposes of this article, a definition of cognitive control will be adopted from Miller and Cohen [2001], where it is defined as a mental state of maintaining cognitive activities that embody specific goals and the means of achieving those specific goals. An important purpose of PFC functioning is to match cognitive demands by evoking established rules or goals when encountering novel sensory information. This “top‐down” processing occurs when the system encounters novel sensory stimuli or tasks and recruitment of cognitive control resources is determined by the strength of the neural pathways present. In cases where neural pathways are only weakly established, greater cognitive control may be needed as internal representations of goals and means to achieve them are generated [Cohen et al.,1992]. In cases of well‐learned and established tasks, PFC mediation thus may be minimal to achieve optimal performance. In contrast, during a novel or rapidly changing task or in cases where the task does not have a well‐developed neural representation, greater cognitive control may be required. If this conceptualization is extended to clinical samples, determining what constitutes “suprathreshold demands” is likely dependent upon the interaction between cognitive dysfunction and task difficulty. In individuals with brain injury and disease, we can infer that individuals without an established neural representation of the task or those who are slow to develop such a representation due to disruption in the neural network, will require additional cognitive control. This last point has important implications throughout the article as the relationship between task performance and PFC activation during functional imaging studies is discussed.
WM AND FMRI IN CLINICAL SAMPLES: FINDINGS AND INTERPRETATIONS
A small body of literature has now emerged employing functional neuroimaging techniques to examine WM functioning in neurologic samples. To examine the influences of cerebral challenge in this article, the focus was on disorders with a distinct onset, before which the neural system could be considered “normal.” There is a growing literature using functional imaging to examine WM deficits in brain trauma and brain diseases such as multiple sclerosis (MS) and human immunodeficiency virus (HIV) infection. Although pathophysiologically distinct, these forms of neurologic insult have similar consequences for frontal lobe functioning resulting in deficits in WM and, more generally, processing speed. One common mechanism for the cognitive deficits observed in each of these syndromes is the disruption of frontal white matter. To date, functional imaging studies of WM in these clinical samples have been largely cross sectional in nature and their results have indicated that clinical samples show altered brain activation patterns, typically increased prefrontal cortical activation. In addition, these altered patterns of brain activation in clinical samples have occasionally been interpreted as “brain reorganization” or “compensatory,” with the latter indicating that increases in brain activation have facilitative influences on task behavior. These interpretations are tenuous for several reasons discussed below.
The first use of fMRI methods to examine cognition in individuals with multiple sclerosis was carried out by Staffen et al. [2002], who examined processing speed and WM with a visual analogue of the paced auditory serial addition test (PASAT). When comparing individuals with MS to healthy controls (HCs), these examiners noted increased right prefrontal activation (BA 8, 6, and 9) as well as left parietal lobe activation in individuals with MS during this task. Task performance was not measured in this study, so the relationship between task performance and activation is not known, which greatly reduces the potential for interpretation. Even so, the data in this study provided an important first application of fMRI to the study of WM in MS and revealed altered brain activation (increased right prefrontal cortex) in a neurologically impaired sample. Later, Hillary et al. [2003] examined the neural mechanisms associated with WM rehearsal in MS through the use of a delayed response task and correlated brain activation with the behavioral performance on the task. These examiners observed increased right prefrontal cortex (defined as middle and superior frontal gyri) and right temporal lobe activation in individuals with MS compared to HCs on this task of verbal WM. Importantly, these investigators noted a negative correlation between right prefrontal cortex activation and task accuracy in individuals with MS. In contrast, no consistent relationship between task performance and left prefrontal cortex activation was detected, indicating that although left DLPFC was involved in this task, it was the degree of right DLPFC recruitment that predicted diminished performance. Similarly, Chiaravalloti et al. [2005] examined individuals with MS with both low and high cognitive impairment, where impairment was determined before scanning using measures of neuropsychological functioning. Functional imaging results in this study revealed greater right PFC recruitment in individuals with MS with high WM impairment compared to that in individuals with MS with low WM impairment. The results revealed incremental right DLPFC recruitment, with HCs showing the least activation, individuals with MS and low WM impairment showing more activation than HCs, and individuals with MS and high WM impairment showing the greatest activation. This finding again reveals an important relationship between WM performance and increased right DLPFC activation. Separately, in a study of WM using an n‐back task, findings by others [Wishart et al.,2004] revealed decreased activation in individuals with MS in areas of activation common across healthy adults, but again found increased prefrontal activation in regions surrounding sites commonly activated by the healthy adult sample.
Other investigators have noted similar findings on speeded WM tasks like the PASAT in MS. For example, in separate investigations of individuals with either mild or an equivocal diagnosis of MS examiners noted increased bilateral prefrontal cortex activation, with the greatest increases occurring in the right frontopolar (BA 10) and right PFC (BA 45/46) [Audoin et al.,2003,2004]. Importantly, these authors used magnetic transfer imaging to examine WM integrity and noted a significant negative relationship between right PFC activation (BA 45) and diminished transfer ratios in their MS sample. This relationship between brain pathophysiology and brain activation was also noted by Mainero et al. [2004], who observed a positive correlation between T2 lesion load and prefrontal brain activation in individuals with MS. Even mild cases of MS thus display increased brain activation during tasks of WM and there seems to be a relationship between brain pathology and degree of PFC recruitment. Because task accuracy was not significantly different between healthy adults and individuals with MS, however, these authors interpreted increased PFC involvement as “brain reorganization” or “compensatory” and associated with improved performance. Although this explanation remains a possibility, there are several important considerations. First, in each of these studies, response accuracy as opposed to reaction time was used to detect performance decrements in the MS sample, yet prior work has established a relationship between slowed reaction time and increased brain activation [Bergerbest et al.,2004; Durston et al.,2003; Rypma et al.,2002; Rypma and D'Esposito,2000]. Second, these studies presumed that a failure to find statistically significant between group differences in task performance was indicative of a positive performance/brain activation relationship within the MS sample. This method relies upon detecting potentially subtle between‐group differences with a small sample size as opposed to directly examining the relationship between brain activation and performance within each group. Importantly, in both studies by Audoin et al. [2003,2004], the MS group performed worse than the HCs (albeit not significantly) on the behavioral task, but again no direct comparisons between activation and performance were made. In addition, in Audoin et al. [2004] when examining a subset of the MS sample, the authors noted that increased right PFC activation (BA 44) was most marked in subjects with the worst PASAT performance. These findings are important because they again indicate that when cognitive deficits were large enough to be detected using response accuracy, the negative relationship between WM performance and brain activation was apparent.
When considering cases of brain trauma, McAllister et al. [1999] were the first to use fMRI to examine WM deficits in individuals with mild traumatic brain injury (mTBI). When comparing healthy adults to individuals with mTBI on an n‐back task, these examiners observed increased right dorsolateral prefrontal and right parietal lobe activation in individuals with mTBI during higher versus lower WM load conditions. McAllister et al. [2001] replicated their findings with a separate mTBI sample, again showing a significant relationship between task load and right prefrontal recruitment. Similar to Audoin et al. [2004], however, the authors did not directly examine the relationship between behavioral performance on the task and the increased brain activation observed in the mTBI samples [McAllister et al.,1999,2001]. In both studies by McAllister et al. [1999,2001], the examiners were unable to detect any significant between‐group differences in accuracy on the fMRI task that could explain the observed right PFC recruitment in the mTBI sample. Again, because of this, the increased right PFC activation in the mTBI sample was interpreted as “compensatory,” or operating to facilitate task performance. Similar to Audoin et al. [2003,2004], McAllister et al. [1999,2001] did not report subject reaction times on the n‐back task, which limited their sensitivity in detecting between group differences in the behavioral data. Given the mild nature of the injuries in the mTBI sample, the behavioral task in these studies may have limited sensitivity to detect a potentially small difference in performance between groups. In both studies by McAllister et al. [1999,2001], formal neuropsychological evaluations outside the scanner revealed that reaction time was the one variable that was significantly diminished in their mTBI sample compared to that in healthy adults. These findings further emphasize that processing speed deficits may have gone undetected in the scanner and may help to explain the increased right prefrontal cortex activation in the mild TBI sample. For these reasons, the increased activation in the mTBI group may not be indicative of neural compensation as defined by McAllister et al. and remains difficult to interpret in their study.
In the first fMRI examination of individuals with moderate to severe brain trauma, Christodoulou et al. [2001] also noted increased right PFC recruitment on a modified paced auditory serial addition task (mPASAT), a task requiring significant processing speed and WM demand [Christodoulou et al.,2001]. Again, this right PFC recruitment was most evident in DLPFC (middle frontal gyrus, translating to BA 9 and 46). In the most recent study of moderate and severe TBI, examiners used the n‐back task to examine parametric manipulation of WM load in individuals with moderate and severe TBI [Perlstein et al.,2004]. The TBI sample in this study showed WM impairments both within and outside the scanner, and again, when compared to healthy adults in this study, individuals with TBI showed greater right dorsolateral PFC activation. In fact, both the healthy adults and the TBI sample showed sustained increases in right DLPFC activation (BA 9 and 46) in response to increasing task load (this consistency with healthy adults is important and is elaborated upon below). The samples studied in Christodoulou et al. [2001] and Perlstein et al. [2004] were injured more severely and exhibited greater cognitive deficits than did those examined in either study by McAllister et al. [1999,2001]; therefore, significant performance differences between individuals with TBI and matched HCs on the fMRI task were easily detected. In contrast to the interpretation proposed by McAllister et al. [200], the data provided by Christodoulou et al. [2001] and Perlstein et al. [2004] revealed that increased right PFC activation was associated with poorer performance on the task and was thus unlikely to be indicative of neural mechanisms that facilitated performance. Even considering the differences in interpretation between these studies, most investigations of WM reviewed here revealed increased right PFC activation that is consistent with those observations made in studies of MS. This consistent finding across distinct clinical samples has important implications for the role of PFC in modulating WM performance during periods of cerebral challenge.
Yet another example of similar PFC activation during tasks of WM after brain disease has been illustrated in examinations of HIV infection [Chang et al.,2001; Ernst et al.,2002]. Although healthy adults and individuals infected with HIV maintained similar accuracy rates on a WM task, individuals with HIV infection showed slower reaction times that were correlated with the magnitude of increased bilateral frontal lobe activation [Chang et al.,2001]. These data are similar to those discussed above and this finding again emphasizes the importance of examining several levels of behavioral performance in response to brain activation including response accuracy and reaction time. Clarifying the relationship between behavioral performance and brain activation in studies of WM in neurologically impaired samples will remain important to characterize fully the role of the PFC in modulating WM.
Taken together, functional imaging studies examining WM in separate neurologic populations reveal strikingly similar findings. These studies reveal a general pattern of increased prefrontal activation in clinical samples and a greater relationship between DLPFC, as opposed to VLPFC, and task performance. Moreover, many of the findings discussed reveal that increased right PFC, as opposed to left PFC, may be more likely to be recruited when cerebral resources are challenged. Analysis of behavioral performance in many studies reveals a negative correlation between task performance and increased prefrontal recruitment. What remains unclear are the causes for increased DLPFC activation across clinical samples. As noted, several examiners have interpreted these alterations in brain activation as “cerebral reorganization” whereas others have used the term “compensatory” to describe increased PFC activation that facilitates task performance. Because of significant methodological shortcomings in the literature to date, interpretation of increased PFC activation as “brain reorganization” may be premature (see Explanations for DLPFC Recruitment below). Separately, use of the term “compensatory” to describe PFC recruitment is potentially more problematic and worth discussing here. In several clinical studies reviewed above, the term “compensatory” has been used to describe activation that directly improves performance, i.e., recruitment of resources in PFC is necessary for clinical samples to perform at a level commensurate to healthy adults. Based upon negative activation/performance relationships observed within much of this literature, however, it is unlikely that recruitment of PFC directly enhances performance. It is more plausible that, in a damaged or inefficient system, recruitment of DLPFC resources occurs in response to increased task load or slowed processing. In other words, in a slow or inefficient system increased PFC activation temporarily provides additional resources for sustained processing of novel information. We propose that these transient alterations in the represented neural network reflect recruitment of cognitive control mechanisms to tolerate various forms of cerebral challenge. If correct, this hypothesis suggests that as task representations are formalized, PFC regions originally recruited would be incrementally unnecessary for efficient task processing. Although this hypothesis has not been tested directly in clinical samples, it is consistent with the current data in regards to activation/performance relationships. Moreover, decreased activation has been observed in healthy adults acclimating to task demands over repeated trials [Landau et al., 2004]. The term “compensation,” as it has been used in the clinical WM literature to describe a direct link between increased activation and improved task performance, therefore may not describe accurately the role of PFC during periods of cognitive challenge. To best account for the current clinical data, we propose that recruitment of PFC regions in cases of brain injury and disease: (1) is associated with poor performance; (2) temporarily facilitates the development of task representations; and (3) will diminish as task representations are finalized and performance increases.
CEREBRAL CHALLENGE IN HEALTHY ADULTS
It has been shown thus far that in clinical studies of WM, DLPFC (and, commonly, right DLPFC) is recruited. It has also been argued that increases in DLPFC activation are negatively correlated with performance and represent increased cognitive control mechanisms allowing for sustained processing of novel sensory information. If DLPFC recruitment is a standard response to cerebral challenge we might expect that, compared to clinical samples, healthy adults would show a similar pattern of DLPFC activation in response to changing task conditions. Indeed, prior examinations of young, healthy adults have revealed very similar patterns of DLPFC recruitment on WM tasks when performance diminishes secondary to increasing task load. For example, Manoach et al. [1997] observed increased activation in right DLPFC in response to increasing task demand on a WM task. These findings that were corroborated by investigators, who noted a negative correlation between right DLPFC activation and behavioral performance and recruitment of right DLPFC in response to increasing task load [Rypma and D'Esposito,1999]. This “dose‐responsive” relationship between right DLPFC recruitment and increasing task demand has been observed in other studies of healthy adults [D'Esposito et al.,1999; Mostofsky et al.,2003; Rypma et al.,1999,2002] suggesting that right DLPFC recruitment during tasks of WM may represent a general response to cerebral challenge. These findings are nearly identical to those observed in neurologically impaired samples and provide an important context for interpreting findings across samples.
It is interesting to note that in separate examinations of healthy adults experiencing total sleep deprivation, sleep‐deprived subjects exhibited decreases in brain activation for some brain regions, but increased right prefrontal activation on tasks of verbal learning and divided attention [Drummond et al.,2000,2001]. Similar to these findings, more recently, investigators examined healthy adults after 48 hr of sustained wakefulness and through measures of covariance, also noted decreases in activation in posterior regions, but increased activation in anterior regions (e.g., anterior cingulate) [Habeck et al.,2004]. Through the use of a total sleep deprivation paradigm, these examiners provided a transient challenge to the prefrontal system resulting in similar findings observed in studies of clinical populations and studies of younger adults engaging in tasks requiring heavy task loads. That is, as performance diminishes, there is an increase in prefrontal cortical activation. Table I summarizes the investigations discussed here and demonstrates the consistent recruitment of prefrontal cortex and, quite commonly, right DLPFC in cases of cerebral challenge.
Table I.
Summary of WM and fMRI findings
| Sample and authors | Task/manipulation | Findings |
|---|---|---|
| TBI | ||
| Christodoulou et al.,2001 | mPASAT | Increased R PFC, R temporal |
| McAllister et al.,1999 | n‐back, high load | Increased R DLPFC, R parietal |
| McAllister et al.,2001 | n‐back, high load | Increased R fup frontal, L mid frontal |
| Perlstein et al., 2003 | n‐back | Increased R DLPFC |
| MS | ||
| Audoin et al.,2003 | PASAT | Increased bilateral PFC (<R) |
| Audoin et al., 2005 | PASAT | Increased R VLPFC, bilateral PFC |
| Chiaravalloti et al.,2005 | PASAT | Increased R PFC, R parietal |
| Hillary et al.,2003 | DMS | Increased R DLPFC, |
| Rehearsal | R temporal | |
| Mainero, et al.,2004 | PASAT | R cingulate, bilateral PFC, bilateral temporal/parietal |
| Staffen et al.,2002 | vPASAT | Increased R PFC, R parietal |
| Wishart et al.,2004 | n‐back | Diffuse prefrontal, parietal |
| HIV | ||
| Chang et al.,2001 | n‐back | Increased bilateral PFC |
| Ernst et al.,2002 | n‐back | Increased PFC |
| HC | ||
| Barch et al., 1997 | CPT/delay | Increased L DLPFC, L Broca's/parietal |
| Braver et al., 1997 | n‐back | Increased bilateral PFC, L inferior frontal |
| D'Esposito et al.,1999 | DRT/high load | Increased DLPFC |
| Manoach et al.,1997 | CPT/high load | Increased R DLPFC |
| Mostofsky et al.,2003 | Counting go/no go, high load | Increased R DLPFC |
| Rypma et al.,2002 | DMS/high load | Increased DLPFC |
| Rypma et al.,1999 | DMS/high load | Increased R DLPFC |
| Rypma and D'Esposito,1999 | DMS/high load | Increased R DLPFC |
| Stern et al., 2000 | PWMT, high monitoring | Increased R DLPFC |
Summary of working memory (WM) and fMRI findings for healthy control (HC) participants during load or task demand manipulations and in individuals with brain injury (TBI) and disease (multiple sclerosis [MS], HIV). L, left; R, right; PFC, prefrontal cotex; DLPFC, dorsolateral prefrontal cortex; mPASAT, modified paced serial addition test (auditory); vPASAT, visual paced serial addition test; DMS, delayed match to sample; DRT, delayed response task; PWMT, pattern working memory task; CPT, continuous performance task.
A review of the current WM literature reveals that increased activation in PFC is consistently observed during periods of cerebral challenge in both clinical samples and studies of healthy adults. It has been hypothesized that these changes represent increases in cognitive control mechanisms, and there is some support for this in the healthy adult literature. For example, examiners have described DLPFC, and specifically areas translating to BA 9 and 46, as providing “supracapacity” resources during periods of increased task load [D'Esposito et al.,1998,1999; Rypma et al.,2002; Rypma and D'Esposito,1999]. Separately, recent work has shown that areas translating to BA 46 and 8 may play an important role in overcoming distractions while processing incoming information [Sakai et al.,2002] and may be important for implementing structure in anticipation task onset [Sakai and Passingham,2003]. Although this level of specificity has yet to be investigated in clinical samples using parametric manipulations and event‐related designs, these findings may have implications for the clinical literature reviewed here. For example, it may be the case that increased activation in BA 46 is required in cases of brain injury and disease to reduce distraction while the task is being processed over a longer period of time. In other words, individuals with brain trauma or disease exhibit slowed processing speed and require more time to perform tasks due slower development of task representations. With a diminished capacity for rapid development of task representations, dorsal PFC areas such as BA 9 and 46 thus may be recruited to handle supracapacity demands or sustain attention during the development of task routines over a protracted processing period.
EXPLANATIONS FOR DLPFC RECRUITMENT
It has been posited that PFC activation commonly increases during periods of cerebral challenge, that DLPFC as opposed to VLPFC plays a critical role in modulating cognitive functioning during cerebral challenge, and that commonly right DLPFC, as opposed to left DLPFC, is more likely to be recruited as performance diminishes. These observations were made across multiple studies examining WM and in most cases, the resultant brain activation patterns reflect the neural networks responsible for not only working memory components (i.e., rehearsal, manipulation) but also basic processing speed/efficiency. The following describes several tenable explanations for these observations.
Increases in DLPFC During Cognitive Challenge
One common explanation for the increased PFC activation observed in clinical samples is that there may be a basic difference in the strategy employed by members of each group while engaging in the experimental task. That is, compared to healthy adults, neurologically impaired samples may use an alternative method while engaging in WM tasks that capitalizes upon residual cognitive resources. Considering the range of functional imaging studies discussed here, the use of different task strategies is unlikely to account for the consistency of the findings across several samples and under distinct testing conditions (e.g., different tasks, varying task load). In addition, strategy differences typically offer explanation for divergent as opposed to convergent findings like those discussed in this article.
An alternative explanation for increased activation in clinical samples could be that increased PFC activation represents widespread cortical disinhibition or neural inefficiency within the network due to the pathophysiological processes related to brain injury or disease. Cortical disinhibition might help to explain the generally greater bilateral activation observed in some studies [Chiaravalloti et al.,2005; Hillary et al.,2003]. This explanation, however, does not easily account for other findings including the similar pattern of increased activation observed in HCs during trials of increased task load. Generalized cortical disinhibition also does not easily explain the specific relationship between right and not left prefrontal activation and diminished performance reported in Hillary et al. [2003] and the specific relationship between brain pathology and right DLPFC observed by Audoin et al. [2004]. Generalized disinhibition secondary to brain pathology thus does not account adequately for the specificity and consistency in the findings in the current literature.
A potentially more plausible explanation for the increased DLPFC activation observed on tasks of WM and processing speed is that the level of prefrontal activation is directly proportional to the relative task difficulty for each participant. In other words, PFC recruitment may be directly related to task demand and occurs at some threshold across all samples, albeit a lower threshold in neurologically impaired individuals. This explanation evokes the idea of cognitive control mechanisms that are recruited based upon individual differences in task performance. Much of the data presented thus far is consistent with this explanation and this finding can be confirmed or refuted by more closely monitoring the relationship between task performance and increases in brain activation in future studies. This explanation is explored further below in the discussion regarding the specific role of right DLPFC in modulating WM performance.
Finally, it remains possible that the observed changes are due to some permanent brain reorganization. According to this explanation, the increased activation observed in these samples is indicative of more formal alterations in the represented neural network and should be observable regardless of task demand or task performance. According to a brain reorganization hypothesis, the same pattern of activation that is apparent only transiently in HCs (e.g., during increased task load, sleep deprivation) is therefore incorporated more permanently into the baseline neural network in clinical samples. Figure 1 reveals a reanalysis of data from prior studies in our laboratory [Chiaravalloti et al.,2005; Christodoulou et al.,2001] showing a positive correlation between duration since diagnosis and right DLPFC recruitment. These findings indicate that the increased right prefrontal recruitment observed in cases of brain injury and disease may be time dependent and therefore may be related to chronic alterations in the represented neural network. Ultimately, if the altered brain activation observed in cases of brain injury and disease represents permanent brain reorganization, then we would expect the aberrant activation (in this case increased PFC activation) to be apparent on repeat testing and not solely accounted for by changing task conditions (i.e., low and high load). As discussed, this hypothesis has not been tested directly in WM studies to date and future investigations will require collection of longitudinal data over the disease/recovery course and methods that allow for tight control over task difficulty to test the viability of this explanation.
Figure 1.
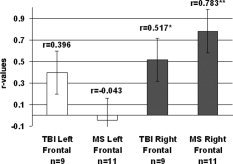
R‐values for correlational analysis between duration since diagnosis and extent of frontal cortex activation (number of active voxels) for TBI and MS samples (original data presented in Christodoulou et al. [2001] and Chiaravalloti et al. [2005], respectively).
Role of Right DLPFC in WM
As noted, many of the current functional imaging studies examining processing speed and WM have revealed increased right DLPFC recruitment during periods of cerebral challenge and, in many cases, investigators have noted a negative correlation between right DLPFC recruitment and behavioral performance. Two potential explanations for the observed right PFC recruitment may help to explain convergent findings, heretofore referred to as the right hemisphere novelty hypothesis and the material‐specific hypothesis.
Right hemisphere novelty hypothesis
Traditionally, the right hemisphere has been thought to be responsible for processing novel sensory information before it is transferred to the left hemisphere for comprehension and action [Goldberg and Costa,1981]. Extensions of this conceptualization of right hemisphere functioning remain salient today, and a contemporary view of this model has been articulated by Gazzaniga [2000] whereby the right hemisphere plays a critical role in collecting information about novel sensory input that is later interpreted by the left hemisphere.
In the right hemisphere novelty hypothesis proposed here, the right PFC may play a unique role in cognitive control mechanisms during tasks of WM in clinical samples. That is, right PFC may be differentially involved in initiating and developing subroutines to facilitate learning and to enhance performance when a novel WM task is encountered. In support of this, examiners have noted that the right PFC may be differentially involved in cognitive control mechanisms during WM tasks in healthy adults [Wagner et al.,2001] and may play an asymmetric role in sustaining attention to novel sensory input [Pardo et al.,1991]. Separately, behavioral studies have shown that brain injury may influence successful integration of multimodal sensory information [Sarno et al.,2003]. Taken together, after disruption of established neural networks secondary to brain injury or disease, right DLPFC may predominate due to a failure to develop efficient task subroutines resulting in continued demand on cognitive control resources. This explanation can also account for the increased right DLPFC activation observed in healthy adults during periods of higher task load and suggests that at some threshold, recruitment of additional attentional resources is a common adaptive mechanism. In sum, according to the right hemisphere novelty hypothesis proposed here, the diminished processing efficiency observed in clinical samples demands greater cognitive control resources during WM tasks, resulting in increased right DLPFC recruitment as the damaged system more slowly accommodates novel sensory input.
Material‐specific hypothesis
The increased right DLPFC recruitment reported in this article is based upon a literature that has examined predominantly verbally mediated stimuli. Several studies have revealed lateralization in WM functioning, with left PFC processing verbally mediated material and right PFC processing nonverbal/spatially mediated material [Gazzaniga,2000; Glahn et al.,2002; McCarthy et al.,1994]. Because of this, a material‐specific hypothesis would explain the increases in right DLPFC activation observed in the current literature as recruitment of a contralateral homologue elicited from experimental designs that emphasize functions traditionally considered to be mediated by the left hemisphere. If a material‐specific hypothesis was accurate, then tasks of nonverbal or spatial processing in clinical samples should result in recruitment of the left DLPFC (a contralateral homologue). For example, Reuter‐Lorenz et al. [2000] observed a “paradoxical” activation pattern in an older sample, with verbally mediated WM tasks revealing greater right PFC activation and nonverbally mediated WM tasks revealing greater left PFC activation compared to that in younger adults. Based upon these findings, it could be argued that brain activation during tasks of WM is dependent upon the material/task being examined and that right DLPFC does not play a unique role in modulating WM performance. Even in the data presented by Reuter‐Lorenz et al. [2000], however, recruitment of PFC was more highly lateralized to the right during the verbal task than it was to the left during the nonverbal task. Separately, an investigation of healthy adults using a spatial WM task revealed increasing right superior frontal gyrus activation in response to increased task load and not a left DLPFC recruitment as would be expected by a material‐specific explanation [Glahn et al.,2002]. Further examinations of nonverbal WM in clinical samples are necessary to determine if DLPFC recruitment is material specific/task dependent (material‐specific hypothesis) or if, regardless of the task, right DLPFC has a unique role in supporting cognitive control mechanisms during suprathreshold WM demands (right hemisphere novelty hypothesis).
ADDITIONAL CONSIDERATIONS
It is important to consider that not all samples have been shown to exhibit PFC recruitment as performance diminishes during WM tasks. The most important exception is normal aging. It is now well established in the aging literature that healthy older subjects show a propensity toward recruiting additional cerebral resources in the PFC compared to younger healthy adults when engaged in tasks of WM [Cabeza,2002; Reuter‐Lorenz et al.,2000; Rypma and D'Esposito,2000]. An important departure in the aging literature compared to studies discussed above, however, is in the relationship between brain activation and task performance. Work provided by Reuter‐Lorenz et al. [2000] and Rypma et al. [2000] independently demonstrated that increased prefrontal activation in healthy, older adults is commonly associated with better performance. Unlike healthy adults and clinical samples, increased PFC activation on WM tasks in the elderly operates to enhance performance directly. This difference may be at least partially due the integration of recruited regions into relevant neural networks responsible for the task. For example, compared with young adults an older sample showed significant alterations in brain activation across several regions outside PFC and both increases and decreases in activation within prefrontal areas [Grady et al.,1998] These findings indicate that although DLPFC may play a critical role in modulating processing speed and WM performance, in the case of aging the success of neural compensation may be more determined by the integration of increased DLPFC involvement into distributed neural network that is changing over the course of a lifetime. Related to this, it is also possible that although anatomically identical, the DLPFC recruitment in older subjects is functionally distinct to that observed in other samples. Ultimately, when compared to other samples discussed above, the activation/performance relationships observed in aging indicate that PFC (either alone or due in its role within an altered neural network) may be modulating WM differently.
There have also been inconsistent findings in regards to prefrontal cortical activation in studies of cortical dementia and schizophrenia. Although several investigators have observed increased activation in response to poorer performance on a task of WM in schizophrenia [Manoach et al.,2000; Walter et al.,2003] other studies have actually noted decreased prefrontal activation [for review, see Manoach,2003]. There are several potential explanations for these inconsistent findings. First, in the case of schizophrenia, because it is has been construed as a neurodevelopmental disorder, there likely exists widespread functional and neuroanatomical differences compared to healthy adults. Secondly, not only is there great heterogeneity in both the progression and symptomatology within schizophrenia, but the chronicity of pharmacologic intervention is rarely controlled [Walter et al.,2003]. In the case of degenerative disorders, the stage or severity of the illness may greatly influence the pattern of brain activation, with recruitment occurring early in the disease and widespread reductions in activation occurring much later in the disease course after significant cortical atrophy has occurred. In addition, most degenerative disorders occur in older populations, so altered brain activation in these samples is in response to a complicated interaction between aging and pathophysiology. For these reasons, the role of DLPFC in WM may be more difficult to characterize in samples with abnormal neurodevelopment or in cases of older adults with moderate to severe neural degeneration.
CONCLUSIONS
The current article has argued that DLPFC plays an important role in modulating WM task performance during periods of cerebral challenge. In cases of brain injury and disease it seems that DLPFC (as opposed to VLPFC) is commonly recruited in response to cerebral challenge. It has also been shown that, in cases of neurologic insult, right DLPFC (as opposed to left DLPFC) activation is correlated with degree of brain pathology and poorer task performance. It has also been argued that right DLPFC recruitment represents increases in cognitive control mechanisms in response to failing performance and is therefore unlikely to be a compensatory mechanism that directly bolsters performance. Finally, it has been maintained that results in the current literature may not represent genuine brain reorganization and that methods to date have been inadequate for making this determination. Further characterization of the role of PFC in modulating WM performance in clinical samples will require the use of longitudinal examinations, parametric manipulations with tight control over task load/performance relationships, and both verbal and nonverbal WM paradigms.
Acknowledgements
We thank Stephen T. Moelter for his invaluable input to this article.
REFERENCES
- Archibald CJ, Fisk JD (2000): Information processing efficiency in patients with multiple sclerosis. J Clin Exp Neuropsychol 22: 686–701. [DOI] [PubMed] [Google Scholar]
- Audoin B, Ibarrola D, Ranjeva JP, Confort‐Gouny S, Malikova I, Ali‐Cherif A, Pelletier J, Cozzone P (2003): Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp 20: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audoin B, Van Au Duong M, Ranjeva JP, Ibarrola D, Malikova I, Confort‐Gouny S, Soulier E, Viout P, Ali‐Cherif A, Pelletier J and others. (2004): Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp 24: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (1992): Working memory. Science 255: 556–559. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Ghahremani DG, Gabrieli JD (2004): Neural correlates of auditory repetition priming: reduced fMRI activation in the auditory cortex. J Cogn Neurosci 16: 966–977. [DOI] [PubMed] [Google Scholar]
- Bradley VA, Welch JL, Dick DJ (1989): Visuospatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatry 52: 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R (2002): Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T (2001): Neural correlates of attention and working memory deficits in HIV patients. Neurology 57: 1001–1007. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N, Hillary F, Ricker J, Christodoulou C, Kalnin A, Liu WC, Steffener J, DeLuca J (2005): Cerebral activation patterns during working memory performance in multiple sclerosis using FMRI. J Clin Exp Neuropsychol 27: 33–54. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, Kalnin AJ, Liu WC, Steffener J, Diamond BJ, Ni AC (2001): Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry 71: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar KO, Barch DM, Braver TS (1997): Issues concerning relative speed of processing hypotheses, schizophrenic performance deficits, and prefrontal function: comment on Schooler et al. (1997). J Exp Psychol Gen 126: 37–41. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan‐Schreiber D, McClelland JL (1992): A parallel distributed processing approach to automaticity. Am J Psychol 105: 239–269. [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Bechet S, Salmon E (1999): Phonological loop and central executive functioning in Alzheimer's disease. Neuropsychologia 37: 905–918. [DOI] [PubMed] [Google Scholar]
- Courtney SM (2004): Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci 4: 501–516. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV (1996): Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 6: 39–49. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res 7: 1–13. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J (1999): Maintenance versus manipulation of information held in working memory: an event‐related fMRI study. Brain Cogn 41: 66–86. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Chelune GJ, Tulsky DS, Lengenfelder J, Chiaravalloti ND (2004a): Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 26: 550–562. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH (2004b): Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc 10: 101–109. [DOI] [PubMed] [Google Scholar]
- Demaree HA, DeLuca J, Gaudino EA, Diamond BJ (1999): Speed of information processing as a key deficit in multiple sclerosis: implications for rehabilitation. J Neurol Neurosurg Psychiatry 67: 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB (2000): Altered brain response to verbal learning following sleep deprivation. Nature 403: 655–657. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Brown GG (2001): Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res 10: 85–92. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ (2003): Parametric manipulation of conflict and response competition using rapid mixed‐trial event‐related fMRI. Neuroimage 20: 2135–2141. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S (2002): Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59: 1343–1349. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS (2000): Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123: 1293–1326. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TG, Manninen M, Huttunen M, Lonnqvist J, Standertskjold‐Nordenstam CG, Cannon TD (2002): Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage 17: 201–213. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Costa LD (1981): Hemisphere differences in the acquisition and use of descriptive systems. Brain Lang 14: 144–173. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV (1998): Age‐related changes in regional cerebral blood flow during working memory for faces. Neuroimage 8: 409–425. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y (2004): An event‐related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed‐match‐to‐sample task. Brain Res Cogn Brain Res 18: 306–321. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM (2000): Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage 11: 380–391. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Chiaravalloti ND, Ricker JH, Steffener J, Bly BM, Lange G, Liu WC, Kalnin AJ, DeLuca J (2003): An investigation of working memory rehearsal in multiple sclerosis using fMRI. J Clin Exp Neuropsychol 25: 965–978. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Anderson AW (2003): FMRI evidence for an organization of prefrontal cortex by both type of process and type of information. Cereb Cortex 13: 265–273. [DOI] [PubMed] [Google Scholar]
- Kail R (1998): Speed of information processing in patients with multiple sclerosis. J Clin Exp Neuropsychol 20: 98–106. [DOI] [PubMed] [Google Scholar]
- Li Y, Chiaravalloti ND, Hillary FG, Deluca J, Liu WC, Kalnin AJ, Ricker JH (2004): Differential cerebellar activation on functional magnetic resonance imaging during working memory performance in persons with multiple sclerosis. Arch Phys Med Rehabil 85: 635–639. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grafman J, Vendrell P, Martinez JM (1988): Slowed information processing in multiple sclerosis. Arch Neurol 45: 281–285. [DOI] [PubMed] [Google Scholar]
- Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, Bozzao L, Pantano P (2004): fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 21: 858–867. [DOI] [PubMed] [Google Scholar]
- Manoach DS (2003): Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res 60: 285–298. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL (2000): Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 48: 99–109. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S (1997): Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport 8: 545–549. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N (1999): Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 53: 13004–1308. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ (2001): Differential working memory load effects after mild traumatic brain injury. Neuroimage 14: 1004–1012. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman‐Rakic P, Shulman RG (1994): Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA 91: 8690–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman‐Rakic P (1996): Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex 6: 600–611. [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D'Esposito M (1997): Working memory impairments in traumatic brain injury: evidence from a dual‐task paradigm. Neuropsychologia 35: 1341–1353. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Morris RG, Baddeley AD (1988): Primary and working memory functioning in Alzheimer‐type dementia. J Clin Exp Neuropsychol 10: 279–296. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ (2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17: 41924–430. [DOI] [PubMed] [Google Scholar]
- Owen AM (1997): The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci 9: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW (2004): Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. J Int Neuropsychol Soc 10: 724–s24 741. [DOI] [PubMed] [Google Scholar]
- Petrides M (1996): Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1455–1461. [DOI] [PubMed] [Google Scholar]
- Petrides M (2000): The role of the mid‐dorsolateral prefrontal cortex in working memory. Exp Brain Res 133: 44–54. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E (1993): Dissociation of human mid‐dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci USA 90: 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (1999): Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11: 1011–1036. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Haughton VM, St Aubin‐Faubert P, Bernardin L (1989a): Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 39: 161–166. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, St Aubin‐Faubert P (1989b): On the nature of memory disturbance in multiple sclerosis. J Clin Exp Neuropsychol 11: 699–712. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA (2000): Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 12: 174–187. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M (2002): The influence of working‐memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci 14: 721–731. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M (1999): The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA 96: 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M (2000): Isolating the neural mechanisms of age‐related changes in human working memory. Nat Neurosci 3: 509–515. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD (1999): Load‐dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage 9: 216–226. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE (2003): Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE (2002): Active maintenance in prefrontal area 46 creates distractor‐resistant memory. Nat Neurosci 5: 479–484. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1992): Influence of processing speed on adult age differences in working memory. Acta Psychol (Amst) 79: 155–170. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996a): The processing‐speed theory of adult age differences in cognition. Psychol Rev 103: 403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996b): General and specific speed mediation of adult age differences in memory. J Gerontol B Psychol Sci Soc Sci 51: P30–42. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Coon VE (1993): Influence of task‐specific processing speed on age differences in memory. J Gerontol 48: 245–255. [DOI] [PubMed] [Google Scholar]
- Sarno S, Erasmus LP, Lipp B, Schlaegel W (2003): Multisensory integration after traumatic brain injury: a reaction time study between pairings of vision, touch and audition. Brain Inj 17: 41324–426. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P (1991): Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry 48: 618–624. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC (1994): Neuropsychological deficits in neuroleptic naive patients with first‐episode schizophrenia. Arch Gen Psychiatry 51: 124–131. [DOI] [PubMed] [Google Scholar]
- Staffen W, Mair A, Zauner H, Unterrainer J, Niederhofer H, Kutzelnigg A, Ritter S, Golaszewski S, Iglseder B, Ladurner G (2002): Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain 125: 1275–1282. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Ely P, Hugenholtz H, Richard MT, LaRochelle S, Poirier CA, Bell I (1985): Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery 17: 41–47. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL (2001): Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage 14: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Walter H, Wunderlich AP, Blankenhorn M, Schafer S, Tomczak R, Spitzer M, Gron G (2003): No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res 61: 175–184. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McDonald BC, Mamourian AC, Flashman LA, Schuschu KR, Ryan KA, Fadul CE, Kasper LH (2004): Brain activation patterns associated with working memory in relapsing‐remitting MS. Neurology 62: 234–238. [DOI] [PubMed] [Google Scholar]


