Abstract
Previous studies have led to the proposal that patients with Alzheimer's disease (AD) may have disturbed functional connectivity between different brain regions. Furthermore, recent resting‐state functional magnetic resonance imaging (fMRI) studies have also shown that low‐frequency (<0.08 Hz) fluctuations (LFF) of the blood oxygenation level‐dependent signals were abnormal in several brain areas of AD patients. However, few studies have investigated disturbed LFF connectivity in AD patients. By using resting‐state fMRI, this study sought to investigate the abnormal functional connectivities throughout the entire brain of early AD patients, and analyze the global distribution of these abnormalities. For this purpose, the authors divided the whole brain into 116 regions and identified abnormal connectivities by comparing the correlation coefficients of each pair. Compared with healthy controls, AD patients had decreased positive correlations between the prefrontal and parietal lobes, but increased positive correlations within the prefrontal lobe, parietal lobe, and occipital lobe. The AD patients also had decreased negative correlations (closer to zero) between two intrinsically anti‐correlated networks that had previously been found in the resting brain. By using resting‐state fMRI, our results supported previous studies that have reported an anterior–posterior disconnection phenomenon and increased within‐lobe functional connectivity in AD patients. In addition, the results also suggest that AD may disturb the correlation/anti‐correlation effect in the two intrinsically anti‐correlated networks. Hum Brain Mapp 2006. © 2006 Wiley‐Liss, Inc.
Keywords: Alzheimer's disease, fMRI, resting‐state, low‐frequency blood oxygenation level‐dependent fluctuations, functional connectivity
INTRODUCTION
Abnormalities in connectivity between different brain areas have been thought as plausible explanations for many of the cognitive function deficits observed in Alzheimer's disease (AD), such as working memory, episodic memory, and attention/executive function impairments [De Lacoste et al., 1993; Delbeuck et al., 2003; Gòmez‐Isla and Hyman, 1997; Perry and Hodges, 1999]. There has been much anatomical evidence pointing toward AD as a disconnection syndrome [Delbeuck et al., 2003]. For example, some neuropathological studies have shown a disruption of the efferent and afferent linkages between the hippocampal formation and other brain areas [Damasio et al., 1990; De Lacoste et al., 1993; Morrison et al., 1986; Van Hoesen, 1990; Van Hoesen et al., 1997]. Rose et al. [2000] evaluated the white matter integrity of mild to moderate AD patients using magnetic resonance diffusion tensor imaging and found that AD patients showed a significant decrease in associative white matter fibers such as the splenium of the corpus callosum, the superior longitudinal fasciculus, and the cingulum.
In addition to these investigations into anatomical connectivity, analysis of functional connectivity, which refers to a measurement of temporal synchrony and correlations between distinct brain regions [Friston et al., 1993], has become more and more popular. Some functional connectivity studies on AD using positron emission tomography (PET) have been done [Azari et al., 1992; Grady et al., 2001, 2003; Horwitz et al., 1987, 1995]. These studies found disrupted functional connectivity between the anterior and posterior regions [Azari et al., 1992; Horwitz et al., 1987, 1995]. A number of studies also found increased functional connectivity within the prefrontal lobe or between prefrontal regions and other brain regions [Grady et al., 2001, 2003; Horwitz et al., 1995]. However, most of these studies were based on task conditions and only focused on the functional connectivity associated with one or a few preselected seed regions of interest (ROIs). To our knowledge, there have only been two PET studies that investigated the altered functional connectivity throughout the entire brain in AD patients [Azari et al., 1992; Horwitz et al., 1987]. They both selected ROIs throughout the entire brain and calculated the correlation coefficients of the regional cerebral metabolic rates for glucose consumption between each pair of them. They found a reduced number or smaller amplitude in frontal–parietal positive correlations. Azari et al. [1992] also showed that AD patients had larger positive correlations between occipital ROIs and stronger negative correlations in general. On the whole, these PET studies gave some support for the hypothesis that AD patients could have widespread disturbance in functional connectivity.
Recently, blood oxygenation level‐dependent contrast functional magnetic resonance imaging (fMRI) studies have found that spontaneous low‐frequency (<0.08 Hz) fluctuations (LFF) measured during the resting‐state showed high temporal coherence between spatially distinct but functionally related regions [Biswal et al., 1995]. This result suggested that LFF may also be appropriate for examining the functional connectivity between different brain regions. Following that study, the LFF connectivity has been found within the primary motor, auditory, visual cortices, as well as some other regions such as the hippocampus, thalamus, and so on. [Cordes et al., 2001; Greicius et al., 2003; Hampson et al., 2002; Jiang et al., 2004; Lowe et al., 1998; Stein et al., 2000]. With respect to AD, some studies have found that the pathophysiology of AD may be associated with abnormality in resting‐state LFF [Grecius et al., 2004; Li et al., 2002; Maxim et al., 2005]. Li et al. [2002] found a decreased functional synchrony of LFF within the hippocampus in AD patients. Maxim et al. [2005] found that AD patients had greater noise in resting fMRI signals in the temporal lobe, dorsal cingulate/medial premotor cortex, and insula. Greicius et al. [2004] suggested a disrupted resting‐state activity within the default‐mode network in AD patients. All these studies showed that the resting‐state LFF in some brain regions were abnormal in AD patients. Therefore, it is reasonable to presume that the LFF connectivity between different brain regions might also be disturbed. However, to date, there have been very few studies examining the disturbed LFF connectivity of different brain areas in AD. On the basis of a ROIs method, Wang et al. [2006] found disturbed functional connectivity between the hippocampus and some other brain areas such as the medial prefrontal cortex, ventral anterior cingulate cortex, and posterior cingulate cortex (PCC). It should be noted that studies based on ROI only provide limited information. Considering the possibility that distributed abnormality of functional connectivity may exists in the resting brain of AD, we think it will be helpful to study functional connectivity from the perspective of whole brain for a better understanding of the pathophysiology of AD.
In the present study, by using resting‐state fMRI, we tested the hypotheses that patients with early AD may have widespread alterations in functional connectivity and analyzed the global distribution of abnormal connectivities throughout the entire brain. To this end, we divided the whole brain into 116 regions and then analyzed the correlations between each pair of them. Using an unbiased approach similar to that used by Horwitz et al. [1987] in a PET study, we evaluated the global distribution of abnormal functional connectivities throughout the entire brain, and sought to find if there are interesting differences that have not been previously reported. As well as positive correlations, we also paid much attention to the differences in negative correlations between AD patients and healthy controls. In the end, we used a ROI‐based functional connectivity method to further test one of our new findings of this unbiased approach, i.e., disturbed functional connectivity in the two intrinsically anti‐correlated networks that were proposed by Fox et al. [2005].
METHODS
Subjects
The medical research ethics committee of Xuanwu Hospital of Capital University of Medical Sciences approved this study and all participants gave written, informed consent prior to taking part in the study. Seventeen healthy elderly controls and seventeen early AD patients participated in our study. We recruited the AD patients from a memory outpatient clinic at Xuanwu Hospital and the healthy controls by advertisement from the local community. The diagnosis of AD patients fulfilled the Diagnostic and Statistic Manual of Mental Disorders, 4th ed. [DSM‐IV, American Psychiatric Association, 1994] criteria for dementia, and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) [McKhann et al., 1984] criteria for AD. The AD patients were free of other diseases and the healthy controls were free of any known medical, neurological, and psychiatric disorders. Prior to this study, none of the patients had been on any medications for the cognitive impairments of AD. We discarded two normal controls and three AD patients that had greater than 1 mm maximum displacement in any of the x, y, z directions or greater than 1° of angular rotation about three axes. In the residual 14 AD patients and 15 normal controls, because of age‐matching and gender‐matching consideration, we used 14 AD patients and 14 healthy controls in the subsequent analysis. Seven of the 14 patients had a Clinical Dementia Rate (CDR) [Morris, 1993] score of 1 and six patients had a CDR score of 0.5. The remaining one patient had a CDR score of 2. We considered those patients with a CDR score of 1 to be mild AD patients and those with a CDR score of 0.5 to be very mild AD patients [Morris et al., 2001].
Data Acquisition
All the images were scanned on a Siemens 1.5‐T Magnetom Sonata system using a standard headcoil. During data acquisition, the subjects were instructed to keep their eyes closed, relax their minds, and move as little as possible. A foam pad and headphones were also used to reduce head motion and scanner noise. The blood oxygenation level‐dependent images of the entire brain were acquired in 20 axial slices by using an echo‐planar imaging sequence [TR/TE = 2000/60 ms, FA = 90°, field of view = 24 cm, matrix = 64 × 64, thickness = 5 mm, gap = 2 mm]. The fMRI scanning lasted for 6 min. Other images not used in the present study were not described here.
Data Preprocessing
Most of the preprocessing steps were carried out using statistical parametric mapping (SPM2, http://www.fil.ion.ucl.ac.uk/spm/). Because of the instability of the initial signal and the subjects' adaptation to the situation, the first 10 images were discarded. The remaining fMRI images were first corrected for within‐scan acquisition time differences between slices and then realigned to the first volume to correct for interscan head motions. In the next, we spatially normalized the realigned images to the standard echo‐planar imaging template and resampled them to 3 × 3 × 3 mm3. Subsequently, the functional images were spatially smoothed with a Gaussian kernel of 4 × 4 × 4 mm3 FWHW to decrease spatial noise. Besides discarding the subjects with large amounts of head motions, we also used a linear regression process to further remove the effects of head motion and other possible sources of artifacts [Fox et al., 2005]: (1) six motion parameters, (2) whole‐brain signal averaged over the entire brain, (3) linear drift. In the end, the fMRI waveform of each voxel was temporally band‐pass filtered (0.01 Hz < f < 0.08 Hz) using the AFNI (http://afni.nimh.nih.gov/).
Anatomical Parcellation
The fMRI datasets that had been registered with the MNI template were segmented using the anatomically labeled template previously reported by Tzourio‐Mazoyer et al. [2002]. This parcellation divided the whole brain into 116 regions: 90 regions in the cerebra and 26 regions in the cerebella. The regions and their abbreviations used in this paper can be seen in Table I.
Table I.
Anatomical parcellation of the entire brain and their abbreviations used in this paper
| Prefrontal Lobe | |
| Superior frontal gyrus | SFG |
| Superior frontal gyrus, dorsolateral | SFGD |
| Superior frontal gyrus, orbital | SFGO |
| Superior frontal gyrus, medial | SFGM |
| Superior frontal gyrus, medial orbital | SFGMO |
| Middle frontal gyrus | MFG |
| Middle frontal gyrus, orbital | MFGO |
| Inferior frontal gyrus, opercular | IFGOP |
| Inferior frontal gyrus, triangular | IFGT |
| Inferior frontal gyrus, orbital | IFGO |
| Gyrus rectus | RECT |
| Anterior cingulate gyrus | ACC |
| Olfactory cortex | OLF |
| Other parts of frontal lobe | |
| Precentral gyrus | PRECG |
| Supplementary motor area | SMA |
| Median cingulated and paracingu‐ ‐late gyrus | MCC |
| Rolandic operculum | ROL |
| Parietal lobe | |
| Postcentral gyrus | POSTCG |
| Superior parietal gyrus | SPG |
| Inferior parietal gyrus | IPG |
| Supramarginal gyrus | SMG |
| Angular gyrus | ANG |
| Paracentral lobule | PCL |
| Precuneus | PCU |
| Posterior cingulate gyrus | PCC |
| Occipital lobe | |
| Calcarine fissure and surrouding cortex | CAL |
| Cuneus | CUN |
| Lingual gyrus | LING |
| Superior occipital gyrus | SOG |
| Middle occipital gyrus | MOG |
| Inferior occipital gyrus | IOG |
| Insula | INS |
| Thalamus | THA |
| Temporal lobe | |
| Superior temporal gyrus | STG |
| Superior temporal gyrus, temporal pole | STGP |
| Middle temporal gyrus | MTG |
| Middle temporal gyrus, temporal pole | MTGP |
| Inferior temporal gyrus | ITG |
| Heschl gyrus | HES |
| Hippocampus | HIP |
| Parahippocampal gyrus | PHIP |
| Amygdala | AMYG |
| Fusiform gyrus | FG |
| Corpus striatum | |
| Caudate nucleus | CAU |
| Lenticular nucleus, putamen | PUT |
| Lenticular nucleus, pallidum | PAL |
| Cerebellum | |
| Cerebellum crus I | CERCR1 |
| Cerebellum crus II | CERCR2 |
| Cerebellum III | CER3 |
| Cerebellum IV & V | CER4&5 |
| Cerebellum VI | CER6 |
| Cerebellum VIIB | CER7b |
| Cerebellum VIII | CER8 |
| Cerebellum IX | CER9 |
| Cerebellum X | CER10 |
| Vermis | |
| Vermis I & II | VER1&2 |
| Vermis III | VER3 |
| Vermis IV & V | VER4&5 |
| Vermis VI | VER6 |
| Vermis VII | VER7 |
| Vermis VIII | VER8 |
| Vermis IX | VER9 |
| Vermis X | VER10 |
The cerebra include 90 regions (45 in each hemisphere) and the cerebella include 26 regions (9 in each cerebellar hemisphere and 8 in the vermis). More details for the cerebral parcellation can be seen in [Tzourio‐Mazyer et al., 2002], and more details for the cerebella parcellation can be seen in [Schmahamann et al., 1999]
Functional Connectivity Analysis
Functional connectivity of the whole brain
We obtained the mean time series of each of the 116 regions by averaging the fMRI time series over all voxels in the region [Achard et al., 2006; Salvador et al., 2005]. Correlation coefficients were computed between each pair of them. Then a Fisher's r‐to‐z transformation was applied to improve the normality of these correlation coefficients. The individual z values were entered into a random effect one‐sample two‐tailed t‐test to determine brain regions showing significant correlations within each group. These z values were also entered into a random effect two‐sample two‐tailed t‐test to determine the regions showing differences in correlations between the groups. The functional correlations which were considered as significantly different between AD patients and the control group must satisfy two criterions: (1) there were significantly different z values between the two groups at the threshold of P < 0.01 (uncorrected); (2) the z values of the correlations were significantly different from zero in at least one group at a threshold of P < 0.01 (uncorrected).
Functional connectivity associated with the PCC
To evaluate the new findings obtained by using the above unbiased approach, we further analyzed the positive and negative correlations associated with the PCC, as follows:
-
1
A seed reference time course was obtained by averaging the time series over the voxels in the left and right PCC of the template used in this paper. Correlation analysis was carried out between the seed reference and the whole brain in a voxel‐wise manner. Then a Fisher's r‐to‐z transformation was applied to improve the normality of these correlation coefficients.
-
2
The individual z value was entered into a random effect one‐sample t‐test in a voxel‐wise manner to determine the brain regions showing significant functional connectivity to the PCC in each group under a combined threshold of P < 0.001 and cluster size ≫540 mm3. This yield a corrected threshold of P < 0.001, determined by Monte Carlo simulation using the AlphaSim program written by D. Ward in AFNI software (Parameters were: FWHM = 4 mm, with a mask of the whole brain tissues). In each group, this procedure provided two sets of regions: one included regions that were significantly correlated with the PCC (“task negative” set) and the other included regions that were significantly anti‐correlated with the PCC (“task positive” set).
-
3
The z values were also entered into a random effect two‐sample t‐test in a voxel‐wise manner to determine the brain regions showing significantly different connectivity to the PCC between AD patients and elderly healthy controls. The significantly different positive correlations were determined with a corrected threshold of P < 0.001 (single voxel threshold of P < 0.01 and cluster size ≫405 mm3, using the AlphaSim program with parameters: FWHM = 4 mm and a mask including voxels in the “task negative” sets of either elderly healthy controls or AD patients). The significantly different negative correlations were also determined with a corrected threshold of P < 0.001 (single voxel threshold of P < 0.05 and cluster size ≫540 mm3, using the AlphaSim program with parameters: FWHM = 4 mm and a mask including voxels in the “task positive” sets of either elderly healthy controls or AD patients).
RESULTS
The demographic characteristics and neuropsychological scores of the subjects analyzed in this study are shown in Table II. No significant differences in gender, age, handedness, and education level were found between the two groups. However, the MMSE scores of AD patients were significantly lower than those of the healthy controls.
Table II.
Characteristics of the AD patients and the healthy controls
| Characteristics | AD (n = 14) | Controls (n = 14) | P value |
|---|---|---|---|
| Gender (female/male) | 7/7 | 7/7 | >0.99a |
| Age (year) | 70.2±6.3 | 69.6±5.5 | 0.80b |
| Handedness (right/left) | 14/0 | 14/0 | >0.99a |
| Education (year) | 9.8±5.0 | 10.1±4.1 | 0.89b |
| MMSE | 23.1±2.6 | 28.8±1.0 | <0.00001b |
MMSE, Mini‐Mental State Examination.
The P value was obtained by Pearson χ2 two‐tailed test with continuity correction.
The P value was obtained by two‐sample two‐tailed t‐test.
Functional Connectivity of the Whole Brain
In all, there were 104 interregional correlations that showed significant differences between the AD patients and the healthy controls (P < 0.01, uncorrected). These included 36 different positive correlations and 68 different negative correlations.
Altered positive correlations
Compared with healthy controls, 15 positive correlations showed a significant decrease in AD patients. Details can be seen in Table III. The results showed decreased correlations between the anterior and posterior regions, mostly between the prefrontal and parietal lobe. There were also some decreased positive correlations within the posterior regions, either within the parietal lobe or between parietal lobe and occipital lobe. In addition, we also found some decreased positive correlations between the cerebellum and other brain regions. In comparison, as shown in Table IV, there were 21 increased positive correlations in AD patients. These increased correlations were mainly limited to intralobes including the prefrontal lobe, parietal lobe, occipital lobe, temporal lobe, and cerebellum. There were also a few increased correlations between frontal regions and other brain regions.
Table III.
The decreased positive interregional correlations in AD patients compared with elderly healthy controls
| Region 1 | Classification | Region 2 | Classification | P value |
|---|---|---|---|---|
| L.SFGO | Prefrontal | L.IPG | Parietal | 0.0073 |
| L.MFG | Prefrontal | L.IPG | Parietal | 0.0025 |
| L.MFGO | Prefrontal | L.IPG | Parietal | 0.0003 |
| R.SFGM | Prefrontal | R.PCU | Parietal | 0.0060 |
| L.IFGO | Prefrontal | R.SMG | Parietal | 0.0010 |
| R.RECT | Prefrontal | R.MTGP | Temporal | 0.0005 |
| L.PCC | Parietal | L.PCU | Parietal | 0.0072 |
| L.PCC | Parietal | R.PCU | Parietal | 0.0028 |
| L.PCC | Parietal | L.CUN | Occipital | 0.0013 |
| R.ANG | Parietal | R.CUN | Occipital | 0.0013 |
| R.ANG | Parietal | R.SPG | Occipital | 0.0090 |
| VER8 | Cerebellum | L.ROL | Other frontal | 0.0001 |
| VER8 | Cerebellum | L.CAL | Occipital | 0.0070 |
| VER6 | Cerebellum | L.POSTCG | Parietal | 0.0048 |
| L.OLF | Prefrontal | R.RECT | Prefrontal | 0.0029 |
L, left; R, right.
Table IV.
The increased positive interregional correlations in AD patients compared with elderly healthy controls
| Region 1 | Classification | Region 2 | Classification | P value |
|---|---|---|---|---|
| R.SFG | Prefrontal | L.CAU | Prefrontal | 0.0056 |
| L.SFGO | Prefrontal | L.ACC | Prefrontal | 0.0066 |
| R.IFGO | Prefrontal | R.SFGM | Prefrontal | 0.0005 |
| R.IFGO | Prefrontal | R.ACC | Prefrontal | 0.0021 |
| R.HIP | Temporal | R.STG | Temporal | 0.0061 |
| R.HIP | Temporal | L.STGP | Temporal | 0.0092 |
| L.HES | Temporal | R.STG | Temporal | 0.0080 |
| L.HES | Temporal | L.MTG | Temporal | 0.0048 |
| L.STG | Temporal | R.STG | Temporal | 0.0034 |
| L.SPG | Parietal | R.PCU | Parietal | 0.0040 |
| R.SPG | Parietal | L.PCU | Parietal | 0.0078 |
| L.CAL | Occipital | R.LING | Occipital | 0.0073 |
| L.SOG | Occipital | R.SOG | Occipital | 0.0078 |
| L.SOG | Occipital | L.MOG | Occipital | 0.0088 |
| VER9 | Cerebellum | R.CER9 | Cerebellum | 0.0097 |
| VER9 | Cerebellum | VER6 | Cerebellum | 0.0062 |
| VER7 | Cerebellum | L.THA | Thalamus | 0.0004 |
| L.OLF | Prefrontal | L.MTG | Temporal | 0.0023 |
| L.ROL | Other frontal | R.STG | Temporal | 0.0024 |
| L.ROL | Other frontal | L.MTG | Temporal | 0.0084 |
| R.POSTCG | Parietal | R.SOG | Occipital | 0.0002 |
L, left; R, right.
Altered negative correlations
There were 68 negative correlations that were significantly different between the healthy controls and the AD patients. As shown in Table V, 26 negative correlations significantly decreased (closer to zero) in AD patients. Most of these negative correlations were associated with the parietal lobe. More interestingly, nearly half of these decreased negative correlations occurred between the two intrinsically anti‐correlated networks that were found during the resting‐state by Fox et al. [2005]. The regions needed to satisfy two criterions in order to be considered as parts of the two intrinsically anti‐correlated networks: (1) the coordinates of the peak foci of the two intrinsically anti‐correlated networks described by Fox et al. [2005] lie in the regions that we considered; (2) the regions that we considered overlapped with those in the paper of Fox et al. [2005] for a large part through visual inspection. In addition, 42 pairs of regions showed increased negative correlations (farther from zero). More details can be seen in Table VI. In contrast with the decreased negative correlations, over half of the increased negative correlations were associated with the occipital lobe. Some increased negative correlations were also associated with the two intrinsically anti‐correlated networks. However, only three of these increased negative correlations were between the two networks. Sometimes the two regions of one correlation were even from the same network, either the “task positive” one or the “task negative” one, which suggests that some anti‐correlations not existing in healthy controls may appeared in AD patients.
Table V.
The decreased negative interregional correlations (closer to zero) in AD patients compared with elderly healthy controls
| Region 1 | Classification | Region 2 | Classification | P value |
|---|---|---|---|---|
| L.MFGa | Prefrontal | L.PCCb | Parietal | 0.0090 |
| R.MFGa | Prefrontal | L.PCUb | Parietal | 0.0010 |
| R.IFGO | Prefrontal | R.PCCb | Parietal | 0.0041 |
| R.IFGT | Prefrontal | R.HIPb | Temporal | 0.0089 |
| R.IFGT | Prefrontal | R.MFGO | Prefrontal | 0.0041 |
| L.SMAa | Other frontal | R.PCCb | Parietal | 0.0024 |
| L.SMAa | Other frontal | L.PCCb | Parietal | 0.0004 |
| L.SMAa | Other frontal | L.PCUb | Parietal | 0.0058 |
| L.SMAa | Other frontal | L.ANGb | Parietal | 0.0094 |
| R.PRECGa | Other frontal | R.PCCb | Parietal | 0.0040 |
| R.PRECGa | Other frontal | L.PCUb | Parietal | 0.0043 |
| R.PRECGa | Other frontal | R.PCUb | Parietal | 0.0018 |
| R.POSTCG | Parietal | L.PCUb | Parietal | 0.0014 |
| R.POSTCG | Parietal | R.PCUb | Parietal | 0.0009 |
| R.IOGa | Occipital | R.PCCb | Parietal | 0.0086 |
| R.CUN | Occipital | R.PHIPb | Temporal | 0.0054 |
| L.CUN | Occipital | R.POSTCG | Parietal | 0.0048 |
| R.CUN | Occipital | R.POSTCG | Parietal | 0.0002 |
| R.CAL | Occipital | L.SMG | Parietal | 0.0033 |
| R.INSa | Insula | R.PCCb | Parietal | 0.0003 |
| VER3b | Cerebellum | R.SPGa | Parietal | 0.0052 |
| VER8 | Cerebellum | R.IPGa | Parietal | 0.0079 |
| VER8 | Cerebellum | L.IFGT | Prefrontal | 0.0067 |
| R.CER6 | Cerebellum | R.SPGa | Parietal | 0.0059 |
| R.CER7bb | Cerebellum | R.INSa | Insula | 0.0056 |
| R.CERCR2b | Cerebellum | R.RECT | Prefrontal | 0.0004 |
Table VI.
The increased negative interregional correlations (farther from zero) in AD patients compared with elderly healthy controls
| Region 1 | Classification | Region 2 | Classification | P value |
|---|---|---|---|---|
| R.IFGOPa | Prefrontal | R.ITGb | Temporal | 0.0087 |
| R.RECT | Prefrontal | L.HIPb | Temporal | 0.0069 |
| L.IFGO | Prefrontal | L.SPGa | Parietal | 0.0090 |
| R.IFGO | Prefrontal | L.SPGa | Parietal | 0.0026 |
| R.IFGO | Prefrontal | R.SPGa | Parietal | 0.0049 |
| L.SFGb | Prefrontal | L.LING | Occipital | 0.0085 |
| L.SFGb | Prefrontal | R.LING | Occipital | 0.0015 |
| R.SFGb | Prefrontal | R.LING | Occipital | 0.0016 |
| R.SFGO | Prefrontal | L.LING | Occipital | 0.0043 |
| R.IFGT | Prefrontal | L.IOGa | Occipital | 0.0030 |
| R.SFGM | Prefrontal | L.IOGa | Occipital | 0.0027 |
| R.SFGMO | Prefrontal | R.SOG | Occipital | 0.0061 |
| R.RECT | Prefrontal | R.LING | Occipital | 0.0013 |
| R.ACCb | Prefrontal | R.SOG | Occipital | 0.0068 |
| R.ACCb | Prefrontal | L.MOG | Occipital | 0.0056 |
| R.ACCb | Prefrontal | L.IOGa | Occipital | 0.0016 |
| L.SMAa | Other frontal | R.LING | Occipital | 0.0089 |
| L.SMAa | Other frontal | L.IOGa | Occipital | 0.0046 |
| L.MCC | Other frontal | R.MOG | Occipital | 0.0041 |
| R.MCC | Other frontal | L.IOGa | Occipital | 0.0096 |
| L.HIPb | Temporal | L.PCUb | Parietal | 0.0060 |
| L.PHIPb | Temporal | L.CAU | Corpus striatum | 0.0023 |
| R.PHIPb | Temporal | L.ANGb | Parietal | 0.0068 |
| R.AMYG | Temporal | R.IPGa | Parietal | 0.0057 |
| R.AMYG | Temporal | L.ANGb | Parietal | 0.0034 |
| R.PCCb | Parietal | R.SOG | Occipital | 0.0042 |
| R.SMG | Parietal | R.ITGb | Temporal | 0.0018 |
| L.PAL | Corpus striatum | L.SOG | Occipital | 0.0037 |
| L.PAL | Corpus striatum | R.SOG | Occipital | 0.0099 |
| L.PAL | Corpus striatum | L.MOG | Occipital | 0.0006 |
| R.PAL | Corpus striatum | L.MOG | Occipital | 0.0029 |
| R.PAL | Corpus striatum | L.IOGa | Occipital | 0.0064 |
| VER7 | Cerebellum | R.MFGO | Prefrontal | 0.0026 |
| R.CER3 | Cerebellum | R.RECT | Prefrontal | 0.0075 |
| R.CERCR2b | Cerebellum | R.HIPb | Temporal | 0.0073 |
| R.CER7bb | Cerebellum | L.MTGa | Temporal | 0.0030 |
| VER9 | Cerebellum | R.POSTCG | Parietal | 0.0072 |
| VER9 | Cerebellum | L.SPGa | Parietal | 0.0089 |
| R.CER7bb | Cerebellum | L.CAL | Occipital | 0.0017 |
| R.CER7bb | Cerebellum | R.LING | Occipital | 0.0013 |
| VER9 | Cerebellum | R.SOG | Occipital | 0.0039 |
Functional Connectivity Associated With the PCC
In the elderly healthy controls, the PCC showed significant connectivities to a number of brain regions. The detailed results can be seen in Figure 1A,B and Table SI in the supplementary materials. Among those regions that were significantly correlated with the PCC, the precuneus (BA 31/7), lateral parietal cortex (BA 39/40), angular gyrus (BA 39), superior frontal gyrus (BA 8/9), inferior temporal gyrus (BA 20/21), and cerebellar tonsils partially overlapped with those regions lying in the “task negative” network that was proposed by Fox et al. [2005]. Among those regions that were significantly anti‐correlated with the PCC, the precentral gyrus (BA 6/44), inferior parietal lobule (BA 40), supplementary motor area (SMA)/pre‐SMA (BA 6/32), inferior frontal gyrus (BA 45), and insula (BA 13) partially overlapped with those regions lying in the “task positive” network [Fox et al., 2005]. The results from the early AD patients are shown in Figure 1C,D and Table SII of the supplementary materials. Among the regions that were significantly correlated with the PCC, the precuneus (BA 31/24/7), lateral parietal cortex (BA 39), angular gyrus (BA 39), middle frontal gyrus (BA 8/10), superior frontal gyrus (BA 8/9), and cerebellar tonsils partially overlapped with those regions lying in the “task negative” network [Fox et al., 2005]. Among those regions that were significantly anti‐correlated with the PCC, the precentral gyrus (BA 6), middle temporal gyrus (BA 6/22), and insula (BA 38/22) partially overlapped with those regions underlying the “task positive” network [Fox et al., 2005]. However, the results also showed that the number of regions that were significantly correlated/anti‐correlated with the PCC was fewer than the number found for the elderly healthy controls, especially in the case of significantly anti‐correlated regions.
Figure 1.
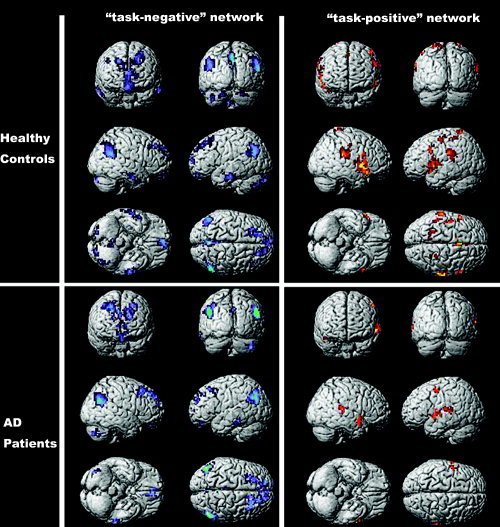
Maps of brain regions showing significant positive or negative correlations with the PCC in elderly healthy controls (A and B) and early AD patients (C and D). The statistical threshold was P 0.001 (corrected). Most of the regions that were significantly correlated with the PCC overlapped with those regions lying in the “task negative” network, and most of the regions that were significantly anti‐correlated with the PCC overlapped with those regions lying in the “task positive” network proposed by Fox et al. [2005].
When comparing the connectivity between AD and control groups, a set of regions, including the left/right precuneus (BA 7/30/31), the right supramarginal gyrus (BA 40), and the left superior frontal gyrus (BA 6/8), showed significantly decreased positive correlations with the PCC in the AD group (Table VII and Fig. 2A). Another set of regions, including the left/right SMA/Pre‐SMA (BA 6/32), the left precentral gyrus (BA 44), the left superior temporal gyrus (BA 22), and the left insula (BA 13/47), showed significantly decreased negative correlations (closer to zero) with the PCC in the AD group (Table VIII and Fig. 2B). The results did not show any cluster having significantly increased positive or negative correlations (farther from zero) with the PCC.
Table VII.
Brain regions exhibiting significantly decreased positive correlations with the PCC in AD patients compared with healthy controls
| Region | Hem | BA | (x, y, z) | T value |
|---|---|---|---|---|
| PCC/PCU | L | 29/30/31 | (−6,−55,5) | 4.26 |
| (−6,−57,28) | 4.19 | |||
| (−6,−57,19) | 3.32 | |||
| PCC/PCU | R | 7/31 | (3,−62,50) | 5.39 |
| (15,−55,14) | 4.17 | |||
| (9,−63,22) | 3.98 | |||
| Supramarginal gyrus | R | 40 | (50,−48,22) | 3.92 |
| Superior frontal gyrus | L | 6/8 | (−27,20,57) | 3.68 |
| (−30,29,31) | 3.33 |
Hem, hemisphere; BA, Brodmann's area; (x, y, z), coordinates of primary peak locations in the space of Talairach [Talairach and Tournoux, 1988].
Figure 2.
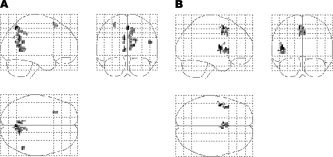
The glass brain representation of the brain regions showing significantly different positive or negative correlations with the PCC between early AD patients and elderly healthy controls. The statistical threshold was P < 0.001 (corrected). (A) Brain regions showing significantly decreased positive correlations with the PCC in early AD patients compared with elderly healthy controls. (B) Brain regions showing significantly decreased negative correlations (closer to zero) with the PCC in early AD patients compared with elderly healthy controls.
Table VIII.
Brain regions exhibiting significantly decreased (closer to zero) negative correlations with the PCC in AD patients compared with healthy controls
| Region | Hem | BA | (x, y, z) | T value |
|---|---|---|---|---|
| SMA/Pre‐SMA | L | 6/32 | (−6,8,49) | 3.35 |
| R | 6/32 | (6,5,38) | 2.72 | |
| (0,20,46) | 2.43 | |||
| Precentral gyrus | L | 44 | (−45,−1,10) | 3.29 |
| (−53,−6,3) | 2.31 | |||
| Superior temporal gyrus | L | 22 | (−48,0,−2) | 2.01 |
| Insula | L | 13/47 | (−12,12,8) | 3.06 |
| (−45,17,−3) | 2.35 |
Hem, hemisphere; BA, Brodmann's area; (x, y, z), coordinates of primary peak locations in the space of Talairach [Talairach and Tournoux, 1988].
DISCUSSION
We examined the altered functional connectivity of AD patients from the following aspects: (1) We analyzed the functional connectivity during the resting‐state by fMRI; (2) In comparison with conventional functional connectivity analysis based on ROIs, we used an unbiased approach to examine the distributions of abnormal functional connectivity throughout the entire brain; (3) In addition to positive correlations, we also paid much attention to abnormal negative correlations; (4) Since there has been no previous study reporting abnormalities in the two intrinsically anti‐correlated networks in AD, we further analyzed the abnormal positive and negative correlations associated with the PCC, which is one of the most important nodes of the two networks [Fox et al., 2005; Fransson, 2005].
To date, there have been very few resting‐state fMRI studies on AD, especially with respect to the analysis of functional connectivity between different brain areas. Most functional connectivity analyses on AD have been based on PET. To our knowledge, this is the first study examining abnormal functional connectivity throughout the entire brain using resting‐state fMRI. Unlike the PET and single‐photon emission computerized tomography methods, fMRI is noninvasive. In addition, resting‐state fMRI has the advantage of easier manipulations, especially for patients, compared with task‐driven methods. Hence the resting‐state fMRI may have potential in clinical applications.
In our study, the results showed that AD patients had decreased positive correlations between prefrontal and parietal regions (Table III). This result is compatible with previous studies that have suggested an anterior–posterior disconnection, either in task conditions or in the resting‐state [Azari et al., 1992; Grady et al., 2001; Horwitz et al., 1987, 1995]. Both frontal and parietal association areas showed marked neuropathological abnormalities in AD patients [Wenk, 2003]. In addition, some studies have also shown that language and visuoconstructive abilities, whose integrity depended on frontal and parietal lobe interactions, were significantly impaired in AD patients [Backman et al., 2005; Brouwers et al., 1984; Henry et al., 2004]. Therefore, we suggest that the fontal–parietal disconnection could be an important biomarker for AD patients.
Our results also showed that AD patients had some increased positive correlations either within the prefrontal regions or between the prefrontal regions and other brain regions (Table IV). Some neuroimaging studies of early AD have found increased prefrontal activity during certain cognitive tasks compared with elderly controls [Becker et al., 1996; Woodard et al., 1998; Pariente et al., 2005]. Increased functional connectivity associated with prefrontal regions has also been reported in AD patients compared with healthy controls [Grady et al., 2001, 2003; Horwitz et al., 1995]. These increases in prefrontal activities and functional connectivity have been interpreted as a compensatory reallocation or recruitment of cognitive resources. Besides those related to prefrontal regions, there were also some increased positive correlations in other brain areas. Most of them were within the temporal lobe, parietal lobe, or occipital lobe (Table IV). Bokde et al. [2006] found increased linear correlations in the parietal lobe when the subjects with mild cognitive impairment participated in a face‐matching task. Azari et al. [1992] found that AD patients had larger positive correlations of regional cerebral metabolic rates for glucose consumption between occipital ROIs. When considered with our results, this suggests that the increased functional connectivity of within‐lobe regions might be not only restricted in the prefrontal lobe but also distributed in other lobes. When considered together with the decreased positive correlations, the results showed that in the brain of early AD patients the decreased connectivities were mainly between lobes, especially between the prefrontal lobe and parietal lobe, but the increased connectivities were mainly within‐lobes. This could possibly imply that the increased within‐lobe connectivities reflect a compensatory effect for the reduced connectivities between lobes. The previous studies mentioned above have shown that the compensatory effect can be found in different tasks, and our current results suggests that even during the resting‐state the increased recruitment still exists and may reflect a more general adaptation to the deficits of AD.
It is important to note that almost all previous fMRI studies of functional connectivity only focused on positive correlations. Negative correlations between different brain regions and their abnormalities in diseases have seldom been reported [Bokde et al., 2006]. Recently, two pioneering studies by Fox et al. [2005] and Fransson [2005] began to focus on the presence of negative correlations among brain regions. Fox et al. [2005] identified two diametrically opposed brain networks on the basis of both correlations within each network and anti‐correlations between networks during the resting‐state. They suggested that the presence of anti‐correlated networks may reveal the intrinsic organization of brain function and the anti‐correlations may serve a differentiating role segregating neuronal processes subserving opposite goals or competing representations. By examining the positive and negative correlation maps of the precuneus cingulate cortex (PCU)/PCC during the resting‐state, Fransson [2005] revealed two networks that were similar to those found by Fox et al. [2005], and suggested that the default mode of brain function [Raichle et al., 2001] might be augmented with a second mode of brain function which reflected the existence of an extrospectively oriented attention network.
In our study, as shown in Table V, there were 26 significantly decreased negative correlations in AD patients. Many regions were adjacent to those that have been found in the two anti‐correlated networks, though they were unlikely to be exactly the same because we used a template to automatically segment the whole brain. Some of these regions, including the supplementary motor area, the precentral gyrus, the middle frontal gyrus, insula, and the inferior occipital gyrus, were very similar to those that were found in the “task positive” network by Fox et al. [2005]. It was very interesting that the counterparts of those regions mentioned above were mostly those regions that were very similar to those that have been found in the “task negative” network, such as the posterior cingulate gyrus, the precuneus, the angular gyrus, the parahippocampal gyrus. Together with the increased negative correlations, these abnormal negative correlations implied that the anti‐correlation effect, which was intrinsically existent between the two opposed networks in healthy controls, might be disturbed in AD patients. Fox et al. [2005] suggested that the anti‐correlations of the two networks might be interpreted as competition between focused attention and process subserving stimulus‐independent thought. From the perspective of the neural networks governing control of attention [Corbetta and Shulman, 2002], Fransson [2005] found that those regions in the top‐down (goal‐directed) and bottom‐up (stimulus‐driven) control systems of attention largely lay within the network of cortical regions that were anti‐correlated with the PCU/PCC, and thus proposed that the anti‐correlations between the two networks reflected a recurring switch between an introspectively versus an extrospectively oriented attention state‐of‐mind. From this point of view, the disturbance of the anti‐correlation effect between the two intrinsic networks may be a partial reason for the attention deficits of AD patients. AD patients have often been described as having difficulty in concentrating, or being easily distractible [Perry and Hodges, 1999]. A number of studies have suggested that attention and executive impairments may be the first nonmemory domain to be affected [Grady et al., 1988; Lafleche and Albert, 1995; Reid et al., 1996]. Perry and Hodges [1999] have proposed that the attention deficits of AD patients might possibly be associated with the disconnection problem. Our results may offer a new perspective to interpret the attention deficits associated with AD. In fact, two task‐based studies by Lustig et al. [2003] and Grady et al. [2006] have found that there was reduced default mode suppression in older adults. Lustig et al. [2003] also found that between AD patients and age‐matched healthy controls there were significant differences in activation of the PCC, which was an important node in the default mode network. Consistent with our hypothesis, they suggested that an alteration in the balance between default mode and task‐related activity could account for increased vulnerability to distraction from irrelevant information, and thereby could affect multiple cognitive domains. To review, we suggest that the disturbance between the two intrinsically anti‐correlated networks may be associated with the attention deficits of AD patients. Of course, further neuropsychology studies are still required to confirm this hypothesis.
Note that the results of our unbiased approach should be taken as descriptive. Since there are so many comparisons (116 × 115/2 = 6670), correction for multiple comparisons may make most of the differences not statistically significant. However, from the perspective of the entire brain, this unbiased approach offers a descriptive representation of the global distribution of the abnormal connectivity in early AD patients. Among these abnormal correlations, the decreased positive correlations between the frontal and parietal regions and the increased positive correlations within the prefrontal lobe, parietal lobe, and occipital lobe have been reported in previous studies, either in the task state or in the resting state [Azari et al., 1992; Bokde et al., 2006; Grady et al., 2001, 2003; Horwitz et al., 1987, 1995]. In addition, we paid much attention to alterations in the negative correlations in AD patients, and the results showed a disruption of the two intrinsically anti‐correlated networks [Fox et al., 2005; Fransson, 2005].
Since there has been no previous study reporting the abnormal negative correlations between the two networks in AD, we further analyzed the abnormalities of the functional connectivity associated with the PCC to evaluate this new finding. Considering that the PCC is one of the most important regions in the two intrinsically anti‐correlated networks, the alterations in functional connectivity associated with it can partially reflect the disturbance of the two networks. As shown in Figure 1, in elderly healthy controls most of the regions that showed significant positive/negative correlations with the PCC overlapped with those in the “task negative” network and “task positive” network, respectively [Fox et al., 2005]. This result suggests that in elderly individuals the two intrinsically anti‐correlated networks still exist, and this further supports the idea that the two networks may reflect an intrinsic organization in human brains [Fox et al. 2005]. However, many of these regions did not appear in the functional connectivity map of the early AD patients. This may suggest that the two intrinsically anti‐correlated networks have been disrupted in early AD patients. The direct comparison of the positive/negative correlations associated with the PCC between the two groups showed that the positive correlations between the PCC and the precuneus, supramarginal gyrus, and superior frontal gyrus, which were also in the “task negative” network, were significantly decreased in early AD patients (Table VII and Fig. 1A). The negative correlations between the PCC and the precentral gyrus, SMA/Pre‐SMA, superior temporal gyrus, and insula, which were in the “task positive” network, were significantly decreased (closer to zero) in early AD patients (Table VII and Fig. 1B). These results were partially consistent with those of the above unbiased approach, and both of them suggested that not only the correlations within the default mode network [Grecius et al., 2003] but also the anti‐correlations between the “task positive” network and the “task negative” network [Fox et al., 2005] were abnormal in early AD patients.
One limitation of our present study lies in the effects of physiological noises such as cardiac and respiratory rhythms. We used a relatively low sampling rate (TR = 2 s) for multislice acquisitions. According to the Nyquist sampling theorem, high frequency noises such as cardiac rhythm can not be completely removed just by band‐pass filtering of 0.01–0.08 Hz. In the future, these physiological effects may be estimated and removed by simultaneously recording the respiratory and cardiac cycle during the data acquisition. In addition, as a resting‐state fMRI study, we did not examine the special cognitive dysfunctions in AD patients. Further studies are needed to investigate the relation between resting‐state functional connectivity and the neuropsychological measurements, and to examine whether disturbed functional connectivity can be a biomarker for cognitive dysfunctions in AD patients.
In summary, we examined the disturbed functional connectivity throughout the entire brain in AD patients by analyzing resting‐state fMRI data. Our results provided some new evidence for the anterior–posterior disconnections and increased within‐lobe connectivities that had been reported in previous studies. In addition, we paid much attention to negative correlations for the first time, and the results implied a disruption between two intrinsically anti‐correlated networks. As a whole, our study gave a descriptive representation for the global distribution of abnormal functional connectivities throughout the entire brain of AD patients, and may suggest directions for further detailed studies.
Supporting information
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat
Acknowledgements
The authors thank Yuan Zhou for her helpful comments for this work. The authors also thank Drs. Rhoda E. and Edmund F. Perozzi of Beijing University of Technology for editing and English language assistance.
Contributor Information
Kuncheng Li, Email: li_cums@yahoo.com.cn.
Tianzi Jiang, Email: jiangtz@nlpr.ia.ac.cn.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore ED ( 2006): A resilient, low‐frequency, small‐world human brain functional network with highly connected association cortical hubs. J Neurosci 26: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association ( 1994): DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- Azari NP, Rapoport SI, Grady CL, Schapiro MB, Salerno JA, Gonzales‐Aviles A, Horwitz B ( 1992): Patterns of interregional correlations of cerebral glucose metabolic rates in patients with dementia of the Alzheimer type. Neurodegeneration 1: 101–111. [Google Scholar]
- Backman L, Jones S, Berger AK, Laukka EJ, Small BJ ( 2005): Cognitive impairment in preclinical Alzheimer's disease: A meta‐analysis. Neuropsychology 19: 520–531. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST ( 1996): Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology 46, 692–700. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Bokde ALW, Lopez‐Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Moller HJ, Hampel H. ( 2006): Functional connectivity of the fusiform gyrus during a face‐matching task in subjects with mild cognitive impairment. Brain 129: 1113–1124. Advance Access published on March 6, 2006. [DOI] [PubMed] [Google Scholar]
- Brouwers P, Cox C, Martin A, Chase T, Fedio P ( 1984): Differential perceptual‐spatial impairment in Huntington's and Alzheier's dimentias. Arch Neurol 41: 1073–1076. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–214. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW, Hyman BT ( 1990): Reflections on the selectivity of neuropahological changes in Alzheimer's disease In: Schwartz MN, editor. Modular Deficits in Alzheimer Type Dementia. Cambridge: MIT Press; pp 83–100. [Google Scholar]
- De Lacoste MC, White CL III( 1993): The role of cortical connectivity in Alzheimer's disease pathogenesis: A review and model system. Neurobiol Aging 14: 1–16. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M, Collete F ( 2003): Alzheimer's disease as a disconnection syndrome? Neuropsychol Rev 13: 79–92. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations—An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ ( 1993): Functional connectivity: The principal component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14. [DOI] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, Schapiro M, Friedland RP, Rapoport SI ( 1988): Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol 10: 576–596. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI ( 2001): Altered brain functional connectivity and impaired short‐term memory in Alzheimer's disease. Brain 124: 739–756. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE ( 2003): Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci 23: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G ( 2006): Age‐related changes in brain activity across the adult lifespan. J Cogn Neurosci 18: 227–241. [DOI] [PubMed] [Google Scholar]
- Gòmez‐Isla T, Hyman BT ( 1997): Connections and cognitive impairment in Alzheimer's disease In: Hyman BT, Duyckaerts C, Christen Y, editors. Connections, Cognition, and Alzheimer's Disease. Berlin: Springer; pp 149–166. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V ( 2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC ( 2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH ( 2004): Verbal fluency performance in dementia of the Alzheimer's type: A meta‐analysis. Neuropsychologia 42: 1212–1222. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Grady CL, Schageter NL, Duara R, Rapoport SI ( 1987): Intercorrelations of regional glucose metabolic rates in Alzheimer's disease. Brain Res 407: 294–306. [DOI] [PubMed] [Google Scholar]
- Horwitz B, McIntosh AR, Haxby JV, Furey M, Salerno J, Schapiro MB, Rapoport SI, Grady CL ( 1995): Network analysis of PET‐mapped visual pathways in Alzheimer's type dementia. Neuroreport 6: 2287–2292. [DOI] [PubMed] [Google Scholar]
- Jiang TZ, He Y, Zang YF, Weng XC ( 2004): Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp 22: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleche G, Albert MS ( 1995): Executive function deficits in mild Alzheimer's disease. Neuropsychology 9: 313–320. [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG ( 2002): Alzheimer disease: Evaluation of a functional MRI index as a marker. Radiology 225: 253–259. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA ( 1998): Functional connectivity in single and multislice echoplanar imaging using resting state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, OBrien KC, McAvoy M, Raichle ME, Morris JC,Buckner RL ( 2003): Functional deactivations: Change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 100: 14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim V, Xendur L, Fadili J, Suckling J, Gould R, Howard R, Bullmore E ( 2005): Fractional Gaussian noise, functional MRI and Alzheimer's disease. Neuroimage 25: 141–158. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of the department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Morris JC ( 1993): The clinical dementia rating (CDR): current version and scoring rules. Neurology 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L ( 2001): Mild cognitive impairment represents early‐stage Alzheimer disease. Arch Neurol 58: 397–405. [DOI] [PubMed] [Google Scholar]
- Morrison J, Scherr S, Lewis D, Campbell M, Bloom F, Rogers J, Benoit R ( 1986): The laminar and regional distribution of neocortical somatostatin and neuritic plaques: Implications for Alzheimer's disease as a global neocortical disconnection syndrome In: Scheibel A, Wechsler A, Brazier M, editors. The Biological Substrates of Alzheimer's Disease. Orlando: Academic Press. pp 115–131. [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipoloti L, Puel M, Demonet JF, Chollet F, Frackowiak RS ( 2005): Alzheimer's patients engage an alternative network during a memory task. Ann Neurol 58: 870–879. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR ( 1999): Attention and executive deficits in Alzheimer's disease: A critical review. Brain 122: 383–404. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Synder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Broe G, Creasey H, Grayson D, McCusker E, Bennett H, Iongley W, Sulway MR ( 1996): Age at onset and pattern of neuropsychological impairment in mild early‐stage Alzheimer's disease. A study of a community‐based population. Arch Neurol 53: 1056–1061. [DOI] [PubMed] [Google Scholar]
- Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM ( 2000): Loss of connectivity in Alzheimer's disease: An evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry 69: 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore ED ( 2005): Neurophysiological architecture of functional magnetic resonance image of human brain. Cereb Cortex 15: 1332–1342. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M ( 1999): Three‐dimentional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10: 233–260. [DOI] [PubMed] [Google Scholar]
- Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E ( 2000): Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol 21: 1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux PA ( 1988): Coplanar stereotactic atlas of the human brain, Thieme, Stuttgart. [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. ( 2002): Automated anatomical labelling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW ( 1990): The dissection by Alzheimer's disease of cortical and limbic neural systems relevant to memory In: McGaugh JL, Weinberger NM, Lynch G, editors. Brain Organization and Memory: Cells, System and Circuits. Oxford: Oxford University Press; pp 234–261. [Google Scholar]
- Van Hoesen GW( 1997): Ventromedial temporal lobe anatomy, with comments on Alzheimer's disease and temporal injury. J Neuropsychiatry Clin Neurosci 9: 331–341. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang YF, He Y, Liang M, Zhang XQ, Tian LX, Wu T, Jiang TZ, Li K ( 2006): Changes in hippocampal connectivity in the early stages of Alzheimer's disease: Evidence from resting state fMRI. Neuroimage 31: 496–504. [DOI] [PubMed] [Google Scholar]
- Wenk GL ( 2003): Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry 64( Suppl 9): 7–10. [PubMed] [Google Scholar]
- Woodard JL, Grafton ST, Voltaw JR, Green RC, Dobraski ME, Hoffman JM ( 1998): Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer's disease. Neuropsychology 12: 491–504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat


