Abstract
Previous findings have shown that the human somatosensory cortical systems that are activated by passive nonpainful electrical stimulation include the contralateral primary somatosensory area (SI), bilateral secondary somatosensory area (SII), and bilateral insula. The present study tested the hypothesis that these areas have different sensitivities to stimulation frequency in the condition of passive stimulation. Functional MRI (fMRI) was recorded in 24 normal volunteers during nonpainful electrical median nerve stimulations at 0.5, 1, 2, and 4 Hz repetition rates in separate recording blocks in pseudorandom order. Results of the blood oxygen level‐dependent (BOLD) effect showed that the contralateral SI, the bilateral SII, and the bilateral insula were active during these stimulations. As a major finding, only the contralateral SI increased its activation with the increase of the stimulus frequency at the mentioned range. The fact that nonpainful median‐nerve electrical stimuli at 4 Hz induces a larger BOLD response is of interest both for basic research and clinical applications in subjects unable to perform cognitive tasks in the fMRI scanner. Hum Brain Mapp 2006. © 2006 Wiley‐Liss, Inc.
Keywords: functional magnetic resonance imaging (fMRI), median nerve electrical stimulation, stimulus frequency, secondary somatosensory cortex (SII)
INTRODUCTION
The organization of the human somatosensory cortex has been intensively studied with magnetoencephalography (MEG) and functional MRI (fMRI). It has been shown that the contralateral primary (SI) and bilateral secondary (SII) somatosensory cortices are involved in the processing of transient nonpainful and painful stimuli [Del Gratta et al.,2000,2002; Ferretti et al.,2003,2004; Ibanez et al.,1995; Kakigi et al.,2000,2003; Mauguiere et al.,1997a,b; Torquati et al.,2002,2003]. SI is presumed to process and encode the type and intensity of the sensory inputs, whereas SII has a multifaceted role, including sensorimotor integration [Huttunen et al.,1996; Narici et al.,1991a,b], integration of information from the two body halves [Hari et al.,1998], and cognitive functions such as attention [Burton et al.,1999; Mima et al.,1998], learning [Diamond et al.,2002], memory [Diamond et al.,2002; Ridley and Ettlinger,1976], integration, and emotional coding of nonpainful and painful stimuli.
A major scientific issue is the physiological activity of the cortical somatosensory systems as a function of the frequency of somatosensory stimulation, i.e., an important physical parameter of the stimulus. Two major skin receptors are involved. Meissner corpuscles are most sensitive to the frequencies of stimulation under 50 Hz (flutter), while Pacinian corpuscles are most sensitive to faster stimulation frequency (100–300 Hz; Harrington and Downs [2001]). High‐frequency stimulations of the index finger at 50, 100, 200, and 400 Hz evoked magnetic fields with similar localization in contralateral SI [Hashimoto et al.,1998]. Stimulation at 150 Hz and 300 Hz elicited an fMRI response in the contralateral SI. Additionally, activity of the primary and supplementary motor cortex was observed using 150 Hz stimuli, that activity being minimal at 300 Hz [Gizewski et al.,2005]. Increasing stimulation from 30 to 80 Hz caused a significant increase in the number of fMRI voxels activated in SII and the posterior insula, while the number of voxels activated in the contralateral SI declined. No significant change in signal intensity with frequencies was found in any of the activated areas [Francis et al.,2000]. In contrast, increasing the interstimulus interval of vibrotactile stimulation at 200 Hz from 1 s to 5 s did not affect the fMRI response in SI and SII [Tuunanen et al.,2003]. On the other hand, increasing median nerve stimulation frequencies at 5, 15, 40, and 100 Hz induced a linear increase of the fMRI response in the primary sensorimotor cortex [Kampe et al.,2000]. On the contrary, stimulation of rat forepaw in the range 1.5–9 Hz showed a decrease of the fMRI response in contralateral SI for frequencies above 3 Hz. [Gyngell et al.,1996]. Furthermore, stimulation in the range 1–8 Hz showed maximal fMRI response at 3 Hz in rodent contralateral SI, bilateral SII, thalamus, and cerebellum [Keilholz et al.,2004].
Other results on SI and SII responses to repetitive stimulation come from MEG‐EEG studies. Electric stimulation of the median nerve elicited somatosensory evoked magnetic fields in the contralateral SI and bilateral SII. In the contralateral SI, the earliest magnetic fields (+20 ms poststimulus peak) and temporal discrimination of the stimuli remained unaffected with increasing stimulus repetition rates at 0.25, 0.5, 1, and 2 Hz [Schnitzler et al.,1999]. Delberghe et al. [1990], in a study with electric median nerve stimulation from 1.6–5.7 Hz, observed no amplitude change of the early somatosensory evoked electric potentials (SEPs), and an amplitude decrease of the later SEPs with increasing stimulation frequency. In a study with vibrotactile stimulation at 200 Hz [Tuunanen et al.,2003], the intensity of the current dipoles in SI, used to model the MEG responses, increased with increasing interstimulus intervals (from 1–5 s). However, it should be stressed, when comparing fMRI with MEG‐EEG, that these two techniques observe different physiological phenomena and may therefore yield apparently contrasting results.
Keeping these data in mind, the present study on normal subjects tested the hypothesis that contralateral SI and bilateral SII have different frequency sensitivity to passive somatosensory stimulations at low frequencies (0.5–4 Hz). This is an important issue not only for basic research but also for clinical applications. fMRI recordings during passive sensory stimulation have been invoked to be useful in the functional evaluation of sensorimotor cortex prior to surgical intervention and in patients with difficulties in performing cognitive or motor tasks [Kampe et al.,2000].
SUBJECTS AND METHODS
Subjects and Stimulation Procedures
Twenty‐four healthy volunteers ranging in age from 19–25 years (11 males, 13 females) were enrolled in this study. All of them were right‐handed according to the Edinburgh Inventory [Oldfield,1971]. All subjects gave written informed consent according to the Declaration of Helsinki [World Medical Association Declaration of Helsinki,1997] and could request an interruption of the investigation at any time. The general procedures were approved by the local Institutional Ethics Committee.
The electric stimulus was a rectangular pulse of 200 μs duration and was delivered to the right median nerve at the wrist via nonmagnetic AgCl electrodes. The stimulation current was set at a level eliciting a sustained, but painless, thumb twitch and was assessed outside the scanner just before the fMRI session. The current level varied across subjects in the range of 5–18 mA (mean value 10.1 ± 3.8 mA). Four frequencies of stimulation were used (0.5, 1, 2, 4 Hz), each frequency in a separate run. The order of the stimulation frequency was varied pseudorandomly across participants. Each participant was given a brief training session in which he/she had to keep their gaze on a fixation point while minimizing eye movements.
fMRI Recordings
Blood oxygen level‐dependent (BOLD) contrast fMRI was performed with a Siemens Magnetom Vision (Erlangen, Germany) scanner at 1.5 T by means of T2*‐weighted echo planar imaging (EPI) free induction decay (FID) sequences with the following parameters: TR, 3 s; TE, 60 ms; matrix size 64 × 64; field of view (FOV), 256 mm; in‐plane voxel size, 4 × 4 mm; flip angle, 90°; slice thickness, 4 mm; and no gap. A standard headcoil was used and the subject's head was fixed by foam pads to reduce involuntary movement. Functional volumes consisted of 22 transaxial slices parallel to the anterior–posterior commissural (AC‐PC) line and covering a brain region extending from the vertex to the middle temporal gyrus. The experimental paradigm was a block design alternating a state of stimulation of 36 s (corresponding to the acquisition of 12 functional volumes) with a control state having the same duration. For each stimulus frequency a run of 100 volumes was acquired starting with a control period. A high‐resolution structural volume was acquired at the end of the session via a 3D MPRAGE sequence with the following features: axial, matrix 256 × 256, FOV 256 mm, slice thickness 1 mm, no gap, in‐plane voxel size 1 × 1 mm, flip angle 12°, TR = 9.7 ms, TE = 4 ms.
Data Analysis
Raw data were analyzed by means of BrainVoyager 4.9 (Brain Innovation, The Netherlands). Due to T1 saturation effects, the first four scans of each run were discarded from the analysis. Preprocessing of functional scans included motion correction and removal of linear trends from voxel time series. A 3D motion correction was performed by means of a rigid body transformation to match each functional volume to the reference volume (the fifth volume) estimating three translation and three rotation parameters. These parameters were stored in log‐files and inspected to check that estimated movement was not larger than approximately half a voxel for each functional run. Two of the subjects did not meet this criterion and were discarded from further analysis. Preprocessed functional volumes of a subject were coregistered with the corresponding structural dataset. Since the 2D functional and 3D structural measurements were acquired in the same session, the coregistration transformation was determined using the slice position parameters of the functional images and the position parameters of the structural volume. Structural and functional volumes were transformed into the Talairach space [Talairach and Tournoux, 1988] using a piecewise affine and continuous transformation. Functional volumes were resampled at a voxel size of 3 × 3 × 3 mm. No spatial or temporal smoothing was performed.
Statistical analysis was performed for each subject and stimulus frequency using the General Linear Model (GLM) [Friston et al.,1995] with correction for temporal autocorrelation [Bullmore et al.,1996; Woolrich et al.,2001]. To account for the hemodynamic delay, the boxcar waveform representing the rest and task conditions was convolved with an empirical hemodynamic response function [Boynton et al.,1996]. Individual statistical maps were thresholded at P < 0.0004 at the voxel level and a cluster size of at least four voxels was required. These thresholds and an estimate of the spatial correlation of voxels [Forman et al.,1995; 3dFWHM routine of AFNI package, Cox,1996] were used as input in a Monte‐Carlo simulation [AlphaSim routine of AFNI package, Cox,1996; Forman et al.,1995] in order to assess the overall significance level (the probability of a false detection for the entire functional volume). In this way we obtained P < 0.05 as the significance level corrected for multiple comparisons. Individual thresholded statistical maps were then superimposed on the respective structural scans for the localization of significantly activated areas.
Regions of Interest
Cortical regions of interest (ROIs) corresponding to contralateral SI and bilateral SII were traced independently by two expert experimenters along the lines outlined in previous literature [Baraldi et al.,1999; Picard and Strick,1996]. Anatomical landmarks included the posterior bank of the central sulcus and the postcentral gyrus at the omega zone [Yousry et al.,1997] for the contralateral SI, and the upper bank of the lateral sulcus near the posterior pole of the insula for the bilateral SII. The mediolateral extension of SI was based on the omega zone landmark, while the mediolateral extension of SII ranged from the lip of the upper bank of the lateral sulcus to its fundus. The omega fold served to delimitate the “hand” area in SI, following the lead of several fMRI studies showing marked “hand” sensorimotor responses in the omega zone [Pizzella et al.,1999; Puce,1995; Rumeau et al.,1994; Toyokura et al.,1999; Wood et al.,1988]. The bilateral region of SII was delineated taking into account that it is located laterally to the representation of the face in SI, within the upper bank of the lateral sulcus in the region of the parietal operculum (approximately from the lip of the lateral sulcus to its fundus) [Magnus et al.,1952; Woolsey et al.,1979].
The bilateral insula was also taken into account. The region of the insular cortex was delineated taking into account that it is located deep within the Sylvian fissure and runs from anterior to posterior deeply to the “opercularis” portions of the frontal, temporal, and parietal lobes. To avoid confounding results with the anterior frontal operculum and SII region, here we considered only the portion of the insula delimitated in the hemispheric surface by the pre‐ and postcentral gyri.
The mentioned ROIs were determined by considering the whole mask obtained from activated voxels at each stimulus frequency. The mean time course of the fMRI signal from the voxels belonging to a given ROI was analyzed to inspect the effect of different stimulus conditions. Attention was devoted to distinguish activated areas in the posterior insula from activated areas in SII. The subject's responses to different stimulation frequencies were characterized by evaluating the BOLD signal intensity variation in each ROI. The relative signal variation between baseline (rest period) and activation (stimulation period) was calculated from the fitted parameters of the GLM:
where betai (i = 0.5, 1, 2, 4 Hz) represents the estimated amplitude of the mean variation of the fMRI signal during the stimulation with respect to baseline. Analysis of the initial overshoot phase of the positive BOLD effect was performed as well by considering the peak response.
The regional comparison of activation (BOLD % change) was undertaken by means of the analysis of variance (ANOVA) for repeated measures. Mauchley's test was used to evaluate the sphericity assumption. The number of degrees of freedom was corrected by means of the Greenhouse–Geisser procedure. The dependent variable of the ANOVA analysis was the BOLD signal relative variation between the stimulation (considering first the mean response and then the peak response) and rest conditions. Six ANOVA analyses were performed. The first was focused on the activity (mean response) of SI and the factor was the stimulus frequency (0.5, 1, 2, 4 Hz). The second and third ANOVAs were focused on the activity (mean response) of bilateral SII and bilateral insula, respectively. These two ANOVAs used stimulus frequency (0.5, 1, 2, 4 Hz) and hemisphere (left, right) as factors. The above three ANOVAs were repeated considering the peak response as the dependent variable.
Group Analysis
In addition to individual subject analysis, a GLM for the entire group of subjects was calculated as well by means of a fixed‐effect group analysis. In this analysis the time series from each run and subject were z‐normalized and concatenated prior to the GLM computation. Group activation maps were thresholded at P < 0.05 (Bonferroni‐corrected) and were superimposed on the (Talairach‐transformed) structural scan of one of the subjects.
RESULTS
Group Analysis
A consistent activation across subjects was observed during electrical stimulation at the four frequencies (0.5, 1, 2, 4 Hz) in contralateral SI, bilateral SII, and bilateral insular cortex. The location of SI, ipsilateral SII (iSII), contralateral SII (cSII), ipsilateral Insula (i Insula), and contralateral Insula (c Insula) activation did not change when considering different stimulation frequencies. Talairach coordinates of activated areas in SI, SII, and insula were derived from the centroids of clusters of activation and are listed in Table I. The group statistical map for the 4‐Hz stimulation is shown in Figure 1 superimposed on an individual (Talairach‐transformed) structural image.
Table I.
Group analysis of the BOLD cortical activation after the galvanic median nerve stimulations: Talairach coordinates (centroids of activated clusters) of the activated areas within SI, SII, and insula
| Area | x | y | z |
|---|---|---|---|
| Contralateral SI | −36 | −31 | 50 |
| Contralateral SII | −48 | −22 | 18 |
| Ipsilateral SII | 50 | −24 | 20 |
| Contralateral insula | −34 | −4 | 16 |
| Ipsilateral insula | 33 | 0 | 15 |
Figure 1.
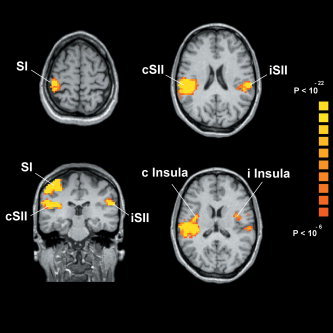
Results of the group analysis showing the activated areas in the somatosensory cortex at 4 Hz electrical stimulation superimposed onto axial and coronal sections of an individual brain. Top, axial view: activation occurring in contralateral SI (left) and bilateral SII (right). Bottom, coronal view (left): activation occurring in contralateral SI and bilateral SII; axial view (right): activation occurring in bilateral insula.
Single‐Subject Analysis
The aforementioned BOLD activation in SI, cSII, and iSII was observed in all individual subjects. Activation in bilateral insular cortex was observed in 15 out of 22 subjects. Activated areas in SI, SII, and insula for two subjects are shown in Figure 2, superimposed onto the inflated cortex obtained from the corresponding structural images. The fMRI signal time course in these areas during the rest and task conditions, averaged across epochs, is shown as well.
Figure 2.
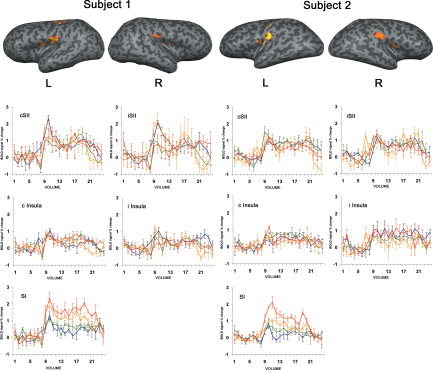
Activated areas in SI, SII, and insula in two subjects. BOLD signal time courses (averaged across epochs) at the different stimulation frequencies are shown (0.5 Hz: blue; 1 Hz: green; 2 Hz: orange; 4 Hz: red) below the corresponding hemisphere. Note the increasing BOLD response in SI as a function of the stimulus rate and the constant response in the other areas. Values on the vertical axis are signal percent change with respect to the rest period, whereas values on the horizontal axis represent the fMRI volumes. Error bars are standard errors.
For each ROI the BOLD response at the different frequencies of stimulation was compared by means of ANOVA. For the first ANOVA design, focused on SI, a significant main effect for the factor stimulus frequency was observed (F(3,63) = 12.67; P < 0.000001), indicating that for this area the mean BOLD activation increased with the increment of the stimulus frequency. For the second ANOVA design, focused on bilateral SII, a significant main effect for the factor hemisphere was observed (F(1,21) = 22.79; P < 0.0001), indicating a larger mean BOLD response for cSII with respect to iSII. For the third ANOVA design, focused on bilateral insula, no significant main effects were observed.
The second set of ANOVAs, considering the peak response, revealed: 1) a significant main effect in SI for the factor frequency (F(3,63) = 15.22; P < 0.0000001), indicating that for this area the peak BOLD activation increased with the increment of the stimulus frequency; 2) a significant main effect for the factor hemisphere in SII (F(1,21) = 10.19; P < 0.004), indicating a larger BOLD response for cSII with respect to iSII; and 3) no significant main effects in the insula. Note that the peak response showed the same dependence on the stimulus frequency as that observed for the mean response.
The mean and peak BOLD responses as a function of the stimulus frequency are reported in Figure 3 for each ROI (mean values across subjects). The Pearson correlation between the stimulus frequency and the BOLD response was calculated for each area. The correlation coefficients and the related significance values are reported in Table II. Note that only for SI did the BOLD response show a significant correlation with stimulation frequency.
Figure 3.
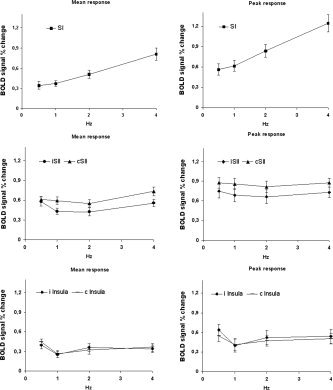
Mean and peak BOLD responses (averaged across subjects) as a function of the stimulus frequency in activated areas in SI, SII, and insula. Error bars are standard errors.
Table II.
Pearson correlation factors (r) and significance of correlation (P) between the stimulus frequency and the BOLD response in SI, iSII, cSII, ipsilateral insula, and contralateral insula
| Area | Mean response | Peak response | ||
|---|---|---|---|---|
| r | P | r | P | |
| SI | 0.998 | <0.004 | 0.995 | <0.002 |
| ISII | 0.152 | <0.85 | 0.044 | <0.955 |
| CSII | 0.739 | <0.262 | 0.032 | <0.968 |
| i Insula | −0.002 | <0.998 | −0.026 | <0.975 |
| c Insula | −0.103 | <0.897 | 0.072 | <0.928 |
DISCUSSION
In the present study, contralateral SI, bilateral SII, and bilateral insula were active during nonpainful electrical median nerve stimulation at 0.5, 1, 2, 4 Hz, in line with the existing evidence of the neural network active during somatosensory stimulus processing [Del Gratta et al.,2000; Ferretti et al.,2003,2004; Frot and Mauguiere,2003; Lin and Forss,2002; Mauguiere et al.,1997a,b; Schnitzler and Ploner,2000; Timmermann et al.,2001; Torquati et al.,2002]. Indeed, the tactile system is characterized by a hierarchical organization in which sensory information is sequentially processed by contralateral SI, contralateral SII, and ipsilateral SII. This is in contrast with the organization of the nociceptive system, which is characterized by a parallel flow of sensory information bilaterally processed by the two SII areas [Ploner et al.,1999; Treede et al.,2000]. Furthermore, the insula comprises a posterior sector (posterior dysgranular and granular insula) in which multimodal sensory inputs including somatosensory information converge to form a somatosensory mapping of the body [Mesulam et al.,1982a,b].
The responses of SII and the insula to the present nonpainful stimuli are in agreement with experimental findings obtained with microelectrode recordings, showing that several areas located in the parietal operculum and functionally connected to SII take part in the processing of painful and nonpainful inputs, namely, retroinsular, granular insula [Burton and Fabri,1995], and associative parietal [namely, 7b; Robinson and Burton,1980a,b] areas. In humans, studies measuring intracranial evoked potentials and fMRI responses have demonstrated that the parasylvian cortex (particularly SII) include separate cortical relays for processing of nonpainful and painful stimuli [Ferretti et al.,2003,2004; Frot et al.,2001,2003; Ploner et al.,1999; Treede et al.,2000]. The present study found higher activation in SII than in insula. A reasonable explanation is that SII activation might reflect mainly sensory stimulus recognition, whereas insular activation would mainly relay a further processing stage related to the emotional and attentional coloring of the stimulus [Brooks et al.,2002; Frot and Mauguiere,2003; Treede et al.,2000], the latter stimulus features being modest in the present type of stimulation.
In the present study, the analysis of the BOLD effect showed that the SII activation in response to different frequencies was characterized by a stronger activation in the contralateral compared to the ipsilateral hemisphere. This result could reasonably be explained by considering the organization of the tactile system as differently converging somatosensory inputs to the contralateral and ipsilateral SII areas. It is well known from previous MEG [Forss et al.,1999; Hari et al.,1993; Mauguiere et al.,1997a,b] and intracranial EEG studies [Frot and Mauguiere,1999] that the tactile somatosensory system has a serial structure. SI is presumed to receive the peripheral afferents, to be involved in the encoding of spatial and sensory‐discriminative aspects, and to dispatch the received input to higher‐order cortical areas, such as SII. This circuit follows a strictly hierarchical processing scheme, in which contralateral SII receives inputs from contralateral SI [Barba et al.,2001; Barbaresi et al.,1994; Forss et al.,2001], while ipsilateral SII mainly receives inputs via transcallosal fibers from the contralateral SII [Frot and Mauguiere,2003; Schnitzler and Ploner,2000]. The stronger activation of the contralateral compared to the ipsilateral SII might be due to the compression of the information through the callosal pathway. An alternative explanation is that the callosal pathway introduces some slight delay in the signal transfer, reducing the synchronization of the target neural population and then the evoked cortical responses.
As a major result of the analysis of the BOLD effect, the contralateral SI was characterized by an activation that progressively increased with the increase of the stimulus frequency. In contrast, both the activation of bilateral SII and of the bilateral insula were not modified significantly by changes of the stimulus frequency in the explored frequency range. This behavior was observed for both the mean and the peak BOLD response. The increasing responses of the contralateral SI as a function of the stimulus frequency (0.5–4 Hz) would depend on the total energy carried by the stimulus trains and on the ability of the SI neurons to account for it at the frequency range in question. This explanation is in line with some previous evidence in animals and humans [Gyngell et al.,1996; Keilholz et al.,2004]. They also complete previous fMRI results in humans obtained with electrical stimulation of the median nerve at higher frequencies (5–100 Hz) and limited to SI [Kampe et al.,2000]. Furthermore, the explanation agrees with previous evidence showing that the magnitude of evoked magnetic fields increased in the contralateral SI in line with the stimulus energy [Jousmaki and Forss,1998; Peresson et al.,1992; Torquati et al.,2002; Tsutada et al.,1999]. Our results on SI are different but not in contrast with the work of Tuunanen et al. [2003], in which no variation of the BOLD response in SI to tactile stimulation at 200 Hz was observed when the interstimulus interval was increased from 1 s to 5 s. Indeed, it should be noted that those authors used a different type of stimulus (tactile rather than electric), and a different experimental paradigm (event‐related rather than block design). In addition, they used much longer stimulus durations (0.1–2 s) and considered a different range of repetition rates.
The present results are apparently in contrast with some EEG and MEG studies on the somatosensory system. In the same work of Tuunanen et al. [2003] cited above, the intensity of the current dipoles in SI, used to model the MEG responses, was seen to increase with increasing interstimulus interval. Again, it should be noted that that study was different from the present one in many respects. In SI, previous findings showed no amplitude change of the early evoked magnetic or electric fields (+20 ms poststimulus peak) to median nerve stimulation from 0.25 to 2 Hz [Schnitzler et al.,1999] or from 1.6 to 5.7 Hz [Delberghe et al.,1990]. In SII, an amplitude decrease of the later electric fields to median nerve stimulation from 0.6 to 5.7 Hz was observed [Delberghe et al.,1990]. These contrasting results can be reconciled taking into account the different temporal resolutions of the EEG‐MEG and fMRI techniques. The EEG‐MEG techniques have a high temporal resolution (milliseconds) able to probe synchronous excitatory and inhibitory (gating, refractoriness, etc.) processes sequentially induced in pyramidal SI neurons by the stimulation train [Hoshiyama and Kakigi,2001,2002,2003]. Instead, the fMRI technique is characterized by a low temporal resolution (seconds) just capturing the slow hemodynamic response (BOLD) related to the cortical activation or deactivation. Indeed, (active) inhibitory processes reducing the temporal synchronization of the cortical neurons (i.e., and the amplitude of the evoked electromagnetic fields) are still related to an increase of the BOLD effect. This explanation deserves to be tested in the present experimental condition with the combined use (separate sessions) of EEG‐MEG, fMRI, and optical imaging, a technique capable of investigating cortical regional blood flow with high temporal resolution (tens of milliseconds; Maclin et al. [2004]).
CONCLUSIONS
The present fMRI study showed that the contralateral SI, bilateral SII, and bilateral insular cortices were active during nonpainful electrical median nerve stimulation at 0.5, 1, 2, and 4 Hz. As a major result, only the contralateral SI increased its level of activation with the increasing rate of the stimulus within the explored range. The present results are of interest for the physiology of somatosensory systems. Furthermore, the indication of a larger somatosensory cortical response for nonpainful stimuli at 4 Hz is of interest for both basic research and clinical applications. In particular, the present methodological approach may be useful for presurgical functional mapping of primary somatosensory cortex and for the study of its plastic reorganization following tumor resection or during dementia processes. In these cases, passive stimulations overcome the difficulties in the standardization of task performance in patients unable to perform sensorimotor and cognitive tasks in the fMRI scanner.
Acknowledgements
The authors thank Massimo Caulo for helpful discussion and suggestions.
REFERENCES
- Apkarian AV, Shi T 1994: Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 14: 6779–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi P, Porro CA, Serafini M, Pagnoni G, Murari C, Corazza R, Nichelli P 1999: Bilateral representation of sequential finger movements in human cortical areas. Neurosci Lett 269: 95–98. [DOI] [PubMed] [Google Scholar]
- Barba C, Frot M, Guenot M, Mauguiere F 2001: Stereotactic recordings of median nerve somatosensory‐evoked potentials in the human pre‐supplementary motor area. Eur J Neurosci 13: 347–356. [DOI] [PubMed] [Google Scholar]
- Barbaresi P, Minelli A, Manzoni T 1994: Topographical relations between ipsilateral cortical afferents and callosal neurons in the second somatic sensory area of cats. J Comp Neurol 343: 582–596. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ 1996: Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N 2002: fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage 15: 293–301. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Williams S, Rabe‐Hesketh S, Janot N, David A, Mellers J, Howard R, Sham P 1996: Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M 1995: Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol 355: 508–538. [DOI] [PubMed] [Google Scholar]
- Burton H, Videen TO, Raichle ME 1993: Tactile‐vibration‐activated foci in insular and parietal‐opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans. Somatosens Mot Res 10: 297–308. [DOI] [PubMed] [Google Scholar]
- Burton H, Abend NS, MacLeod AM, Sinclair RJ, Snyder AZ, Raichle ME 1999: Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cereb Cortex 9: 662–674. [DOI] [PubMed] [Google Scholar]
- Cox RW 1996: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Delberghe X, Mavroudakis N, Zegers de Beyl D, Brunko E 1990: The effect of stimulus frequency on post‐ and pre‐central short‐latency somatosensory evoked potentials (SEPs). Electroencephalogr Clin Neurophysiol 77: 86–92. [DOI] [PubMed] [Google Scholar]
- Del Gratta C, Della Penna S, Tartaro A, Ferretti A, Torquati K, Bonomo L, Romani GL, Rossini PM 2000: Topographic organization of the human primary and secondary somatosensory areas: an fMRI study. Neuroreport 11: 2035–2043. [DOI] [PubMed] [Google Scholar]
- Del Gratta C, Della Penna S, Ferretti A, Franciotti R, Pizzella V, Tartaro A, Torquati K, Bonomo L, Romani GL, Rossini PM 2002: Topographic organization of the human primary and secondary somatosensory cortices: comparison of fMRI and MEG findings. Neuroimage 17: 1373–1383. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Harris JA, Petersen RS 2002: Sensory learning and the brain's body map In: Nelson RJ, editor. The somatosensory system. Boca Raton, FL: CRC Press; p 183–195. [Google Scholar]
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T 2001: Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci 13: 1071–1079. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Quattrini A, Salvolini U 2002: Mechanical noxious stimuli cause bilateral activation of parietal operculum in callosotomized subjects. Cereb Cortex 12: 446–451. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Babiloni C, Del Gratta C, Caulo M, Tartaro A, Bonomo L, Rossini PM, Romani GL 2003: Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: an fMRI study. Neuroimage 20: 1625–1638. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Del Gratta C, Babiloni C, Caulo M, Arienzo D, Tartaro A, Rossini PM, Romani GL 2004: Functional topography of the secondary somatosensory cortex for nonpainful and painful stimulation of median and tibial nerve: an fMRI study. Neuroimage 23: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC 1995: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Forss N, Hietanen M, Salonen O, Hari R 1999: Modified activation of somatosensory cortical network in patients with right‐hemisphere stroke. Brain 122: 1889–1899. [DOI] [PubMed] [Google Scholar]
- Forss N, Narici L, Hari R 2001: Sustained activation of the human SII cortices by stimulus trains. Neuroimage 13: 497–501. [DOI] [PubMed] [Google Scholar]
- Francis ST, Kelly EF, Bowtell R, Dunseath WJ, Folger SE, McGlone F 2000: fMRI of the responses to vibratory stimulation of digit tips. Neuroimage 11: 188–202. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ 1995: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 173–181. [Google Scholar]
- Frot M, Mauguiere F 1999: Timing and spatial distribution of somatosensory responses recorded in the upper bank of the sylvian fissure (SII area) in humans. Cereb Cortex 9: 854–863. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguiere F 2003: Dual representation of pain in the operculo‐insular cortex in humans. Brain 126: 438–450. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia‐Larrea L, Guenot M, Mauguiere F 2001: Responses of the supra‐sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra‐cerebral recordings. Pain 94: 65–73. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Koeze O, Uffmann K, de Greiff A, Ladd ME, Forsting M 2005: Cerebral activation using a MR‐compatible piezoelectric actuator with adjustable vibration frequencies and in vivo wave propagation control. Neuroimage 24: 723–730. [DOI] [PubMed] [Google Scholar]
- Gyngell ML, Bock C, Schmitz B, Hoehn‐Berlage M, Hossmann KA 1996: Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha‐chloralose anesthetized rats. Magn Reson Med 36: 13–15. [DOI] [PubMed] [Google Scholar]
- Hari R, Karhu J, Hamalainen M, Knuutila J, Salonen O, Sams M, Vilkman V 1993: Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur J Neurosci 5: 724–734. [DOI] [PubMed] [Google Scholar]
- Hari R, Hanninen R, Makinen T, Jousmaki V, Forss N, Seppa M, Salonen O 1998: Three hands: fragmentation of human bodily awareness. Neurosci Lett 240: 131–134. [DOI] [PubMed] [Google Scholar]
- Harrington GS, Hunter Downs J 3rd 2001: fMRI mapping of the somatosensory cortex with vibratory stimuli. Is there a dependency on stimulus frequency? Brain Res 897: 188–192. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Mashiko T, Kimura T, Imada T 1998: Human somatosensory evoked magnetic fields to vibratory stimulation of the index finger: is there frequency organization in SI? Electroencephalogr Clin Neurophysiol 109: 454–461. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R 2002: New concept for the recovery function of short‐latency somatosensory evoked cortical potentials following median nerve stimulation. Clin Neurophysiol 113: 535–541. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R 2001: Two evoked responses with different recovery functions in the primary somatosensory cortex in humans. Clin Neurophysiol 112: 1334–1342. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R 2003: Changes in somatosensory evoked responses by repetition of the median nerve stimulation. Clin Neurophysiol 114: 2251–2257. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Wikstrom H, Korvenoja A, Seppalainen AM, Aronen H, Ilmoniemi RJ 1996: Significance of the second somatosensory cortex in sensorimotor integration: enhancement of sensory responses during finger movements. Neuroreport 7: 1009–1012. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Deiber MP, Sadato N, Toro C, Grissom J, Woods RP, Mazziotta JC, Hallett M 1995: Effects of stimulus rate on regional cerebral blood flow after median nerve stimulation. Brain 118: 1339–1351. [DOI] [PubMed] [Google Scholar]
- Jousmaki V, Forss N 1998: Effects of stimulus intensity on signals from human somatosensory cortices. Neuroreport 9: 3427–3431. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Hoshiyama M, Shimojo M, Naka D, Yamasaki H, Watanabe S, Xiang J, Maeda K, Lam K, Itomi K, Nakamura A 2000: The somatosensory evoked magnetic fields. Prog Neurobiol 61: 495–523. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Tran TD, Qiu Y, Wang X, Nguyen TB, Inui K, Watanabe S, Hoshiyama M 2003: Cerebral responses following stimulation of unmyelinated C‐fibers in humans: electro‐ and magneto‐encephalographic study [Review]. Neurosci Res 45: 255–275. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Jones RA, Auer DP 2000: Frequency dependence of the functional MRI response after electrical median nerve stimulation. Hum Brain Mapp 9: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP 2004: Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med 52: 89–99. [DOI] [PubMed] [Google Scholar]
- Lin YY, Forss N 2002: Functional characterization of human second somatosensory cortex by magnetoencephalography [Review]. Behav Brain Res 135: 141–145. [DOI] [PubMed] [Google Scholar]
- Maclin EL, Low KA, Sable JJ, Fabiani M, Gratton G 2004: The event‐related optical signal to electrical stimulation of the median nerve. Neuroimage 21: 1798–1804. [DOI] [PubMed] [Google Scholar]
- Magnus O, Penfield W, Jasper H 1952: Mastication and consciousness in epileptic seizures. Acta Psychiatr Neurol Scand 27: 91–115. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R 1997a: Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. I. Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 104: 281–289. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R 1997b: Activation of a distributed somatosensory cortical network in the human brain: a dipole modelling study of magnetic fields evoked by median nerve stimulation. II. Effects of stimulus rate, attention and stimulus detection. Electroencephalogr Clin Neurophysiol 104: 290–295. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ 1982a: Insula of the old world monkey. I. Architectonics in the insulo‐orbito‐temporal component of the paralimbic brain. J Comp Neurol 212: 1–22. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ 1982b: Insula of the old world monkey. III. Efferent cortical output and comments on function. J Comp Neurol 212: 38–52. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Campbell JN, Raja SN 1994: Peripheral neural mechanisms of nociception In: Wall PD, Metzack R, editors. Textbook of pain. London: Churchill Livingstone; p 13–44. [Google Scholar]
- Mima T, Nagamine T, Nakamura K, Shibasaki H 1998: Attention modulates both primary and second somatosensory cortical activities in humans: a magnetoencephalographic study. J Neurophysiol 80: 2215–2221. [DOI] [PubMed] [Google Scholar]
- Narici L, Modena I, Opsomer RJ, Pizzella V, Romani GL, Torrioli G, Traversa R, Rossini PM 1991a: Neuromagnetic somatosensory homunculus: a non‐invasive approach in humans. Neurosci Lett 121: 51–54. [DOI] [PubMed] [Google Scholar]
- Narici L, Liberati D, Cerutti S, Santoni A 1991b: Analysis of the neuromagnetic data on the frequency responsivity of the human brain: a progress report. Clin Phys Physiol Meas 12( Suppl A): 43–47. [DOI] [PubMed] [Google Scholar]
- Oldfield RC 1971: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Peresson M, Casciardi S, Del Gratta C, Di Luzio S, Macri MA, Pizzella V, Romani GL, Rossini PM 1992: Neuromagnetic mapping under mixed median nerve stimulation: infIuence of stimulus intensity on source parameters In: Hoke M, Ernd SN, Okada YC, Romani GL, editors. Biomagnetism, clinical aspects. Amsterdam: Excerpta Medica; p 241–245. [Google Scholar]
- Picard N, Strick PL 1996: Motor areas of the medial wall: a review of their location and functional activation [Review]. Cereb Cortex 6: 342–353. [DOI] [PubMed] [Google Scholar]
- Pizzella V, Tecchio F, Romani GL, Rossini PM 1999: Functional localization of the sensory hand area with respect to the motor central gyrus knob. Neuroreport 10: 3809–3814. [DOI] [PubMed] [Google Scholar]
- Ploner M, Schmitz F, Freund HJ, Schnitzler A 1999: Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol 81: 3100–3104. [DOI] [PubMed] [Google Scholar]
- Ploner M, Schmitz F, Freund HJ, Schnitzler A 2000: Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol 83: 1770–1776. [DOI] [PubMed] [Google Scholar]
- Puce A 1995: Comparative assessment of sensorimotor function using functional magnetic resonance imaging and electrophysiological methods [Review]. J Clin Neurophysiol 12: 450–459. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Ettlinger G 1976: Impaired tactile learning and retention after removals of the second somatic sensory projection cortex (SII) in the monkey. Brain Res 109: 656–660. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H 1980a: Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol 192: 69–92. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H 1980b: Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol 192: 93–108. [DOI] [PubMed] [Google Scholar]
- Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, Villringer K, Kurth R, Villringer A 2001: Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex 11: 463–473. [DOI] [PubMed] [Google Scholar]
- Rumeau C, Tzourio N, Murayama N, Peretti‐Viton P, Levrier O, Joliot M, Mazoyer B, Salamon G 1994: Location of hand function in the sensorimotor cortex: MR and functional correlation. AJNR Am J Neuroradiol 15: 567–572. [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Ploner M 2000: Neurophysiology and functional neuroanatomy of pain perception [Review]. J Clin Neurophysiol 17: 592–603. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Volkmann J, Enck P, Frieling T, Witte OW, Freund HJ 1999: Different cortical organization of visceral and somatic sensation in humans. Eur J Neurosci 11: 305–315. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P 1998: Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A 2001: Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol 86: 1499–1503. [DOI] [PubMed] [Google Scholar]
- Torquati K, Pizzella V, Della Penna S, Franciotti R, Babiloni C, Rossini PM, Romani GL 2002: Comparison between SI and SII responses as a function of stimulus intensity. Neuroreport 13: 813–819. [DOI] [PubMed] [Google Scholar]
- Torquati K, Pizzella V, Della Penna S, Franciotti R, Babiloni C, Romani GL, Rossini PM 2003: “Gating” effects of simultaneous peripheral electrical stimulations on human secondary somatosensory cortex: a whole‐head MEG study. Neuroimage 20: 1704–1713. [DOI] [PubMed] [Google Scholar]
- Toyokura M, Muro I, Komiya T, Obara M 1999: Relation of bimanual coordination to activation in the sensorimotor cortex and supplementary motor area: analysis using functional magnetic resonance imaging. Brain Res Bull 48: 211–217. [DOI] [PubMed] [Google Scholar]
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA 2000: Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus [Review]. Pain 87: 113–119. [DOI] [PubMed] [Google Scholar]
- Tsutada T, Tsuyuguchi N, Hattori H, Shimada H, Shimogawara M, Kuramoto T, Haruta Y, Matsuoka Y, Hakuba A 1999: Determining the appropriate stimulus intensity for studying the dipole moment in somatosensory evoked fields: a preliminary study. Clin Neurophysiol 110: 2127–2130. [DOI] [PubMed] [Google Scholar]
- Tuunanen PI, Kavec M, Jousmaki V, Usenius JP, Hari R, Salmelin R, Kauppinen RA 2003: Comparison of BOLD fMRI and MEG characteristics to vibrotactile stimulation. Neuroimage 19: 1778–1786. [DOI] [PubMed] [Google Scholar]
- Wood CC, Spencer DD, Allison T, McCarthy G, Williamson PD, Goff WR 1988: Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg 68: 99–111. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM 2001: Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Erickson TC, Gilson WE 1979: Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg 51: 476–506. [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki 1997: Recommendations guiding physicians in biomedical research involving human subjects. JAMA 277: 925–926. [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P 1997: Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157. [DOI] [PubMed] [Google Scholar]


