Abstract
Relative to individuals who do not have addictive disorders, drug abusers exhibit greater devaluation of rewards as a function of their delay (“delay discounting”). The present study sought to extend this finding to methamphetamine (MA) abusers and to help understand its neural basis. MA abusers (n = 12) and control subjects who did not use illicit drugs (n = 17) participated in tests of delay discounting with hypothetical money rewards. We then used a derived estimate of each individual's delay discounting to generate a functional magnetic resonance imaging probe task consisting of three conditions: “hard choices,” requiring selections between “smaller, sooner” and “larger, later” alternatives that were similarly valued given the individual's delay discounting; “easy choices,” in which alternatives differed dramatically in value; and a “no choice” control condition. MA abusers exhibited more delay discounting than control subjects (P < 0.05). Across groups, the “hard choice > no choice” contrast revealed significant effects in the ventrolateral prefrontal cortex, dorsolateral prefrontal cortex (DLPFC), dorsal anterior cingulate cortex, and areas surrounding the intraparietal sulcus (IPS). With group comparisons limited to these clusters, the “hard choice > easy choice” contrast indicated significant group differences in task‐related activity within the left DLPFC and right IPS; qualitatively similar nonsignificant effects were present in the other clusters tested. Whereas control subjects showed less recruitment associated with easy than with hard choices, MA abusers generally did not. Correlational analysis did not indicate a relationship between this anomaly in frontoparietal recruitment and greater degree of delay discounting exhibited by MA abusers. Therefore, while apparent inefficiency of cortical processing related to decision‐making in MA abusers may contribute to the neural basis of enhanced delay discounting by this population, other factors remain to be identified. Hum. Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: decision‐making, delay discounting, functional magnetic resonance imaging, methamphetamine
INTRODUCTION
Rewards and punishments are more potent reinforcers when they are immediate than when delayed. The relationship between the delay and the value of reinforcers is referred to as “delay discounting” and has been hypothesized to be the primary basis of impulsivity [Ainslie, 1975]. Delay discounting may be especially relevant to the phenomenon of addiction. The rewards from drug use are relatively immediate and the adverse consequences tend to be delayed; were the reverse true, it is unlikely there would be problem drug use. Given the nature of the contingencies involved in drug use behavior, it has been hypothesized that individuals who develop drug abuse problems may, on average, exhibit more delay discounting than those who do not have drug problems [Bickel et al., 1998].
While there are important caveats to this reasoning, especially with respect to the inconsistency of delay discounting across different domains of reward [Chapman, 1996; Petry, 2003], the predicted association between drug abuse and delay discounting has been repeatedly observed. In the typical task employed, participants must make choices between pairs of rewards (usually monetary) that differ in amount and immediacy (e.g., Would you prefer $10 today or $15 in 1 month?). Compared to research subjects without drug abuse disorders, greater delay discounting was observed among individuals who chronically abuse cocaine [Coffey et al., 2003; Kirby and Petry, 2004], opioids [Wallace, 1979; Kirby et al., 1999; Kirby and Petry, 2004], alcohol [Vuchinich and Simpson, 1998], tobacco [Mitchell, 1999; Cairns and van der Pol, 2000; Reynolds et al., 2004], and a variety of substances [Petry, 2003]. Some of these studies used actual rewards (typically one trial randomly chosen for payment) while others used hypothetical rewards.
Neural Substrates of Performance on Delay Discounting Task
Perhaps because delay discounting assessment has grown out of economic and behaviorist traditions, little work has explored the neuropsychological substrates that underlie performance on the task. The clinical literature indirectly suggests the possibility that the ventromedial prefrontal cortex (VMPFC), including the orbitofrontal cortex (OFC), plays a role in the valuation of delayed rewards. Patients with lesions in the VMPFC exhibit impairments in decision‐making, having been described as suffering from “a profound exaggeration of what may be a normal basic tendency, to go for the now rather than bank on the future” [Bechara et al., 1996, 1999; Rogers et al., 1999a]. Consistent with this idea, lesions of the OFC in rats produce increased preference for smaller, more immediate rewards over larger later rewards [Mobini et al., 2002]. However, delay discounting tasks typically employed in human studies differ substantially from those used in studies of rats. Perhaps most critically, in human discounting experiments, alternatives are presented as verbal/numeric information and outcomes are not experienced during the completion of the procedure. In animal studies, by contrast, contingencies are presented to the organism solely as the accumulation of experiences during the training and testing procedure, thus requiring the organism to learn reward contingencies. Given extensive literature implicating the OFC in tracking and updating reward value [Rolls et al., 1994; Rolls, 2000; Kringelbach et al., 2003; Wallis and Miller, 2003], it is plausible that the effects observed in rat delay discounting experiments do not generalize to human delay discounting tasks, which do not require reward tracking. Indeed, a recent human study revealed no significant difference in delay discounting among VMPFC (inclusive of OFC) lesion patients, though VMPFC lesion patients did demonstrate shorter time horizons, as measured in a task requiring them to generate lists of events that would occur in their own futures [Fellows and Farah, 2005].
Recently, McClure et al. [2004] conducted the first study to pair functional magnetic resonance imaging (fMRI) with a delay discounting task similar to those described above. Across trials, activation during decision‐making occurred in the lateral prefrontal and posterior parietal cortices and secondary motor areas (especially during selection of choices in which the delay discounted values of the alternatives were similar, thus making the choices more difficult). In contrast, limbic and paralimbic areas were active selectively during trials in which receipt of an immediate reward was possible. Furthermore, on trials with an immediate alternative, when participants chose the later alternative, signal change was significantly greater in those areas associated with decision‐making in general than in limbic and paralimbic regions, while conversely, when participants chose the immediate alternative, signal change was (nonsignificantly) greater in the limbic and paralimbic regions than in areas associated with decision‐making in general. These data were interpreted as demonstrating dissociation between two decision‐making systems: a corticolimbic system that is activated by immediate opportunities for reward and a second system that includes lateral prefrontal and parietal areas associated with more abstract decision‐making [for discussion of these results, see Ainslie and Monterosso, 2004].
Neural Activity of Substance Abusers Performing Decision‐Making Tasks
Although fMRI studies have not previously compared brain activity among drug abusers and nondrug abusers performing a delay discounting task, drug abuse vs. control group comparisons have been conducted using other decision‐making tasks. These studies have suggested anomalies among drug abusers in frontoparietal circuitry. In a study that used a task that evaluated, among other things, preference for risk [Rogers et al., 1999b], a heterogeneous group of drug abusers exhibited both greater risk‐taking than controls and less task‐related activation (indexed by regional increases in relative perfusion, measured by positron emission tomography, PET) in the left pregenual anterior cingulate cortex (ACC) [Fishbein et al., 2005]. Using the same task, Ersche et al. [2005] compared chronic amphetamine users, chronic opiate users, former drug users, and matched control subjects. Relative to the group with no history of drug abuse, the other three groups (combined) exhibited less activation of the right dorsolateral prefrontal cortex (DLPFC) and significantly greater activation in the left (OFC [Ersche et al., 2005]).
In PET studies that paired measurements of relative cerebral blood flow during performance of the Iowa Gambling Task, which measures learned avoidance of uncertain punishments [Bechara et al., 1994, 1997], participants who abused marijuana [Bolla et al., 2005] or cocaine [Bolla et al., 2003] exhibited less activation in the right DLPFC relative to controls. Cocaine abusers also showed less recruitment (relative to control subjects) in the right superior parietal lobule, the left medial frontal gyrus, left middle temporal gyrus, and the right cerebellum, but greater activation in the right OFC, the putamen, and the left postcentral gyrus. Marijuana users exhibited less activation than control subjects in the right lateral OFC and left parietal lobe [Bolla et al., 2003, 2005]. Although these data suggest possible substrates of deficits in decision‐making, their interpretation is complicated by performance differences on the tasks; participants who made more bad (i.e., high‐risk) decisions also encountered, among other things, more high penalties. As such, differences in task‐related activity may reflect responses to different stimuli rather than substrates of behavioral differences.
Paulus et al. [2002, 2003] compared behavior and brain activity of methamphetamine (MA)‐dependent individuals and control subjects performing a simple two‐choice guessing task in which random contingencies assured equality of outcomes irrespective of strategy. Relative to control subjects, MA‐dependent participants made selections that were more frequently consistent with a win‐stay/lose‐switch strategy based entirely on the outcome of the trial immediately preceding the choice [Paulus et al., 2002, 2003]. Both groups showed task‐related activity bilaterally in the prefrontal, parietal, and insular cortices, while the MA abusers exhibited significantly less activation in the inferior prefrontal cortex and DLPFC [Paulus et al., 2002, 2003]. Taken as a whole, the literature comparing neural recruitment during decision‐making in subjects who have drug abuse disorders and control subjects suggests that there are differences within the prefrontal cortex, most consistently in the DLPFC, with less decision‐related recruitment in drug abusers [Paulus et al., 2002, 2003; Bolla et al., 2003, 2005; Ersche et al., 2005].
In the first part of the present study, we assessed delay discounting in a group of MA‐dependent participants and a group of comparison subjects who did not use illicit drug of abuse (except for light marijuana abuse). In the second part of the study, we paired a variant of the task used in the first part of the study with fMRI. Based on previous comparisons of drug‐abusing populations, we anticipated greater delay discounting in MA abusers than in comparison participants. Given the significant association between greater activity in the lateral prefrontal and posterior parietal areas and preference for larger but more delayed alternatives [McClure et al., 2004], taken with several reports of abnormally low activation in the prefrontal cortex during decision‐making in samples of drug abusers (especially in the DLPFC [Paulus et al., 2002, 2003; Bolla et al., 2003, 2005; Ersche et al., 2005]), we also hypothesized that MA abusers would demonstrate less task‐related signal change in the prefrontal cortex paired with anomalously high willingness to trade reward value for reward immediacy.
MATERIALS AND METHODS
Participants
We recruited a group of control subjects (n = 17) and a group of MA‐dependent participants who were not seeking treatment for their dependence (n = 12). Because of the very high rate of cigarette smoking typically observed in MA abusers [London et al., 2004], we predominantly recruited smokers in our control group. All participants gave written informed consent after receiving a detailed explanation of the study and its procedures (approved by the University of California at Los Angeles Office for Protection of Research Subjects). Inclusion in the MA group required testing positive for recent use of MA (and not other illicit drugs), reporting 1 or more years of using ≥ 1 g of MA per week, and meeting DSM‐IV criteria for MA dependence (according to the Structured Clinical Interview for DSM‐IV [First et al., 1996]). Other current axis I diagnoses were exclusionary, except for nicotine dependence. Participants across groups were excluded if they reported current use of psychotropic medications, use of any other medications known to affect cognitive functioning (e.g., clonidine), were in counseling, were taking medication for psychological problems, or had a history of hospitalization for psychiatric illness. Participants across groups were also excluded if they reported a history of head trauma involving loss of consciousness and/or requiring hospitalization, or if they scored ≥ 46 on the Wender Utah Rating Scale [Ward et al., 1993], suggesting childhood attention deficit‐hyperactivity disorder. Urine samples were collected from all participants at enrollment and were tested using a five‐panel rapid test from Alfa Scientific (testing for cocaine, MA, opioids, tetrahydrocannabinol, and benzodiazepines). A positive drug test (other than for MA in the MA abuse group or marijuana for either group) resulted in exclusion from participation. A self‐report of light marijuana use (≤ 1 joint per week) was not exclusionary for either group.
All of the MA users had used other illicit drugs at least once in their lifetimes, but none met diagnostic criteria for current abuse or dependence (other than related to MA). The most frequent drugs other than MA ever used in the MA group were alcohol (11 subjects; 91.7%), marijuana (10 subjects; 83.3%), and cocaine (9 subjects; 75.0%). One of the MA abusers met criteria for past alcohol abuse, and another met criteria for past alcohol dependence.
The groups did not differ significantly in gender (MA, 33.3% female; controls, 29.4% female), age (MA, 33.8 ± 8.1 years; controls, 29.7 ± 7.2 years), race (MA, 66.67% white non‐Hispanic; controls, 50.0% white non‐Hispanic), the proportion who were cigarette smokers (MA, 75% smokers; controls, 94.1% smokers), or IQ as estimated by the vocabulary subscale of the Shipley Institute of Living Scale (MA, 104.7 ± 9.1; controls, 109.7 ± 8.1). MA abusers reported recent use (during the month before testing) averaging 4.8 ± 7.9 g of MA per week and had used the drug for an average of 7.7 ± 8.9 years. Six of the 12 MA participants (50.0%) reported smoking as their primary route of MA administration (3 injecting, 2 snorting, 1 oral).
Procedure
MA participants resided at the University of California at Los Angeles General Clinical Research Center for 5–7 days of abstinence (verified by urine test) prior to participation, and control subjects participated on a nonresidential basis. Urine samples were collected every day from MA abusers and were tested randomly, at least twice a week, to ensure abstinence during participation. One participant (not included in the sample described) was excluded after remaining positive for MA after 1 week of inpatient stay, suggesting unusually slow elimination or continued use of MA.
Behavioral Assessment of Delay Discounting
The delay discounting task, which was administered by computer, was a version of the Monetary‐Choice Questionnaire developed by Kirby et al. [1999]. Participants were presented with a fixed set of 27 choices between smaller immediate rewards (ranging from $11 to $80) and larger delayed rewards (ranging in amount from $20 to $85 and in delay from 7 to 186 days). The method for computing individual discount functions from the delay discount procedure is described below. Participants were instructed to indicate their choice by clicking a mouse on the preferred option.
Intertemporal Choice Task Paired With fMRI
In the fMRI choice probe task, each test block was composed of three “hard choice” trials, three “easy choice” trials, or three “no choice” trials. Hard choices presented participants with an immediate alternative (between $5 and $50) and a larger alternative delayed by between 1 week and 3 months. The amount of the delayed alternative was computer‐generated on the basis of the participant's responses during the prescanning assessment of individual delay discounting, such that the discounted value of both alternatives (see equation below) was approximately equivalent. For easy choices, the procedure was identical, except that the amounts of the larger delayed alternative were generated on the basis of a k‐parameter that was one order of magnitude larger or smaller (50% of each) than the participant's best‐fit k‐value, resulting in very disparately valued alternatives. No choice trials were generated using the same procedure as hard‐choice trials, except that only one of the two alternatives (randomly selected) was presented.
Each trial lasted 7 s. During the hard‐choice and easy‐choice trials, the two reward alternatives were presented on either side of view separated by a line (e.g., “$10 today” on one side of the screen, and “$15 in 10 days” on the other side). The side of the immediate and delayed alternatives was randomized. On no‐choice trials, the single alternative was presented on one side of the display, with side randomized. After 5 s, if a response had not been made, the instruction “Please Respond” appeared at the bottom of the screen. The participant indicated his or her response by pressing the buttons on a two‐button pad that corresponded to the preferred option. An arrow appeared over the option selected, and the text changed from white to yellow. For the no‐choice trials, only one option was presented, and participants were instructed to select that option in the same manner as on other trials. After the 7‐s trial, the screen was cleared for 1 s, resulting in a fixed trial length of 8 s. Trials were distributed in blocks, each composed of three trials of the same type (hard choice, easy choice, or no choice). During each run of the task, each type of block was presented four times, resulting in a total run time of 4 min 48 s. One of four block orders was used for each participant, and each participant performed two consecutive task runs, separated by a 1‐min rest period.
Image Acquisition
Data were acquired on an Allegra 3T MRI device (Allegra, Siemens). Localizing scans were acquired first to verify head position and to identify the AC‐PC line for purposes of establishing the acquisition plane. Next, we acquired a high‐resolution T2‐weighted echo‐planar anatomical image covering the entire brain volume (26 slices, aligned to the AC‐PC line, 4 mm thick/1 mm skip, pixels 1.56 mm2, TR/TE 4,000/28, four averaged acquisitions), which was used for spatial alignment [Woods et al., 1999] and as a background image to display statistical results. Functional images were acquired using a gradient‐echo EPI sequence (45 slices, 2 mm thick/0.5 mm skip, pixels = 3.125 mm2, TR/TE = 2500/28 ms, flip angle = 90°).
Data Analysis
Analysis of delay discounting
Delay discounting during behavioral testing was estimated by fitting data to the discount function equation: V = A/(1 + kD), in which V is the value of the amount A at delay D (in days), and the best‐fit value for parameter k provides the index of delay discounting [Ainslie and Haendel, 1983; Mazur, 1987; Kirby, 1997]. Estimates of the best‐fit k‐parameters for individual subjects were computed on the basis of “indifference points,” implied by each choice presented to the subjects. The “indifference point” is the k‐value at which both options of the choice would be equally preferred. For example, the indifference point k‐value for the choice between an immediate $27 and $50 delayed by 21 days is 0.041, meaning that someone who discounted the future accordingly would be indifferent to the choice between these two options.
Because the task required subjects to express preferences, k‐values were estimated as the geometric mean between the lowest implied indifference k‐value in which subjects chose the delayed option, and the highest implied indifference k‐value in which subjects chose the immediate option. If subjects' choices were inconsistent, k‐values were estimated as the point that was consistent with the highest number of expressed preferences. In the case of ties, the geometric mean of the equally good k‐values was used. Group k‐values were compared by t‐test. For hard choices in the fMRI probe task, each participant's computed k‐values was used to generate maximally difficult choice pairs, while a k‐value one order of magnitude larger or smaller was used to generate easy choices. Thus, for a participant with a k‐value estimate of 0.041, easy choices were generated as pairs that would be equally valued given a k‐value of 0.41 or 0.0041. For such a participant, the choice between an immediate $27 and $50 delayed by 21 days would qualify as a hard choice, while the choice between an immediate $27 and $259 delayed by 21 days, and the choice between an immediate $27 and $29 delayed by 21 days, would qualify as easy choices.
Image processing and analysis
Brain activation was assessed using tools from the FMRIB Software Library, FSL 3.2 (FMRIB, Oxford, U.K.; http://www.fmrib.ox.ac.uk/fsl) [Smith et al., 2004]. All functional scans were motion‐corrected using MCFLIRT [Jenkinson and Smith, 2001]. Any scan that showed a maximum displacement of > 2 mm was removed from further analysis. The data sets were smoothed with a nonlinear algorithm designed to preserve image structure by only smoothing over voxels classified as the same tissue type (5 mm kernel) [Smith and Brady, 1997]. Data were subjected to a multiple‐regression analysis using a prewhitening technique [Woolrich et al., 2001] to account for the intrinsic temporal autocorrelation of BOLD imaging [Zarahn et al., 1997]. Regressors or explanatory variables (EVs) were binary except for the temporal discount parameter k. For this EV, data were normalized by log transformation and then demeaned. Registration of functional EPI images to high‐resolution images, and of high‐resolution images into standard MNI space, was carried out using FLIRT (FMRIB's Lineat Image Registration Tool [Jenkinson and Smith, 2001]).
Group analysis and group comparisons were performed using random‐effects analyses. Cluster detection was applied to the group Z (Gaussianized T) statistic images determined by Z > 2.3 and a corrected cluster extent significance threshold of P = 0.05 [Worsley et al., 1992; Forman et al., 1995]. All task runs were analyzed individually in the first level of analyses. For each participant, a second‐level analysis was then performed in order to combine the Z‐statistical maps from the two task run. The resulting statistical maps from these second‐level analyses (also Z‐statistic maps) were the inputs for all group analyses (third level).
Four analyses were conducted. First, we combined data from MA and control participants in a single group analysis to identify clusters in which MRI signal was significantly greater during hard‐choice relative to no‐choice blocks. We considered these to be inclusive of regions that we could identify as recruited by the task in general. A mask of all such regions was constructed and other analyses were conducted only within this mask. We next conducted between‐group comparisons to assess group differences in task‐related activity (including only the clusters identified in combined group analysis described above). Next, we assessed correlations (across groups) between individual indexes of temporal discounting and task‐related signal change (again including only the clusters identified in the initial contrast). Finally, we conducted an exploratory event‐related analysis in which hard‐choice trials were divided into those trials in which the more immediate alternative was selected and those in which the delayed alternative was selected. Signal during these two events (from the time of stimulus presentation until response selection) was contrasted for each task run. As with other analyses, contrast maps for each subject's two task runs were combines in second‐level analyses, and the resulting subject‐level contrast maps were combined to obtain a random‐effects group‐level contrast image. We considered this analysis exploratory since the study was not designed to optimize power for this comparison, either in terms of event timing or, more importantly, in terms of the number of relevant events; since only 12 hard‐choice trials were presented in each task run, individual contrast maps were, on average, based on just six trials.
RESULTS
Behavioral Assessment of Delay Discounting (Tests Outside Scanner)
The geometric mean k‐parameter estimate for MA‐dependent subjects was 0.045; for the comparison participants, it was 0.013. Placing this difference in context, a subject with a k‐value of 0.013 was approximately indifferent in choosing between $20 immediately and $28 delayed by 1 month, while a subject with a k‐value of 0.045 was approximately indifferent between the choice of $20 immediately and $47 delayed by 1 month. After these data were normalized by natural log transformation, the group difference indicated more delay discounting in MA abusers relative to controls (t(27) = 2.06; P < 0.05). The mean reaction times for MA abusers and control subjects (3.16 ± 1.45 and 2.86 ± 0.83 s, respectively) did not differ significantly (t(27) = 0.62; P = 0.57). Of the 27 trials, 3 or fewer responses were inconsistent with the best‐fit k‐value for 9 of 12 MA‐dependent participants (75.0%) and 13 of 17 control participants (76.5%).
Delay Discounting Choice Task Paired With fMRI
Responses in the easy‐choice condition were predominantly in accord with the k‐parameter estimates made outside the scanner and did not differ significantly across groups (MA abusers, 91.5% ± 4.41% consistent; controls, 93.8% ± 3.89% consistent; t(21) = 1.28; P = 0.21). Consistent with the study manipulation, selection of the larger‐later alternative was near 50% during the hard‐choice condition; MA abusers selected the larger‐later alternative on 49.6% ± 5.71% of the trials, and controls on 52.3% ± 6.29% of the trials (t(21) = 1.07; P = 0.30). Latency of MA abusers to respond on easy‐choice trials was 2.50 ± 0.71 s; on hard‐choice trials, it was 3.26 ± 1.10 s. Among control subjects, latency to respond on easy‐choice trials was 2.13 ± 0.33 s; on hard‐choice trials, it was 2.96 ± 0.40 s. These data were analyzed by a repeated‐measures analysis of variance (ANOVA), which included group as a between‐subject variable, and condition (hard choice vs. easy choice) as a within‐subject variable. Condition was a highly significant predictor of response time, with participants taking longer to respond during hard choices (F(1,21) = 36; P < 0.001). Neither group (F(1,21) = 1.46; P = 0.24) nor the interaction between group and condition (F1,21) = 0.8; P = 0.78) was significant predictors of response time.
fMRI Analysis
Imaging data from three control subjects and two MA abusers were excluded due to either excessive motion or the presence of remarkable artifacts (radiofrequency [RF] leak). Imaging data from a fourth control participant was not collected due to a scheduling error. The remaining data (from 10 MA abusers and 13 control subjects) were included in all imaging analyses.
Analysis With Groups Combined
Analysis comparing hard‐choice blocks with no‐choice blocks (hard choice > no choice) identified six active clusters (Fig. 1 and Table I). Bilateral activation was observed in both ventrolateral prefrontal cortex (VLPFC; BA 10, 11, 47) and DLPFC (BA 9, 46), as well as in the region surrounding the intraparietal sulcus (IPS; BA 40 and 7). On the left but not right, the cluster of activation within the VLPFC extended beyond the junction of the frontal operculum and into the anterior insula. A large cluster of activation was also observed in the anterior cingulate and supplementary motor cortices (BA 8).
Figure 1.
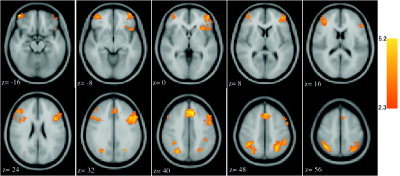
Radiological presentation of areas in which significantly greater signal was observed in hard‐choice blocks relative to nonchoice blocks (P < 0.05 cluster extent, whole brain analysis). The six clusters are characterized in Table I.
Table I.
Clusters showing significant task‐related activity: hard choice > no choice
| x, y, z | Max Z | Voxels | |
|---|---|---|---|
| R VLPFC/DLPFC | 42, 37, 12 | 5.03 | 1,441 |
| L DLPFC | −47, 15, 33 | 4.65 | 1,364 |
| R IPS | 34, −57, 48 | 4.65 | 1,131 |
| L VLPFC/insula | −41, 39, −2 | 4.30 | 1,090 |
| L IPS | −34, −54, 49 | 4.76 | 1,010 |
| AC and SMA | −3, 26, 42 | 5.13 | 913 |
Groups combined, whole‐brain analysis. Cluster extent threshold of P < 0.05, corrected for multiple comparisons in whole brain. Coordinates (x, y, z) indicate Montreal Neurological Institute coordinates of the voxel with maximum Z‐score.
R and L VLPFC, right and left ventrolateral prefrontal cortices; R and L DLPFC, right and left dorsolateral prefrontal cortices; R and L IPS, right and left intraparietal sulci; AC, anterior cingulate; SMA, supplementary motor cortex.
Third‐level analysis comparing easy‐choice blocks to no‐choice blocks (conducted only within clusters identified in hard choice > no choice) identified three active clusters (Table II). Activation was observed bilaterally in the IPS and in the left DLPFC.
Table II.
Clusters showing significant task‐related activity
| x, y, z | Max Z | Voxels | |
|---|---|---|---|
| Easy choice > no choices | |||
| L IPS | −31, −55, 48 | 3.72 | 495 |
| L DLPFC | −43, 12, 33 | 4.02 | 462 |
| R IPS | 31, −62, 44 | 4.00 | 435 |
| Hard choice > easy choice | |||
| R VLPFC | 43, 42, 19 | 3.64 | 307 |
| AC, SMA | −7, 27, 40 | 3.89 | 289 |
Analysis comparing hard‐choice blocks to easy‐choice blocks (hard choice > easy choice) identified two active clusters (Table II). One of these clusters extended across either side of the border between the anterior cingulate and the supplementary motor area, and the other fell primarily within the right VLPFC.
Comparisons of MA vs. Controls
The contrast MA > control subjects yielded no group differences in the contrasts considered above. The contrast of controls > MA did yield two clusters that were more active in the “hard choice > easy choice” contrast. These clusters were in the left DLPFC (−44, 22, 24) and right IPS (40, −54, 50). To provide a qualitative summary of the data, the percent signal change (relative to no choice) was computed for each subject for both the easy‐choice and the hard‐choice blocks in each of the six clusters identified in the combined group analysis (Table I). These data, presented in Figures 2, suggest a pattern of signal change that was largely consistent across clusters; while fMRI signal was generally greater in the hard‐choice blocks than the easy‐choice blocks, the difference between the two conditions was considerably greater for control subjects than for the MA abusers. Among control subjects, recruitment during easy‐choice blocks was minimal, whereas among MA abusers, recruitment during easy‐choice blocks was near the level observed in hard‐choice blocks.
Figure 2.
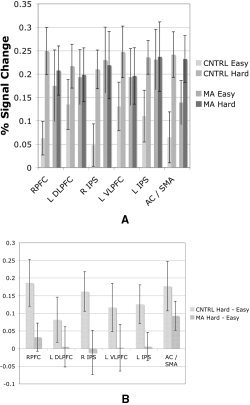
A presents the signal change (mean ± standard error) for each group for both the easy‐choice condition and the hard‐choice condition, both relative to the no‐choice trials. Among control subjects, very little signal change is present in the easy‐choice condition relative to what is observed in the hard‐choice condition. For MA abusers, however, similar signal change was observed in both conditions. B directly presents the differences between the hard‐choice and easy‐choice conditions, highlighting the group effect. Although the effect was only significant in two clusters, the tendency for signal change in these two conditions to be more different among control participants than among MA participants appears to be uniform across the six clusters.
Correlational analyses were conducted to identify statistical relationships between signal change and delay discounting (using the log‐transformed k‐parameter) across groups, again considering just those regions that demonstrated task‐related differences in signal in the combined group analysis (Fig. 1). No significant relationship was observed between signal differences in either the “easy choice > nonchoice” or “hard choice > easy choice” conditions. However, in one cluster within the left VLPFC (BA 10), there was an inverse correlation between signal change in the “hard choice > no choice” contrast and delay discounting (greater increase in signal intensity associated with less delay discounting; Fig. 3).
Figure 3.
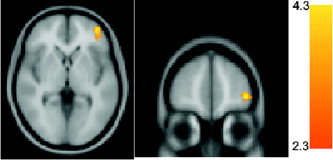
Radiological presentation of cluster in right VLPFC in which greater signal change (P < 0.05 cluster extent) in the “hard choice > no choice” contrast was associated with less trading of amount for immediacy (less delay discounting). Analysis was limited to clusters identified as active in the same contrast (Table I).
The event‐related comparison of signal during hard‐choice trials in which the later‐larger alternative was chosen and those in which the smaller‐sooner alternative was chosen did not identify any significant differences within the mask used in accordance with the a priori data analysis plan. An exploratory analysis repeated this contrast without limiting the analysis to clusters identified in the hard choice > no choice contrast. Again, no significant differences were observed.
DISCUSSION
Behavioral Data
The level of delay discounting observed in our study groups indicates that, like other substance‐abusing populations considered in a laboratory setting, MA abusers exhibit a greater than normal willingness to trade amount for immediacy (MA, k = 0.045; control, k = 0.013; P < 0.05). This difference was observed despite the fact that the control group consisted predominantly of cigarette smokers: a population observed to have a higher level of delay discounting than nonsmokers [e.g., Mitchell, 1999]. Indeed, the discount rate exhibited by this small group of MA abusers was, in the context of relevant literature, quite high. Kirby et al. [1999] reported a geometric mean parameter of k = 0.025 for heroin abusers (n = 56), while Monterosso et al. [2001] reported a discount parameter of k = 0.019 for cocaine abusers (n = 32).
Inferred Neural Recruitment During Delay Discounting Task
As noted in the introduction, McClure et al. [2004] reported on a nonclinical sample choosing between smaller‐sooner and later‐larger rewards, with the selected alternative from one trial to be paid out. Dissociation was observed between limbic/paralimbic regions that were recruited during selection of choices involving immediate alternatives, and a frontoparietal network (primarily in the lateral prefrontal cortex and IPS) that were inferred to be recruited during choice selection in general. The pattern of task‐related signal change observed in our study strongly resembles this second network of activation. The robust bilateral recruitment in the IPS may reflect both the calculations required by the task and the response selection component of the task. Participants must somehow combine the delay and amount parameters and compare the alternatives. The IPS has been widely implicated in numerical calculations in both lesion [Dehaene and Cohen, 1997; Butterworth, 1999] and imaging studies [Gruber et al., 2001; Zago et al., 2001; Simon et al., 2002]. Indeed, simple number comparison recruits activity in this region [Pesenti et al., 2000; Fias et al., 2003], and there is also evidence that response selection, even in the absence of numerical comparison, may recruit activation in the IPS [Bunge et al., 2002].
The observed frontal activation, particularly in the DLPFC, which is widely implicated in working memory [Baddely, 1986], is also consistent with behavioral experiments linking the delay discounting task to working memory. Hinson et al. [2003] observed both that working memory capacity was correlated with greater preference for later‐larger alternatives, and that placing participants under a task‐extrinsic working memory load (using a dual‐task paradigm) resulted in a shift in preference toward smaller‐sooner alternatives [Hinson et al., 2003].
Unlike McClure et al. [2004], we did not observe activity in limbic/paralimbic regions. Although failures to reject the null hypothesis should be interpreted with caution, this discrepancy is worth some consideration, particularly given that limbic and paralimbic activity in the McClure et al. [2004] study was observed on trials that included an immediate alternative, and all trials in the current study included an immediate alternative. One plausible basis for the discrepancy (beyond myriad methodological differences) is the reliance in the present study on purely hypothetical choices. The reduced hedonic import of choices that are purely hypothetical may have minimized the limbic recruitment in the present study. In light of this, the fact that group differences in delay discounting were nevertheless observed is particularly interesting, as it suggests that the limbic recruitment that has been posited to function in opposition to the cooler judgment produced by the frontoparietal network [McClure et al., 2004] may not be the source, or at least not the only source, of high levels of delay discounting in drug‐abusing populations relative to controls. Indeed, most studies demonstrating greater delay discounting in drug‐abusing populations have used purely hypothetical rewards [Vuchinich and Simpson, 1998; Bickel et al., 1999; Kirby et al., 1999; Mitchell, 1999; Cairns and van der Pol, 2000; Moeller and Dougherty, 2002; Coffey et al., 2003; Petry, 2003; Kirby and Petry, 2004]. Although it is possible that limbic activation that was below the level of detection in the present study nevertheless mediated performance, it is also possible that repeated hypothetical choices about money do not robustly recruit limbic activity, and limbic activation during response selection does not reflect the circuitry, or not all of the circuitry, that mediates high delay discounting among substance abusers.
Neural Activation in Methamphetamine‐Dependent vs. Control Subjects
Based on observations that drug‐abusing participants exhibit less neural recruitment in frontoparietal clusters during decision‐making than comparison subjects who do not abuse drugs (especially in the DLPFC [Paulus et al., 2002, 2003; Bolla et al., 2003, 2005; Ersche et al., 2005]), and on the fact that lateral prefrontal cortical activity has been related to preference for later‐larger rewards [McClure et al., 2004], we hypothesized that relative to control participants, MA abusers would demonstrate lower task‐related signal change in a frontoparietal network, and that this difference would be associated with greater delay discounting. As we did not observe evidence of lower task‐related activity in the prefrontal cortex among MA abusers, however, this hypothesis was not supported.
The parametric manipulation of decision difficulty did reveal a significant difference in neural recruitment (as evidenced by MRI signal change) among MA abusers relative to control participants during the task. There were two clusters (one in the left DLPFC and the other in the right posterior parietal cortex) in which the difference between the hard‐choice and easy‐choice conditions itself differed between groups. Exploratory analysis of signal change in all clusters identified as active in the task suggested that, although only significant in these clusters, a similar pattern was present in all regions recruited by the task. In general, the tendency for neural recruitment to be greater in response to hard choices than easy choices (as also reported in McClure et al. [2004]) was more robust in control subjects than MA abusers. Inspection of percent signal changes (Fig. 2A) suggests that this pattern was principally driven by the easy‐choice condition, in which minimal signal change was observed in control subjects, but in which MA abusers demonstrated recruitment that was near to the level observed with difficult choices. Interestingly, a similar pattern of BOLD activity was observed among cocaine abusers relative to control subjects in an fMRI study of inhibitory control in which working memory load was parametrically varied [Hester and Garavan, 2004]. While control subjects in that study demonstrated greater BOLD response with increased working‐memory demand, BOLD response did not vary with working‐memory load among cocaine abusers.
The observed pattern of signal change in hard choices versus easy choices may reasonably be interpreted as inefficiency in the MA‐dependent group. The hard‐choice condition presented subjects with alternatives that, given the participant's own tendency to trade off amount and immediacy, were maximally difficult. As such, the observed signal change during this condition was likely the maximal change that would be observed given the general parameters of the task (e.g., pace, perceived import). In contrast, the easy‐choice condition presented subjects with disparately valued alternatives. For control participants but not MA participants, the neural signal change corresponding to the easy‐choice condition was just a small fraction of the signal change corresponding to the hard‐choice condition, suggesting resolution of easy choices with a relatively low expenditure in neural activity.
Correlation Between Activation and Delay Discounting
Correlational analyses did not identify any cluster of activation during either the “easy choice–no choice” or “hard choice–easy choice” contrasts that was related to individual level of delay discounting. The lack of relationship between discounting and signal change in the “hard choice–easy choice” contrast is noteworthy in that it casts doubt on the possibility that the observed group differences in activation associated with this contrast are related to the behavioral group difference in delay discounting among MA abusers. It appears possible, then, that the apparent inefficiency in neural response observed in MA abusers is not closely tied to the tendency toward greater delay discounting, although a larger sample might reveal such a relationship.
A significant relationship was observed between mean signal change in a cluster within the left VLPFC in the hard‐choice vs. no‐choice contrast and the delay discounting parameter estimates. Across groups, participants with greater signal difference in this cluster exhibited less delay discounting (Fig. 3). The mid‐ventrolateral prefrontal cortex, in interaction with posterior cortical association areas, has been widely hypothesized to subserve active selection, comparison, and judgment of stimuli held in short‐term and long‐term memory [Petrides, 1996, 2000]. Future imaging studies using similar methodologies may indicate whether the observed relationship between activation in the left VLPFC during maximally difficult tradeoffs and degree of delay discounting is robust, and if so, whether the relationship is specific to the region, or whether it is present throughout the frontoparietal network recruited by the task.
Event‐Related Analysis Comparing Recruitment Based on Choice
Finally, we did not observe any significant difference between fMRI signal in the exploratory analysis comparing hard‐choice trials in which the more immediate alternative was selected, and those in which the larger but more delayed alternative was selected. In light of previous work associating individual choices with the relative amount of frontoparietal and limbic/paralimbic recruitment, the lack of observed difference may again be related to the fact that, perhaps owing to the use of hypothetical choices, we did not observe significant limbic recruitment in this study. Notably, the study was not designed to optimize the power of this contrast either in terms of the onset timing of events, or in terms of the number of relevant events (on average only six of each type per task run).
Study Limitations
One limitation of the present study is the presence of significant signal loss due to susceptibility artifact in the region of the ventral prefrontal cortex that includes the OFC. This effect was determined by comparing functional scan data with structural T1 images. Because of the OFC signal loss, we were unable to assess findings in this potentially task‐relevant region. Another limitation is the lack of statistical power in the present study given the modest sample sizes may have led to type II errors. In terms of imaging data, we observed signal change that indicated a consistent trend toward a group difference in the “hard choice–easy choice” contrast, yet possibly due to our sample size, the null hypothesis could be rejected in only two small clusters. Furthermore, although inspection of signal change data suggested greater recruitment among MA abusers in the “easy choice–no choice” contrast, differences did not reach statistical significance.
MA abusers exhibited an attenuation of the relationship between the difficulty of decisions and the amount of frontoparietal signal change observed. This was principally driven by the tendency among control subjects to select between disparately valued alternatives (easy choices) with very little frontoparietal recruitment while MA abusers exhibited nearly as much signal change when selecting between disparately valued alternatives as compared to choosing between similarly valued alternatives. Although correlational analysis did not suggest a relationship between this apparent inefficiency and the greater delay discounting observed among MA abusers, inefficiency in decision‐making may contribute to the difficulty of cessation from MA abuse by effectively increasing the extent to which processes other than decision‐making guide behavior. Clinically, the chronic substance abuser presents as one who “engages in behavior, especially drug‐taking behavior, without thinking” [O'Brien et al., 1975: p. 116]. While our experimental task required participants to make well‐defined hypothetical choices, thus explicitly placing them in a decision‐making mode, ordinarily behavior is only sometimes guided by explicit decision‐making processes. Behavior is alternatively guided by more stimulus‐bound processes that result from drive states, conditioned associations, or unconscious/reflexive responses [Jentsch and Taylor, 1999]. Given the competition that exists ordinarily between deliberate decision‐making processes and more automatic processes (e.g., the rapid conditioned response to a cue indicating drug availability), it is plausible that inefficiency in resolving decisions would result in behavior that is predominated by automatic processes. Indeed, both animal and human research has suggested an association between stimulus‐bound behavior and chronic drug abuse [Jentsch and Taylor, 1999; Paulus et al., 2003]. With respect to this hypothesis, the critical manifestation of frontoparietal inefficiency may not be its effect on what choices are made during explicit decision‐making, but rather a diminishment in the extent to which decision‐making is engaged in contexts that do not explicitly require it (i.e., not what decision is made, but whether a decision is made). On this hypothesis, the primary relevance of the observed frontoparietal inefficiency with respect to chronic MA abusers may be orthogonal to the repeatedly observed tendency among substance abusers to select more immediate alternatives during explicit decision‐making tasks.
Acknowledgements
Supported by the National Institutes of Health grant K01 DA0051‐01A1 (to J.R.M.) and 3R01 DA015179‐02S1 (to E.D.L.) and NIH grant P20RR020750. The General Clinical Research Center at the University of California at Los Angeles is supported by the National Institutes of Health grant 5M01RR000865‐31.
REFERENCES
- Ainslie G (1975): Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82: 463–496. [DOI] [PubMed] [Google Scholar]
- Ainslie G (2004): Gods are more flexible than resolutions. Behav Brain Sci 27: 18–19. [Google Scholar]
- Ainslie G, Haendel V (1983): The motives of the will In: Gottheil E, Druley K, Skodola T, Waxman H, editors. Etiology Aspects of Alcohol and Drug Abuse. Springfield, IL: Charles C. Thomas: 119–140. [Google Scholar]
- Baddely A (1986): Working memory. London: Oxford University Press. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW (1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR (1996): Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 6: 215–225. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR (1997): Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–1295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP (1999): Different contributions of the human amygdala and ventromedial prefrontal cortex to decision‐making. J Neurosci 19: 5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ, Petry NM (1998): The price of change: the behavioral economics of drug dependence. Behav Ther 29: 545–565. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ (1999): Impulsivity and cigarette smoking: delay discounting in current, never, and ex‐smokers. Psychopharmacology 146: 447–454. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, et al. (2003): Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision‐making task. Neuroimage 19: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL (2005): Neural substrates of faulty decision‐making in abstinent marijuana users. Neuroimage 26: 480–492. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD (2002): Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Butterworth B. 1999. The mathematical brain. London: Macmillan. [Google Scholar]
- Cairns J, van der Pol M (2000): Valuing future private and social benefits: the discounted utility model versus hyperbolic discounting models. J Econ Psychol 21: 191–205. [Google Scholar]
- Chapman GB (1996): Temporal discounting and utility for health and money. J Exp Psychol Learn Memory Cogn 22: 771–791. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003): Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine‐dependent individuals. Exp Clin Psychopharmacol 11: 18–25. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L (1997): Cerebral pathways for calculation: double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex 33: 219–250. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks‐Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ (2005): Abnormal frontal activations related to decision making in current and former amphetamine‐ and opiate‐dependent individuals. Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ (2005): Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia 43: 1214–1221. [DOI] [PubMed] [Google Scholar]
- Fias W, Lammertyn J, Reynvoet B, Dupont P, Orban GA (2003): Parietal representation of symbolic and nonsymbolic magnitude. J Cogn Neurosci 15: 47–56. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1996): Structured Clinical Interview for DSM‐IV Axis I Disorders, Clinician Version (SCID‐CV). Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Fishbein D, Eldreth D, Hyde C, Matochik J, London ED, Contoreggi C, Varughese K, Kimes A, Breeden A, Grant S (2005): Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Cogn Brain Res 23: 119–136. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A (2001): Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 11: 350–359. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H (2004): Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24: 11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P (2003): Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn 29: 298–306. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM (2001): A global optimisation method for robust affine registration ofbrain images. Med Image Analysis 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999): Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward‐related stimuli. Psychopharmacology (Berl) 146: 373–390. [DOI] [PubMed] [Google Scholar]
- Kirby KN (1997): Bidding on the future: evidence against normative discounting of delayed rewards. J Exp Psychol Gen 126: 54–70. [Google Scholar]
- Kirby KN, Petry NM, Bickel WK (1999): Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128: 78–87. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM (2004): Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non‐drug‐using controls. Addiction 99: 461–471. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C (2003): Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 13: 1064–1071. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, et al. (2004): Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61: 73–84. [DOI] [PubMed] [Google Scholar]
- Mazur J (1987): An adjusting procedure for studying delayed reinforcement In: Commons M, Mazur J, Nevin J, Rachlin H, editors. The effect of delay and of intervening events on reinforcement value. Hillsdale, NJ: Lawrence Erlbaum Associates; p 55–73. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004): Separate neural systems value immediate and delayed monetary rewards. Science 306: 503–507. [DOI] [PubMed] [Google Scholar]
- Mitchell S (1999): Measures of impulsivity in cigarette smokers and non‐smokers. Psychopharmacology 146: 455–464. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM (2002): Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 160: 290–298. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM (2002): Impulsivity ad substance abuse: what is the connection? Addict Disord Their Treat 1: 3–10. [Google Scholar]
- Monterosso J, Ehrman R, Napier K, O'Brien CP, Childress AR. Three decision‐making tasks in cocaine‐dependent patients: Do they measure the same construct? Addiction 2001;96: 1825–1837. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, O'Brien TJ, Mintz J, Brady JP (1975): Conditioning of narcotic abstinence symptoms in human subjects. Drug Alcohol Depend 1: 115–123. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA (2002): Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine‐dependent subjects. Neuropsychopharmacology 26: 53–63. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA (2003): Decision making by methamphetamine‐dependent subjects is associated with error‐rate‐independent decrease in prefrontal and parietal activation. Biol Psychiatry 53: 65–74. [DOI] [PubMed] [Google Scholar]
- Pesenti M, Thioux M, Seron X, De Volder A (2000): Neuroanatomical substrates of arabic number processing, numerical comparison, and simple addition: a PET study. J Cogn Neurosci 12: 461–479. [DOI] [PubMed] [Google Scholar]
- Petrides M (1996): Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1455–1461. [DOI] [PubMed] [Google Scholar]
- Petrides M (2000): Impairments in working memory after frontal cortical excisions. Adv Neurol 84: 111–118. [PubMed] [Google Scholar]
- Petry NM (2003): Discounting of money, health, and freedom in substance abusers and controls. Drug Alcohol Depend 71: 133–141. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K (2004): Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes 65: 35–42. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, et al. (1999a): Dissociable deficits in the decision‐making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan‐depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999b): Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19: 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J (1994): Emotion‐related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 57: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2000): The orbitofrontal cortex and reward. Cereb Cortex 10: 284–294. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S (2002): Topographical layout of hand, eye, calculation, and language‐related areas in the human parietal lobe. Neuron 33: 475–487. [DOI] [PubMed] [Google Scholar]
- Smith S, Brady J (1997): SUSAN‐a new approach to low level image processing. Int J Comp Vision 23: 45–78. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: 208–219. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA (1998): Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol 6: 292–305. [DOI] [PubMed] [Google Scholar]
- Wallace C (1979): The effects of delayed rewards, social pressure, and frustration on the response of opiate addicts. NIDA Monogr Ser 25: 6–25. [PubMed] [Google Scholar]
- Wallis JD, Miller EK (2003): Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci 18: 2069–2081. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW (1993): The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150: 885–890. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dapretto M, Sicotte NL, Toga AW, Mazziotta JC (1999): Creation and use of a Talairach‐compatible atlas for accurate, automated, nonlinear intersubject registration, and analysis of functional imaging data. Hum Brain Mapp 8: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M, Ripley B, Brady M, Smith S (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]
- Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio‐Mazoyer N (2001): Neural correlates of simple and complex mental calculation. Neuroimage 13: 314–327. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M (1997): Empirical analyses of BOLD fMRI statistics: I, spatially unsmoothed data collected under null‐hypothesis conditions. Neuroimage 5: 179–197. [DOI] [PubMed] [Google Scholar]


