Abstract
Motivated by claims that relegate the syntactic functions of Broca's region to working memory (WM) and not to language‐specific mechanisms, we conducted an fMRI and an aphasia study that featured two varieties of intrasentential dependency relations: One was syntactic movement (e.g., Which boy does the girl think ◂ examined Steven?), the other was antecedent–reflexive binding (e.g., Jill thinks the boy examined himself). In both, WM is required to link two nonadjacent positions. Syntactically, they are governed by distinct rule systems. In health, the two dependencies modulated activity in distinct brain regions within the left inferior frontal gyrus and the left middle temporal gyrus. Binding uniquely modulated activation in the right frontal lobe. Receptive abilities in brain damaged patients likewise distinguished among these syntactic types. The results indicate that sentence comprehension is governed by syntactically carved neural chunks and provide hints regarding a language related region in the right hemisphere. Hum Brain Mapp 2006. © 2006 Wiley‐Liss, Inc.
Keywords: aphasia, broca area, fMRI, linguistics, memory, short‐term
INTRODUCTION
Certain dependency relations in complex sentences force the human syntax analyzer to establish a link between two nonadjacent positions in real‐time. Natural language is replete with such sentences. The most prominent among these, perhaps, is the syntactic dependency found in sentences with displaced elements. The object of a declarative sentence (Table Ia) is found on the left edge when the sentence is turned into a question (Table Ib). Likewise, a subject of an embedded clause (Table Ic) is found on the left of the main clause when turned into a question or focused [Table Id(i,ii)]. This syntactic displacement keeps core meaning unchanged. To account for meaning constancy despite syntactic change, most (if not all) approaches invoke a link between the moved element (“filler”) and the vacated position (“gap” “◂”).
Table I.
Example sentences to demonstrate syntactic movement
| a | Alex pushed the teenage boy. |
| b | Which boy did Alex push ◂? |
| c | Alex supposed that the teenage boy spoke to the girl. |
| d(i) | Which boy did Alex suppose ◂ spoke to the girl? |
| d(ii) | It was the teenage boy that Alex supposed ◂ spoke to the girl. |
Sentences in (a) and (c) do not contain movement, whereas those in (b) and (d) resulted from movement of the object, and the embedded subject in (a) and (c), respectively. Movement can be motivated by question formation [b, d(i)] or in order to place focus on a sentential element [d(ii)]. The symbol ◂ indicates the position of the constituent prior to movement.
The nature of “filler‐gap dependencies”, or syntactic movement, is at the center of linguistic investigation (with references too numerous to cite). Its on‐line computation has its characteristic time‐course [Bever and McElree, 1988; Love and Swinney, 1996; Nakano et al., 2002; Nicol and Swinney, 1989; Traxler and Pickering, 1996], and its reception is impaired in Broca's aphasia, as gleaned from convergent results across multiple sentence types, tasks, and languages [Grodzinsky, 2000]. In health, fMRI studies of adult speakers of English, German, and Hebrew have documented significantly greater activation in Broca's area on trials with filler‐gap dependencies compared to when they are absent [Ben‐Shachar et al., 2003, 2004; Caplan, 2001; Caplan et al., 1998; Constable et al., 2004; Fiebach et al., 2005; Just et al., 1996; Röder et al., 2002; Stromswold et al., 1996].
This dense body of empirical results has led to a view of Broca's region as a critical locus of highly specialized processes underlying syntactic movement in natural language [Grodzinsky, 2000]. Whether and how these relate to Working Memory (WM) is less clear. Several possibilities exist: First, Broca's region might house a WM specialized for movement. Indeed, increased distance between the filler and its gap in a sentence produces greater activation in the region of Broca's area [Cooke et al., 2001; Fiebach et al., 2005]. Second, Broca's area may actually support a syntactic WM that is not specialized for a particular syntactic relation but used generally in syntactic processing, as has been proposed by Caplan and Waters [1999]. Finally, the WM in Broca's area may yet have a general cognitive character [Just and Carpenter, 1992]. Neuroimaging studies that stretch WM with such tasks as n‐back have found activation correlates to verbal WM load within Broca's area [Braver et al., 1997; Jonides et al., 1998; Smith and Jonides, 1999], leading to the conclusion that the syntactic role of Broca's area is only apparent, where in fact the syntactically induced activations and deficits are due to the ubiquitous involvement of a generic WM in syntactic tasks [Kaan and Swaab, 2002].
This experimental series sought to tease apart the specific syntactic and the more general WM alternatives through both an fMRI and aphasia experiment using minimally different sentence pairs. If Broca's area supports a syntactic (or even simply a verbal) WM store that does not differentiate between different types of syntactic dependencies, then any sentence that requires the linking of nonadjacent elements in a string should activate it, with link length modulating signal intensity. If, however, Broca's area's role in the analysis of syntactic movement is specific, then a neurological association between movement and other intrasentential dependency relations (such as reflexive binding, see methods for a description) that require linking need not be expected.
Since the hypothesis we are testing concerns whether or not there is overlapping activation due to a general WM, the exact nature of the syntactic differences between the dependency relations that we study here are of no concern to us in the present context. And, while the intrasentential dependencies we compare are different in certain syntactic respects (see Methods), they are identical from the general WM perspective we are testing: they equally require a link of varying length between elements in a stimulus sequence (even though link length is not modulated in the current study, see later). Thus, the general WM perspective expects overlap in the areas activated by the two dependencies.
METHODS
fMRI Stimuli
Natural language syntax avails us of linking relations other than movement. A reflexive pronoun [himself in (1)] receives its referential identity from an antecedent (the cunning man) to which it is “bound”, and which must be (i) overt and local (i.e., structurally close) and (ii) agree with the reflexive in gender and number, or else the result is ungrammatical (2)–(3):
-
1
The girl supposes the cunning man hurt himself. [grammatical]
-
2
*The girl supposes the cunning man hurt herself. [ungrammatical (nonlocal antecedent)]
-
3
*The girl supposes the cunning men hurt himself. [ungrammatical (local antecedent number mismatch)]
This relation, called binding, bears important similarities to movement: both require a link between (potentially) nonadjacent constituents, and hence their analysis and interpretation rely critically on verbal working memory (WM). In movement sentences, the semantic role of the “Filler” is determined at its “Gap” (“◂”): Which boy asks about the recipient of action in (Table Ib), and about the embedded agent in [Table Id(i)]; in binding sentences, the reference of the reflexive depends on the identity of the antecedent (himself = the cunning man). Yet despite these similarities, movement and binding fall under rather different syntactic constraints [Lasnik and Uriagereka, 1988] and demonstrate different processing time courses [Nicol and Swinney, 1989]. These two linking relations are thus similar in placing greater demands on WM than sentences without dependencies, yet they are syntactically distinct. Again, as discussed in the introduction, the various differences between the dependencies is not a concern given that we are testing for overlap in activation as predicted by a general WM theory.
Informed by initial observations on Broca's aphasic patients who suffered from a movement deficit, yet seemed exempt from a deficit in binding [Grodzinsky et al., 1993], we constructed a grammaticality judgment task that served as our testing ground: If on‐line linking of Filler‐Gap and antecedent–reflexive is supported by an overarching verbal or syntactic WM, the reception of these sentences should merely be a WM task (perhaps an instance of the n‐back task). Broca's region should therefore be activated in both cases, with signal intensity modulated by the distance between the two linked elements. If, however, WM‐based linking is not a neurally instantiated generalization, and each syntactic type is driven by a different rule, then there is no reason to expect activation overlap.
The stimuli had a 2 (±MOV) × 2 (±BIND) × 2 (±GRAM) design (see Supplementary Material at http://freud.tau.ac.il/~yosef1/). The eight conditions each had 16 unique sentences. On average the sentences were 3,383 ms long. A grammaticality judgment task was used to verify that the subjects were actively processing the stimuli. All 128 sentences were presented in two different runs in different orders in an event‐related design. The total number of trials (256) were divided into two runs in order to give the subjects a rest. Possible extraneous effects within each run are eliminated through calculating a weighted average of the two runs. Each run consisted of eight blocks of 16 sentences. Half the grammatical and half the ungrammatical sentences of each type were included in each block. The sentences were randomized within the block. Each block ended with two frames of silence and three frames of silence were inserted prior to the first block of both runs. Therefore, each run was composed of eight blocks with a total of 147 events/scans.
fMRI Subjects
Eleven neurologically intact (seven females;
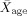 = 26 years, s.e.m.age = 1.74 years), right‐handed subjects with normal hearing participated in the study. Most subjects had taken at least two courses in linguistics. Since the study involved a grammaticality judgment task, it was preferred to have subjects with a linguistics background to be assured of their confidence and comfort with the task, and thereby reduce noise in the data. Although one might argue that the subjects had some expertise at the task (i.e., grammaticality judgment), they were not experts with regards to the specific rules of these two syntactic constructions. Informed consent was provided in accordance with guidelines approved by the Montreal Neurological Institute (MNI) Ethics and Research Committee.
= 26 years, s.e.m.age = 1.74 years), right‐handed subjects with normal hearing participated in the study. Most subjects had taken at least two courses in linguistics. Since the study involved a grammaticality judgment task, it was preferred to have subjects with a linguistics background to be assured of their confidence and comfort with the task, and thereby reduce noise in the data. Although one might argue that the subjects had some expertise at the task (i.e., grammaticality judgment), they were not experts with regards to the specific rules of these two syntactic constructions. Informed consent was provided in accordance with guidelines approved by the Montreal Neurological Institute (MNI) Ethics and Research Committee.
fMRI Procedure
One to two days prior to imaging the subject was read the experimental instructions and given six practice sentences to be sure they were comfortable with the task. In the magnet, subjects lay supinely with pneumatic earphones and an air vacuum cushion placed around their head to help prevent head motion. A computer mouse was placed under their left hand for their grammaticality judgments. The left hand was chosen in case motor activation was detected. If it was detected, it would be in the right hemisphere and dissociable from the typically left lateralized language processing regions. However, given the dynamics of each event, no motor activation was expected to be detected (i.e., the hemodynamic response in motor cortex should not occur until after the scan). Presentation® software (Version 0.53) was used for stimuli presentation. A localizer and a 15 min anatomical scan were conducted prior to functional imaging. A warm‐up of six sentences was first presented to be sure everything was working appropriately (e.g. button presses and scanner pulse are being acknowledged by software) and to give the subject some practice at the task. Two runs of stimuli were then presented with the run order being balanced across subjects.
fMRI Parameters
Image acquisition was performed with a 1.5T Siemens Vision imager at the MNI in Montreal, Canada. A localizer was performed followed by whole‐brain T1‐weighted imaging for anatomical localization (256 × 256 matrix; 176 continuous 1.00 mm sagittal). Each functional volume was acquired with a 64 × 64 matrix size and a total volume acquisition time of 2,000 ms with an acquisition delay of 6,500 ms. Each imaging run produced 147 acquisitions of the brain volume (TE = 50 ms, TR = 2.0 s, FA = 40°, FOV = 320 × 320 mm).
The temporal dynamics of each event was the following. One functional volume was acquired every 8,500 ms. Intermittent scanning was used in order to exempt the subjects from magnet noise as they listened to the stimulus sentences. The sentence was programmed to end at 2,000, 1,500, or 2,500 ms prior to the onset of the scan. A 2,000 ms delay from end of sentence to onset of scan allowed for a 3,000 ms delay from the end of sentence to mid‐scan. WM load will begin to build at the antecedent and then peak at the point just prior to the gap or reflexive. WM for the dependency will cease at the point of the gap or reflexive. The time from the peak in WM to mid scan is approximately 3.5–4.0 s. More precisely, the average time from the onset of the reflexive to the end of the sentence was 0.630 s (i.e., corresponding to a 3.630 s delay from the peak in WM to mid scan) and the average time from the position of the gap to the end of the sentence was 1.064 s (corresponding to a 4.064 s delay from the peak in WM to mid scan). Given that prefrontal regions show a 4–6 s delay to peak [Buckner et al., 1996; Ranganath et al., 2003], we will catch either the peak of the hemodynamic response function (HRF) corresponding to the peak in WM load or, at a minimum, the peak in the HRF that corresponds to WM load just prior to the point its load is greatest. Jitter in the delay by ±500 ms was used to increase the probability of capturing the peak of the hemodynamic response since the exact timing of the peak is unknown. The difference between the average time from gap to sentence end and the average time from reflexive onset to sentence end was 0.434 s. This difference is compensated for by the jitter in the position of the scan by overall a 1.0 s interval, which is more than twice as long as the difference. Therefore, none of our results can be accounted for by appealing to this difference.
fMRI Behavioral Data Analysis
The number of correct (i.e., acceptances of +gram and rejection of −gram) and incorrect judgments (the opposite) in each of the eight conditions was pooled across the two runs for each subject. A percent correct judgment score was then computed per condition [No. Correct/(No. Correct + No. Incorrect)] and per subject. The percent correct judgment data was entered into a 2 (±BIND) × 2 (±MOV) × 2 (±GRAM) ANOVA. An alpha of 0.05 was used.
fMRI Data Analysis
Functional data were processed with a spatial filter (FWHM = 6 mm) and corrected for motion artifacts. Statistical analyses were based on the general linear model [Worsley et al., 2002] (see http://www.bic.mni.mcgill.ca/~keith/). A map analysis, opposed to an ROI analysis, was performed since it does not bias the localization of an effect, but simply identifies where conditional effects lie. For the primary analysis, all frames corresponding to silence or ungrammatical sentences were excluded. The design matrix was not convolved with a hemodynamic response function (HRF) since there was one acquisition per event and in fact assumptions about the HRF are embedded within the design itself. In fitting the linear model, linear drift in the data was removed. The design matrix coded for a main effect of movement and a main effect of binding by collapsing across binding or movement, respectively. Additionally, the interaction of these two variables was coded within a design matrix. For each subject, a t‐statistical map was generated for the contrast. For each subject, the statistical maps (effect and standard deviation) from the two runs were combined into a weighted average. The combined runs were then transformed into standardized space (MNI coordinate system) using in‐house software [Collins et al., 1994] and entered into a group mixed effects analysis to produce group maps. The group t‐map was searched for clusters, whereby each voxel had an associated P < 0.005 (uncorrected). For each ROI, the percent signal change (PSC) values for each subject, broken down by condition, were visualized to better understand the effects (i.e. relative to baseline).
Analyses were also computed to determine the probability with which our activation within LIFG lies within Broca's area. Amunts et al.'s (1999) probability map of BA44 and BA45 were used in the calculation for the LIFG ROI of the movement effect and the LIFG ROI of the binding effect, respectively. The MNI Coordinates of our LIFG ROIs were converted into the voxel coordinates of the probability maps. These coordinates were then used in extracting values from these voxels in the probability maps. The values were then averaged. The values at each voxel in the [Amunts et al., 1999] map corresponds to the number of subjects with overlapping cytoarchitectonic structure, therefore, the average value for the ROI needed to be divided by the number of brains (n = 10) in order to derive a percentage.
fMRI Results
While being scanned, subjects listened to, and judged the grammaticality (GRAM) of, sentences which contained a binding relation (+BIND), movement (+MOV), both (+MOV +BIND), or none (−MOV−BIND; Table II; See Supplementary Material at http://freud.tau.ac.il/~yosef1/for instructions). Although response accuracy was ≫90% in all conditions, differences could still be discerned: the 2 (±MOV) × 2 (±BIND) × 2 (±GRAM) Repeated‐Measures ANOVA revealed a main effect of binding (F(1,10) = 16.66, P = 0.002) and a main effect of movement (F(1, 10) = 8.37, P = 0.016). The difficulty of the binding and movement trials was equal ‐ accuracy was lower when binding (
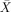 = 96.09%, s.e.m. = 0.60%) or movement (
= 96.09%, s.e.m. = 0.60%) or movement (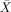 = 96.58%, s.e.m. = 0.52%) was present compared to when they were not (
= 96.58%, s.e.m. = 0.52%) was present compared to when they were not (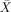 = 98.93%, s.e.m. = 0.34% and
= 98.93%, s.e.m. = 0.34% and 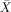 = 98.44%, s.e.m. = 0.41%, respectively). RT was not measured, as we had no theoretical or experimental motivation to justify the imposition of time–pressure on the subjects, which might have affected the BOLD response. Ungrammatical sentences (−GRAM) were presented for the sole purpose of giving the subjects a task that ensures that they attend to the stimuli. Since we are interested in teasing apart linking processes during normal sentence analysis, which does not include ungrammatical strings, all fMRI analyses pertain to grammatical sentences (+GRAM) only.
= 98.44%, s.e.m. = 0.41%, respectively). RT was not measured, as we had no theoretical or experimental motivation to justify the imposition of time–pressure on the subjects, which might have affected the BOLD response. Ungrammatical sentences (−GRAM) were presented for the sole purpose of giving the subjects a task that ensures that they attend to the stimuli. Since we are interested in teasing apart linking processes during normal sentence analysis, which does not include ungrammatical strings, all fMRI analyses pertain to grammatical sentences (+GRAM) only.
Table II.
Example sentences from the fMRI experiment
| No Link | a. −MOV−BIND | +GRAM | The girl supposes the cunning man hurt Christopher |
| −GRAM | *The girl supposes the cunning man swam Christopher | ||
| Link | b. −MOV+BIND | +GRAM | The girl supposes the cunning man hurt himself |
| −GRAM | *The girl supposes the cunning man hurt herself | ||
| c. +MOV−BIND | +GRAM | Which older man does Julia suppose ◂ hurt the child | |
| −GRAM | *Which older man does Julia suppose ◂ swam the child | ||
| d. +MOV+BIND | +GRAM | Which older man does Julia suppose ◂ hurt himself | |
| −GRAM | *Which older man does Julia suppose ◂ hurt herself |
The NoLink|Link partition distinguished conditions with and without a dependency relation (whether Binding or Movement). Each example sentence (n = 16 per condition) features a grammatical (+GRAM) and an ungrammatical (smaller font, –GRAM) counterpart (where the latter is later excluded from analysis). (a) No Link, baseline (−MOV−BIND). (b) No Movement, Binding (−MOV+BIND). (c) Movement, no Binding (+MOV−BIND). (d) Both Movement and Binding (+MOV+BIND; see Supplementary Materials at http://freud.tau.ac.il/~yosef1/ for more details about stimulus construction, and a complete list of stimuli).
To focus on similarities or differences between the two types of dependencies (movement and binding), maps of their main effects and interaction were calculated. In order for us to consider a region as demonstrating an interaction between the two types of constructions, the region must not only be present in the interaction map but also in at least one of the main effect maps. The binding effect map produced six ROIs, whereas the movement effect map produced four ROIs (see Tables III and IV, respectively). Each subject's preprocessed (motion corrected, spatially filtered, and trend corrected) data was used in the calculation of PSC values. The PSC values were used to better understand the nature of the effects in each ROI by examining their position relative to baseline.
Table III.
Binding Effect
| ROI no. | Landmark | Volume (mm3) | X | Y | Z | BA |
|---|---|---|---|---|---|---|
| 1 | RMFG | 1472 | 30 | 47 | 27 | 9/10 |
| 2 | RMFG | 704 | 28 | 53 | 13 | 10 |
| 3 | LIFG/LOG | 272 | −53 | 38 | −3 | 45/47 |
| 4 | MCG | 464 | 3 | 27 | 31 | 24 |
| 5 | LMTG | 256 | −56 | −52 | −6 | 21/37 |
| 6 | RMTG | 120 | 64 | −22 | 0 | 21 |
ROIs from the main effect of Binding map thresholded at t(352) = 2.59, P < 0.005. BA, Brodmann area; mean coordinates (X, Y, Z) for each ROI are in MNI standardized space; RMFG, right middle frontal gyrus; LIFG, left inferior frontal gyrus; LOG, left orbital gyrus; MCG, medial cingulate gyrus; LMTG, left middle temporal gyrus; RMTG, right middle temporal gyrus.
Table IV.
Movement Effect
| ROI no. | Landmark | Volume (mm3) | X | Y | Z | BA |
|---|---|---|---|---|---|---|
| 1 | LSTG | 3456 | −54 | −47 | 13 | 21/22 |
| 2 | LiPCS | 824 | −41 | 0 | 36 | 4/6 |
| 3 | LiPCS | 376 | −48 | 17 | 28 | 4/6 |
| 4 | LIFG | 304 | −42 | 6 | 5 | 44 |
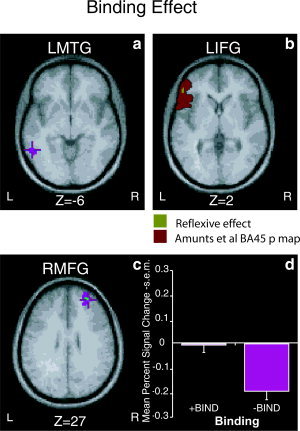
ROIs from the main effect of Movement map thresholded at t(352) = 2.59, P < 0.005. BA, Brodmann area; mean coordinates (X, Y, Z) for each ROI are in MNI standardized space; LSTG, left superior temporal gyrus; LiPCS, left inferior precentral sulcus; LIFG, left inferior frontal gyrus.
The right MFG (Fig. 1) and MTG demonstrated a main effect of binding, implicating the right hemisphere in the on‐line computation of syntactic links. While the right MTG has been reported by other syntactic tasks [Ben‐Shachar et al., 2003; Cooke et al., 2001; Fiebach et al., 2005], the right MFG has not. Additionally, the LIFG/LOG and LMTG demonstrated a main effect of binding (see Fig. 1). Other results were in line with previously reported findings: The LIFG, LSTG, and LiPCS demonstrated a main effect of movement [Ben‐Shachar et al., 2003, 2004; Meyer et al., 2003] (see Fig. 2). None of the areas that demonstrated a main effect demonstrated an interaction effect.
Figure 1.
A main effect of binding was evidenced within (a) LMTG, (b) LIFG/LOG and, (c) RMTG. The overlap of our LIFG ROI with Amunts et al. [1999] probability map of BA 45 is presented in (b). The probability map is color‐coded red and the LIFG ROI is color‐coded green. The LIFG area lies within BA 45 with a probability of 22.08%. The binding effect within RMFG resulted from a decreased blood oxygen level‐dependent (BOLD) response during nonbinding conditions and is presented in (d). Recent studies indicate that a decrease in the BOLD response corresponds better to a neuronal inhibition account than a “blood‐stealing” one [Stefanovic et al., 2004]. All other effects (movement or binding) resulted from a positive BOLD change (relative to silence). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 2.
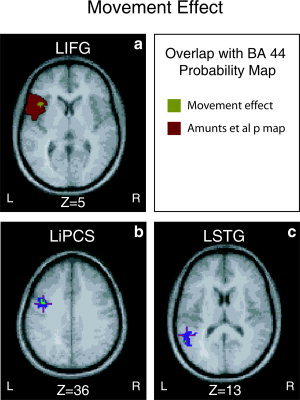
There was a main effect of movement within LIFG (a), LiPCS (b), and LSTG (c). The overlap of our LIFG ROI with Amunts et al.'s [1999] probability map of BA 44 is presented in a. The probability map is color‐coded red and the LIFG ROI is color‐coded green. The LIFG area lies within BA 44 with a probability of 31.90% based on Amunts et al. [1999] probability map (see Methods for a description of the probability calculations). It should be pointed out that the activation lies to the left of the insula since the overlaid probability map makes it difficult to see. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
LESION STUDY METHODS
Lesion Study Stimuli
Multiple studies in many languages and in different laboratories have documented a syntactic movement deficit in Broca's aphasia [cf. Drai and Grodzinsky, 2006ab] for recent quantitative analyses of results of movement tests from n = 100 patients). Wernicke's aphasic patients have also evinced a movement deficit, although the picture for this syndrome seems to be more complex and less stable [e.g., Grodzinsky and Finkel, 1998; Zurif et al., 1993]. No such deficit has ever been documented for patients with right hemispheric damage. In light of this and in analogy to the fMRI experiment, our lesion study sought to determine whether the aphasic deficit at the sentence level is restricted to movement, or rather, extended to other dependency relations. Previous evidence regarding Broca's aphasic patients gives preliminary clues about this question, indicating that at least on a limited set of sentence contexts, Broca's aphasic patients are intact in the domain of binding, that is, they may be capable of carrying out comprehension tasks that involve the establishment of a link between a reflexive and its antecedent [Grodzinsky et al., 1993]. In light of the above, we focused on binding, seeking to extend and solidify previous results in a way that would be amenable to a comparison with our fMRI study of healthy subjects. Specifically, this study looks at reflexives in the context of two potential intrasentential antecedents, a design that has never been used before. This forces the patient to invoke his/her knowledge of the relevant principles in full.
We thus conducted two tests with four groups of participants (see below), in which error rate was the dependent measure. First, to assess the extent of a movement deficit in our participants, we carried out a movement comprehension test in a standard, forced binary‐choice sentence‐to‐picture matching design. Sentences (n = 10 per condition) were ±movement (−movement sentences: actives, subject‐gap relative clauses; +movement: passives, object‐gap relatives).
Next, participants carried out grammaticality judgments on four conditions in a 2(±MOV) by 2(±GRAM; always in the presence of binding; see Table V): sentences contained a binding relation, or both a binding and a movement relation, each with two potential antecedents. There were 20 token sentences per condition (see Supplementary Materials at http://freud.tau.ac.il/~yosef1/for a characterization of the stimuli and a complete list).
Table V.
Example sentences from the Aphasia study
| +Grammatical | −Grammatical | |
|---|---|---|
| −MOV | a. It seems to Sally that the father rewards himself | b. It seems to Sally that the father rewards herself |
| +MOV | c. The father seems to Sally ◂ to reward himself | d. The father seems to Sally ◂ to reward herself |
All sentences contained reflexives, hence a Binding relation. Half the sentences contained a Movement relation. The grammatical status of sentences––which subjects were asked to judge––was manipulated by changing the gender of the reflexive, which led to a Match or a MisMatch in grammatical gender agreement between the reflexive and its correct antecedent. Each sentence featured 2 different‐gender, potential antecedents to the reflexive (see Supplementary Materials at http://freud.tau.ac.il/~yosef1/ for more details about stimulus construction, and a complete list of stimuli). A reflexive requires a local antecedent, hence in the absence of Movement (a), a gender match between the reflexive and the closest potential antecedent leads to grammaticality; a mismatch leads to ungrammaticality (b). When confounded with Movement, the situation is reversed: Now a gender match with a distant antecedent leads to grammaticality [due to the mediation of the gap that is linked to both the antecedent and the reflexive, (c)]; a gender match with the closest antecedent (d) leads to ungrammaticality because of the intervening gap.
Lesion Study Participants
We tested 17 participants (seven Broca's and six Wernicke's aphasic patients, three right hemisphere damaged patients, and two right‐handed neurologically intact, age‐ and education‐matched control participants). All patients were diagnosed on the basis of clinical neurological findings, neuroimaging, and the boston diagnostic aphasia examination (BDAE; see Supplementary Materials at http://freud.tau.ac.il/~yosef1/ for clinical details).
Lesion Study Methods
The standard comprehension test [subject‐ and object‐relative clauses, actives and passives; SOAP; Love and Oster, 2002] involved a binary‐choice sentence‐to‐picture matching and was used to ensure that our groups present with a typical picture in comprehension. For the novel, grammaticality‐judgment test, sentences were presented both auditorily and in writing. Each sentence was printed on a separate sheet of paper in very large font, and read aloud twice to the patient, who operated under no time constraints. Eighty sentences (see Supplementary Materials at http://freud.tau.ac.il/~yosef1/) were presented in random order (mixed with sentences from another experiment) through 2–5 sessions per patient (with many breaks within each session). Instructions and presession training procedures were the same as in a previous aphasia study [Grodzinsky and Finkel, 1998; see Supplementary Materials at http://freud.tau.ac.il/~yosef1/].
Lesion Study Statistical Analysis
The scores for each patient group and condition were submitted to a one‐sample Kolmogorov‐Smirnov Test to determine whether their distributions diverged from normal. Once it was ascertained that they did not significantly differ from normal, the use of standard parametric tests for data analysis was legitimized.
Two analyses were carried out. First, if the patients are unable to perform the linking relation they should simply guess, resulting in a performance level around 50%. Therefore, the patient groups' accuracy scores in the two conditions were compared to chance (50%) using a t test (two tailed, α = 0.05). Second, we tested for a difference between the conditions (±MOV) in each of the patient groups with paired t tests (two tailed, α = 0.05) to be convinced of a distinction between the two conditions.
Lesion Study Results
The comprehension part
All patients were tested on this well established battery. Only patients were tested on this part of the study (as it is a well‐established test). Broca's and Wernicke's aphasics had above‐chance comprehension scores on active sentences (
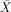 = 91.67%, s.e.m. = 6.54, t(5) = 6.37, P < 0.001;
= 91.67%, s.e.m. = 6.54, t(5) = 6.37, P < 0.001; 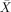 = 91.67%, s.e.m. = 4.01, t(5) = 10.381, P < 0.001) and subject relatives (
= 91.67%, s.e.m. = 4.01, t(5) = 10.381, P < 0.001) and subject relatives (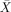 = 90.00%, s.e.m. = 5.16, t(5) = 7.75, P < 0.001;
= 90.00%, s.e.m. = 5.16, t(5) = 7.75, P < 0.001; 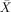 = 80.00%, s.e.m. = 3.65, t(5) = 8.216, P < 0.001), and around chance on object relatives (
= 80.00%, s.e.m. = 3.65, t(5) = 8.216, P < 0.001), and around chance on object relatives (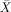 = 60.00%, s.e.m. = 8.56, t(5) 1.17, P < 0.296:
= 60.00%, s.e.m. = 8.56, t(5) 1.17, P < 0.296: 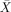 = 41.67%, s.e.m. = 6.54, t(5) = −1.274, P < 0.259) and passives (
= 41.67%, s.e.m. = 6.54, t(5) = −1.274, P < 0.259) and passives (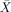 = 66.67%, s.e.m. = 9.89, t(5) = 1.69, P < 0.153;
= 66.67%, s.e.m. = 9.89, t(5) = 1.69, P < 0.153; 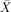 = 56.67%, s.e.m. = 10.54, t(5) = 0.63, P < 0.555). The RH patients had scores significantly above chance on actives (
= 56.67%, s.e.m. = 10.54, t(5) = 0.63, P < 0.555). The RH patients had scores significantly above chance on actives (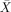 = 100%, s.e.m. = 0), subject‐relatives (
= 100%, s.e.m. = 0), subject‐relatives (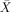 = 100%, s.e.m. = 0), object‐relatives (
= 100%, s.e.m. = 0), object‐relatives (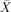 = 96.67%, s.e.m. = 5.77, t(2) = 14.0, P < 0.005) and passives (
= 96.67%, s.e.m. = 5.77, t(2) = 14.0, P < 0.005) and passives (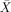 = 100%, s.e.m. = 0).
= 100%, s.e.m. = 0).
The grammaticality judgment part
We first tested the control participants, in order to ensure ceiling performance on the test materials by individuals whose language is intact. We then moved to the patients. The Broca's and Wernicke's patients had scores on the +MOV condition that did not significantly differ from chance (t(5) = 1.99, P = 0.103 and t(5) = −0.29, P = 0.785, respectively) and scores on the −MOV condition that were significantly better than chance (t(5) = 7.60, P = 0.001, t(5) = 3.90, P = 0.011, respectively). RH patient group's performance was significantly above chance on both the +MOV (t(2) = 26, P = 0.001) and the −MOV (t(2) = 58, P < 0.001) conditions (see Fig. 3).
Figure 3.
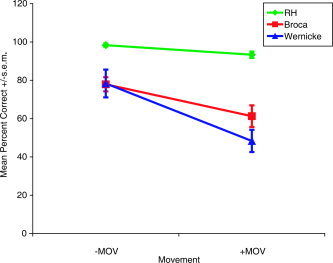
Mean percent correct grammaticality judgment (+SEM) broken down by absence (−MOV) or presence (+MOV) of movement and patient group. RH: right hemisphere lesioned patients. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Broca and Wernicke's aphasics performed worse on +MOV than −MOV (t(5) = −5.10, P = 0.004 and t(5) = −3.42, P = 0.019, respectively), whereas the RH patients' performance did not distinguish between the two conditions (t(2) = −3.46, P = 0.074).
DISCUSSION
This cross‐methodological study, in which both lesion‐based and fMRI results converged in distinguishing between binding and movement, found that neither movement‐driven activation of Broca's area or movement comprehension deficits in Broca's aphasics generalize to other syntactic dependency relations which tax WM. The on‐line analysis of movement and binding activated distinct regions of Broca's area (BA44 and BA45/47, respectively), the analysis of binding activated a right hemispheric region (RMFG), and both analyses activated distinct parts of left Wernicke's area. All three results have important implications.
The binding related activation of LIFG was more anterior and inferior to the region produced by movement. This pattern of distinct activation is inconsistent with either a syntactic WM or a general verbal WM account of Broca's area. It seems that as far as syntactic processing is concerned there is no way to generalize WM to a particular region. Moreover, the Broca's aphasics were not impaired on binding, as they were on movement. Thus, while the anatomical data we have for them does not specify whether or not BA45/47 were damaged, we can assert that the region critical to the comprehension of movement is not critical for the comprehension of binding. This finding implies that indeed the processing roles of these two regions are more distinct than similar.
While this study demonstrates that Broca's area has specificity, this does not mean that its functional role is exclusive to movement processing. Rather, it would appear from the empirical record that as Broca's area represents a large anatomical area, it houses multiple processing modules. Therefore, there could be a general WM module and a movement specific WM one, as well.
The modulation of RMFG by +BIND might suggest a syntactic process related to the ±binding contrast. However, the limited localizing information we have on our RH patients (see Supplementary Material at http://freud.tau.ac.il/~yosef1/) prevents us from making any assertions about whether or not the RMFG is critical to binding processing. Furthermore, the effect is due to significantly lower activations for sentences lacking a bound reflexive compared to those with a reflexive (see Fig. 1d) with the PSC for sentences with a bound reflexive being indistinguishable from rest. Little is known about right frontal lobe activity during rest in the context of on‐line sentence processing tasks, yet at least one previous grammaticality judgment study found RH activations that stand in a similar relation to rest. These results merit serious consideration. Perhaps, regions of the right hemisphere are typically deactivated during sentence processing and binding releases the RMFG from this deactivation. While it is not clear why reflexive binding implicates this area (whether through activation or deactivation), this finding is not accidental, as a parametric study with related contrasts has replicated this result [Santi and Grodzinsky, 2006]. This consistent result calls for more detailed investigation, necessary to understand what aspects of binding influence its activation and why. There are several possibilities for the deactivation, each bearing predictions for future testing. Potential reflexive‐induced prosodic differences between the +BIND and −BIND conditions might modulate the RH, known to be sensitive to sentence‐level prosody [Ross, 2000]. Also, semantic differences between verb‐argument complexes with and without reflexives might lead to this effect [Avrutin, 2006]. Be it as it may, linguistic notions seem necessary in order to specify the observed result.
The role and degree of involvement of Wernicke's region is less clear. It was activated by binding, but the Wernicke's aphasic patients were above chance in detecting binding violations.
All in all, our results indicate that a precise account of brain/language relations must make reference to linguistic theoretic terms. Moreover, it is the use of these in our design which enabled us to expose a new right hemispheric area that supports on‐line syntactic analysis.
Supporting information
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .
Acknowledgements
We would like to thank Robert Zatorre for fMRI design advice, Christine Rogers for fMRI data collection, Jennifer Greene for Aphasia data collection, Keith Worsley and Marc Bouffard for fMRI statistical/technical advice, and Dan Drai for advice on analyzing the aphasia behavioral data.
REFERENCES
- Amunts K, Schleicher A, Börgel U, Mohlberg H, Uylings HBM, Zilles K( 1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- Avrutin S( 2006): Weak syntax In: Grodzinsky Y, Amunts K, editors. Broca's Region. New York: Oxford University Press; pp 49–62. [Google Scholar]
- Ben‐Shachar M, Hendler T, Kahn I, Ben‐Bashat D, Grodzinsky Y( 2003): The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychol Sci 14: 433–440. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Palti D, Grodzinsky Y( 2004): Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336. [DOI] [PubMed] [Google Scholar]
- Bever TG, McElree B( 1988): Empty categories access their antecedents during comprehension. Linguist Inq 19: 35–43. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC( 1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Bandettini RA, O'Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR( 1996): Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Natl Acad Sci USA: 14878–14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D( 2001): Functional neuroimaging studies of syntactic processing. J Psycholinguist Res 30: 297–320. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G( 1998): Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci 10: 541–552. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters GS( 1999): Verbal working memory and sentence comprehension. Behav Brain Sci 22: 77–126. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Constable TR, Pugh KR, Berroya E, Mencl EW, Westerveld M, Ni W, Shankweiler D( 2004): Sentence complexity and input modality effects in sentence comprehension: An fMRI study. Neuroimage 22: 11–21. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M( 2001): Neural basis for sentence comprehension: Grammatical and short‐term memory components. Hum Brain Mapp 15: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drai D, Grodzinsky Y( 2006a): A new empirical angle on the variability debate: Quantitative neurosyntactic analyses of a large data set from Broca's Aphasia. Brain Lang 96: 117–128. [DOI] [PubMed] [Google Scholar]
- Drai D, Grodzinsky Y( 2006b): The variability debate: More statistics, more linguistics. Brain Lang 96: 157–170. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD( 2005): Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp 24: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y( 2000): The neurology of syntax: Language use without Broca's area. Behav Brain Sci 23: 1–21. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Finkel L( 1998): The neurology of empty categories: Aphasics' failure to detect ungrammaticality. J Cogn Neurosci 10: 281–292. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Wexler K, Chien YC, Marakovitz S, Solomon J( 1993): The breakdown of binding relations. Brain Lang 45: 396–422. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumaker EH, Smith EE, Koeppe RA, Awh E, Reuter‐Lorenz PA, Marshuetz C, Willis CR( 1998): The role of parietal cortex in verbal working memory. J Neurosci 18: 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA( 1992): A capacity theory of comprehension: Individual differences in working memory. Psychol Rev 99: 122–149. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR( 1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY( 2002): The brain circuitry of syntactic comprehension. Trends Cogn Sci 6: 350–356. [DOI] [PubMed] [Google Scholar]
- Lasnik H, Uriagereka J( 1988): A Course in GB Syntax: Lectures on Binding and Empty Categories. Cambridge, MA: The MIT Press. [Google Scholar]
- Love T, Oster E ( 2002): On the categorization of Aphasic typologies: The SOAP (A test of syntactic complexity). J Psycholinguist Res 31: 503–529. [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D( 1996): Coreference processing and levels of analysis in object‐relative constructions: Demonstration of antecedent reactivation with the cross‐modal priming paradigm. J Psycholinguist Res 25: 5–24. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici A( 2003): Functional MR imaging exposes differential brain responses to syntax and prosody during auditory sentence comprehension. J Neurolinguist 16: 277–300. [Google Scholar]
- Nakano Y, Felser C, Clahsen H( 2002): Antecedent priming at trace positions in Japanese long‐distance scrambling. J Psycholinguist Res 31: 531–571. [DOI] [PubMed] [Google Scholar]
- Nicol J, Swinney D( 1989): The role of structure in coreference assignment during sentence comprehension. J Psycholinguist Res 18: 5–19. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M( 2003): Prefrontal activity associated with working memory and episodic long‐term memory. Neuropsychologia 41: 378–389. [DOI] [PubMed] [Google Scholar]
- Röder B, Stock O, Neville H, Bien S, Rösler F( 2002): Brain activation modulated by the comprehension of normal and pseudo‐word sentences of different processing demands: A functional magnetic resonance imaging study. Neuroimage 15: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Ross ED( 2000): Affective prosody and the aprosodias In: Mesulam M‐M, editor. Principles of Behavioral and Cognitive Neurology. New York: Oxford University Press; pp 316–331. [Google Scholar]
- Santi A, Grodzinsky Y ( 2006): Interaction of Working Memory and Syntax in Broca's Area. San Francisco: Cognitive Neuroscience Society. [Google Scholar]
- Smith EE, Jonides J( 1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Pike BG( 2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S( 1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Traxler MJ, Pickering MJ( 1996): Plausibility and the processing of unbounded dependencies: An eye‐tracking study. J Mem Lang 35: 454–475. [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC( 2002): A general statistical analysis for fMRI data. Neuroimage 15: 1–15. [DOI] [PubMed] [Google Scholar]
- Zurif EB, Swinney D, Prather P, Solomon J, Bushell C( 1993): An on‐line analysis of syntactic processing in Broca's and Wernicke's aphasia. Brain Lang 45: 448–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .


