Abstract
Several studies report that patients with schizophrenia who experience auditory verbal hallucinations (AVH) tend to misidentify their own speech as that of somebody else. We tested the hypothesis that this tendency is associated with poor functional integration within the network of regions that mediate the evaluation of speech. Using functional magnetic resonance imaging, we measured brain responses from 11 schizophrenics with AVH, 10 schizophrenics without AVH, and 10 healthy controls. Stimuli comprised prerecorded words, which varied for their source (self, alien) and acoustic quality (undistorted, distorted). Participants had to indicate whether each word was spoken in their own or another person's voice via a button press. Using dynamic causal modeling, we estimated the impact of one region over another (“effective connectivity”) and how this was modulated by source and distortion. In controls and in patients without AVH, the connectivity between left superior temporal and anterior cingulate cortex was significantly greater for alien‐ than for self‐generated speech; in contrast, the reverse trend was found in schizophrenic patients with AVH. In conclusion, when patients with AVH appraise their own speech we find impaired functional integration between left superior temporal and anterior cingulate cortex. Although this finding is based on external rather than internal speech, the same mechanism may contribute to the faulty appraisal of inner speech that putatively underlies AVH. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: schizophrenia, auditory verbal hallucinations, speech misattribution, functional neuroimaging, dynamic causal modeling
INTRODUCTION
Healthy individuals have little difficulty in distinguishing their own voice from that of somebody else, even when the acoustic quality of external speech is reduced by the introduction of a pitch change. In contrast, patients with schizophrenia who experience auditory verbal hallucinations (AVH) are more likely to misidentify their own speech as being that of somebody else, especially when it is distorted [Allen et al., 2004; Johns and McGuire, 1999; Johns et al., 2001]. This finding has driven a number of neuroimaging studies, which have measured brain responses during the conscious appraisal of self‐ and alien‐generated speech in healthy individuals and schizophrenic patients with and without AVH [Allen et al., 2005, in press; Fu et al., 2005, under review]. When evaluating the source of distorted speech, healthy controls and nonhallucinating patients typically engage the superior temporal, inferior frontal, and anterior cingulate cortices. In contrast, patients with AVH demonstrate altered activation in the anterior cingulate and superior temporal regions bilaterally, suggesting that their tendency to misattribute their own speech to an external source is associated with abnormal responses in these regions.
The anterior cingulate, inferior frontal, and superior temporal regions are strongly and directly interconnected [Petrides and Pandya, 1988; Vogt and Pandya, 1987]. Furthermore, recent diffusion tensor imaging (DTI) studies have revealed differences in the orientation of white matter fibers between schizophrenic patients with AVH relative to both healthy individuals and patients without AVH [Hubl et al., 2004; Shergill et al., in press]. Abnormal activation in the anterior cingulate and superior temporal cortices in patients with AVH may thus reflect altered functional integration within the network of regions responsible for the appraisal of speech. This hypothesis is consistent with the idea that schizophrenia is better characterized in terms of abnormal neural interactions within a distributed network than in terms of specific, localized neural deficits [Bullmore et al., 1997; Fletcher et al., 1999; Friston and Frith, 1995; Gold and Weinberger, 1991; Honey et al., 2005; Lawrie et al., 2002; McGuire and Frith, 1996; Meyer‐Lindenberg et al., 2005; Schlosser et al., 2003; Shergill et al., 2003; Stephan et al., 2006; Whalley et al., 2005].
The aim of the present study was to investigate the neural interactions that mediate the explicit evaluation of self‐ and alien‐generated verbal stimuli in healthy individuals and schizophrenic patients with and without AVH. Using functional magnetic resonance imaging (MRI), we measured brain responses while participants were presented with a series of prerecorded words, which varied in terms of source (self, alien) and acoustic quality (undistorted, distorted) across trials. Participants were required to indicate whether each word was spoken in their own or another person's voice via a button press. Regional activations in the three experimental groups have been reported elsewhere [Allen et al., in press]. In brief, all three groups engaged a distributed network including bilateral superior temporal, inferior frontal, and anterior cingulate cortices. However, in controls and nonhallucinators relative to hallucinators, successful identification of self‐distorted speech was associated with increased activation in the anterior cingulate cortex. Here, we used dynamic causal modeling(DCM) [Friston et al., 2003; Mechelli et al., 2003] to estimate the influence that one region exerts over another (i.e., “effective connectivity”) and how this is modulated by source and acoustic quality, for each subject independently. The subject‐specific estimates were then combined in order to compare healthy individuals and schizophrenic patients with and without AVH.
Based on the previous reports of abnormal functional integration in schizophrenia [Fletcher et al., 1999; Honey et al., 2005; Lawrie et al., 2002; Meyer‐Lindenberg et al., 2005; Schlosser et al., 2003; Shergill et al., 2003; Whalley et al., 2005], we expected that (i) a number of functional connections would be impaired in patients regardless of the presence or absence of AVH. Such differences may reflect the intrinsic neuropathology of the disorder independent of its symptomatic manifestation. On the basis of the observation that patients with AVH are more likely to misidentify their own distorted speech as being that of somebody else [Allen et al., 2005, in press; Fu et al., 2005, under review], we then predicted that (ii) some functional connections would be specifically impaired in this group of patients. In particular, we tested the hypothesis that there would be altered functional integration between the anterior cingulate cortex and other components of the network of areas involved in discriminating self‐ from alien‐generated speech, including the inferior frontal and the superior temporal cortices [Allen et al., 2005, in press; Fu et al., 2005, under review].
MATERIALS AND METHODS
Subjects
All participants were right‐handed males who spoke English as their first language. All gave written informed consent for the procedure in accordance with protocols approved by the Local Research Ethics Committee (LREC). Ten healthy volunteers were recruited from the local community through advertisements. Those with a history of medical or psychiatric disorders, a drug or alcohol abuse problem, or a family history of psychiatric disorders were excluded. Eleven patients who had prominent and current auditory verbal hallucinations (AVH) and 10 patients who had psychotic symptoms other than verbal auditory hallucinations were recruited via the South London and Maudsley National Healthy Trust (SLAM). Although the two patient groups differed on hallucination ratings, they were matched on other positive symptoms, particularly, delusions (Table I). Hallucinators had been experiencing AVH for at least a week at the time of testing; in contrast, nonhallucinators had no previous history of hallucinations. All patients met the DSM‐IV criteria for a diagnosis of schizophrenia and were on regular doses of antipsychotic medication for at least 1 month prior to testing. Exclusion criteria included: (i) a second DSM‐IV Axis I or Axis II diagnosis (e.g. depression); (ii) neurological disorders (e.g. Parkinson's disease); (iii) significant cognitive deficits (i.e., IQ < 80); (iv) heavy alcohol consumption or illicit drug usage. The information on the demographic and clinical characteristics of the three experimental groups is given in Table I.
Table I.
Demographic and clinical characteristics of the three experimental groups (mean and standard deviation)
| Variable | Controls N = 10 | Nonhallucinators N = 10 | Hallucinators N = 11 | Group comparisons |
|---|---|---|---|---|
| Age (years) | 28.50 (4.37) | 34.78 (11.4) | 35.33 (6.63) | NS |
| Years of education | 13.88 (3.14) | 12.3 (1.64) | 11.22 (4.63) | NS |
| Premorbid IQ | 114 (4.35) | 99 (8.56) | 101 (8.98) | F = 13.50, P < 0.001 |
| Age of first onset | 21.31 (5.63) | 22.5 (5.13) | NS | |
| Duration of illness | 16.32 (12.42) | 12.33 (9.35) | NS | |
| AVH | 0 | 4.44 (0.72) | U = 0, P < 0.001 | |
| Other hallucinations | 0 | 0.82 (0.32) | NS | |
| Delusions | 4.15 (1.37) | 4.33 (0.86) | NS | |
| Formal thought disorder | 1.57 (1.15) | 0.95 (0.42) | NS | |
| Bizarre behavior | 0.73 | 0.55 | NS | |
| SAPS total | 6.38 (2.82) | 10.11 (1.2) | U = 10.5, P = 0.004 | |
| SANS total | 6.75 (5.51) | 6.22 (5.50) | NS | |
| SANS attentional problems | 1.83 (1.25) | 1.55 (1.01) | NS | |
| Typical:Atypical | 3:7 | 4:6 | X2 = 0.11, P = 0.73 | |
| Depression (CDSS) | 5.51 (6.77) | 8.00 (7.22) | NS |
The scale for the assessment of positive symptoms (SAPS) and the scale for the assessment of negative symptoms (SANS) measure symptoms experienced in the month prior to the interview. All subscales on SAPS/SANS are scored from 0 to 5 with five representing the most severe symptomatology. SAPS total refers to the mean of global scores for hallucination, delusion, bizarre behaviour, and formal thought disorder. SANS total refers to the mean of global scores for alogia, anhedonia, inappropriate affect, avolation, and affective flattening. Level of depression and premorbid IQ were assessed using the Calgary depression scale (CDSS) and the NART, respectively. “U” refers to the Mann‐Whitney Test.
NS, not significant.
Experimental Paradigm
The task required the subjects to indicate whether each word was spoken in their own or another person's voice via a button press. The stimulus set comprised 80 adjectives applicable to people (e.g., perfect, foul, and tall). All the words were mono or bisyllabic with a Thorndike‐Lorge frequency of > 50 [Gilhooly and Logie, 1980], and were selected from lists used in a previous study [McGuire et al., 1996]. The source of speech (self versus alien) and the acoustic quality (0 vs. −4 semitones) of the stimuli were manipulated within a factorial design. This resulted in four experimental conditions (self‐undistorted, self‐distorted, alien‐undistorted, and alien‐distorted) each comprising 20 words. The words were balanced for number of syllables and valence (i.e., amounts of emotional and neutral words) across experimental conditions. Stimuli were presented in an event‐related design and with an interstimulus interval between 4 and 12 s to minimize expectation bias.
Procedure
One hour before scanning, participants were asked to read a list of 80 words aloud in a neutral voice and were told that they did not need to remember them. This ensured that the experimental task relied on perceptual discrimination as opposed to source memory. The participants' speech was recorded by a computer on Cool Edit 2000 (for Windows). The experimenter then edited the set of recordings such that 40 of the words were replaced by a recording of the same word spoken in another person's voice, and 40 of the words were pitch‐shifted. The degree of pitch shift was −4 semitones, chosen because it made the speaker's voice harder to recognize without the speech becoming incomprehensible. Once they were in the scanner, participants were told that if they thought the speech they heard was their own, then they were asked to press a button corresponding to “Myself.” If unsure of its identity, they were asked to press a button corresponding to “unsure,” and if they thought the speech belonged to someone else, they were asked to press a button corresponding to “Not myself.” The type of response and the reaction time of each subject were recorded in a computer for subsequent behavioral analysis.
Data Acquisition
Images were acquired in a 1.5 T Magnet (Signa LX‐GE, Milwaukee) using a “compressed” acquisition [Hall et al., 1999] with TR of 1.2 s (0.8 s of silence), flip angle 80°, TE 40 ms, 64 × 64 pixels, field of view of 200 mm, slice thickness 7 mm, and interslice gap 0.7 mm. A total of 482 image volumes were acquired in two runs of 6 min, with each volume consisting of 14 axial slices parallel to the AC‐PC line. The compressed acquisition permitted presentation of each word in the absence of acoustic scanner noise.
Data Analysis
Preprocessing
Preprocessing of the functional volumes was performed using SPM2 software (http://www.fil.ion.ucl.ac.uk/spm), running under Matlab 6.5 (Mathworks, Sherbon, MA). All volumes from each subject were realigned using the first as reference and resliced with sync interpolation. The functional images were spatially normalized [Friston et al., 1995] to a standard MNI‐305 template using nonlinear‐ basis functions. Functional data were spatially smoothened with a 6‐mm full width at half maximum isotropic Gaussian kernel, to compensate for residual variability in functional anatomy after spatial normalization and to permit application of Gaussian random field theory for adjusted statistical inference.
Statistical parametric mapping
We performed a standard statistical analysis of regional responses in order to identify regions expressing significant activation during the evaluation of speech. To remove low‐frequency drifts, the data were high‐pass filtered using a set of discrete cosine basis functions with a cutoff period of 128 s. Each of the four experimental conditions (i.e., self‐undistorted; self‐distorted; alien undistorted; alien undistorted) was modeled independently by convolving the onset times with a synthetic hemodynamic response function. The parameter estimates were calculated for all brain voxels using the general linear model, and statistical parametric maps for each experimental condition were computed in a subject‐specific fashion.
Dynamic causal modeling
We used dynamic causal modeling (DCM) [Friston et al., 2003; Mechelli et al., 2003] as implemented in SPM2 software. The aim of DCM is to estimate, and make inferences about, the influence that one neural system exerts over another and how this is affected by the experimental context. In DCM, a reasonably realistic but simple neuronal model of interacting neural regions is constructed. DCM uses a previously validated biophysical model of functional MRI measurements to estimate the underling neuronal activity from the observed hemodynamic response [Friston et al., 2000]. The estimated underlying neuronal activity is then used to derive the connectivity parameters, as described elsewhere [Friston et al., 2003]. Two sets of parameters were of particular interest: (i) “intrinsic connections” that characterize the coupling between regions irrespective of stimulus type and (ii) “bilinear terms” that characterize changes in associated activity caused by source and acoustic quality manipulations.
For each subject, a dynamic causal model was constructed, which comprised five regions, which expressed significant activation during the evaluation of self‐ and alien‐generated speech when compared with the rest. To account for individual differences, we derived the exact coordinates of the regions from the local maxima of the subject‐specific statistical parametric maps within 16 mm of the group‐maxima. Regions included the left superior temporal [mean coordinates (x, y, z): −60 ± 4, −32 ± 2, 12 ± 4], right superior temporal [mean coordinates (x, y, z): 54 ± 4, −26 ± 4, 6 ± 4], left inferior frontal [mean coordinates (x, y, z): −34 ± 4, 22 ± 2, −2 ± 4], right inferior frontal [mean coordinates (x, y, z): 34 ± 4, 24 ± 4, −4 ± 2], and anterior cingulate cortex [mean coordinates (x, y, z): −3 ± 4, 22 ± 4, 26 ± 4]. Regions were defined as 6‐mm spheres and regional activities were extracted in terms of principal eigenvariate. In addition, the network comprised forward and backward connections (i.e., intrinsic connections) between all regions as depicted in Figure 1. Additional connectivity parameters (i.e., bilinear terms) were specified to look at the influence of source and distortion on all backward and forward connections. The stimulus function, which encoded the auditory presentation of all auditory words, entered the dynamic causal model through the sensory area, i.e., superior temporal cortex. The resulting perturbation was then allowed to propagate throughout the model via interconnections between this region and the remaining nodes.
Figure 1.
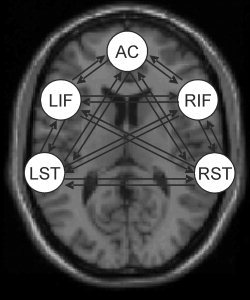
The dynamic causal model that was used to investigate the interregional coupling in hallucinators, nonhallucinators, and healthy controls. The model included five regions (i.e., left superior temporal, right superior temporal, left inferior frontal, right inferior frontal, and dorsal anterior cingulate cortex) and all possible forward and backward connections amongst them.
The forward and backward “intrinsic connections” (which characterize the coupling between regions irrespective of source and distortion level) and the “bilinear terms” (which capture how the intrinsic connections vary as a function of distortion and source) were estimated for each subject independently. In DCM, the units of connections are per unit time and therefore correspond to rates: a strong connection means an influence that is expressed quickly or with a small time constant. A positive (i.e., greater than zero) intrinsic connection indicates that an increase in activity in the “source” region is associated with an increase in activity in the “target” region. Conversely, a negative (i.e., smaller than zero) intrinsic connection indicates that an increase in activity in the “source” region is associated with a decrease in activity in the “target” region. The subject‐specific estimates were then entered into an ANOVA in SPSS in order to identify significant differences amongst the three experimental groups. One potential problem with the investigation of neuronal interactions with DCM and similar approaches such as structural equation modeling is that the number of connectivity variables dramatically scales up as the number of nodes increases. For instance, our dynamic causal model of five regions of interest resulted in a total of 20 intrinsic connections. A Bonferroni correction for multiple comparisons would have been inappropriate, because statistical inferences were highly correlated rather than independent. In the absence of any established procedure, we attempted to control for false positive rate in two ways. First, we used a relatively conservative statistical threshold of P < 0.01, which yield an expected false positive rate of 1%; effects that reached significance at P < 0.05 were also reported for completeness but were not discussed. Second, we restricted our statistical inferences to 12 connections of interest between anterior cingulate and superior temporal cortices; between anterior cingulate and inferior frontal cortices; between superior temporal and inferior temporal cortices (see Table II for complete list). These connections were chosen based on experimental evidence from previous studies of regional responses [Allen et al., 2005, in press; Fu et al., under review] and neuronal interactions [Fletcher et al., 1999; Lawrie et al., 2002] in schizophrenia. Once significant differences amongst the three experimental groups were identified using the ANOVA, post hoc two‐tailed t‐tests in SPSS were used to better characterize the intrinsic connections and bilinear terms.
Table II.
Intrinsic connections in controls, nonhallucinators, and hallucinators
| Intrinsic connection | Controls N = 10 | Nonhallucinators N = 10 | Hallucinators N = 11 | Group comparisons (P‐value) | ||
|---|---|---|---|---|---|---|
| Intrinsic connection | Effect of distortion | Effect of source | ||||
| L inf front → L sup temp | −0.03 (0.28) | −0.01 (0.04) | −0.03 (0.06) | 0.95 | 0.53 | 0.38 |
| L sup temp → L inf front | 0.22 (0.27) | 0.10 (0.12) | 0.12 (0.19) | 0.38 | 0.29 | 0.54 |
| L sup temp → ant cingulate | 0.34 (0.18) | 0.05 (0.11) | 0.11 (0.21) | 0.00 | 0.03 | 0.00 |
| Ant cingulate → L sup temp | 0.14 (0.32) | 0.02 (0.07) | −0.03 (0.07) | 0.11 | 0.40 | 0.70 |
| L inf front → ant cingulate | 0.08 (0.22) | 0.00 (0.08) | 0.04 (0.07) | 0.42 | 0.11 | 0.90 |
| Ant cingulate → L inf front | 0.12 (0.18) | 0.03 (0.10) | 0.03 (0.07) | 0.27 | 0.81 | 0.43 |
| R inf front → R sup temp | −0.04 (0.18) | 0.02 (0.06) | −0.03 (0.07) | 0.49 | 0.08 | 0.06 |
| R sup temp → R inf front | 0.20 (0.21) | 0.10 (0.12) | 0.12 (0.11) | 0.37 | 0.29 | 0.29 |
| R sup temp → ant cingulate | 0.30 (0.28) | 0.04 (0.17) | 0.09 (0.11) | 0.02 | 0.05 | 0.11 |
| Ant cingulate → R sup temp | 0.12 (0.18) | 0.03 (0.09) | 0.03 (0.07) | 0.23 | 0.80 | 0.49 |
| Ant cingulate → R inf front | 0.10 (0.10) | 0.04 (0.13) | 0.06 (0.09) | 0.39 | 0.89 | 0.25 |
| R inf front → Ant cingulate | 0.02 (0.13) | 0.03 (0.14) | 0.04 (0.07) | 0.90 | 0.06 | 0.87 |
We report the group means and standard deviations (in brackets), with values significantly greater than zero highlighted in bold (one‐sample t‐tests; P < 0.05). A series of ANOVAs were used to identify group differences in intrinsic connectivity and in the effects of distortion and source respectively. We report the P‐values of these group differences with significant effects highlighted in bold. R, Right; L, Left; inf, inferior; sup, superior; ant, anterior; font, frontal; temp, temporal.
RESULTS
Behavioral Data
The behavioral results from these subjects have been reported in detail elsewhere [Allen et al., 2005, in press]. In brief, there were significant effects of source (F = 6.00, df = 1.28, P = 0.02), distortion (F = 12.36, df = 1.28, P = 0.002), and group (F = 6.18, df = 1.28, P = 0.006). The interaction between group and distortion did not reach significance (F = 0.61, df = 2.28, P = 0.54). In contrast, there was a significant interaction between source of speech and group (F = 3.50, df = 2.28, P = 0.04). A post‐hoc one‐way ANOVA revealed a significant difference between groups in the self‐speech condition (F = 11.24, df = 2.30, P < 0.001). Specifically, patients with AVH misidentified their own speech as being that of somebody else more often than both healthy controls (P = 0.001) and patients without AVH (P = 0.001).
Dynamic Causal Modeling
We constructed a dynamic causal model that included anterior cingulate, bilateral inferior frontal, and bilateral superior temporal cortices (Fig. 1). We then estimated the influence that one region exerted over another and how this was modulated by the source and acoustic quality of the stimuli, respectively. Although we used a statistical threshold of P < 0.01, we also report (but do not discuss) effects that reached significance at P < 0.05 for completeness.
Intrinsic connections
Intrinsic connections refer to the impact that one region exerts over another irrespective of the level of distortion and the source of speech. Using our statistical threshold of P < 0.01, we found that the intrinsic connection from left superior temporal cortex to the anterior cingulate differed across experimental groups (ANOVA; F = 7.7, df = 2.28, P = 0.002). Post hoc, two‐tailed t‐tests revealed that this connection was stronger in healthy controls than both hallucinators (two‐sample t‐test; P = 0.017) or nonhallucinators (two‐sample t‐test; P < 0.001), but did not differ between patient groups.
When lowering the statistical threshold to P < 0.05, we found that the connection from right superior temporal cortex to anterior cingulate differed across the three experimental groups (ANOVA; F = 4.5, df = 2.28, P = 0.021). This connection was stronger in healthy controls than both hallucinators (two‐sample t‐test; P = 0.044) and nonhallucinators (two‐sample t‐test; P = 0.027), and was not significantly different between patient groups.
We finally report that the connections from left superior temporal to left inferior frontal cortex and from right superior temporal to right inferior frontal cortex were significantly greater than zero in all three experimental groups (one‐sample t‐tests; P < 0.05). However, the strength of these connections did not differ significantly between the groups, even at a less conservative statistical threshold (ANOVA; P > 0.1). Thus, these intrinsic connections were similarly, positively engaged during task performance in the three experimental groups (see Table II and Fig. 2a for details).
Figure 2.
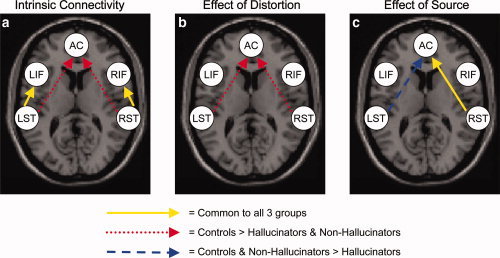
Results of the analysis of effective connectivity with dynamic causal modeling (DCM) (with a statistical threshold of P < 0.05). (a) Significant effective connectivity irrespective of source and distortion; (b) Effect of distortion on the effective connectivity: increased coupling for undistorted than distorted speech; (c) Effect of source on the effective connectivity: increased coupling for alien‐ than self‐generated speech. Solid arrows in yellow indicate effects that are significant in all three experimental groups; dotted arrows in red indicate effects that are stronger in controls than in either group of patients; broken arrows in blue indicate effects, which are stronger in healthy controls and nonhallucinators than in hallucinators. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effect of distortion on the intrinsic connectivity
At the statistical threshold of P < 0.01, we did not detect any group‐dependent effect of distortion on the intrinsic connectivity. At a lower statistical threshold, we found that the effect of distortion on the connections from left and right superior temporal cortex to anterior cingulate differed across the three experimental groups (ANOVA; P < 0.05). Exploration of the group‐specific bilinear terms revealed that these intrinsic connections were significantly stronger for undistorted than distorted stimuli in healthy controls (one‐sample t‐test; P < 0.05) but not in either group of patients. Post hoc two‐sample t‐tests confirmed that the effect of distortion on these connections was significantly stronger in controls than both hallucinators (P < 0.05) and nonhallucinators (P < 0.05), but did not differ between the two groups of patients. Thus, distortion has a greater effect on the functional coupling between these regions in healthy subjects than in schizophrenic patients, irrespective of whether they were prone to AVH (Table II and Fig. 2b).
Effect of source on the intrinsic connectivity
At the statistical threshold of P < 0.01, we found that the effect of source on the left superior temporal cortex to the anterior cingulate differed across the three experimental groups (ANOVA; P = 0.006). Exploration of the group‐specific bilinear terms revealed that this intrinsic connection was stronger for alien‐ than self‐generated speech in healthy controls (one‐sample t‐test; P = 0.030) and in patients without AVH (one‐sample t‐test; P = 0.055) but not in patients with AVH, who expressed the reverse trend (one‐sample t‐test; P = 0.046). Post hoc two‐sample t‐tests confirmed that the effect of source on this connection was significantly stronger in controls relative to hallucinators (P = 0.019) and in nonhallucinators relative to hallucinators (P = 0.046), but did not differ between controls and nonhallucinators. Thus, the effect of source on the functional coupling from left superior temporal cortex to the anterior cingulate was specifically impaired in patients with AVH. At a more liberal statistical threshold (P < 0.05), there were no additional intrinsic connections that were affected by source differentially across the three experimental groups.
We also found that the connection from right superior temporal cortex to the anterior cingulate was stronger for alien‐ than self‐generated speech in all three experimental groups (one‐sample t‐tests; P < 0.05), and this effect did not differ significantly between the groups, even with a less conservative statistical threshold (ANOVA; P > 0.1). Thus, the strength of this intrinsic connection was modulated by source to a similar extent in each experimental group (Table II and Fig. 2c).
DISCUSSION
Patients with AVH tend to make more misattribution errors when they hear their own distorted voice when compared with schizophrenic patients without AVH and healthy controls [Allen et al., 2004; Johns and McGuire, 1999; Johns et al., 2001]. This misattribution is thought to be the fundamental deficit underlying AVH [Frith, 1992; Frith and Done, 1988; Garety et al., 2001]. Here, we combined functional MRI with DCM [Friston et al., 2003; Mechelli et al., 2003] to investigate neural interactions during the conscious appraisal of self‐ and alien‐generated speech in healthy controls and patients with and without AVH.
Our first finding was that the intrinsic connection from left superior temporal cortex to the anterior cingulate was impaired in patients relative to healthy controls irrespective of the presence or absence of AVH. This impairment may thus reflect an intrinsic neuropathology of the disorder, which does not depend on the presence or absence of AVH. An alternative possibility is that it might reflect differences in IQ between controls and patients or neurophysiological changes associated with antipsychotic medication [Honey and Bullmore, 2004]. However, there have been no studies so far reporting that IQ and antipsychotic medication have a specific influence on the coupling between these particular regions.
Our second finding was that the intrinsic connection between left superior temporal and anterior cingulate cortex was modulated by source of speech in patients without AVH and healthy controls but not in patients with AVH who expressed the reverse trend. This difference cannot be explained by potential confounds such as age, age at illness onset, illness duration, premorbid IQ, positive symptoms other than AVH, negative symptoms, and type of medication since the two patient groups were carefully matched for these factors (see Table I). The results are therefore consistent with our hypothesis that the tendency to misattribute one's own distorted speech is associated with altered functional integration between the superior temporal cortex and more rostral parts of the network. The results also suggest that this impairment may be specific to the left hemisphere, consistent with the reports of predominant left‐hemisphere contribution to the generation of AVH [David, 1999; Weiss and Heckers, 1999] and left‐lateralized abnormalities in the white matter fibers of patients with AVH [Hubl et al., 2004; Shergill et al., in press]. Nevertheless, right‐lateralized regions have also been implicated in AVH [Seal et al., 2004].
Both the left superior temporal cortex and the anterior cingulate cortex have previously been implicated in the misattribution of self‐ and alien‐generated speech. For instance, the left superior temporal cortex is more active during the processing of alien‐ relative to self‐generated speech in healthy controls and patients without AVH but not in patients with AVH [Allen et al., 2005, in press; Fu et al., 2005, under review]. This region has also been associated with AVH in severalstructural [Flaum et al., 1995; Levitan et al., 1999; Shapleske et al., 2001] and functional [Lennox et al., 2000; Shapleske et al., 2001; Shergill et al., 2000; Woodruff et al., 1997] neuroimaging studies. Similarly, the anterior cingulate cortex is activated when subjects process distorted relative to undistorted speech in healthy controls and patients without AVH but not in patients with AVH [Allen et al., in press]. This region has been implicated in processing conflict between competing stimuli or responses [Carter et al., 1998] and has been associated with schizophrenia in several neurophysiological [Benes et al., 1987, 1992] and neuroimaging [Dolan et al., 1995; Fletcher et al., 1999] studies. Our investigation extends these findings by demonstrating that the functional integration between superior temporal cortex and the dorsal part of the anterior cingulate cortex is specifically impaired during the evaluation of self‐ and alien‐generated speech in patients with AVH. This finding provides support to the idea that dysfunction within the temporo‐cingulate network might lead to false auditory perceptions in schizophrenia [Hunter et al., 2006]. However, further studies are required to better characterize the putative cognitive function that is subserved by this temporo‐cingulate network.
The present investigation was motivated by the idea that schizophrenia is best characterized in terms of abnormal neuronal interactions within a distributed network of regions rather than localized neuronal deficits. The results are consistent with previous studies that found impaired functional integration in schizophrenia [Fletcher et al., 1999; Honey et al., 2005; Lawrie et al., 2002; Meyer‐Lindenberg et al., 2005; Schlosser et al., 2003; Shergill et al., 2003]. However, our investigation has two novel aspects. First, most of previous studies examined patients recruited on the basis of their diagnosis of schizophrenia, but whose symptom profile was unspecified and thus varied within the group. These studies could not therefore establish the extent to which differences in functional integration between patients and volunteers were related to the disorder or to specific symptoms [Honey et al., 2005]. The results of our investigation indicate that impaired functional integration in schizophrenia may be related to the expression of specific symptoms, such as AVH. Second, previous studies have used methods of “functional connectivity,” which identify temporal correlations between neuronal responses in distinct regions but cannot indicate the direction of the connectivity [Friston et al., 1993a]. Although these studies typically postulated a breakdown in top‐down connectivity in schizophrenia, most could not test this hypothesis with the exception of Schlosser et al. [2003]. In contrast, the use of DCM allowed the investigation of “effective connectivity,” which refers to the influence of one neural system exerts over another either directly or indirectly [Friston et al., 1993b]. Interestingly, our analysis revealed a breakdown in the connectivity from left superior temporal cortex to anterior cingulate cortex. This finding needs to be replicated but nevertheless is conceptually important as it suggests that some of the neuronal abnormalities in schizophrenia may reflect a disruption of bottom‐up as opposed to top‐down neural mechanisms. This appears to be inconsistent with cognitive models, which typically postulate impaired self‐monitoring during speech generation [Frith, 1992; Frith and Done, 1988] or faulty appraisal of auditory experiences [Garety et al., 2001] as the underlying basis of AVH.
It should be noted that inferences about neuronal interactions as revealed by DCM are insensitive to abnormal regional responses per se [Friston et al., 2003]. For example, if a patient group activates two or more regions to half the degree of the activation in the control group, the effective connectivity may be exactly the same. Likewise, if a patient group activates two or more regions to the same degree as the control group, the effective connectivity may differ due to different temporal dynamics of neuronal responses. A previous analysis of the regional activations in the present data revealed abnormal anterior cingulate responses in patients with AVH during successful recognition of self‐distorted speech [Allen et al., in press]. However, this observation alone could not establish whether neuronal interactions were also impaired and, most importantly, which effective connections were implicated in particular.
One potential problem with the investigation of brain responses in terms of neuronal interactions is that the number of connectivity variables dramatically scales up as the number of nodes increases. For instance, here, we used a dynamic causal model, which comprised five regions of interest and a total of 20 intrinsic connections that were tested using a series of one‐way ANOVA. A Bonferroni correction for multiple comparisons would have been inappropriate because statistical inferences were highly correlated. In the absence of any established procedure, we attempted to control for false positive rate by (i) using a statistical threshold of P < 0.01 instead of the “standard” P < 0.05; (ii) restricting our inferences to those (12) connections of interest between anterior cingulate, superior temporal, and inferior frontal cortices within each hemisphere. An alternative approach would be the use of Bayesian rather than classical statistics to compare different experimental groups [see Bitan et al., 2005 for a recent example]. The use of a Bayesian framework avoids the need for multiple comparison adjustment when making inferences about the connection parameters [Friston et al., 2003].
Another potential problem with the present investigation is the relatively small number of subjects within each experimental group. This limitation was due to the difficulty of recruiting patients with stable and active AVH and patients without a history of AVH who were matched on other positive symptoms. The limited number of subjects does not invalidate our significant results because we used a second‐level analysis that identified group‐related differences consistent across subjects. However, we cannot exclude the possibility that the small sample size resulted in false negatives or marginally significant effects.
It also should be emphasized that DCM does not assume direct anatomical connections between regions of interest. For instance, the intrinsic connectivity from the left superior temporal cortex to the anterior cingulate might be mediated by indirect anatomical connections for instance via dorsolateral prefrontal cortex, which was not included in our dynamic causal model. However, this would not reduce the theoretical interest of our findings: even if other brain regions mediate the coupling between left superior temporal cortex and anterior cingulate, our investigation establishes that these two areas are functionally associated when subjects appraise the source of self‐generated and alien speech and that the normal modulation of this association by speech source is impaired in patients with AVH. Although this finding is derived from a study involving external speech, we speculate that the same impairment in effective connectivity may affect the processing of inner speech, and hence contribute to the faulty appraisal of inner speech that is thought to underlie AVH.
REFERENCES
- Allen PP,Johns LC,Fu CH,Broome MR,Vythelingum GN,McGuire PK ( 2004): Misattribution of external speech in patients with hallucinations & delusions. Schizophr Res 69: 277–287. [DOI] [PubMed] [Google Scholar]
- Allen PP,Amaro E,Fu CH,Williams SC,Brammer M,Johns LC,McGuire PK ( 2005): Neural correlates of the misattribution of self‐generated speech. Hum Brain Mapp 26: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PP,Amaro E,Fu CHY,Williams SCR,Brammer M,Johns LC,McGuire P (in press): Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry. [DOI] [PubMed] [Google Scholar]
- Benes FM,Majocha R,Bird ED,Marotta CA ( 1987): Increased vertical axon numbers in cingulate cortex of schizophrenics. Arch Gen Psychiatry 44: 1017–1021. [DOI] [PubMed] [Google Scholar]
- Benes FM,Vincent SI,Alsterberg G,Bird ED,SanGiovanni JP ( 1992): Increased GABAA receptor binding in superficial layers of schizophrenic cingulate cortex. J Neurosci 12: 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T,Booth JR,Choy J,Burman DD,Gitelman DR,Mesulam MM ( 2005): Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci 25: 5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET,Frangou S,Murray RM ( 1997): The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res 28: 143–156. [DOI] [PubMed] [Google Scholar]
- Carter CS,Braver TS,Barch DM,Botvinick MM,Noll D,Cohen JD ( 1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- David AS ( 1999): Auditory hallucinations: Phenomenology, neuropsychology and neuroimaging update. Acta Psychiatr Scand Suppl 395: 95–104. [DOI] [PubMed] [Google Scholar]
- Dolan RJ,Fletcher P,Frith CD,Friston KJ,Frackowiak RSJ,Grasby PJ ( 1995): Dopaminergic modulation of an impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 378: 180–182. [DOI] [PubMed] [Google Scholar]
- Flaum M,Swayze VW II,O'Leary DS,Yuh WT,Ehrhardt JC,Arndt SV,Andreasen NC ( 1995): Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 152: 704–714. [DOI] [PubMed] [Google Scholar]
- Fletcher P,McKenna PJ,Friston KJ,Frith CD,Dolan RJ ( 1999): Abnormal cingulate modulation of fronto‐temporal connectivity in schizophrenia. Neuroimage 9: 337–342. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Frith CD ( 1995): Schizophrenia: A disconnection syndrome? Clin Neurosci 3: 89–97. [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Liddle PF,Frackowiak RSJ ( 1993a): Functional connectivity: The principal component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13: 5–14. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Frith CD,Frackowiak RSJ ( 1993b): Time‐dependent changes in effective connectivity measured with PET. Hum Brain Mapp 1: 69–80. [Google Scholar]
- Friston KJ,Ashburner J,Frith CD,Poline J‐B,Heather JD,Frackowiak RSJ ( 1995): Spatial registration and normalization of images. Hum Brain Mapp 2: 1–25. [Google Scholar]
- Friston KJ,Mechelli A,Turner R,Price CJ ( 2000): Nonlinear responses in fMRI: The Balloon model, Volterra kernels and other hemodynamics. Neuroimage 12: 466–477. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Harrison L,Penny W ( 2003): Dynamic causal modelling. Neuroimage 19: 1273–1302. [DOI] [PubMed] [Google Scholar]
- Frith CD ( 1992): The Cognitive Neuropsychology of Schizophrenia. Hove: Lawrence Earlbaum. [Google Scholar]
- Frith CD,Done DJ ( 1988): Towards a neuropsychology of schizophrenia. Br J Psychiatry 153: 437–443. [DOI] [PubMed] [Google Scholar]
- Fu CH,Vythelingum GN,Brammer MJ,Williams SC,Amaro E Jr,Andrew CM,Yágüez L,van Haren NE,Matsumoto K,McGuire PK ( 2005): An fMRI study of verbal self‐monitoring: Neural correlates of auditory verbal feedback. Cereb Cortex 16: 969–977. [DOI] [PubMed] [Google Scholar]
- Fu CHY,Vythelingum GN,Brammer MJ,Abel KM,Andrew C,Williams SCR,Yágüez L,Allin MPG,van Haren NEM,Matsumoto K,McGuire PK (under review): Who said that? Neural basis of external misattribution in schizophrenia.
- Garety PA,Kuipers E,Fowler D,Freeeman D,Bebbington PE ( 2001): A cognitive model of the positive symptoms of psychosis. Psychol Med 31: 189–195. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ,Logie RH ( 1980): Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1,944 words. Behav Res Methods Instrum 12: 365–427. [Google Scholar]
- Gold JM,Weinberger DR ( 1991): Frontal lobe structure, function and connectivity in schizophrenia In: Kerwin R, Dawbarn D, McCulloch J, Tamminga C, editors. Cambridge Medical Reviews: Neurobiology and Psychiatry. Cambridge: Cambridge University Press; pp 39–59. [Google Scholar]
- Hall DA,Haggard MP,Akeroyd MA,Palmer AR,Summerfield AQ,Elliott MR,Gurney EM,Bowtell RW ( 1999): “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey G,Bullmore E ( 2004): Human pharmacological MRI. Trends Pharmacol Sci 25: 366–374. [DOI] [PubMed] [Google Scholar]
- Honey GD,Pomarol‐Clotet E,Corlett PR,Honey RA,McKenna PJ,Bullmore ET,Fletcher PC ( 2005): Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain 128: 2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D,Koenig T,Strik W,Federspiel A,Kreis R,Boesch C,Maier SE,Schroth G,Lovblad K,Dierks T ( 2004): Pathways that make voices: White matter changes in auditory hallucinations. Arch Gen Psychiatry 61: 658–668. [DOI] [PubMed] [Google Scholar]
- Hunter MD,Eickhoff SB,Miller TW,Farrow TF,Wilkinson ID,Woodruff PW ( 2006): Neural activity in speech‐sensitive auditory cortex during silence. Proc Natl Acad Sci USA 103: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC,McGuire PK ( 1999): Verbal self‐monitoring and auditory hallucinations in schizophrenia. Lancet 353: 469–470. [DOI] [PubMed] [Google Scholar]
- Johns LC,Rossell S,Frith C,Ahmad F,Hemsley D,Kuipers E,McGuire PK ( 2001): Verbal self‐monitoring and auditory verbal hallucinations in patients with schizophrenia. Psychol Med 31: 705–715. [DOI] [PubMed] [Google Scholar]
- Lawrie SM,Buechel C,Whalley HC,Frith CD,Friston KJ,Johnstone EC ( 2002): Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51: 1008–1011. [DOI] [PubMed] [Google Scholar]
- Lennox BR,Park SB,Medley I,Morris PG,Jones PB ( 2000): The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res 100: 13–20. [DOI] [PubMed] [Google Scholar]
- Levitan C,Ward PB,Catts SV ( 1999): Superior temporal gyral volumes and laterality correlates of auditory hallucinations in schizophrenia. Biol Psychiatry 46: 955–962. [DOI] [PubMed] [Google Scholar]
- McGuire PK,Frith CD ( 1996): Disordered functional connectivity in schizophrenia. Psychol Med 26: 663–667. [DOI] [PubMed] [Google Scholar]
- McGuire PK,Silbersweig DA,Frith CD ( 1996): Functional neuroanatomy of verbal self monitoring. Brain 119: 907–917. [DOI] [PubMed] [Google Scholar]
- Mechelli A,Price CJ,Noppeney U,Friston KJ ( 2003): A dynamic causal modelling study of category effects: Bottom‐up or top‐down mediation? J Cogn Neurosci 15: 925–934. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg AS,Olsen RK,Kohn PD,Brown T,Egan MF,Weinberger DR,Berman K ( 2005): Regionally specific disturbance of dorsolateral prefrontal‐hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386. [DOI] [PubMed] [Google Scholar]
- Petrides M,Pandya DN ( 1988): Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol 273: 52–66. [DOI] [PubMed] [Google Scholar]
- Schlosser R,Gesierich T,Kaufmann B,Vucurevic G,Hunsche S,Gawehn J,Stoeter P ( 2003): Altered effective connectivity during working memory performance in schizophrenia: A study with fMRI and structural equation modeling. Neuroimage 19: 751–763. [DOI] [PubMed] [Google Scholar]
- Seal M,Aleman A,McGuire PK ( 2004): Alluring imagery, unanticipated speech and deceptive memory: Neurocognitive models of auditory verbal hallucinations in schizophrenia. Cogn Neuropsychiatry 9: 43–72. [DOI] [PubMed] [Google Scholar]
- Shapleske J,Rossell SL,Simmons A,David AS,Woodruff PW ( 2001): Are auditory hallucinations the consequence of abnormal cerebral lateralization? A morphometric MRI study of the sylvian fissure and planum temporale. Biol Psychiatry 49: 685–693. [DOI] [PubMed] [Google Scholar]
- Shergill SS,Bullmore E,Simmons A,Murray R,McGuire P ( 2000): Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry 157: 1691–1693. [DOI] [PubMed] [Google Scholar]
- Shergill SS,Brammer MJ,Fukuda R,Williams SC,Murray RM,McGuire PK ( 2003): Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry 182: 525–531. [DOI] [PubMed] [Google Scholar]
- Shergill SS,Kanaan RAA,Chitnis XA,O'Daly O,Jones DK,Frangou S,Williams SCR,Horward RJ,Barker GJ,Murray R,McGuire PK (in press): A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. [DOI] [PubMed] [Google Scholar]
- Stephan KE,Baldeweg T,Friston KJ ( 2006): Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry 59: 929–939. [DOI] [PubMed] [Google Scholar]
- Stephan KE,Harrison LM,Kiebel SJ,David O,Penny WD,Friston KJ: Dynamic causal models of neural system dynamics: Current state and future extensions. J Biosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA,Pandya DN ( 1987): Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262: 271–289. [DOI] [PubMed] [Google Scholar]
- Weiss AP,Heckers S ( 1999): Neuroimaging of hallucinations: A review of the literature. Psychiatry Res 92: 61–74. [DOI] [PubMed] [Google Scholar]
- Whalley HC,Simonotto E,Marshall I,Owens DG,Goddard NH,Johnstone EC,Lawrie SM ( 2005): Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain 128: 2097–2108. [DOI] [PubMed] [Google Scholar]
- Woodruff PW,Wright IC,Bullmore ET,Brammer M,Howard RJ,Williams SC Shapleske J,Rossell S,David AS,McGuire PK,Murray RM ( 1997): Auditory hallucinations and the temporal cortical response to speech in schizophrenia: A functional magnetic resonance imaging study. Am J Psychiatry 154: 1676–1682. [DOI] [PubMed] [Google Scholar]


