Abstract
Modern video games represent highly advanced virtual reality simulations and often contain virtual violence. In a significant amount of young males, playing video games is a quotidian activity, making it an almost natural behavior. Recordings of brain activation with functional magnetic resonance imaging (fMRI) during gameplay may reflect neuronal correlates of real‐life behavior. We recorded 13 experienced gamers (18–26 years; average 14 hrs/week playing) while playing a violent first‐person shooter game (a violent computer game played in self‐perspective) by means of distortion and dephasing reduced fMRI (3 T; single‐shot triple‐echo echo‐planar imaging [EPI]). Content analysis of the video and sound with 100 ms time resolution achieved relevant behavioral variables. These variables explained significant signal variance across large distributed networks. Occurrence of violent scenes revealed significant neuronal correlates in an event‐related design. Activation of dorsal and deactivation of rostral anterior cingulate and amygdala characterized the mid‐frontal pattern related to virtual violence. Statistics and effect sizes can be considered large at these areas. Optimized imaging strategies allowed for single‐subject and for single‐trial analysis with good image quality at basal brain structures. We propose that virtual environments can be used to study neuronal processes involved in semi‐naturalistic behavior as determined by content analysis. Importantly, the activation pattern reflects brain‐environment interactions rather than stimulus responses as observed in classical experimental designs. We relate our findings to the general discussion on social effects of playing first‐person shooter games. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: functional magnetic resonance imaging, violent video games, virtual reality, complex behavior, anterior cingulate cortex, amygdala
INTRODUCTION
Ohler and Nieding [2006] suggested that one of the biological functions of playing is to rehearse real situations without risk. Video games use virtual reality (VR) to allow playful rehearsal of semi‐natural situations. Indeed, latest‐generation video games achieve a near to realistic depiction of environments and allow for social interactions. Further, many young members of modern societies spend many hours weekly in these virtual worlds; e.g., in the United States at least 5% of male adolescents play more than 20 hrs/week [Kaiser Family Foundation, 2002]. As such, having a subject play video games within a functional magnetic resonance imaging (fMRI) scanner can be considered an ideal paradigm to study behavior in immobilized subjects
Recently, an increasing number of neuroscience studies have departed from paradigms with simple stimulus response designs. For instance, Touryan et al. [2005] studied responses of cells in the visual cortex of anesthetized cats to natural visual scenes. Complex cells responded faster to natural stimuli than random pattern but kept their spatial tuning. Using fMRI, Hasson et al. [2004] compared neural responses to the viewing of a movie between subjects. The common pattern was thought to reflect activation determined by the viewing of semi‐natural scenes. Notably, when independent component analysis (ICA) was run on the dataset, patterns were extracted that correlated with the appearance of specific objects such as hands. Bartels and Zeki [2004a, b] suggested that during viewing of semi‐natural scenes a degree of neural modularity is maintained that is comparable to more controlled stimulation. This same study emphasized that anatomical connectivity is clearly reflected during such viewing conditions [Bartels and Zeki, 2005]. In conclusion, besides isolated stimuli, complex scenes may also be assessable by analytic approaches such as functional brain mapping.
In contrast to passively viewed movies, interactive VR does not allow for controlling the time course of behavior and stimuli. Essential for interactive environments is that one can decide freely (limited only by the rules of the virtual environment) on the time course of actions, e.g., navigation and social interaction. Interactivity must also involve a certain predictability of consequences [Riva and Wiederhold, 2002]. As a result, the behavioral pattern must be classified individually to relate corresponding neuronal activity directly to single events. In particular, the different time courses may cancel out intersubject correspondence used for the analysis of free‐viewing conditions of the same materials [Hasson et al., 2004]. In terms of more exploratory methods, hypothesis testing is limited. ICA is one example of this [Bartels and Zeki, 2004b]. Moreover, the assumption of spatially independent brain maps may segregate modules and suppress dependent distributed activity. The present study introduces a procedure to describe interactive behavior as observed during playing a first‐person shooter video game in a parametric design.
Content analysis of text and audiovisual stimuli is frequently applied in media research and can be considered a reliable and valid method [Krippendorf, 1980]. Content analysis of video game playing can capture individual interaction patterns within the virtual environment, including violent interactions [Weber et al., 2006]. First‐person shooter games are controversially discussed media products but certainly reflect state‐of‐the‐art VR. Taken together, first‐person shooter games represent a challenging paradigm for interdisciplinary research of media psychology, communication sciences, and social cognitive neuroscience. The present fMRI study investigated neuronal activity during complex virtual behavior as represented by playing a first‐person shooter game with respect to meaningful categories such as violence.
Davidson et al. [2000] found that functional imaging may reveal precursors of aggressive behavior. The authors suggested that a network of orbitofrontal cortex (OFC), amygdala, and anterior cingulate cortex (ACC) regulates aggressive emotions. In psychopaths—mostly characterized by tendencies toward criminal and aggressive behavior—changes in the reactivity of emotional networks were observed [Birbaumer et al., 2005; Schneider et al., 2002; Veit et al., 2002]. They were characterized by reduced involvement of affective networks during potentially harmful situations. One core structure for the linkage of cognitive tasks with affective processing is the ACC, which is part of the medial frontal cortex extending from premotor to basal structures around the corpus callosum. As such, it has been thought of as an interface between cognition and emotion [Allman et al., 2001] and divided the dorsal cognitive part (dACC) and the rostral affective part (rACC) [Bush et al., 2000]. During cognitive interference tasks, increased brain activations were observed in most studies at the dACC, whereas the rACC was found activated if affective information was involved. Thus, the ACC's function might reveal if and how a complex cognitive task such as playing a first‐person shooter game can have an impact on affective processing.
Gaming can be expected to involve reinforcement [Reuter et al., 2005]. The dopaminergic reward system projects from ventral tegmental areas to amygdala and OFC [e.g., in rats: Kelley and Berridge, 2002], was found consistently to inflict ACC and amygdala [Makris et al., 2004], and is activated during playing video games [Koepp et al., 1998]. Thus, in addition to the ACC, the amygdala and OFC must be considered to respond to violent video games. The amygdala mediates affective and—in particular—aversive learning [Davidson, 2001; LeDoux, 2000]. Amygdala activation would be expected, thus, to promote avoidance and to counteract well‐controlled cognitive behavior. Frontal cortical structures, such as the OFC, might reflect more cognitive aspects of social interactions that contribute to feelings such as empathy [Damasio, 2003; Shamay‐Tsoory et al., 2005]. However, the extent and the susceptibility for artifacts of the OFC render it difficult to test a priori hypotheses by means of fMRI.
In the present study, experienced gamers were investigated during unrestrained playing of a first‐person shooter game. A content analysis scheme [Weber et al., 2006] allowed for meaningful categorization of every 100 ms of the game's time course. fMRI recorded brain activity during free game playing without further experimental manipulations. State‐of‐the‐art artifact suppression at 3 T magnetic field strength reliably detected basal brain structures. The construct violence was tested for related brain activity. We focused on limbic structures (ACC and amygdala) to test for reliability and face validity with respect to previously suggested correlates of aggression and violence.
MATERIALS AND METHODS
Subjects
Thirteen male German volunteers (age 18–26, median 23) were recruited on the basis of previous experience with video games (15.1 ± 9.0 hrs/week) with ads posted at the local university and in video game stores. Inclusion criteria were: age between 18 and 26 years, playing at least 5 hours weekly of video games, and right‐handedness. Individuals with contraindication against MR investigations, acute or anamnesis of major neurological, psychiatric, or ophthalmologic disorders were excluded. The study protocol was approved by the Ethics Committee of the University of Tübingen, Germany.
Imaging Paradigm
Each volunteer played five preselected rounds of the first‐person shooter game “Tactical Ops: Assault on Terror” (Infogrames Europe, Villeurbanne, France). Of each session, 12 min of brain activity was obtained by means of fMRI in addition to the video and sound of the game.
fMRI was conducted at 3 T (Magnetom TRIO, Siemens, Erlangen, Germany) by means of triple‐echo single‐shot echo‐planar imaging (EPI; echo times TE = 23, 40, and 62 ms; 64 × 48 matrix with 4 × 4 mm2 resolution; 4 mm slice thickness plus 1 mm gap) with dynamic distortion correction [Weiskopf et al., 2005] and dephasing compensation [Mathiak et al., 2004]. Twenty‐four oblique‐transverse slices obtained whole brain coverage with repetition time TR = 2.25 s (330 volumes per session). Interleaved slice acquisition allowed for spatiotemporal oversampling reconstruction of the volumes, i.e., volume 1 = odd and even slices from acquisition 1, volume 2 = even slices from acquisition 1 and odd slices from acquisition 2, volume 3 = odd and even slices from acquisition 2, etc. The formally obtained TR' of 1.13 s reduced aliasing of frequencies in the range of TR/2 and TR'/2 (0.2–0.4 Hz) after spatial filtering. These frequencies were relevant for respiration and the independent variables in our experiment.
For overlay, anatomical data were acquired from each participant before the functional sessions (T1‐weighted 3D‐MPRAGE, 256 × 224 × 160 matrix with 1‐mm isotropic voxels). The video display of the game play with the audio track was recorded on a Super‐VHS video tape. The analog tape was digitized offline for content analysis.
Content Analysis
An inductive, time‐based content analysis with two independent coders (male graduate students from the Annenberg School for Communication, University of Southern California) and one supervisor (R.W.) was designed. The trained coder rated how and to what extent the subjects interacted violently within specific virtual game environments. The recorded and digitized videos of the subjects' game play were analyzed frame by frame and category changes were noted with 100‐ms time resolution.
The coding scheme consisted of five main categories (phases) that were defined as: A) passive/dead: This phase was clearly defined in the game—a black bar was displayed and no actions could be performed (no violent interactions occurred); B) preparation/search: The player explored the virtual environment without any opponents or nonidentifiable characters in the player's field of view (no immanent danger, no violent interactions); C) potential danger: There are either characters in the player's field of view that are identified as opponents or characters that cannot clearly be identified and may or may not be opponents (violent interactions expected); D) under attack: The player is attacked by an opponent before or after the player used his own weapons (some violent interactions); and E) active fighting: The player uses weapons (many violent interactions) [for details, see Weber et al., 2006]. The coders received a coding training of about 16 hours based on videos not used in the study. The unit of analysis was every single frame of the game playing video (25 frames/s). The coding procedure yielded an intercoder reliability of 0.85 (Cohen's kappa) [Krippendorf, 1980]. Inconsistent ratings were discussed and the supervisor corrected inconsistent codes after coder reliability was determined.
fMRI Data Analysis
The reconstructed images underwent artifact reduction: construction of dynamic distortion maps from triple‐echo EPI with alternating phase‐encoding direction and subsequent matching of the three echoes [Weiskopf et al., 2005]; a combination of the three echoes weighted with TE*STE based on expected contrast from the averaged signal decay [Mathiak et al., 2004]. Statistical parametric mapping was conducted following the standard SPM2 procedures with normalization into the Montreal Neurological Institute (MNI) [Collins, 1994] template space of functional and anatomical data; smoothing with 12‐mm full‐width at half‐maximum Gaussian kernel; general linear model constructed from the five coding phases convoluted with hemodynamic response function as independent variables; and random effect model for group analysis corrected for multiple testing across the entire brain volume. First, we compared brain activity during phases with violent interactions (phases C, D, and E) and phases without violent interactions (phases A and B, T test with volume corrected P < 0.05). Our a priori hypothesis let us focus on the network of ACC structures and amygdala. Second, we described the brain areas that were significantly influenced by the five different game phases (false discover rate of P < 0.05) [Genovese et al., 2002].
Movement parameters as obtained for realignment served to describe involuntary head motion of subjects during scanning. For the exploration of image quality, single‐subject maps were overlaid on the individual EPI average. Moreover, we extracted single‐voxel time courses from the normalized and smoothed functional images at anatomically predefined MNI coordinates (rACC = ±6, 38, –4 mm, amygdala = ±24, 6, –20 mm). These time courses served for display of single event data and the estimation of effect sizes.
RESULTS
Network of ACC and Amygdala
Statistical parametric mapping of signal changes during violent scenes showed a strong deactivation of the rostral part of the ACC (highest T value = 13.1 across the entire brain; blue overlay in Fig. 1A) and activation of dorsal parts of the ACC (red overlay in Fig. 1A). This pattern was in complete agreement with the pattern observed during aggressive thoughts [Pietrini et al., 2000]. In parallel to the suppression of the affective subdivision of the ACC, functional mapping showed signal decreases in the amygdala during virtual violent behavior (Fig. 1B).
Figure 1.

A: Projection of signal changes to violent scenes on averaged anatomy. The dorsal part of the anterior cingulum (dACC) increases, whereas the rostral part (rACC) decreases neuronal activity during virtual violence. B: The amygdala shows suppression of hemodynamic activity during violence as well. C: BOLD signal change during violent scenes. Each bar reflects the estimated signal change in one subject at bilateral rACC (8‐s delay), dACC (0‐s delay), and amygdala (6‐s delay). The signal increase at the dACC and the decrease at the affective areas can be observed with very few exceptions.
Overview of Activation Patterns
Table I summarizes the suppressed clusters during virtual violence as found in the statistical mapping procedure. The cluster with the maximal T value was the largest and encompassed the rACC and amygdala. Parahippocampal place area (PPA) and intraparietal sulcus (IPS) deactivation might be attributed to higher spatial awareness and attention during the navigation task, as opposed to the fighting scenes [Epstein et al., 1999; Kanwisher et al., 2001]. Signal increases in the temporoparietal junction, cerebellum, and precuneus during violent scenes may primarily reflect the high coordinative, attentional, and visual demands during the violent scenes (Table II) [Kanwisher and Wojciulik, 2000]. The dACC was only reflected by a rather small cluster.
Table I.
Inhibited clusters during virtual violence
| MNI coordinates [mm]: x, y, z | Maximal T value | Cluster size [mL] | |
|---|---|---|---|
| Limbic, e.g., rACC, amygdala | 14, 38, −2 | 13.1 | 34.1 |
| IPS L | −50, −60, 54 | 11.6 | 13.0 |
| PPA R | 44, −40, −6 | 10.2 | 9.1 |
| Angular gyrus R | 36, −52, 24 | 8.9 | 2.2 |
| Orbitofrontal L | −26, 56, −16 | 8.4 | 0.4 |
| Posterior insula L | −38, −2, 6 | 8.2 | 13.9 |
| PPA L | −40, −30, −18 | 7.3 | 1.7 |
| IPS R | 54, −54, 48 | 7.2 | 2.5 |
rACC: rostral part of anterior cingulate cortex, IPS: intraparietal sulcus, PPA: parahippocampal place area.
Table II.
Activated clusters during virtual violence
| MNI coordinates [mm]; x, y, z | Maximal T value | Cluster size [mL] | |
|---|---|---|---|
| Cerebellum R | 54, −58, −24 | 3.8 | 55.2 |
| Precuneus | −2, −56, 56 | 8.9 | 4.4 |
| Temporoparietal junction R | 60, −32, 18 | 8.9 | 3.8 |
| Temporoparietal junction L | −56, −38, 36 | 7.6 | 9.9 |
| dACC | −2, −12, 40 | 7.1 | 1.4 |
dACC: Dorsal part of the anterior cingulate cortex.
Effect Sizes
The BOLD (blood oxygen level‐dependent) response of fMRI does not allow for absolute quantification. However, the relative signal change expressed as a percent value is frequently used as a semiquantitative measure for effect size. Signal changes at the a priori defined areas may not reflect maximal signal changes but can be considered across the group as average change within the target structure. The range of observed BOLD signal changes at the rACC was on the order of 0.5% (Fig. 1C). For this structure, this was a rather large effect. Indeed, in a previous study signal changes at the level of the rACC were targeted for maximization by feedback of the signal [Weiskopf et al., 2003]. After extensive training, the subject achieved a median change of only 0.13%.
Explanatory Power of Coding Scheme
The F contrast across the coding of all five phases revealed that large portions of the brain were significantly modulated by the chosen constructs. Indeed, the summed volume of activated clusters was 564 mL of the total 1,833 mL included in the SPM analysis (nongray matter structures were not excluded, making this estimate rather conservative; 5% false discovery rate). Comparisons of each of the four active conditions with the passive viewing condition revealed significant activation in motor areas including cerebellar, premotor, and motor areas throughout the active conditions (Fig. 2). More unexpectedly, the active fighting condition was linked to distributed inhibition (Fig. 2B). Moreover, bilateral temporoparietal junctions were inhibited in “save” active (Fig. 2A) and activated in active fighting phases (Fig. 2B), conceivably reflecting spatial self‐representation [Blanke et al., 2005].
Figure 2.
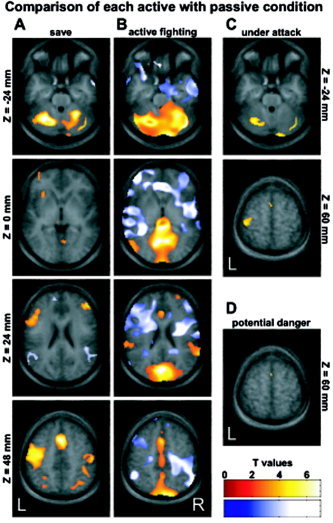
Explorative mapping of active phases as compared to the passive condition (virtual death). A: The save condition (left column) elicited higher activity (orange overlay) at motor (cerebellum, supplemental motor area (SMA), and left‐lateralized motor cortex) and executive areas (dorsolateral prefrontal cortex) in addition to lower activity (blue overlay) in bilateral temporoparietal junction. B: The active fighting condition (middle column) was linked to distributed inhibition of frontal, limbic, auditory, and right motor areas and activation of cerebellar, visual, temporoparietal, and cingular areas. The under attack (C) and the potential danger (D) conditions activated only in motor related areas (under attack: cerebellum, SMA, and motor cortex; potential danger: only SMA).
The interactivity of the game did not allow controlling the duration of the phases. The duration of phases differed since the aforementioned playing phases are not uniformly distributed in unrestrained video game playing: the relative time contributions (±SD across group) were 25.7% ± 7.5% for the passive phase (A = “dead”), 58.6% ± 6.1% for preparation phases (B), 7.4% ± 1.5% for potential danger (C), 1.1% ± 0.3% for under attack (D), and 6.8% ± 2.1% for active fighting (E). As a consequence, the power for conclusions on the under attack phase was much lower than preparation phases and still lower than on potential danger and fighting phases. Therefore, the phases coded as under attack led to only a few significant voxels (Fig. 2C). The phases potential danger hardly differed statistically from passive viewing—probably representing the exploration during the phase (Fig. 2D). Remarkably, as compared to passive viewing the active fighting condition had a slightly lower duration but exhibited much more significant signal changes and, thus, the effect size during the under attack condition must be much lower.
Data Quality and Variability
Intrasession head motion was 0.30 ± 0.15 mm on average. For instance, as compared to a recent report of a standard nonmotor task (0.53 ± 0.23 mm) [Österbauer et al., 2006], these values are low.
Even in single subjects the signal from rACC and amygdala voxels was strongly correlated with the game play as rated by our coding scheme (see exemplary time courses in Fig. 3A–B). Due to the distortion procedure with adaptive filtering and high switching rate of the gradient encoding, the signal at the level of the amygdala was sufficient for activation even in single subjects (Fig. 3C).
Figure 3.
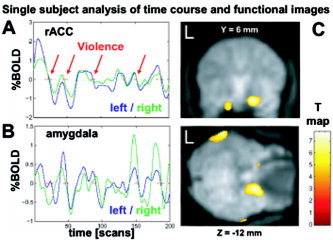
Single‐subject analysis with time course of exemplary raw data at the rACC (A) and the amygdala (B) as compared to violent scenes (marked with red lines). Regardless of the noise, dips after each violent event are clearly delineated. C: Overlay of BOLD signal changes at the level of the amygdala of a single subject on its EPI data. The (unsmoothed) EPI shows a low amount of dephasing and the overlaid activation is clearly located within the right amygdala in this subject.
It has been claimed that different personalities perceive and interpret media violence differently [Potter and Tomasello, 2003]. The observed patterns across subjects were stable, with very few exceptions (Fig. 1C). One subject achieved a correlation coefficient of up to −0.31 with the violence indicator variable and −0.54% BOLD signal change. One subject showed a trend to the opposite pattern, i.e., signal decrease in dACC and increases in left rACC and amygdala. This suggested that the intersubject variability did not merely reflect noise. However, the small sample size did not allow for conclusive statistics on the nature of these heterogeneities.
DISCUSSION
We investigated neuronal activity related to semi‐naturalistic behavior within a virtual environment. A distributed network of neural modules was significantly related to five constructs encoding interaction patterns. During virtual violence, brain activations in cognitive ACC, temperoparietal junction, precuneus, and cerebellum as well as deactivation in limbic‐orbitofrontal areas, IPS, and PPA were consistent with the expected neuronal correlates. As to the limbic structures, statistics and effect size observed during the violence construct can be considered large and seem to differ from pure arousal or cognitive demand effects. Advanced imaging technology supported reliable and sensitive detection of neuronal activity down to the single‐trial level. This is the first study that relates semi‐naturalistic behavior represented by video game playing to specific brain activity.
fMRI during Natural Behavior
Natural behavior is complex. Conceivably, it is impossible to derive a complete set of descriptive variables. Even virtual behavior during video game play cannot be classified completely. Our coding scheme focused on modes of interaction. To test the reliability of one of the constructs, we concentrated on the presence or absence of violent interactions as a contrast of interest. Finally, we modeled the temporal relationship with a canonical hemodynamic response, ignoring possible anticipatory or sustained neuronal activity [Handwerker et al., 2004]. Nevertheless, even this oversimplified model led to a rather high explanation of variance in the BOLD signal. The interactivity of virtual reality may challenge a simplistic interpretation with a causal stimulus‐response model. However, for an initial understanding of the brain function during complex and interactive behavior, such simplifying approaches are indispensable.
One of the problems emerging from the analytical paradigm applied in interactive settings is that the duration of the phases varies. The subjects played the first‐person shooter game without restrictions and they were familiar with the rules and options of this game. In our study, however, the variability between subjects was relatively low, although the five different phases were represented with different proportions. As a consequence of low power, the explorative maps for two conditions (potential danger and under attack) elicited few activated clusters. Nevertheless, under the linearity assumption, estimated effect sizes should be unaffected by these differences and intersubject variability would lead only to a conservative underestimation of activations in the second‐level analysis.
As compared to conventional fMRI, where basal brain areas pose large problems, the advanced imaging procedure may have helped to obtain reproducible results [Weiskopf et al., 2005]. The changes at affective areas—in particular the rACC—were surprisingly consistent and showed high effect sizes. The amygdala showed signal changes observable even at the single trial level. This suggested, first, that virtual environments may lead to a higher motivation and a fuller involvement as compared to standard stimulation paradigms. This motivational effect enhanced self‐control of local brain activity (Brain Pong) [Goebel et al., 2004; Weiskopf et al., 2006]. Second, the coding scheme of the applied content analysis was highly relevant for neurobiological functions. This is remarkable because the scheme referred to complex virtual interactions rather than simple game variables such as gain or loss of money [cf. Reuter et al., 2005]. Thus, interactions like fighting—even when not helpful to score—may serve as an important and independent motivational factor of the game.
Cortical Networks
Neural patterns as measured by the BOLD response are characterized by changes of activity. We found differences between scenes with and scenes without violence in a video game. However, the phases without violence contained more spatial navigation behavior. Indeed, relative PPA deactivation during violence is most plausibly explained by higher activity during navigation tasks [Epstein et al., 1999]. Likewise, IPS and right angular gyrus activation may reflect shifts of spatial attention during nonviolent interactions [Corbetta and Shulman, 2002; Karnath et al., 2001]. Thus, in addition to the virtual violence, the chosen constructs differed in spatial attention and navigation.
Precuneus and temperoparietal junction activations during virtual violence do not directly relate to the construct violence. Enhanced visual analysis and multisensory integration, however, may well explain this pattern [Zhang et al., 2004]. Thus, we must expect that the indicator for violence in our study is associated with certain sensorimotor demands that are less prominent during other phases. Right‐hemispheric cerebellar activity is consistent with activation due to higher sensorimotor integration with the right hand used to control the video game [Ohyama et al., 2003]. In contrast, no significant change of motor activity emerged during the violent scenes. Conceivably, the right cerebellum contributes to rather higher‐order sensorimotor coordination.
Limbic Areas
Brain patterns during complex behavior may be evaluated with respect to a priori knowledge. In the present study we focused on ACC and amygdala activation related to violent interactions. From a large number of experimental studies, we know about the involvement of these structures in monitoring cognitive and affective conflicts: Bush et al. [2000] separated the ACC in a dorsal‐cognitive and a rostral‐affective subdivision based on a review of functional imaging studies. This model underspecifies the complexity of functions to which the ACC seems to contribute; however, for a number of control operations the gradient from cognitive operations at the dorsal part—close to premotor structures—and affective contributions of the rostral part—linking up to classical affective structures (orbitofrontal and amygdala)—seems to hold. This suggestion is consistent with the opposite sign of dACC and rACC activity in the present study. Moreover, a distributed basal network emerged encompassing amygdala and orbitofrontal areas in an extended deactivation cluster with the rostral ACC. Interestingly, the modulation of affective areas reflected the largest effect of virtual violence on the brain. This pattern suggested active suppression of affective processing in favor of the cognitive operation.
We observed suppression of affective areas during violent scenes concomitant with a signal increase in cognitive areas. Pietrini et al. [2000] asked subjects to imagine scenes where they employed unrestrained but justified violence. In comparison to the imagination of affective scenes without violence, dorsal ACC increased activity and the rostral ACC decreased activity. Rostral ACC and amygdala were deactivated in parallel. Siegal and Varley [2002] suggested that the amygdala is part of the core structure for the reflection of others thoughts and emotions (“theory of mind”). Conceivably, avoidance of fear reaction [LeDoux, 2003] or empathy improves the ability to react precisely in a violent situation and virtually kill opponents. Mathews et al. [2005] suggested that violent computer games may reduce reactivity of frontal brain areas. This long‐term effect resembles patterns in subjects prone to violence [e.g., Birbaumer et al., 2005].
Implication for Social Effects of Violent Computer Games
The effect of violent video games and first‐person shooter games in particular on aggressive cognitions, emotions, and behavior is a frequently discussed topic [Anderson and Bushman, 2002; Anderson, 2003, 2004; Sherry, 2001]. The cumulative dose of video games in modern societies is immense: about 10 million hours are played daily by US adolescents (estimated from data provided by the Kaiser Family Foundation [2002]). Thus, even a small effect in terms of increased aggressiveness or decreased prosocial behavior would be influential across the society. The present study targets violent scenes during these games and finds a rather specific pattern that is presumably related to aggressive cognitions and emotions. One might speculate that a frequent training of aggressive neuronal pattern leads to the development of aggressive problem‐solving scripts, hostile attribution biases, and normative beliefs approving of aggression as stated by social‐cognitive theory [Bandura, 2001]. This proposition with its important implications, however, is not a direct conclusion of this study's findings. Future studies might aim at the learning mechanisms of cognitive‐affective networks [Weiskopf et al., 2003] and investigate how the short‐ and long‐term effects of these mechanisms translate into aggressive cognitions, affects, and behavior in real life [see Anderson and Dill, 2000].
CONCLUSION
Virtual reality as presented in first‐person shooter games can be used to study brain activity during semi‐naturalistic behavior. Although we observed patterns of suppressed affective structures induced by virtual violent interactions, the current experiment does not prove whether the rehearsal of such a mechanism can promote aggressive behavior in real life. However, strengthening of affective inhibition during virtual violence may represent a plausible learning mechanism and may explain how playing first‐person shooter video games could cause aggressive reactions. Conversely, learning mechanisms of virtual social interactions can also promote prosocial behavior, e.g., therapeutically. As concerns functional imaging, interactivity may improve sensitivity. The higher motivation and involvement in interactive environments seem to increase neuronal response amplitudes.
Acknowledgements
We thank Maike Borutta for technical assistance, Kibum Park and Alfred Aguilar for the codings, and Silke Anders, Nils Birbaumer, Ute Ritterfeld, Ralf Veith, Peter Vorderer, and Nikolaus Weiskopf for helpful and valuable discussions. We also thank Michael Erb and Uwe Klose for help with MRI. We thank Ute Ritterfeld, University of Southern California Los Angeles, and Katharina Behr, University of Music and Drama Hannover/Germany, for help in designing and conducting the content analyses.
REFERENCES
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001): The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci 935: 107–117. [PubMed] [Google Scholar]
- Anderson CA (2003): Violent video games: myths, facts, and unanswered questions. Psychological Science Agenda: Science Briefs 16: 1–3. Retrieved June 11, 2005, from http://www.apa.org/science/psa/sb-anderson.html [DOI] [PubMed] [Google Scholar]
- Anderson CA (2004): An update on the effects of playing violent video games. J Adolesc 27: 113–122. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ (2002): The effects of media violence on society. Science 295: 2377–2379. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Dill KE (2000): Video games and aggressive thoughts, feelings, and behavior in the laboratory and in life. J Pers Soc Psychol 78: 772–790. [DOI] [PubMed] [Google Scholar]
- Bandura A (2001): Social cognitive theory of mass communication. Media Psychol 3: 265–299. [Google Scholar]
- Bartels A, Zeki S (2004a): Functional brain mapping during free viewing of natural scenes. Hum Brain Mapp 21: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2004b): The chronoarchitecture of the human brain — natural viewing conditions reveal a time‐based anatomy of the brain. Neuroimage 22: 419–433. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2005): Brain dynamics during natural viewing conditions—A new guide for mapping connectivity in vivo. Neuroimage 24: 339–349. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H (2005): Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry 62: 799–805. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual‐Leone A, Brugger P, Seeck M, Landis T, Thut G (2005): Linking out‐of‐body experience and self processing to mental own‐body imagery at the temporoparietal junction. J Neurosci 25: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M I (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Collins DL (1994): Model‐based segmentation of individual brain structures from magnetic resonance imaging data. PhD thesis, McGill University, Montreal.
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Damasio A (2003): Feelings of emotion and the self. Ann N Y Acad Sci 1001: 253–261. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2001): Toward a biology of personality and emotion. Ann N Y Acad Sci 935: 191–207. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL (2000): Dysfunction in the neural circuitry of emotion regulation — a possible prelude to violence. Science 28: 591–594. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999): The parahippocampal place area: recognition, navigation, or encoding? Neuron 23: 115–125. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Goebel R, Sorger B, Kaiser J, Birbaumer N, Weiskopf N (2004): BOLD brain pong: self‐regulation of local brain activity during synchronously scanned, interacting subjects. Program No. 376.2. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience. [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M (2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21: 1639–1651. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R (2004): Intersubject synchronization of cortical activity during natural vision. Science 303: 1634–1640. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation (2002): Key facts: children and video games. http://www.kff.org/entmedia/3271-index.cfm
- Kanwisher N, Wojciulik E (2000): Visual attention: insights from brain imaging. Nature Rev Neurosci 1: 91–100. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Downing P, Epstein R, Kourtzi Z (2001): Functional neuroimaging of visual recognition In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press. [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M (2001): Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 41: 950–953. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002): The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22: 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM (1998): Evidence for striatal dopamine release during a video game. Nature 393: 266–268. [DOI] [PubMed] [Google Scholar]
- Krippendorf K (1980): Content analysis. An introduction to its methodology. Beverly Hills, CA: Sage. [Google Scholar]
- LeDoux JE (2000): Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS Jr, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC (2004): Decreased absolute amygdala volume in cocaine addicts. Neuron 44: 729–740. [DOI] [PubMed] [Google Scholar]
- Mathews VP, Kronenberger WG, Wang Y, Lurito JT, Lowe MJ, Dunn DW (2005): Media violence exposure and frontal lobe activation measured by functional magnetic resonance imaging in aggressive and nonaggressive adolescents. J Comput Assist Tomogr 29: 287–292. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H (2004): Discrimination of temporal information at the cerebellum: functional magnetic resonance imaging of nonverbal auditory memory. Neuroimage 21: 154–162. [DOI] [PubMed] [Google Scholar]
- Ohler P, Nieding G (2006). Why play? An evolutionary perspective In: Vorderer P, Bryant J, editors. Playing video games: motives, responses, and consequences. Mahwah, NJ: Erlbaum. [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD (2003): What the cerebellum computes. Trends Neurosci 26: 222–227. [DOI] [PubMed] [Google Scholar]
- Österbauer RA, Wilson JL, Calvert GA, Jezzard P (2006): Physical and physiological consequences of passive intra‐oral shimming. Neuroimage 29: 245–253. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J (2000): Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry 157: 1772–1781. [DOI] [PubMed] [Google Scholar]
- Potter WJ, Tomasello TK (2003): Building upon the experimental design in media violence research: the importance of including receiver interpretations. J Commun 53: 315–329. [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Büchel C (2005): Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci 8: 147–148. [DOI] [PubMed] [Google Scholar]
- Riva G, Wiederhold BK (2002): Introduction to the special issue on virtual reality environments in behavioral sciences. IEEE Trans Inf Technol Biomed 6: 193–197. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Posse S, Grodd W, Müller‐Gärtner HW (2002): Functional imaging of conditioned aversive emotional responses in antisocial personality disorder. Neuropsychobiology 42: 192–201. [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon‐Peretz J (2005): Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol 18: 55–67. [DOI] [PubMed] [Google Scholar]
- Sherry JL (2001): The effects of violent video games on aggression. A meta‐analysis. Hum Commun Res 27: 409–431. [Google Scholar]
- Siegal M, Varley R (2002): Neural systems involved in “theory of mind”. Nat Rev Neurosci 3: 463–471. [DOI] [PubMed] [Google Scholar]
- Touryan J, Felsen G, Dan Y (2005): Spatial structure of complex cell receptive fields measured with natural images. Neuron 45: 781–791. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N (2002): Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett 328: 233–236. [DOI] [PubMed] [Google Scholar]
- Weber R, Ritterfeld U, Mathiak K (2006): Does playing violent video games induce aggression? Empirical evidence of a functional magnetic resonance imaging study. Media Psychol 8: 39–60. [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N (2003): Physiological self‐regulation of regional brain activity using real‐time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage 19: 577–586. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Klose U, Birbaumer N, Mathiak K (2005): Single‐shot compensation of image distortions and BOLD contrast optimization using multi‐echo EPI for real‐time fMRI. Neuroimage 22: 1068–1079. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K (2006): Self‐regulation of local brain activity using real‐time functional magnetic resonance imaging (fMRI). J Physiol (Paris) 98: 357–373. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Sun X, Li Z, Wang Z, He S, Hu X (2004). Cross‐modal temporal order memory for auditory digits and visual locations: an fMRI study. Hum Brain Mapp 22: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]


