Abstract
Behavioral studies have provided important insights into the mechanisms governing interlimb coordination. In this study, we combined kinematic and functional magnetic resonance imaging (fMRI) analysis to investigate the brain cortical and subcortical areas involved in interlimb coordination and the influence of direction of movement and of body segment position on the activity of those areas. Fifteen right‐handed healthy subjects were studied while performing cyclic in‐phase and antiphase hand and foot movements with the dominant, right limbs, with the upper limb positioned either prone or supine, and in front or behind with respect to the trunk. When contrasting antiphase to in‐phase movements, fMRI analysis demonstrated an increased recruitment of a widespread sensorimotor network (including regions in the frontal and parietal lobes, bilaterally, the cingulated motor area, the thalami, the visual cortex, and the cerebellum) considered to function in motor, sensory, and multimodal integration processing. When contrasting the anterior to the posterior position of the upper limb with respect to the trunk, we found different recruitment patterns in the frontal and parietal regions as well as the preferential recruitment of the basal ganglia, the insula, and the cerebellum during the first condition and of regions located in the temporal lobes during the second one. Different brain areas are engaged at a different extent during interlimb coordination. In addition to the relative difficulty of the movement, the different cognitive and sensorial loads needed to control and perform the motor act might be responsible for these findings. Hum Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: functional magnetic resonance imaging, in‐phase, antiphase, coordination, limb position
INTRODUCTION
Neurophysiological studies in healthy individuals have demonstrated that rhythmic voluntary movements of flexion‐extension of ipsilateral limbs require a different effort and attention if they are performed in the same angular direction (in‐phase, “easy association”) than when they are performed in different directions (antiphase, “difficult association”) [Baldissera et al.,1982,1991]. In addition, in the difficult association, increase in the frequency of task execution leads to shortened performance because of the reversal to the in‐phase movement [Baldissera et al.,1991]. No influence has been reported yet on the role of the of upper limb position (i.e., prone or supine) on the behavioral observations [Baldissera et al.,1982].
Among the mechanisms involved in the maintenance of limb coordination, the role of peripheral control, in particular the H‐reflex modulation [Baldissera et al.,1998], and that of a central regulation of limbs synchronization have been postulated. Although these two mechanisms are likely to be involved at a different extent during the performance of in‐phase and antiphase movements of flexion‐extension of the ipsilateral limbs, this aspect has not been fully elucidated yet. In addition, whereas several studies have investigated the role of peripheral modulation [Baldissera et al.,1998,2002], only a few reports have focused on CNS‐associated changes [Colier et al.,1999; Ehrsson et al.,2000; Debaere et al.,2001]. The results obtained by these latter studies seem to indicate that in‐phase and antiphase movements activate a similar brain network.
The analysis of coordination processes is of particular interest for gaining insight into the organization of voluntary movements. The study of bimanual coordination of ipsilateral hand and foot coordination patterns has allowed the identification of several coordination constrains, which include the mechanical characteristics of joints and muscles, the organization of the neuronal substrates that control movements, and the external sources of feedback. It is worth noting that these constrains are not mutually exclusive and their relative contributions vary according to the task performed. In bimanual movement coordination, the traditional theory that homologous muscle coactivation is mainly responsible for the in‐phase movements symmetry has been confuted by an experiment by Mechsner et al. [2001], who hypothesized that sensorimotor coordination is independent of any muscular constraint, but it is rather exclusively of a spatial/perceptual origin. Although the findings of Mechsner et al. [2001] are still a matter of debate and have not been replicated by other authors [Salter et al.,2004; Riek and Woolley,2005], they brought the attention on the influence of the manipulation of limb spatial arrangement on voluntary movement coordination. Considering ipsilateral hand‐foot coordination, different studies would suggest that directional and spatial rather than muscular constraints have a dominant impact on coordination [Baldissera et al.,1982,1991; Salesse et al.,2005]. However, the only spatial arrangement considered in these studies was the prone or supine position of the forearm. A variable that has not been considered in the assessment of in‐phase and antiphase movements of ipsilateral limbs is the influence of upper limb position, i.e., in front or behind with respect to the trunk, on the performance of these movements.
This study was designed to gain additional insight into the role of central control on coordination of ipsilateral hand and foot movements. In particular, the main questions we wished to address were the following. One, are the behavioral and functional magnetic resonance imaging (fMRI) differences observed by previous studies during in‐phase and antiphase movements of the ipsilateral limbs confirmed when changing the position of the right upper limb? To answer this question, we measured the same kinematic variables previously measured in a seminal study on ipsilateral hand and foot coordination [Baldissera et al.,2001] and we evaluated how these variables and the corresponding brain patterns of cortical activation differed between in‐phase and antiphase ipsilateral hand and foot movements when considering a wide spectrum of positions of the upper limb (i.e., prone or supine, anterior or posterior with respect to the trunk). Two, which are the brain patterns of cortical activations related to the different positions of the right upper limb? In this context, a similar pattern of cortical activations independently of the position of the right upper limb would confirm the role of a “muscular constraint” on the control of hand and foot coordination. Conversely, the change of the movement‐associated brain patterns of cortical activations according to the position of the upper limb would support a role of the central control. Three, does a different position of the upper limb modify the subjects' preferences toward antiphase movements instead of the in‐phase ones. This would support the theory of an influence of postural mechanisms on voluntary movement execution.
PATIENTS AND METHODS
Subjects
We studied 15 right‐handed [Oldfield,1991] healthy individuals (9 women, 6 men; mean age, 21.6 years; range, 20–26 years). Local ethics committee approval and written informed consent from each subject were obtained prior to study initiation.
Experimental Design
The experiment has been performed in two different sessions. During the first one, a detailed instrumental analysis of movement kinematic was performed. On the second occasion, performed within 3 days from the previous one, fMRI was acquired. During both experimental sessions, subjects lied in a lateral left position, with the right leg supported by a cushion, with the hip flexed at 40°, the knee flexed at 40°, and the ankle left free.
During both sessions, the subjects were asked to perform two different movements: cyclic in‐phase flexion‐extension of the hand and foot; and cyclic antiphase flexion‐extension of the hand and foot while maintaining four different positions of the right upper limb, with the arm pointing toward the head of the subject (Fig. 1): prone‐anterior: shoulder 0°, elbow flexion of 90°, forearm prone; supine‐anterior: shoulder 0°, elbow flexion of 90°, forearm supine; prone‐posterior: shoulder extent of 40°, elbow extent, forearm prone; supine‐posterior: shoulder extent of 40°, elbow extent, forearm supine.
Figure 1.

Limb positions during the prone‐anterior (A) and the prone‐posterior conditions of the experiment (B). In panel A, the upper arm is positioned as follows: shoulder in neutral position, elbow flexed at 90°, and forearm in prone position. The lower limb is positioned with both hip and knee flexed at 40°. In B, the upper arm is positioned as follows: shoulder extended at 40°, elbow in maximal extension, and forearm in prone position. The lower limb is positioned with both hip and knee flexed at 40°. The kinematic acquisition was done with markers placed on the head of the fifth metatarsus, the lateral malleolus, the fibula head, the lateral homerus condyle, the styloid ulnae, and the head of the fifth metacarpus. The subjects were asked to move simultaneously their hand and foot, both in phase (black arrows) and antiphase (white arrows). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In‐phase movements implied simultaneous dorsal flexion of the foot and flexion of the hand toward the head of the subject, or plantar flexion of the foot and flexion of the hand away from the head of the subject, when the upper limb was positioned in front with respect to the trunk. Conversely, they implied simultaneous plantar flexion of the foot and flexion of the hand toward the head of the subject, or dorsal flexion of the foot and flexion of the hand away from the head of the subject when the upper limb was positioned behind the trunk (Fig. 1).
Instrumental Study of Kinematic
Hand and foot kinematic movements were derived using a six‐video camera system (Elite; BTS) after placing six passive markers on the right side of the body in the following positions: the head of the fifth metatarsus, the lateral malleolus, the fibula head, the lateral homerus condyle, the styloid ulnae, and the head of the fifth metacarpus. The sampling frequency was 100 Hz. Each of the eight movements studied (in‐phase and antiphase in each of the four positions) was tested at spontaneous and maximum speeds. The sequence of the positions was randomly selected. Each movement lasted 15 s with a 30‐s pause between two consecutive movements. In each position, we considered as first movement the one chosen by the subject at spontaneous speed. We decided to analyze the first movement chosen in the different experimental conditions to test whether the manipulation of the position of the upper limb might influence the performance of coordinated hand and foot movements. This would support the theory of an influence of postural mechanisms on voluntary movement execution.
Analysis of Kinematic
Hand and foot kinematics were shown using curves in which the movement range was reported on the y‐axis and the time on the x‐axis (Fig. 2). These curves were filtered by a low‐pass filter of 6 Hz. During kinematic analysis, the following variables were measured: the frequency of hand and foot movements, expressed in Hz; the delay between hand and foot movement; and the error time. The delay has been analyzed by localizing on the hand and foot curves the median point of the range of movement. The median point of the earlier segment was then projected on the curve of the other segment. The two points resulting on the delayed segment curve were then projected on the y‐axis for showing the delay as a range of movement degree. The delay of each acquisition was computed as mean of the delay of each single movement, with a negative sign given to the foot and a positive one to the hand. The error time was measured as the seconds of errors during the 15‐s trial acquisition, with an interruption of the movement in a segment or with an inversion of the movement direction between hand and foot. The subject's spontaneous movement was considered to be the easiest one in each of the eight conditions.
Figure 2.
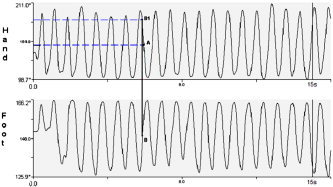
Kinematic curves of hand and foot during an antiphase movement. The x‐axis reports the acquisition time and the y‐axis the movement range in degree. The movement frequency has been computed as the number of flexion‐extension movements during the acquisition time and expressed in Hz. The delay has been analyzed by localizing on the hand and foot curves the median point of the range of movement (A and B). The median point of the former segment (foot) was then projected onto the curve of the latter one (B1). The two points resulting on the delayed segment curve were then projected on the y‐axis to show the delay as a range of movement degree. The delay of each acquisition was computed as mean of the delay for each single movement, with a negative sign given to the foot and a positive one the hand.
Kinematic Statistical Analysis
The comparisons between the movements of hand and foot and between in‐phase and antiphase movements, in relation to the kinematic variables, were performed using the nonparametric Wilcoxon test. The comparisons of the same kinematic variables concerning the same movement in the four different conditions were performed using the nonparametric Friedman test for multiple measurements. The variables related to the supine‐posterior condition were evaluated using the Mann‐Whitney test for independent samples. This condition was analyzed separately from the others because of a between‐subject difference in the choice of the first movement (i.e., in‐phase vs. antiphase; see Results).
Functional MRI Acquisition
Using a block design (ABAB), where five periods of activation were alternated with six periods of rest, the subjects were scanned while performing the eight tasks previously described, split into eight different runs. During the active phases of each run, each subject was instructed to perform only a specified movement, while during the rest phase the subjects lied with the limbs fixed in the previously described positions (see Experimental Design). Upper limbs switched locations during the pause from a run to another. Foam cushions were used to avoid excessive head and shoulder movements. The movements were paced by a metronome at 1 Hz frequency. This frequency was selected after a preliminary analysis of the kinematic data of a group of healthy individuals, who did not take part to the present study (data not shown), in order to have similar between‐subject task performance in the different experimental conditions and therefore to avoid a possible bias on fMRI results related to a different between‐subject task performance. Subjects were trained to perform the tasks until they could make the movements in a relaxed manner before performing the study. They were also instructed to keep their eyes closed during fMRI acquisition and they were monitored visually during scanning by two observers that remained in the scanner room during the entire fMRI acquisition to ensure accurate task performance and to check for additional movements (e.g., mirror movements). The sequence of the runs was randomly selected by the two observers. Motor function assessment was performed for all the subjects at the time of fMRI acquisition using the nine‐hole peg test (9‐HPT) and the maximum finger‐tapping frequency [Herndon,1997]. The maximum finger‐tapping rate was observed for two 30‐s trial periods outside the magnet, and the mean frequency to the nearest 0.5 Hz entered the analysis.
Brain MRI scans were obtained using a 1.5 T machine (Vision; Siemens, Enlargen, Germany). In order to have MRI scans in the radiological convention and to reduce the impact of the acquisition scheme on fMRI data realignement, the subjects were registered on the scanner console as lying in a lateral position. Sagittal T1‐weighted images were acquired to define the anterior‐posterior commissural (AC‐PC) plane. Functional MR images were acquired using a T2*‐weighted single‐shot echo‐planar imaging (EPI) sequence [repetition time (TR) = 3.0 s; echo time (TE) = 66 ms; flip angle (FA) = 90°; matrix size = 128 × 128; field of view (FOV) = 256 × 256 mm]. Twenty‐four axial slices, parallel to the AC‐PC plane, with a thickness of 5 mm, covering the whole brain were acquired during each measurement. Shimming was performed for the entire brain using an auto‐shim routine, which yielded satisfactory magnetic field homogeneity.
Using the same magnet, a brain dual‐echo turbo spin echo (TSE) sequence (TR/TE = 3300/16,98; echo train length = 5; 24 axial slices, 5 mm thickness) with the same orientation as the fMRI data set and a brain sagittal 3D T1‐weighted magnetization‐prepared rapid acquisition gradient echo (MP‐RAGE) sequence (TR/TE = 11.4/4.4; FA = 15°; FOV = 256 × 256; matrix size = 256 × 256; slab thickness 160 mm; voxel size = 1 × 1 × 1 mm3) were also acquired.
fMRI Analysis
All image postprocessing was performed on an independent computer workstation (Sun Sparcstation; Sun Microsystems, Mountain View, CA). fMRI data were analyzed using the statistical parametric mapping (SPM99) software [Friston et al.,1995]. Prior to statistical analysis, all images were realigned to the first one to correct for subject motion, spatially normalized into the standard space of SPM, and smoothed with a 10 mm 3D Gaussian filter. Before proceeding with spatial normalization, realigned images were carefully checked. None of the subjects showed head displacements above 3 mm. Changes in blood oxygenation level‐dependent (BOLD) contrast associated with the performance of the motor tasks were assessed on a pixel‐by‐pixel basis using the general linear model [Friston et al.,1995] and the theory of Gaussian fields [Worsley and Friston,1995]. Specific effects were tested by applying appropriate linear contrasts. Significant hemodynamic changes for each contrast were assessed using t‐statistical parametric maps (SPMt). The activations during the performance of the different tasks and the comparisons between the different tasks were investigated using a random‐effect analysis using a one‐sample t‐test or two‐sample t‐test for unpaired data as appropriate. Cluster of voxels with a height threshold P < 0.001 (uncorrected) and an extent threshold P < 0.05 (corrected) were considered as significant. The following comparisons were decided a priori: in‐phase vs. antiphase movements, prone vs. supine position of the right upper limb, anterior vs. posterior position of the right upper limb with respect to the trunk, and evaluation of first chosen movement in the supine‐posterior condition.
To achieve a more precise definition of the anatomical locations of activated regions in each individual, MP‐RAGE images from each subject were coregistered to the corresponding fMRI data sets and normalized into the same standard space. Then, fMRI results were superimposed onto these high‐resolution images. Within each region of statistical significance, local maxima of signal increase were determined and their location was expressed in terms of x‐, y‐, and z‐coordinates into standard SPM space (Montreal Neurological Institute coordinates). A 3D anatomical atlas was also used to increase confidence in the definition of the anatomical locations of the activated areas [Duvernoy,1999].
RESULTS
Kinematic Analysis
In the conditions of prone‐anterior, supine‐anterior, and prone‐posterior, all the subjects chose as first movement the in‐phase hand and foot movement. In the condition of supine‐posterior, six subjects chose the in‐phase association, whereas nine subjects preferred the antiphase association.
The analysis of the movement frequency, performed by combining the four conditions, showed significantly higher frequency during the in‐phase compared with the antiphase condition (in‐phase = 1.63 ± 0.53 Hz, mean ± SD; antiphase = 1.36 ± 0.50 Hz; P < 0.001). The same results were obtained when considering the four conditions separately (Table I). In‐phase movements had the maximum frequency with the forearm in prone position (positioned both anterior and posterior to the trunk) both at spontaneous and at maximum speed (P < 0.001; Table I). Conversely, antiphase movements had the maximum frequency with the forearm in supine position (positioned both anterior and posterior to the trunk) both at spontaneous and at maximum speed (P < 0.001; Table I).
Table I.
Kinematic results showing the mean frequency (±2 standard deviations) of hand and foot movements in the four experimental conditions
| Prone‐anterior | Supine‐anterior | Prone‐posterior | Supine‐posterior | |
|---|---|---|---|---|
| In‐phase spontaneous frequency (Hz) | 1.30 ± 0.32 | 1.19 ± 0.36 | 1.50 ± 0.34 | 1.18 ± 0.41 |
| In‐phase maximum frequency (Hz) | 2.07 ± 0.43 | 1.86 ± 0.38 | 2.11 ± 0.39 | 1.84 ± 0.52 |
| Antiphase spontaneous frequency (Hz) | 1.02 ± 0.25 | 1.17 ± 0.35 | 1.00 ± 0.37 | 1.35 ± 0.38 |
| Antiphase maximum frequency (Hz) | 1.37 ± 0.37 | 1.68 ± 0.5 | 1.40 ± 0.4 | 1.89 ± 0.51 |
The movements performed in the two conditions with the upper limb in front of the trunk had the same frequency of those performed with the upper limb behind the trunk (in front = 1.46 ± 0.54 Hz; behind = 1.53 ± 0.51 Hz; P = NS).
In the supine‐posterior condition, the frequency of the movements performed as first choice did not differ between the two groups of subjects doing different associations (in‐phase as first choice = 1.68 ± 0.74 Hz; antiphase as first choice = 1.77 ± 0.48 Hz; P = NS).
The analysis of the delay between hand and foot movement showed a delay of the hand in all the conditions (6.8 ± 11.9°). In Table II, the number of subjects with a delay of hand or foot in the four conditions is reported. Considering the delay during a given movement (i.e., in‐phase and antiphase movements at a spontaneous and maximum speed) in the four different conditions, a significant difference was found only during the antiphase movement at maximum speed (P < 0.05). The delays were greater with the upper limb positioned behind the trunk than in front of the trunk (P < 0.05).
Table II.
Kinematic results showing the number of subjects having a hand and foot delay in the different test conditions
| Prone‐anterior | Supine‐anterior | Prone‐posterior | Supine‐posterior | |||||
|---|---|---|---|---|---|---|---|---|
| Hand | Foot | Hand | Foot | Hand | Foot | Hand | Foot | |
| In‐phase spontaneous frequency | 8 | 2 | 8 | 3 | 7 | 2 | 6 | 3 |
| In‐phase maximum frequency | 5 | 2 | 4 | 4 | 5 | 3 | 6 | 2 |
| Antiphase spontaneous frequency | 7 | 4 | 9 | 4 | 7 | 2 | 9 | 3 |
| Antiphase maximum frequency | 8 | 2 | 7 | 2 | 10 | 4 | 4 | 2 |
The analysis of the error times did not show differences between error times of hand and those of foot. The error time, during the 15‐s acquisition, was significantly higher during the antiphase movements than during the in‐phase ones (antiphase = 1.41 ± 2.1 s; in‐phase = 0.21 ± 1.2 s; P < 0.001; Table III). No difference was found in the error times when considering the anterior or the posterior position of the upper limbs with respect to the trunk.
Table III.
Mean error times (±2 standard deviations), expressed in seconds, in the different test conditions
| Prone‐anterior | Supine‐anterior | Prone‐posterior | Supine‐posterior | |
|---|---|---|---|---|
| In‐phase spontaneous frequency | 0.00 | 0.00 | 0.13 ± 0.53 | 0.07 ± 0.43 |
| In‐phase maximum frequency | 0.04 ± 0.18 | 0.19 ± 0.53 | 0.00 | 1.2 ± 3.1 |
| Antiphase spontaneous frequency | 1.13 ± 1.63 | 1.06 ± 2.09 | 1.61 ± 2.2 | 0.70 ± 2.16 |
| Antiphase maximum frequency | 1.71 ± 1.74 | 2.11 ± 2.71 | 1.69 ± 1.79 | 1.23 ± 2.16 |
| Mean error time | 0.72 ± 0.84 | 0.84 ± 0.96 | 0.86 ± 0.91 | 0.8 ± 0.54 |
Each condition was acquired for 15 s.
Structural MRI
No abnormalities were detected on brain dual‐echo scans of all the subjects.
fMRI
During fMRI acquisition, all subjects performed the tasks correctly and no additional movements were noted.
Between‐Task Comparisons
In‐phase vs. antiphase movements
No areas showed a more significant activation during in‐phase vs. antiphase movements. On the contrary, the comparison of antiphase vs. in‐phase movements showed a more significant activation of the ipsilateral cingulated motor area (CMA; SPM space coordinates: 14, −28, 48; P < 0.001), ipsilateral middle frontal gyrus (MFG; SPM space coordinates: 50, 20, 30; P < 0.001), bilateral precentral gyrus (SPM space coordinates: −48, −6, 38 and 42, −8, 30; P < 0.001), bilateral inferior frontal gyrus (IFG; SPM space coordinates: −32, 38, −6 and 50, 28, 2; P < 0.001), bilateral thalamus (SPM space coordinates: −18, −20, 8 and 18, −22, 6; P < 0.001), bilateral precuneus (SPM space coordinates: −14, −66, 42 and 30, −50, 48; P < 0.001), contralateral infraparietal sulcus (IPS; SPM space coordinates: −26, −46, 58; P < 0.001), ipsilateral inferior parietal lobule (SPM space coordinates: 54, −38, 34; P < 0.001), ipsilateral cuneus (SPM space coordinates: 14, −72, 12; P < 0.001), and posterior lobe of the contralateral cerebellar hemisphere (SPM space coordinates: −28, −64, −22; P < 0.001; Fig. 3, Table IV).
Figure 3.
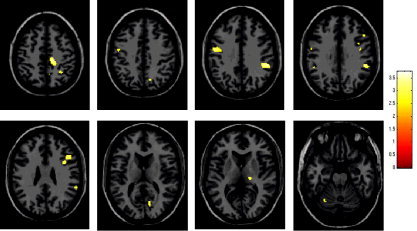
Random effect analysis showing, on a high‐resolution T1‐weighted image in the standard SPM space (neurological convention), regions of relative increased cortical activations (color‐coded for t‐values) in healthy individuals during the performance of cyclic antiphase vs. in‐phase flexion‐extension of right dominant hand and foot. Several areas are visible located in the frontal and parietal lobes, bilaterally, the visual cortex, the thalamus, and the cerebellum.
Table IV.
Summary of the fMRI results obtained from the comparison between the antiphase vs. in‐phase movements of the dominant hand and foot and from the comparisons between the in front vs. the behind position of the dominant upper limb with respect to the trunk (and vice versa) in healthy subjects
| Anatomical regions | Sidea | Antiphase vs. in‐phase movement (SPM coordinates) | Anterior vs. posterior position of the right upper limb (SPM coordinates) | Posterior vs. anterior position of the right upper limb (SPM coordinates) |
|---|---|---|---|---|
| Precentral gyrus | R | 42, −8, 30 | ||
| Precentral gyrus | L | −48, −6, 38 | ||
| Cingulated motor area | R | 14, −28, 48 | 8, 14, 38 | |
| Middle frontal gyrus | R | 50, 20, 30 | 32, 38, 22 | |
| Middle frontal gyrus | L | –32, 50, 6 | 0, 50, 22 | |
| Inferior frontal gyrus | R | 50, 28, 2 | 52, 18, 4 | |
| Inferior frontal gyrus | L | −32, 38, −6 | ||
| Postcentral gyrus | R | 34, −40, 50 | ||
| Infraparietal sulcus | R | 14, −68, 46 | ||
| Infraparietal sulcus | L | −26, −46, 58 | −22, −62, 40 | |
| Angular gyrus | L | −50, −68, 34 | ||
| Precuneus | R | 30, −50, 48 | 2, −56, 30 | |
| Precuneus | L | −14, −66, 42 | −32, −80, 43 | |
| Inferior parietal lobule | R | 54, −38, 34 | ||
| Caudate head | R | 8, 10, 2 | ||
| Caudate tail | R | 34, −42, 6 | ||
| Thalamus | R | 18, −22, 6 | ||
| Thalamus | L | −18, −20, 8 | −22, −24, 14 | |
| Insula | R | 34, 20, 6 | ||
| Insula | L | −46, 8, 6 | ||
| Middle temporal gyrus | R | 50, 12, 34 | ||
| Middle temporal gyrus | L | −58, −60, 22 | ||
| Cuneus | R | 14, −72, 12 | ||
| Vermis | B | 0, −54, −6 | ||
| Cerebellar hemisphere | L | −28, −64, −22 | −32, −68, −28 |
R, right; L, left; B, bilateral.
Prone vs. supine position of right upper limb
No significant differences were detected.
Anterior vs. posterior position of right upper limb with respect to trunk
The comparison between the anterior vs. the posterior position of the right upper limb with respect to the trunk showed a more significant activation of the ipsilateral CMA (SPM space coordinates: 8, 14, 38 and 6, 22, 22; P < 0.001), ipsilateral postcentral gyrus (SPM space coordinates: 34, −40, 50; P < 0.001), bilateral MFG (SPM space coordinates: 32, 38, 22 and −32, 50, 6; P < 0.001), ipsilateral IFG (SPM space coordinates: 52, 18, 4; P < 0.001), bilateral IPS (SPM space coordinates 14, −68, 46 and −22, −62, 40; P < 0.001), ipsilateral caudate tail (SPM space coordinates: 34, −42, 6; P < 0.001), bilateral insula (SPM space coordinates: 34, 20, 6 and −46, 8, 6; P < 0.001), contralateral thalamus (SPM space coordinates: −22, −24, 14; P < 0.001), bilateral vermis (SPM space coordinates: 0, −54, −6; P < 0.001), and the posterior lobe of the contralateral cerebellar hemisphere (SPM space coordinates: −32, −68, −28; P < 0.001; Fig. 4). On the contrary, the comparison between the posterior vs. the anterior position of the right upper limb with respect to the trunk showed a more significant activation of the contralateral MFG (SPM space coordinates: 0, 50, 22; P < 0.001), contralateral angular gyrus (SPM space coordinates: −50, −68, 34; P < 0.001), ipsilateral head of caudate nucleus (SPM space coordinates: 8, 10, 2; P < 0.001), bilateral precuneus (SPM space coordinates: 2, −56, 30 and −32, −80, 42; P < 0.001), ipsilateral middle temporal gyrus (SPM space coordinates: 50, 12, 34; P < 0.001), and contralateral MT/V5 complex (SPM space coordinates: −58, −60, 22; P < 0.001; Fig. 5, Table IV).
Figure 4.
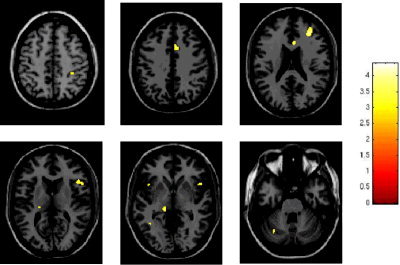
Random effect analysis showing, on a high‐resolution T1‐weighted image in the standard SPM space (neurological convention), regions of relative increased cortical activations (color‐coded for t‐values) in healthy individuals during the performance of cyclic flexion‐extension of right dominant hand and foot with the right upper limb positioned in front vs. behind with respect to the trunk. Several areas are visible located in the frontal and parietal lobes, bilaterally, the caudate tail, the thalamus, and the cerebellum.
Figure 5.
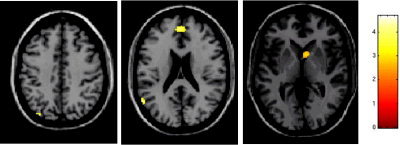
Random effect analysis showing, on a high‐resolution T1‐weighted image in the standard SPM space (neurological convention), regions of relative increased cortical activations (color‐coded for t‐values) in healthy individuals during the performance of cyclic flexion‐extension of right dominant hand and foot with the right upper limb positioned behind vs. in front with respect to the trunk. Several areas are visible located in the frontal, parietal and temporal lobes, and the caudate head.
Evaluation of first chosen movement in supine‐posterior condition
During the performance of the first chosen movement, an increased activation of the ipsilateral CMA (SPM space coordinates: 8, 36, 24; P < 0.001) was observed when compared with the performance of the second chosen movement (Fig. 4).
Interaction of spatially congruent vs. spatially incongruent movements
This analysis was performed since kinematic analysis showed an influence of pronation‐supination on the strength of the symmetry effect (maximum frequency with the forearm in prone position during in‐phase movements and with the forearm in supine position for antiphase movements). During spatially incongruent movements, a more significant activation of the contralateral CMA (SPM space coordinates: −4, −16, 26 and −8, −6, 44; P < 0.001), bilateral MFG (SPM space coordinates: 30, 44, −4 and −30, 38, −10; P < 0.001), bilateral thalamus (SPM space coordinates: −20, −22, 16 and 6, −18, 14; P < 0.001), and ipsilateral inferior parietal lobule (SPM space coordinates: 52, −40, 34; P < 0.001) was detected when contrasted to spatially congruent movements.
DISCUSSION
In this study, we used kinematic analysis and fMRI to evaluate the influence of limb position on the performance of cyclic in‐phase and antiphase movements of the dominant limbs and the neural pathways responsible for these movements.
In agreement with previous studies [Baldissera et al.,1982,1991], kinematic analysis showed that, on average, in‐phase movements were performed at higher frequency with lower time errors than antiphase ones. However, contrary to previous findings [Baldissera et al.,1982; Carson et al.,1995], we showed that the position of the limbs can modify these findings. In particular, in‐phase movements had the maximum frequency with the forearm in prone position, whereas antiphase movements had the maximum frequency with the forearm in supine position. Conversely, the position of the upper limb with respect to the trunk did not influence movement speed. A common finding of previous works aimed at investigating in‐phase and antiphase hand and foot movements was that in healthy subjects, in‐phase movements represented the preferential coupling, independently of the prone or supine position of the forearm [Baldissera et al.,1982,1991]. In this study, we found that this behavior tends to change when the upper limb is positioned behind the trunk. In this case, the majority of the subjects studied indeed preferred the antiphase coupling with the forearm in the supine position. This finding would suggest that body and body segment positions might influence between‐limb coupling and coordination, thus supporting the hypothesis of an interaction of postural mechanisms with voluntary movements. In particular, these findings strengthen the role of spatial and direction‐dependent constrains in establishing accurate hand and foot coordination patterns [Baldissera et al.,1982,1991,2005; Salesse et al.,2005].
fMRI analysis demonstrated an increased recruitment of the sensorimotor network (including several regions in the frontal and parietal lobes, bilaterally, the CMA, the thalami, the visual cortex, and the cerebellum) usually considered to function in motor, sensory, and multimodal integration processing during antiphase compared with in‐phase movements. Similar results, except for the activation of the visual cortex and cerebellum, were obtained when considering hand position influence on the previous contrast. Since all the subjects were carefully monitored during task execution and all the tasks were performed at a similar speed, which was below the spontaneous speed chosen by the subjects in the different experimental conditions, we can reasonably rule out any influence related to the different task performance on these findings. In addition, we carefully tried to avoid possible influences of head and shoulder movements on the observed patterns of brain activation by monitoring carefully subjects' performance during fMRI acquisition and by checking the degree of head movement on realigned images before fMRI statistical analysis.
In‐phase movements of the ipsilateral hand and foot represent the intrinsic behavior of the motor system. Conversely, antiphase association requires a continuous attentive effort to inhibit conflicting neuronal commands and to maintain coordination. This increased attentional demand is witnessed by the increased activation of regions in the frontal lobes during antiphase movements, including the CMA. In normal subjects, CMA activation has been found to be related to the presentation of new motor tasks and perhaps reflects relative task difficulty [Paus et al.,1993; Rao et al.,1993; Jenkins et al.,1994]. In addition, the CMA has been suggested to play an important role in conflict monitoring [Carter et al.,1998; Botvinick et al.,1999]. Therefore, the increased activity of the CMA observed in our study might indicate that antiphase as compared with in‐phase movements were more difficult and required a higher level of cognitive control, presumably to direct attention between tasks or to monitor potential conflicts. More specifically, increased cognitive control is mandatory when habitual behavior needs to be blocked to allow the execution of less familiar actions, as was the case in our experiment. The role of the CMA in the execution of spatially complex coordination tasks has been recently underlined by a study of Wenderoth et al. [2005], where the increased CMA activation was found during the performance of a bimanual task. Similar to our results, this study also described an increased activation of the precuneus, which was related to shifting attention between different locations in space, which was necessary for monitoring the trajectories of the left and right wrists when both limbs moved in parallel.
The activation of other brain areas when contrasting the antiphase to the in‐phase movement also agrees with the notion of the subject's perception of antiphase movements as a more complex/novel task. The thalamus [Brooks,1995; Parent and Hazrati,1995] and the cerebellum have extensive connections with the motor and somatosensory cortices and are involved in motor programming, execution, and control [Ehrsson et al.,2002]. Interestingly, the posterior lobe of the cerebellum has been related to motor imagery [Grafton et al.,1996], movement coordination [Ramnani et al.,2001], and motor learning [Sakai et al.,1998]. Despite the fact that all the subjects were trained to perform the tasks before fMRI acquisition, we cannot rule out learning as a factor influencing our results. Activation changes related to motor learning have been shown not only during the performance of complex tasks [Karni et al.,1998], but also following simple finger movements [Tracy et al.,2001; Morgen et al.,2004]. The parietal cortex is usually involved in the elaboration of somatosensory inputs [Rizzolatti et al.,1997] and in movement preparation and planning [Cohen and Andersen,2002; Leuthold and Jentzsch,2002]. Furthermore, it also has extensive connections with regions of the frontal lobes, to which it sends rich sensory information for movement control. The recruitment of a parietofrontal circuit has been recently demonstrated in healthy individuals during implicitly imagined movements of the hands [Johnson et al.,2002]. As a consequence, mental imagery might yet be another mechanism engaged during antiphase movement performance. The possible role of mental imagery during antiphase movements is also strengthened by the observed increased recruitment of areas located in the occipital lobes, which are known to be part of the visual network and have extensive connections with motor areas [Pelphrey et al.,2005]. A previous study [Debaere et al.,2001] found exclusively an increased activation of the SMA and of the cingulate cortex during the performance of antiphase than in‐phase movements of the dominant limbs. The discrepancy with our findings might be related either to the different number of subjects recruited or to the different set of experimental conditions, our data being obtained from the average of four different conditions.
Although the kinematic analysis did not show differences in kinematic variables when considering the anterior and posterior positions of the upper limb with respect to the trunk, we investigated the brain patterns of activation related to the different positions of the upper limb with respect to the trunk in order to test whether the activations of different brain networks, related to the processing of different aspects of the motor act, might be responsible for the observed kinematic results. When comparing the anterior vs. the posterior position of the upper limb with respect to the trunk, we found that different frontal and parietal regions were recruited during the two tasks, with a preferential recruitment of the basal ganglia, the insula, and the cerebellum during the first condition and of regions located in the temporal lobes during the second one. A possible explanation of these observations is that the activation of different frontal and parietal regions related to the different position of the upper limb with respect to the trunk might be related to a different processing of sensorial information to control the motor act. The insular cortex has been shown to play a role in cross‐modal transfer of information [Hadjikhani and Roland,1998], which would be required in this task to integrate effectively the felt position of the limbs with their movements. In addition, the insular cortex has also been implicated in the synchronization of movement kinematic [Mosier and Bereznaya,2001] and has connections with numerous cortical and subcortical motor regions [for a review, see Augustine,1996]. The temporal lobes contain several areas involved in motion processing, in particular, the MT/V5 complex [Barton et al.,1996]. Their increased activations with the upper limb positioned behind the trunk suggests that the perceived limb motion pattern is relevant in order to generate fine hand‐foot coordination.
The analysis of the first movement chosen in the supine‐posterior condition (the only condition showing a between‐subject difference in phase preference) exclusively demonstrated an increased activation of the CMA. The role of the CMA, in particular of the anterior portion of this region, in action selection has been demonstrated by several studies [Deiber et al.,1991; Cunnington et al., 2005]. This region has been shown to play a crucial role in initiation, motivation, and goal‐directed behaviors [Devinsky et al.,1995].
All together, these results indicate that during unilateral hand and foot movements, different neuronal pathways are recruited at different extent according to the type of movement (i.e., in‐phase or antiphase) and the position of the upper limbs. This is likely to occur to process the cognitive and sensorial information needed to control and perform the motor act.
REFERENCES
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P (2001); Neural compensation for mechanical loading of the hand during coupled oscillations of the hand and foot. Exp Brain Res 139: 18–29. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Borroni P, Cavallari P, Cerri G (2002): Excitability changes in human corticospinal projections to forearm muscle during voluntary movements of ipsilateral foot. J Physiol 15: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Civaschi P (1982): Preferential coupling between voluntary movements of ipsilateral limbs. Neurosci Lett 34: 95–100. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Marini G, Tassone G (1991): Differential control of in‐phase and anti‐phase coupling of rhythmic movements of ipsilateral hand and foot. Exp Brain Res 83: 375–380. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Leocani L (1998): Cyclic modulation of the H‐reflex in a wrist flexor during rhythmic flexion‐extension movements of the ipsilateral foot. Exp Brain Res 118: 427–430. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Esposti R (2005): Postural constraints to coupling of ipsilateral hand‐foot movements. Neuroreport 16: 1615–1619. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Guthrie B, Wood M, Mikulis D (1996): Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Ann Neurol 40: 387–398. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Brooks DJ (1995): The role of the basal ganglia in motor control: contributions from PET. J Neurol Sci 128: 1–13. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Kelso JA, Elliott D (1995): Phase transitions and critical fluctuations in rhythmic coordination of ipsilateral hand and foot. J Mot Behav 27: 211–224. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA (2002): A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci 3: 553–562. [DOI] [PubMed] [Google Scholar]
- Colier WNJM, Quaresima V, Oeseburg B, Ferrari M (1999): Human motor‐cortex oxygenation changes induced by cyclic coupled movements of hand and foot. Exp Brain Res 129: 457–461. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Robinson S, Moser E (2006): The selection of intended actions and the observation of others' actions: a time‐resolved fMRI study. NeuroImage 24: 1294–1302. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Van Hecke P, Duysens J (2001): Brain areas involved in interlimb coordination: a distributed network. NeuroImage 14: 947–958. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS (1991): Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1999): The human brain: surface, blood supply, and three‐dimensional sectional anatomy. New York: Springer. [Google Scholar]
- Ehrsson HH, Naito E, Geyer S, Amunts K, Zilles K, Forssberg H, Roland PE (2000): Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: a PET study. Eur J Neurosci 12: 3385–3398. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Kuhtz‐Buschbeck JP, Forssberg H (2002): Brain regions controlling nonsynergistic versus synergistic movement of the digits: a functional magnetic resonance imaging study. J Neurosci 22: 5074–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. NeuroImage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (1996): Localization of grasp representations in humans by positron emission tomography: II, observation compared with imagination. Exp Brain Res 112: 103–111. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE (1998): Cross‐modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. J Neurosci 18: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon RM (1997): Handbook of neurologic rating scales. New York: Demos Vermande. [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: a study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ (2002): Selective activation of a parietofrontal circuit during implicitly imagined prehension. NeuroImage 17: 1693–1704. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey‐Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG (1998): The acquisition of skilled motor performance: fast and slow experience‐driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold H, Jentzsch I (2002): Distinguishing neural sources of movement preparation and execution: an electrophysiological analysis. Biol Psychol 60: 173–198. [DOI] [PubMed] [Google Scholar]
- Mechsner F, Kerzel D, Knoblich G, Prinz W (2001): Perceptual basis of bimanual coordination. Nature 414: 69–73. [DOI] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, Frank J, McFarland H, Martin R, Cohen LG (2004): Kinematic specificity of cortical reorganization associated with motor training. NeuroImage 21: 1182–1187. [DOI] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I (2001): Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 140: 280–289. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1991): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN (1995): Functional anatomy of the basal ganglia: I, the cortico‐basal ganglia‐thalamo‐cortical loop. Brain Res Brain Res Rev 20: 91–127. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E (1993): Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G (2005): Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cereb Cortex 15: 1866–1876. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Toni I, Passingham RE, Haggard P (2001): The cerebellum and parietal cortex play a specific role in coordination: a PET study. NeuroImage 14: 899–911. [DOI] [PubMed] [Google Scholar]
- Rao SM, Binder JR, Bandettini PA, et al. (1993): Functional magnetic resonance imaging of complex human movements. Neurology 43: 2311–2318. [DOI] [PubMed] [Google Scholar]
- Riek S, Woolley D (2005): Hierarchical organisation of neuro‐anatomical constraints in interlimb coordination. Hum Mov Sci 24: 798–814. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (1997): Parietal cortex: from sight to action. Curr Opin Neurobiol 7: 562–567. [DOI] [PubMed] [Google Scholar]
- Sakai K, Takino R, Hikosaka O, Miyauchi S, Sasaki Y, Putz B, Fujimaki N (1998): Separate cerebellar areas for motor control. Neuroreport 9: 2359–2363. [DOI] [PubMed] [Google Scholar]
- Salesse R, Temprado JJ, Swinnen SP (2005): Interaction of neuromuscular, spatial and visual constraints on hand‐foot coordination dynamics. Hum Mov Sci 24: 66–80. [DOI] [PubMed] [Google Scholar]
- Salter JE, Wishart LR, Lee TD, Simon D (2004): Perceptual and motor contributions to bimanual coordination. Neurosci Lett 363: 102–107. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Faro SS, Mohammed F, Pinus A, Christensen H, Burkland D (2001): A comparison of “early” and “late” stage brain activation during brief practice of a simple motor task. Brain Res Cogn Brain Res 10: 303–316. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP (2005): The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci 22: 235–246. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited—again. NeuroImage 2: 173–181. [DOI] [PubMed] [Google Scholar]


