Abstract
Neuroimaging studies of painful stimuli in humans have identified a network of brain regions that is more extensive than identified previously in electrophysiological and anatomical studies of nociceptive pathways. This extensive network has been described as a pain matrix of brain regions that mediate the many interrelated aspects of conscious processing of nociceptive input such as perception, evaluation, affective response, and emotional memory. We used functional magnetic resonance imaging in healthy human subjects to distinguish brain regions required for pain sensory encoding from those required for cognitive evaluation of pain intensity. The results suggest that conscious cognitive evaluation of pain intensity in the absence of any sensory stimulation activates a network that includes bilateral anterior insular cortex/frontal operculum, dorsal lateral prefrontal cortex, bilateral medial prefrontal cortex/anterior cingulate cortex, right superior parietal cortex, inferior parietal lobule, orbital prefrontal cortex, and left occipital cortex. Increased activity common to both encoding and evaluation was observed in bilateral anterior insula/frontal operculum and medial prefrontal cortex/anterior cingulate cortex. We hypothesize that these two regions play a crucial role in bridging the encoding of pain sensation and the cognitive processing of sensory input. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: cognitive, pain, fMRI, anterior insula, cingulate cortex
INTRODUCTION
Intensity is one of the most salient characteristics of pain. It is evaluated mainly by a person's subjective description of a private experience. The conscious perception of pain, however, does not always directly reflect incoming signals from primary sensory neurons [Melzack and Katz,1994; Petrovic and Ingvar,2002]; as incoming sensory input becomes part of conscious awareness, it undergoes extensive associative elaboration and modulation [Mesulam,1998].
Conscious awareness of pain includes not only an appreciation of the quantitative and qualitative descriptive attributes of a noxious sensation but also the evaluation of the emotional meaning of the sensation. Previous literature suggests that the evaluation of a painful sensation involves at least two components. The first is an initial, automatic feeling of unpleasantness. The second, termed “secondary pain affect,” represents the emotional response associated with the anticipation of future implications of pain [Gracely,1992; Price,2000; Price and Harkins,1992]. An alternative interpretation is that the initial feeling of unpleasantness is encoded as part of the noxious sensation and that secondary pain affect is an outcome of the cognitive processing that occurs as part of the conscious awareness of the sensation.
The goal of this study was to test the hypothesis that spontaneous brain processing of variable intensities of noxious stimuli could be distinguished from the cognitive evaluation of pain intensity (pain rating). Previous reports led us to hypothesize that the brain regions that would be activated during the experience of different levels of pain would include contralateral primary somatosensory cortex (SI), bilateral secondary somatosensory cortex (SII), insular cortex/operculum, anterior cingulate cortex (ACC), and thalamus [Alkire et al.,2004; Bornhovd et al.,2002; Buchel et al.,2002; Peyron et al.,2000]. We will refer to these regions as the pain sensory intensity‐encoding network. Cognitive evaluation of pain intensity (pain‐rating tasks) requires mapping the nociceptive experience to some sort of semantic construct like a word or number on a scale and is only one part of the evaluative component of pain, which may include additional components such as pain quality, unpleasantness, and cognitive constructs such as the implication of pain [Price,2000]. We hypothesized that the performance of a rating task requires the involvement of both brain regions supporting general cognitive evaluation tasks (i.e., deciding how loud a sound is or how bright a light is) as well as additional regions specific to evaluation of pain intensity. We specifically hypothesized that the anterior insular cortex, ACC, and dorsolateral prefrontal cortex would be activated during performance of a pain‐rating task [Craig,2002; Craig et al.,2000; Gollub et al., unpublished results; Maihofner et al.,2004].
SUBJECTS AND METHODS
Subjects
Sixteen right‐handed subjects (8 males; mean age, 27 ± 6.0 years ± standard deviation [SD]) were recruited into this study as approved by the Human Research Committee of the Massachusetts General Hospital. All subjects gave written informed consent after the experimental procedures had been fully explained.
Experimental Procedures
Calibrated thermal pain stimuli were delivered to the right medial aspect of the forearm using a TSA‐2001 Thermal Sensory Analyzer with a 3 cm × 3 cm probe (Medoc Advanced Medical Systems, Rimat Yishai, Israel) running proprietary computerized visual analog scale software [COVAS; Becerra et al.,1999; Peyron et al.,1999]. Thermal stimuli were 10 s in duration, including the 2.5‐s ramp up and down from baseline, initiated from a baseline resting temperature of 32°C.
Subjects participated in two behavioral testing sessions followed by one functional magnetic resonance imaging (fMRI) scanning session. Each session was separated by a minimum of 1 week. The behavioral sessions were used to determine appropriate stimuli intensities, to minimize anticipatory anxiety, and to control for rating strategy and learning effects. The subjects were instructed in the use of 0–20 Sensory and Affective scales [Gracely et al.,1978a,b].
In the behavioral sessions, two heat pain stimulus intensities were selected for each subject; one to elicit responses in the strong range (HIGH PAIN; 14–17 on the Sensory Box Scale) and one to elicit responses in the mild to moderate range (LOW PAIN; 8–11 on the Sensory Box Scale). A warm, non‐painful stimulus of 34°C (WARM) was used as a control for all subjects. To separate the sensory experience of the stimuli from the cognitive evaluative effort of the rating task, subjects were explicitly asked to focus their attention on their arm during each heat stimulus and then to wait for the scales to be displayed before performing the rating task.
In session 1, subjects first experienced an ascending series of calibrated heat stimuli (starting from 38°C and increasing by 1°C to 52°C or up to the subjects' tolerance). Temperatures that elicited subjective intensity ratings in the range of LOW pain and HIGH pain were selected for each subject. The ascending series was followed by randomized sequences of two repetitions of the three stimulus intensities (HIGH pain, LOW pain, and WARM). In session 2, the same randomized sequences were applied again and the temperature was adjusted if necessary to ensure that subjective ratings were in the desired range for each stimulus type.
In the fMRI scanning session, six different sequences of 12 stimuli trials were applied to six different regions of the right medial lower arm so that each region received one sequence. A sequence was applied during one functional run. A trial consisted of a presentation of a stimulus and a scale (Fig. 1). Three levels of stimuli (HIGH pain, LOW pain, and WARM) were presented during each stimulus sequence. Each level was presented four times and the order of presentation was ordered randomly. To begin each trial, a red fixation cross was displayed to cue the start of the stimulus administration. After the 10‐s stimulus administration, the red fixation cross was replaced by a black fixation cross for 4 s, followed by one of the two types of scales (rating or control) for 10 s. Subjects were instructed to perform the appropriate scaling tasks as described below during the scale presentation. A black fixation cross was displayed during the intertrial interval, which varied with a mean interval of 6 s.
Figure 1.
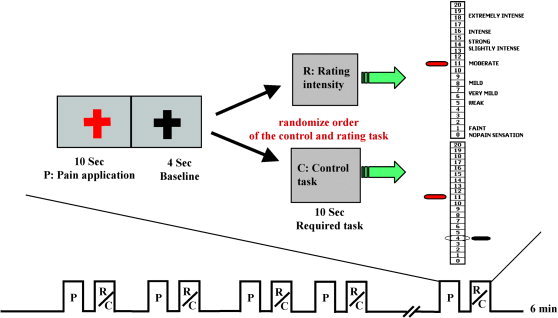
Schematic representation of the experimental paradigm. Each 6‐min scan was composed of a series of 12 pain stimuli trials followed by either a rating or control task in a single trial design. Each trial started with the fixation cross turning to red to cue the subject to attend to the administration of the thermal stimulus (10‐s duration). There then was a 4‐s delay during which a black fixation cross was displayed. This delay was followed by the display of either the Gracely Sensory Box scale with anchor words (Rating task) or of the same 0–20 number scale with a bar pointing to a circled target number (Control task). For either task, the subject used their left index and middle fingers to push buttons that moved a red pointer from midscale to the target number for the Control task or to the appropriate response number for the Rating task. The interval between two trials was randomized, with average of 6 s.
Subjects were instructed to focus on the stimulus but to wait until the scale was displayed to perform the poststimulus task. After half of the stimulus presentations, the 0–20 Sensory scale was displayed with a red pointer positioned at the middle number on the scale. In this condition, the subjects were required to rate the sensory intensity of the preceding stimulus by pressing buttons with the left index and middle fingers to move the red pointer up and down (Fig. 1). In the other trials, the usual anchor words were omitted, replaced by a target bar pointing to one of the numbers on the scale (Fig. 1). To respond to this type of scale, subjects had to press a button to move the red pointer to the target number instead of giving their own rating of the intensity of the preceding stimuli. The choice of target numbers for the control task ensured that the two tasks required a comparable number of button presses. This target‐matching task was used as control for the sensory intensity rating.
The order of two rating tasks and level of the previous noxious stimuli were ordered randomly and counterbalanced. Before scanning, subjects participated in a practice session, which included stimuli administration on the left arm and both scale tasks. Subjects were allowed to practice the tasks until they were confident about the process.
Functional MRI Data Acquisition and Analysis
All brain imaging was carried out with a three‐axis gradient head coil in a 3 Tesla MRI system (Siemens Allegra; Siemens Medical Systems, Erlagen, Germany). Thirty axial slices (4‐mm thick with 1‐mm skip) parallel to the anterior and posterior commissure, encompassing the entire brain, were imaged with 2,000‐ms repetition time (TR), 40‐ms echo time (TE), 90‐degree flip angle, and 3.13 × 3.13 mm in‐plane spatial resolution. A high‐resolution 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence was also collected.
Preprocessing and statistical analysis were carried out using SPM2 software (Wellcome Department of Cognitive Neurology). Preprocessing included motion correction, normalization to the MNI305 stereotactic space, and spatial smoothing with an 8‐mm Gaussian kernel and default high‐pass filtering to remove low‐frequency noise. Global signal scaling was not applied to prevent spurious deactivations.
A random‐effects analysis was carried out with SPM2. A separated general linear model (GLM) was specified for each subject with regressors for each of nine conditions tested: three intensity levels of HIGH pain, LOW pain, and WARM by three epochs of 10 s: administration of stimuli, sensory intensity rating, and matching target control. To examine the network that encodes pain intensity, we tested two contrasts for each subject: HIGH pain > LOW pain and LOW pain > WARM. Regions mediating the cognitive evaluation of heat pain intensity were identified by comparing the effects of the sensory rating task to the matching target control task for all noxious stimuli (HIGH and LOW pain intensity stimuli only), and also separately for each of the three stimulus intensities.
Group analysis was carried out using a random‐effects model. Contrast images for each subject and each effect of interest were generated as described above. These contrast images were analyzed using a GLM to determine voxel‐wise t‐statistics. A one‐way t‐test was used to determine group activation for each contrast. Voxel‐wise activation at P < 0.001 uncorrected and cluster activation P < 0.05 corrected with 10 contiguous voxels were considered to be statistically significant. In addition, a conjunction analysis was carried out to specifically identify brain regions activated by both sensory intensity encoding and evaluation.
RESULTS
Pain‐Rating Data
Average ratings on the Sensory scale in response to the HIGH pain, LOW pain, and WARM intensities of the thermal stimuli were within the intended ranges (mean ± SD) 14.8 ± 2.0, 8.5 ± 2.2, and 0.5 ± 0.7, respectively. In exit interviews, all subjects reported following the suggested strategy of focusing on the sensation during stimulus administration and waiting for the appearance of the scale to perform either the rating or target‐matching task.
Functional MRI Results
Nociceptive and sensory intensity encoding
Table I shows significant regions of activation found in contrasts tested to elucidate the networks involved in sensory intensity encoding. Comparison between the two painful conditions (HIGH pain > LOW pain) yielded highly significant activations in the entire predicted network including bilateral insular and opercular cortices, ACC/medial prefrontal cortex (MPFC), SII, lingualis gyrus, thalamus, cerebellum and left SI (contralateral) in the arm region. These robust activations required an increase in the statistical threshold to voxel‐wise uncorrected P < 0.0001 to separate clusters, but no new regions of activation were found even in the default threshold. The comparison of LOW pain > WARM provided no significant activations at the default threshold. At a lower threshold of voxel‐wise uncorrected P < 0.005 with 10 continuous voxels, however, activations were observed in bilateral insular and frontal opercular cortices, and ACC/MPFC. These results indicate that the LOW pain stimuli elicited increases in brain activity throughout most of the network that were only slightly greater than those in the WARM condition, whereas the HIGH pain stimuli elicited much greater increases in activation.
Table I.
Brain areas activated during encoding of heat pain intensity
| Comparison | Brodmann area | Z score | Cluster P value (corrected) | Voxels in cluster (n) | Peak coordinate (x,y,z) | Ratio |
|---|---|---|---|---|---|---|
| High > low pain | Left primary somatosensory cortex (1, 2)a | 3.58 | 0.042 | 199 | 14, −42, 72 | 15/16 |
| Left postcentral cortex, SII (1,2,43) | 4.97 | 0.000 | 325 | 66, −22, 22 | 15/16 | |
| Right postcentral cortex, SII (1,2,43) | 4.89 | 0.004 | 108 | −66, −26, 20 | 15/16 | |
| Left/right anterior cingulate/medial prefrontal cortex (24,32) | 4.33 | 0.000 | 234 | 4, 2, 46 | 14/16 | |
| Left/right lingualis gyrus (18) | 4.24 | 0.000 | 285 | 20, −70, −2 | 13/16 | |
| Left posterior/middle/anterior insular cortex | 4.64 | 0.000 | 669 | 36, −20, 16 | 16/16 | |
| Right middle/posterior insular cortex | 6.58 | 0.039 | 55 | −36, −16, 14 | 12/16 | |
| Right middle insular cortex/frontal operculum | 6.43 | 0.000 | 197 | −52, 10, −4 | 15/16 | |
| Right middle insular cortex/putamen | 4.32 | 0.008 | 90 | −32, 0, 2 | 12/16 | |
| Right thalamus | 6.84 | 0.000 | 386 | −14, −8, 8 | 14/16 | |
| Left thalamus | 4.45 | 0.000 | 438 | 16, −14, 8 | 14/16 | |
| Left/right cerebellum | 4.79 | 0.000 | 339 | 0, −48, −26 | 15/16 | |
| Right cerebellum | 4.27 | 0.006 | 99 | −8, −78, 18 | 13/16 | |
| Low pain > warm | No suprathreshold clustersa |
The threshold is set to voxel‐wise P < 0.001 uncorrected and cluster P < 0.05 corrected with 10 contiguous voxels. Ratio indicates the number of subjects to the total (N = 16) who showed activation (P < 0.01 voxels, uncorrected) in each region of interest. Peak coordinates refer to the MNI305 atlas.
Original threshold of voxel‐wise uncorrected P < 0.001 and cluster corrected P < 0.05 with 10 contiguous voxels.
SII, secondary somatosensory cortex; MNI, Montreal Neurological Institute.
Cognitive pain intensity evaluation
Table II shows significant regions of activation for the contrast of the sensory rating task to the matching target control task for all noxious stimuli, which included bilateral anterior insula/frontal operculum, MPFC/ACC, right middle/inferior/orbital prefrontal gyrus, superior/inferior parietal lobule, angular gyrus, left inferior frontal gyrus, putamen, and occipital cortex. The separate analyses of the contrasts for the HIGH pain, LOW pain, and WARM trials reveal a strong influence of stimulus intensity on activity in this network (LOW pain rating > WARM rating > HIGH pain rating). Comparison of the rating to the control task after the LOW pain stimuli resulted in the most significant and widespread activation in regions, which include bilateral anterior insula and frontal operculum, right middle/inferior prefrontal gyrus, superior parietal cortex, inferior parietal lobule, MPFC/ACC, middle frontal gyrus, and left occipital cortex. Comparison of the rating to the control task after WARM stimuli trials revealed a significant activation only in bilateral occipital cortex at the threshold we set; lowering the threshold to P < 0.005 uncorrected with 10 continuous voxels revealed activation in bilateral occipital cortex, dorsolateral prefrontal cortex, insula, right superior parietal gyrus/precuneus, ACC/MPFC, and left precuneus. For the trials after HIGH pain stimuli, lowering the threshold to P < 0.005 uncorrected with 10 continuous voxels revealed activations in bilateral occipital cortex, left middle/inferior prefrontal gyrus, anterior insula, precuneus, superior/inferior parietal gyrus, right operculum/anterior insula and superior frontal gyrus. These results suggest that the activation patterns are quite similar for the ratings of different stimulus intensity levels, albeit with different significance.
Table II.
Brain areas activated during cognitive evaluation of heat pain intensity
| Comparison | Area (Broadmann area) | Z score | Cluster P value (corrected) | Number of voxels in cluster | Peak coordinate (x,y,z) | Ratio |
|---|---|---|---|---|---|---|
| Pain rating control task | Right middle/inferior/orbital prefrontal gyrus (9/10/11/45/46) | 5.17 | 0.000 | 6830 | −28, 52, 16 | 16/16 |
| Right anterior insular cortex/frontal operculum | 5.09 | −30, 20, 8 | 16/16 | |||
| Right/left medial prefrontal G./anterior cingulate cortex (24/32/6) | 4.90 | −12, 16, 56 | 14/16 | |||
| Right superior/inferior parietal lobule (7) | 5.02 | 0.000 | 2666 | −14, 74, 52 | 14/16 | |
| Right angular gyrus (39) | 4.31 | ‐ | ‐ | −32, −80, 34 | 15/16 | |
| Left anterior insular cortex/frontal operculum | 4.33 | 0.000 | 930 | 42, 14, 2 | 15/16 | |
| Left inferior prefrontal gyrus (45)/putamen | 4.27 | 36, 32, 4 | 14/16 | |||
| Left occipital gyrus (17,18) | 5.15 | 0.000 | 794 | 14, −98, −6 | 16/16 |
The threshold is set to voxel‐wise uncorrected P < 0.001 and cluster P < 0.05 corrected with 10 continuous voxels. Ratio indicates the number of subjects of the total (n = 16) who showed activation (voxel‐wise P < 0.01 uncorrected) in each region of interest.
Inspection of the statistical maps from individual subjects supports the results from the group analysis. Most subjects (at least 75%) showed significant activation in brain regions reported for the group analysis for conditions testing both sensory intensity encoding and cognitive pain intensity evaluation.
Overlap and difference between sensory intensity encoding and cognitive intensity evaluation
Figure 2 compares noxious sensory intensity encoding (HIGH pain > LOW pain) to cognitive sensory intensity evaluation (pain rating compared to target matching control task for all painful stimuli). Regions that were significantly activated in both contrasts include bilateral anterior insula and ACC/MPFC. Conjunction analysis using the minimum statistic compared to the conjunction null method [Friston et al.,2005; Nichols et al.,2005] was applied to investigate further the brain regions activated in common by the above two contrasts. The results showed the same regions of activation in bilateral anterior insula and ACC/MPFC (voxel‐wise P < 0.001 uncorrected).
Figure 2.
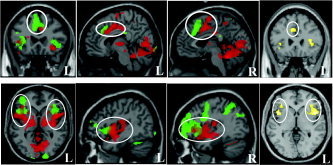
Overlapping brain activations evoked by encoding of heat pain intensity (HIGH pain > LOW pain indicated by red color), cognitive evaluation of heat pain (PAIN rating > Control task indicated by green color) and the common regions of the two contrasts from conjunction analysis (indicated by yellow color). Threshold was set to P < 0.001 uncorrected with 10 continuous voxels. L, left; R, right.
For sensory intensity encoding, the activation extended from anterior insula to the middle/posterior insula; for cognitive intensity evaluation, the activation extended from anterior insula to lateral and orbital (right) prefrontal area. In MPFC/ACC, the activation for sensory intensity encoding was localized posterior to the activation found for cognitive intensity evaluation.
The direct comparison of the above two contrasts to reveal differences between them yielded concordant results. Greater activations (P < 0.001 uncorrected and cluster activation P < 0.05 corrected with 10 contiguous voxels) were observed in right posterior insula/operculum (−40, −18, 4), right rostral ACC (−8, 54, −6), left posterior cingulate cortex (−10, −48, 34), right supramarginal gyrus (−46, −66, 34) and bilateral cerebellum (24, −18, −24; −14, −64, 14) during pain sensory encoding than during cognitive sensory intensity evaluation. Greater activation was observed in left superior frontal gyrus (24, 6, 60), left middle frontal gyrus (32, 58, 14), left superior parietal lobule and precuneus (14, −72, 54), right occipital cortex (−14, −98, −4), and bilateral medial prefrontal cortex (2, 24, 48) during cognitive sensory intensity evaluation than during pain sensory encoding.
DISCUSSION
This study used fMRI to dissociate the neural processes of sensory encoding from the cognitive evaluation of heat pain intensity. The results confirm that the process of encoding pain sensation in the human brain uses multiple and parallel brain regions in both the lateral and medial pain systems, including the contralateral SI, bilateral insula/frontal operculum, MPFC/ACC, SII, and thalamus [Coghill et al.,1999]. These results are also consistent with recent studies that used the stimulus‐response function to define the brain regions responsible for intensity encoding [Alkire et al.,2004; Bornhovd et al.,2002; Buchel et al.,2002].
Our sensory rating task identified a brain network active during the cognitive evaluation of pain intensity that includes bilateral anterior insula and frontal operculum, right middle/inferior prefrontal gyrus, superior parietal cortex, inferior parietal lobule, MPFC/ACC, middle frontal gyrus, and left occipital cortex. Importantly, the reactivation of the bilateral anterior insula occurred after the stimulus had ended, consistent with the fact that the sensory rating task required somatosensory imagery for retrieval of previous sensory information about both the pain to be rated and prior experiences for comparison. It also required matching the somatic sensation to a numerical scale, making a decision about a sensory rating, and more attention than that required for the control task. The brain regions in which we observed increased activity during pain intensity rating are similar to those activated by response selection, executive control, working memory, episodic memory, and perceptual and problem‐solving tasks that involve high levels of mental effort [Duncan and Owen,2000]. The activation in the occipital regions is most likely a consequence of the additional visual stimulation provided by the presence of the anchor words.
The results from the separate analysis of the ratings of LOW and HIGH pain stimulus intensities showed that less intense pain stimuli are more potent in the activation of the evaluative network. This finding is consistent with post‐study oral reports from subjects indicating that stimuli in the mild to moderate range were the most difficult to rate whereas high‐intensity stimuli were the easiest to rate. This self‐report is supported by further analysis of the behavioral data, which shows that the LOW pain ratings are more variable than are the HIGH pain ratings within subjects. The mean and SD of the within‐subject SDs are 2.6 ± 1.2 and 2.0 ± 1.1, for LOW and HIGH pain respectively. For 12 of 16 subjects, the LOW pain SD was greater. A paired t‐test on the SDs for HIGH and LOW pain showed that the LOW pain SDs were significantly larger than that of HIGH pain (P < 0.04).
The different effects of task difficulty (less intense pain stimuli are more potent in the activation of the evaluative network whereas stronger pain stimuli are more potent in the activation of the encoding network) provide strong experimental support for the concept that sensory intensity encoding and cognitive evaluation of pain intensity involve different neural processing mechanisms.
In a recent fMRI study, Singer et al. [2004] reported anterior insula and ACC activation when female subjects experienced pain and when they witnessed a loved one experiencing pain. The two different conditions yielded overlapping areas of activation in the anterior insula and ACC that closely resemble what we observed in our study (see their Fig. 1 and our Fig. 2). The brain areas activated when subjects experienced their own pain match the brain areas activated in our subjects during pain encoding. In contrast, the areas activated by empathy with another's pain closely match areas activated by cognitive evaluation. Our interpretation of these results is that a common cognitive intensity evaluation process underlies assessment of the intensity of pain experienced by the self or by another person and that these processes are involved in the act of empathy.
A new finding from this study is the identification of overlapping regions in the bilateral MPFC/ACC and anterior insula/frontal operculum that are activated during both encoding and evaluation of pain intensity. Activation in both regions was extensive and the overlap was only partial. The ACC regions uniquely involved in evaluation of pain intensity were located in the cognitive subdivision of the ACC as defined by Bush et al. [2000] and anterior to those involved in sensory intensity encoding. We hypothesize that these two regions play a crucial role in bridging the sensory encoding of pain and the cognitive processing of sensory input.
The neuroanatomical literature provides evidence of the requisite pathways required to support the hypothesis of this bridging role for the anterior insula. The insular cortex is divided into four interconnected subdivisions; from rostral to caudal, they are: insular proisocortex, agranular subdivision, dysgranular subdivision, and the granular insular areas [Cipolloni and Pandya,1999]. Agranular insula (primarily located in anterior portion of insula) receives projections from SI, retroinsular area, superior temporal sulcus, and the amygdaloid and entorhinal cortex and projects to frontal areas, retroinsular area, and SII [Augustine,1996; Cipolloni and Pandya,1999]. Further studies have shown that agranular insula also projects fibers into cingulate areas [Augustine,1996]. All these innervations link the anterior insula with limbic, emotional arousal, and working memory system [Cahill and McGaugh, 1998]. This link provides the anatomical basis for the multiple functions of anterior insula.
A limitation in this study is the unknown extent to which we were able to separate the pain sensory encoding from cognitive sensory intensity evaluation. In this study, we made several efforts to separate these two procedures. First, we used a Gracely Sensory Box scale with anchor words (see Fig. 1 for details of anchor words). This scale is more complicated than a simple 0–10 scale with no anchor words. Second, we trained the subject from the very beginning of their participation to feel the pain first and then to look at the scale to perform the ratings. Finally, during the fMRI scanning, the subjects only had to rate half of the total stimuli. This should have compelled them to follow the strategies we suggested and to pay attention to the pain and then wait for the scale to be displayed to do the sensory rating or button press to move the cursor to the target. All subjects reported that they followed these procedures. Nevertheless, subjects may confound the sensory encoding and cognitive evaluation to a certain extent.
In summary, we found two dissociated but overlapping brain networks involved in pain sensory intensity encoding and cognitive evaluation of pain intensity. The overlap of these two processes is localized to bilateral anterior insula and MPFC/ACC. A neural network that includes anterior insula/frontal operculum, MPFC/ACC, lateral/orbital prefrontal, and parietal cortex may represent a second level pain information processing circuit that supports the active, conscious cognitive evaluation of pain sensation.
Acknowledgements
This study was supported by the National Institutes of Health (R21 DA11229, R21AT00949 and PO1‐AT002048‐A‐01 to R.L.G.) and by a Harvard Medical School Osher pilot grant (to J.K.).
REFERENCES
- Alkire MT, White NS, Hsieh R, Haier RJ (2004): Dissociable brain activation responses to 5‐hz electrical pain stimulation. Anesthesiology 100: 939–946. [DOI] [PubMed] [Google Scholar]
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comie AR, Gonzales RG, Barsook D (1999): Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 41: 1044–1057. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C (2002): Painful stimuli evoke different stimulus‐response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single‐trial fMRI study. Brain 125: 1326–1336. [DOI] [PubMed] [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C (2002): Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single‐trial laser functional magnetic resonance imaging study. J Neurosci 22: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN (1999): Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol 403: 431–458. [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadorola MJ (1999): Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 82: 1934–1943. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM (2000): Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE (2005): Conjunction revisited. Neuroimage 25: 661–667. [DOI] [PubMed] [Google Scholar]
- Gracely RH (1992): Affective dimensions of pain: how many and how measured? APS Journal 1: 243–247. [Google Scholar]
- Gracely RH, McGrath PA, Dubner R (1978a): Ratio scales of sensory and affective verbal pain descriptors. Pain 5: 5–18. [DOI] [PubMed] [Google Scholar]
- Gracely RH, McGrath PA, Dubner R (1978b): Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain 5: 19–29. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Schmelz M, Forster C, Neundorfer B, Handwerker HO (2004): Neural activation during experimental allodynia: a functional magnetic resonance imaging study. Eur J Neurosci 19: 3211–3218. [DOI] [PubMed] [Google Scholar]
- Melzack R, Katz J (1994): Pain measurement in persons in pain In: Wall PD, Melzack R, editors. Textbook of pain. Edinburgh: Churchill Livingstone; p 337–351. [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M (2002): Imaging cognitive modulation of pain processing. Pain 95: 1–5. [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia‐Larrea L, Gregoire M, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B (1999): Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain 122: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L (2000): Functional imaging of brain responses to pain. A review and meta‐analysis (2000): Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Price DD (2000): Psychological and neural mechanisms of the affective dimension of pain. Science 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- Price DD, Harkins SW (1992): The affective‐motivational dimension of pain: a two stage model. ASP Journal 1: 229–239. [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD (2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]


