Abstract
Internal senses of the position of the eye in the orbit may influence the cognitive processes that take into account gaze and limb positioning for movement or guiding actions. Neuroimaging studies have revealed eye position‐dependent activity in the extrastriate visual, parietal, and frontal areas, but, at the earliest vision stage, the role of the primary visual area (V1) in these processes remains unclear. Functional MRI (fMRI) was used to investigate the effect of eye position on V1 activity evoked by a quarter‐field stimulation using a visual checkerboard. We showed that the amplitude of V1 activity was modulated by the position of the eye, the activity being maximal when both the eye and head positions were aligned. Previous studies gave impetus to the emerging view that V1 activity is a cortical area in which contextual influences take place. The present study suggests that eye position may affect an early stage of visual processing. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: primary visual area, V1, eye position, humans, fMRI
INTRODUCTION
Recent reviews of the processes that underlie sensorimotor integration emphasized the influence of gaze coding for the transformations from retinal‐ to arm‐centered reference frame coordinates [Carey, 2003; Desmurget et al., 1998; Pouget et al., 2002]. Such influences were initially hypothesized to occur in parietal areas [Andersen et al., 1993] and may modify the visual response depending on where the position of the eye is in the orbit. However, these processes may also occur anywhere an eye position signal is present in the brain. Indeed, signals related to the position of the eye have been found in many brain regions in nonhuman studies, namely, in the primary visual area [Guo and Li, 1997; Trotter and Celebrini, 1999; Weyand and Malpeli, 1993] and through higher visual areas such as V3A [Galletti and Battaglini, 1989], V4 [Bremmer, 2000], V6 [Galletti et al., 1995; Nakamura et al., 1999], MT/MST areas [Bremmer et al., 1997b; Squatrito and Maioli, 1996, 1997], as well as in parietal regions [Andersen et al., 1985; Bremmer et al., 1997a, 1999] and in the frontal premotor cortex [Boussaoud et al., 1998; Boussaoud and Bremmer, 1999]. Functional MRI (fMRI) studies have localized human homologs of these cortical areas and have shown that the signal, related to the position of the eye, modulates activity in the extrastriate visual areas [DeSouza et al., 2002; Deutschlander et al., 2005] and in the parieto‐frontal network where hand‐arm movements are coordinated [Baker et al., 1999; DeSouza et al., 2000].
However, less information is known about the earliest stage of cortical visual processing, namely, in the primary visual area (V1). Previous electrophysiological [Guo and Li, 1997; Trotter and Celebrini, 1999; Weyand and Malpeli, 1993] and modeling studies [Pouget et al., 1993] have described that eye position‐dependent activity in V1 neurons occurs to a lesser extent than that reported in parietal and premotor cortices, but the findings are still consistent with the idea that both retinal and eye position signals may also converge at an early vision stage.
Accordingly, we recently showed in humans that the amplitude of the early C1 component of visual‐evoked potentials (VEPs) was modulated by eye position [Andersson et al., 2004]. Unfortunately, this previous study was conducted with only two occipital intermediate electrodes of the modified 10–20 system EEG montage and, thus, did not allow us to compute any direct location of V1 activity. The C1 component is a reliable index of V1 electrical activity [Di Russo et al., 2002; Martinez et al., 2001; Noesselt et al., 2002] and it led us to conclude, albeit indirectly, that eye position may influence V1 activity in humans. The purpose of the present study, therefore, is to investigate the eye position‐dependent activity of V1 in humans by conducting an fMRI experiment in which activity in the V1 area can be localized.
SUBJECTS AND METHODS
Subjects
Twelve subjects (six female, six male) recruited from an academic environment, ages 20–26 years, participated in this study. All subjects were right‐handed as assessed by the Edinburgh inventory questionnaire [Oldfield, 1971], free of any neurological disorder, and showed a normal brain MRI scan. All subjects gave written informed consent to participate in this study and the Basse‐Normandie Ethics Committee approved all of the procedures.
Task Design
Subjects were placed in a supine position in the bore of the magnet and instructed to continuously gaze at a red fixation point with both eyes. In order to keep their visuospatial attention on the fixation point, the subjects had to count the total number of decreases in light intensity emitted from the screen. The fixation point was placed either at 0° or 7° to the left, or 7° to the right of the center of the screen. Subjects had to keep their head still and, depending on the MRI scan protocol, to direct and to maintain their eyes either straight forward (0°) or 7°‐leftward or 7°‐rightward throughout the different scan series (Fig. 1). Nine MRI scans were obtained in each subject, three for each deviated eye position. Each block‐designed scan series alternated five 30‐s fixation periods with four 30‐s fixation periods together with a quarter‐field visual stimulation. The latter stimulation consisted of a black and white square checkerboard flashed against a black background (3.5° of visual angle, 0.6 cycle.deg–1 of spatial frequency, ISI = 500–1000 ms). Each scan series included the four 30‐s quarter‐field stimuli randomly alternated with the projection of a single fixation point. The total visual quarter‐field stimulation on the retina was equivalent regardless of the deviated eye position.
Figure 1.
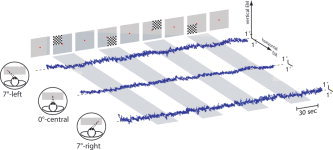
Eye tracking measurements observed in one subject for each deviated eye position (7°‐leftward on the top, 0°‐forward in the middle, and 7°‐rightward on the bottom). Both horizontal and vertical eye movements (EM) are represented for each series alternating a 30‐s fixation period and a 30‐s fixation period with visual quarter‐field stimulus (see Subjects and Methods for details). [Color figure can be viewed in the online issue, which is available at www. interscience.wiley.com.]
Both the fixation point and checkerboard images were delivered by a STIM software package (Neuroscan, El Paso, TX). They were projected with a magnetically shielded LCD video onto a translucent tangent screen and viewed through a dichroic cold mirror that was also dedicated for the eye tracking system (see below). The tangent screen was 100 cm wide and 40 cm high at a viewing distance of 260 cm, for a maximum visual angle of 22°.
Eye Tracking
The position of the eye was monitored during the MR scanning procedure through the use of an infrared eye tracking video system (Model 504, Applied Sciences Laboratory, Waltham, MA) adapted to the MR environment [Gitelman et al., 2000]. The subject's right eye was filmed (60 Hz) through a dichroic cold mirror (Optoprim, France) that allows unobtrusive viewing of the eye by the video system and an unimpeded view of the visual stimuli by the subject at the rear and front openings of the magnet. Eye tracking data were analyzed using ILAB 3.8 (D. Gitelman, http://www.brain.northwestern.edu/ilab/) and in‐house ILAB plugins written in Matlab (MathWorks, Natick, MA). Figure 1 illustrates some examples of the eye tracking procedure recorded in one subject for each eye position. During each fixation point the eye position was measured and averaged for each subject for 0°, 7°‐leftward, and 7°‐rightward scan series. An analysis of variance (ANOVA) was then performed in order to calculate the differences between the three averaged measurements and the three experimental eye positions in the 12 subjects.
MRI Procedures
Subjects were examined using a Signa 1.5 T MR scanner (General Electric, Milwaukee, WI) with a volume transmit and receive coil that provided whole head coverage. At the beginning of each scan session a high‐resolution anatomical scan was acquired (whole‐brain T1‐weighted spoiled‐grass; 256 × 256 × 124; 0.9375 × 0.9375 mm2 in‐plane resolution, 1.5 mm slice thickness). The protocol also included the acquisition of an interlaced proton density (PD)/T2‐weighted anatomical scan of 21 axial 5‐mm thick slices designed to cover the entire cortex and to delineate the field of view (FOV) of the functional scan series. For each subject nine functional BOLD series of 100 scans each were collected using a gradient‐echo echo‐planar sequence with a repetition time of 3000 ms, an echo time of 60 ms, a flip angle of 90°, an in‐plane resolution of 3.75 × 3.75 mm2 and 21 axial 5‐mm thick slices.
MRI Data Analyses
Data were analyzed using both in‐house and public software (SPM99, Welcome Department of Cognitive Psychology, London, UK; AIR, UCLA, USA; MPITOOL, Max‐Planck‐Institute, Germany). The first three volumes in each series, collected before equilibrium magnetization was reached, were discarded. The differences in timing of the functional slice acquisition were corrected to consider that the volumes were sampled at the middle of each repetition time period (SPM99). The fourth volume of the first scan series was then taken as the functional reference volume (fMRI0). For registration of the fMRI0 onto the stereotaxic Montreal Neurological Institute (MNI) template, two rigid (fMRI0 onto the T2‐volume and the PD‐volume onto the T1‐volume) and one nonlinear (the T1‐volume onto the MNI template) registration matrices were computed and combined into one matrix. The registration of the fMRI0 and T1‐volume in the MNI template space were thereafter verified visually with MPI Tool (Max Planck Institute, Germany) and corrected manually if necessary. The second step consisted in the registration of each fMRI volume onto the fMRI0 volume using SPM99 and the spatial normalization of each volume in the MNI template space using the registration matrix computed in the first step (see above). Amplitude normalization was also performed to compensate for any variations in intensity across the series. Finally, data were spatially smoothed with an 8 × 8 × 8‐mm3 full‐width at half‐maximum (FWHM) Gaussian kernel.
The statistical analysis of the BOLD signal, based on the General Linear Model, was computed with SPM99. The individual data were first analyzed separately for each subject. Twelve experimental conditions were considered (4 quarter‐fields × 3 eye positions = 12 contrast maps). The fixation point period between two quarter‐field visual stimulations were considered as the baseline and, therefore, implicitly included in the model. Significant activations were sought for in the 12 experimental conditions. We then performed a second level group analysis using the 12 contrast maps generated in the individual analysis. Since our goal was to specifically investigate the eye position‐dependent activity of V1, which lies on quarter‐field retinotopic properties, we proceeded in three steps. The first two steps were designed to define four calcarine‐weighted quarter‐field retinotopic volumes of interest (VOIs). Thereafter, these VOIs were used to compute activity in V1 for the 12 contrast maps and to perform an ANOVA between the different eye positions for each VOI and for each quarter‐field stimulus.
Definition of functional VOIs
We opted for a combining and intersecting approach using the former 12 contrast maps in order to select, in a functional manner, the visual activity with quarter‐field retinotopic properties regardless of the eye position.
We first computed four contrast maps (INF, SUP, LEFT, RIGHT), each of them dealing with one quarter‐field visual stimulus, again regardless of eye position. For example, the contrast map “INF” revealed the brain areas, which were activated by both left and right inferior quarter‐field visual stimuli (i.e., visual areas dealing with whole‐, hemi‐, and quarter‐field retinotopic properties). Second, we defined four maps of differences (subtraction maps, i.e., INF‐SUP, SUP‐INF, LEFT‐RIGHT, and RIGHT‐LEFT), which in turn revealed the brain areas that were activated during inferior, superior, left, and right stimuli, respectively, regardless of the eye position. As such, the INF‐SUP contrast map revealed the brain areas that were more activated by both left and right inferior quarter‐field visual stimuli than both superior ones, namely, visual areas dealing with hemi‐ and quarter‐field retinotopic properties. Finally, four conjunction analyses between these subtraction maps (INF‐SUP ∩ LEFT‐RIGHT; INF‐SUP ∩ RIGHT‐LEFT; SUP‐INF ∩ LEFT‐RIGHT; SUP‐INF ∩ RIGHT‐LEFT) led us to isolate brain areas that showed quarter‐field retinotopic properties (Table I). Indeed, the conjunction analysis (INF‐SUP ∩ LEFT‐RIGHT) revealed brain areas that were activated during any inferior and any left visual quarter‐field for any eye position, namely, brain areas showing a quarter‐field retinotopy for the left inferior quarter‐field.
Table I.
Activation peaks retinotopically related to each visual quarter‐field stimulus identified by conjunctionanalysis (see Subjects and Methods for details)
| Conjunction analysis | Quarter‐field stimulus | Retinotopic related‐ calcarine region | Voxels | MNI coord. | t value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| INF‐SUP ∩ LEFT‐RIGHT | Inferior left | Sup. Right (green) | 384 | +18 | −98 | +14 | 8.1 |
| INF‐SUP ∩ RIGHT‐LEFT | Inferior right | Sup. Left (red) | 428 | −20 | −98 | +8 | 8.0 |
| SUP‐INF ∩ LEFT‐RIGHT | Superior left | Inf. Right (orange) | 123 | +20 | −84 | −8 | 4.5 |
| SUP‐INF ∩ RIGHT‐LEFT | Superior right | Inf. Left (blue) | 155 | −20 | −82 | −12 | 4.9 |
Each color in parentheses corresponds to a retinotopic cluster illustrated in Figure 2A.
This approach yielded a single quarter‐field retinotopic cluster of activation per quarter‐field visual stimulus with a corresponding local maximum along the calcarine fissure (Table I, Fig. 2). As expected, each quarter‐field visual stimulus activated, at the level of V1, the opposite corners of the cross‐shaped calcarine fissure in the hemisphere contralateral to the stimulation. According to the visual angle of the present quarter‐field stimuli (3.5°), such retinotopic V1 activations were also localized at the most posterior part of the calcarine fissure with respect to the classical retinotopy of the primary visual cortex [Horton and Hoyt, 1991]. Although their local maxima were localized in V1 (Table I), these clusters may encompass V1 activity but also additional brain areas with quarter‐field retinotopic properties, namely, V2, V3/VP, and V4v [DeYoe et al., 1996; Sereno et al., 1995; Tootell et al., 1998].
Figure 2.
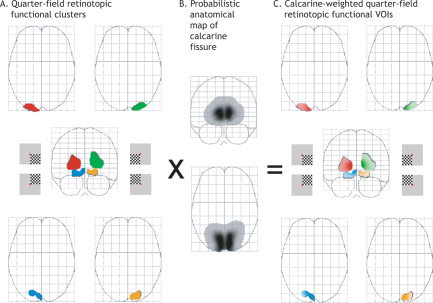
Definition of the calcarine‐weighted quarter‐field retinotopic VOIs. See Subjects and Methods for details. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Probabilistic anatomical weighting
In order to filter out the impact of extrastriate visual activity, we computed four calcarine‐weighted quarter‐field retinotopic VOIs (Fig. 2C). A binary version of the four quarter‐field retinotopic clusters was created (Fig. 2A) and multiplied with the probabilistic anatomical map of the calcarine fissure of the 12 subjects (Fig. 2B). This was obtained by identifying both calcarine fissures of each subject according to Ono's atlas guides [Ono et al., 1990] on high‐resolution anatomical MR images. One calcarine volume per hemisphere was defined as the major sulcus extending from the occipital pole to the junction with the parieto‐occipital sulcus (including the cortex above and below the fissure) and traced onto multiple sagittal slices through the volume up to the fundus of the calcarine branches. Both left and right calcarine volumes defined in the 12 subjects were averaged and spatially smoothed with an 8 × 8 × 8‐mm3 FWHM Gaussian kernel as performed for functional MR data (Fig. 2B).
Use of the calcarine‐weighted quarter‐field retinotopic VOIs
For each subject the mean amplitude of activated voxels was computed in each VOI for each original contrast map, (i.e., 4 quarter‐field stimuli × 3 eye positions). According to the present definition of a calcarine‐weighted functional VOI, the higher the probability that activated voxels belonged to the calcarine fissure, the stronger were their weights in the calculation of the mean amplitude of activation within a VOI. In other words, activity from other quarter‐field retinotopic areas (especially V3/VP and V4v distant from the calcarine fissure) was minimized by their low weights in the calculation. At the very worst, part of the quarter‐field V2 activity bordering the calcarine fissure was included with substantial weights in the calculation.
Finally, we performed an ANOVA between the different eye positions on the mean amplitude of activations for each VOI and for each quarter‐field stimulus.
RESULTS
Eye Tracking
The position of the eye was monitored during MR scanning procedures using an infrared eye tracking video system (see Subjects and Methods). The results showed that subjects were able to accurately maintain their eye position during each fixation period and over the duration of the scan (Table II). ANOVA failed to reveal any significant difference between both measured and expected eye positions (F(2,10) = 0.94, P = 0.4). The absence of any noticeable eye movement during the fixation period ensured that the retinotopic projection of a given quarter‐field was equivalent for each eye position.
Table II.
Mean (± standard deviation) of the averaged eye position during MR scanning
| Deviated eye position | Theoretical value | Experimental Value | |
|---|---|---|---|
| Leftward | x | −7 | −6.9 ± 0.8 |
| y | 0 | 0.1 ± 0.6 | |
| Central | x | 0 | 0.0 ± 0.4 |
| y | 0 | 0.0 ± 0.8 | |
| Rightward | x | 7 | 6.8 ± 0.8 |
| y | 0 | −0.2 ± 0.5 |
Eye Position Signals in V1 duringQuarter‐Field Stimulation
ANOVA was performed between the different eye positions for the mean amplitude of activations measured in each weighted‐calcarine functional VOI and for each quarter‐field stimulus. Considering one quarter‐field visual stimulus, two types of activation were observed in a given functional VOI.
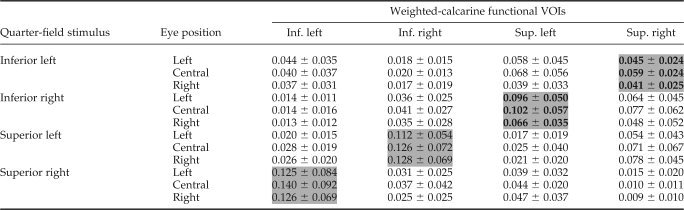
First, with respect to the cross‐shaped retinotopy of the primary visual cortex, V1 activation was evoked by a quarter‐field visual stimulation in its relevant crossed functional VOI, and second, further V1 activations (although to a lesser degree) were measured in the three other functional VOIs nonretinotopically related to the quarter‐field stimulation (Table III).
Table III.
BOLD signal measured in each weighted‐calcarine functional volume of interest (VOI) for each quarter‐field stimulus and each eye position
|
Activities retinotopically related to each quarter‐field stimulus are shaded with their corresponding VOI.
Considering the retinotopically evoked activity, we observed a significant effect of eye position on the mean amplitude of the V1 activation for both left (F(2,11) = 5.97, P < 0.001) and right (F(2,11) = 5.60, P < 0.02) inferior quarter‐field stimulations (Fig. 3).
Figure 3.
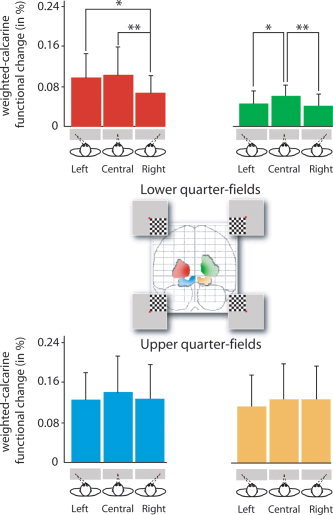
Mean amplitude of visually evoked activity in the four weighted‐ calcarine functional VOIs related to their retinotopic visual quarter‐fields. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For the left inferior quarter‐field, visually evoked activity was significantly decreased for each deviated eye position as compared to the central eye position (post‐hoc PLSD Fisher, leftward‐deviated: P < 0.05, rightward‐deviated: P < 0.01). For the right inferior quarter‐field, the mean amplitude of the visual evoked activity was also significantly decreased for rightward‐deviated eye position as compared to both central (P < 0.005) and leftward‐deviated (P < 0.02) eye position. Although this was only a trend, the mean amplitude of V1 activation evoked by left and right superior quarter‐field stimulations was also higher for central eye position than for deviated eye positions.
Considering V1 activations observed in the three functional VOIs nonretinotopically related to the quarter‐field stimulation, ANOVA did not show any effect of eye position regardless of the quarter‐field stimulation.
DISCUSSION
The present study showed that visually evoked activity in V1 was decreased when the eyes were deviated both 7°‐left or ‐rightward. We will first discuss the present findings by considering some possible experimental reasons for observing a weaker response to nonaligned vision, and then discuss the functional significance of enhanced aligned central vision in humans.
Eye Position‐Dependent V1 Evoked Activity
The absence of noticeable eye movements during fixation, as revealed by the eye‐tracking measurements, ensured that the retinotopic projection of a given quarter‐field was equivalent, whatever the position of the eye. Therefore, the present findings could not be related to a change in the selectivity of the visual receptive fields with respect to the location of the visual stimulus.
The question arose whether the changes of amplitude in V1 evoked activity may be due to oculomotor signals and/or retinal disparity, i.e., to the difference in the position of the visual stimulus on each retina related to the relative monitor. As reported elsewhere [Andersson et al., 2004], we evaluated the difference of both horizontal and vertical disparity between the different deviation of the eye. The difference between the magnitude of disparity for the central eye position and each deviated eye condition depends on both eye deviation and the distance of the visual stimulus from the fixation point. Due to the distance between the eye and the monitor in the present study (260 cm, see Subjects and Methods), the maximal difference in disparity between the deviated and central eye position was 0.0175°, an amount that appears to be too small to link the present results to a single disparity effect.
We observed, albeit to a lesser extent, further V1 activation in the three functional VOIs nonretinotopically related to a given quarter‐field. However, one may argue that the present quarter‐field stimulations were adjacent to both horizontal and vertical meridians that led to a partial overlap of a given quarter‐field beyond the borders of its retinotopic representation, as reinforced by the 8 × 8 × 8‐mm3 filtering of the functional data. Nevertheless, such further V1 activation was not modulated by the position of the eye per se.
The present findings suggest that the position of the eye influenced the amplitude of activation in V1 by modulating the activity of neurons related to the visual stimulation and not the selectivity of their receptive field. Our results also showed that the eye position modulated the V1 evoked activity all the more as for lower visual field. Concerning this latter point, several neurophysiological and behavioral studies have shown that human performance on various visual tasks may change according to whether stimuli are presented in the lower or in the upper visual field. Consistent with the present findings, MEG responses to visual pattern onset are larger when stimuli are presented in the lower field, rather than in the upper visual field [Portin et al., 1999]. Behavioral studies also showed vertical visual field asymmetry. For instance, the segmentation of an image into “figure” and “background” is performed better in the lower visual field [Rubin et al., 1996]. Furthermore, attentional resolution was reported to be greater in the lower than in the upper visual field [Bilodeau and Faubert, 1997], and neglect symptoms were reported to be more evident in the lower visual field [He et al., 1996].
Such a functional heterogeneity between lower and upper visual fields has been previously addressed: a distinction between near (peri‐personal) and far (extra‐personal) space are biased toward the lower and upper visual fields, respectively [Previc, 1990]. Processing of environmental information in the lower visual field is believed to be more global because of its involvement in reaching and other manipulations performed in a peri‐personal space, whereas processing in the upper visual field is primarily local and linked to a visual search and recognition mechanism directed towards the extra‐personal space. Indeed, the functional differences between near and far visual space are correlated with their disproportionate representations in the dorsal and ventral divisions of the visual cortex, respectively, and in the magnocellular and parvocellular pathways that project to them [Previc, 1990]. Many of the visual areas in the dorsal pathway of the monkey receive inputs from across the entire retina, including the far periphery. In contrast, the ventral pathway, from V1 to the inferotemporal cortex, receives most of its input from foveal and parafoveal retina, reflecting its role in object recognition and scene perception [Milner and Goodale, 1993]. The differences in visual field representation between dorsal and ventral streams are, therefore, also evident across the upper and lower portions of the visual field. Visually guided pointing movements with the hand are both faster and more accurate when performed in the lower visual field when compared with the same movements made in the upper visual field [Danckert and Goodale, 2001]. This finding provides evidence in support of the idea that the visuomotor systems in the human brain show a bias toward processing information in the lower visual field. An internal sense of eye position in the orbit influences the processes that take into account gaze and limb positioning for a guiding action. As described in the present study, the influence of eye position on V1 activity is larger for the lower visual field than for the upper field. This observation suggests the role of a functional specialization of the lower visual field for global processing in reaching and other manipulations performed in a peri‐personal space. However, although this was only a trend, the mean amplitude of V1 activation evoked by left and right superior quarter‐field stimulations was also higher for a central eye position than for deviated eye positions. The fact that the area of the cortex that was activated, with respect to the quarter‐field retinotopy, was twice as great than that for the lower, when compared to the upper, visual fields (Table I) may explain, in part, why a significant eye position effect was observed only for the lower field conditions. Further investigations are required to better understand if the functional specialization of the lower visual field for global processing makes it more sensitive to eye position than the upper visual field.
Eye Positional Benefit for Aligned Vision: A Bottom‐up Process
We observed that V1 activity showed a stronger retinotopic response for standard vision, i.e., when both eyes and head orientations were centrally aligned. Our findings agree with a previous electrophysiological study that recorded signals of eye position in V1 neurons [Guo and Li, 1997]. It is noteworthy that Guo and Li observed an effect of eye position on the activity of 47 out of 91 V1 neurons, several of which showed a maximal activity for a central visual stimulation, while the same visual stimulation presented for a deviated eye position resulted in lower neuronal activity.
Our results are also in line with recent psychometric results that showed that eye position affects orientation of visuospatial attention [Craighero et al., 2004]. Using a classical Posner paradigm, these latter authors showed that attentional benefits are always present in a standard aligned vision but not when both eyes were deviated to the left or rightward. In the present study, we observed a benefit of the aligned central vision at the macroscopic level of V1 activity. One may argue that such a benefit may be explained by the summation of the intrinsic neuronal activity that was modulated by the position of the eye. Indeed, in its strongest form a neuron‐exhibiting gaze coding may respond to a visual target only when the eyes are in a certain position in the orbit. On the contrary, retinotopic neuron activity would grow (or weaken) depending on the position of the eyes within the orbit, even though the visual stimulus, from a retinal point of view, is kept constant [Guo and Li, 1997; Weyand and Malpeli, 1993]. As a matter of fact, eye position‐dependent V1 neurons do not change the location of their receptive field.
The present fMRI data did not allow one to validate the top‐down and/or bottom‐up origin of the eye position effect on V1 activity. One may first argue that V1 activity may be modulated by eye position through delayed feedback signals originating from extrastriate and/or oculomotor structures in a similar manner that attention‐related signals affect V1 activity [for review, see Di Russo et al., 2002; Kastner and Ungerleider, 2000]. But one may also hypothesize that the eye position may influence V1 activity through early feedforward signals. Indeed, we recently showed that the initial C1 sensory response (50–90 ms) of visually evoked potentials originating from the V1 area was modulated by eye position [Andersson et al., 2004]. Our previous findings, together with recent studies, give impetus to an emerging view that V1 activity is modulated by visual attention through delayed feedback signals and suggest that both eye position and attention‐related signals may affect the early stage of visual processing in a different manner. The former point of view suggests an effect related to extraocular muscle afferents and/or corollary discharges [Buisseret and Maffei, 1977; Toyama et al., 1984], while the latter is considered as a late top‐down process [Martinez et al., 1999; Noesselt et al., 2002].
Acknowledgements
We thank Dr. Alan Young for the English proofing of the final version of article and the anonymous reviewer for helpful comments.
REFERENCES
- Andersen RA, Essick GK, Siegel RM 1985: Encoding of spatial location by posterior parietal neurons. Science 230: 456–458. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Li CS, Stricane B 1993: Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol 3: 171–176. [DOI] [PubMed] [Google Scholar]
- Andersson F, Etard O, Denise P, Petit L 2004: Early visual evoked potentials are modulated by eye position in humans induced by whole body rotations. BMC Neurosci 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Donoghue JP, Sanes JN 1999: Gaze direction modulates finger movement activation patterns in human cerebral cortex. J Neurosci 19: 10044–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau L, Faubert J 1997: Isoluminance and chromatic motion perception throughout the visual field. Vision Res 37: 2073–2081. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Bremmer F 1999: Gaze effects in the cerebral cortex: reference frames for space coding and action. Exp Brain Res 128: 170–180. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Jouffrais C, Bremmer F 1998: Eye position effects on the neuronal activity of dorsal premotor cortex in the macaque monkey. J Neurophysiol 80: 1132–1150. [DOI] [PubMed] [Google Scholar]
- Bremmer F 2000: Eye position effects in macaque area V4. Neuroreport 11: 1277–1283. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Distler C, Hoffmann KP 1997a: Eye position effects in monkey cortex. II. Pursuit‐ and fixation‐related activity in posterior parietal areas LIP and 7a. J Neurophysiol 77: 962–977. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Ilg UJ, Thiele A, Distler C, Hoffmann KP 1997b: Eye position effects in monkey cortex. I. Visual and pursuit‐related activity in extrastriate areas MT and MST. J Neurophysiol 77: 944–961. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Graf W, Ben Hamed S, Duhamel JR 1999: Eye position encoding in the macaque ventral intraparietal area (VIP). Neuroreport 10: 873–878. [DOI] [PubMed] [Google Scholar]
- Buisseret P, Maffei L 1977: Extraocular proprioceptive projections to the visual cortex. Exp Brain Res 28: 421–425. [DOI] [PubMed] [Google Scholar]
- Carey DP 2003: Neuropsychological perspectives on sensorimotor integration: eye‐hand coordination and visually‐guided reaching In: Kanwisher N, Duncan J, editors. Functional neuroimaging of visual cognition. Attention and performance XX. Oxford: Oxford University Press; p 481–502. [Google Scholar]
- Craighero L, Nascimben M, Fadiga L 2004: Eye position affects orienting of visuospatial attention. Curr Biol 14: 331–333. [DOI] [PubMed] [Google Scholar]
- Danckert J, Goodale MA 2001: Superior performance for visually guided pointing in the lower visual field. Exp Brain Res 137: 303–308. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Pelisson D, Rossetti Y, Prablanc C 1998: From eye to hand: planning goal‐directed movements. Neurosci Biobehav Rev 22: 761–788. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Dukelow SP, Gati JS, Menon RS, Andersen RA, Vilis T 2000: Eye position signal modulates a human parietal pointing region during memory‐guided movements. J Neurosci 20: 5835–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza JF, Dukelow SP, Vilis T 2002: Eye position signals modulate early dorsal and ventral visual areas. Cereb Cortex 12: 991–997. [DOI] [PubMed] [Google Scholar]
- Deutschlander A, Marx E, Stephan T, Riedel E, Wiesmann M, Dieterich M, Brandt T 2005: Asymmetric modulation of human visual cortex activity during 10 degrees lateral gaze (fMRI study). Neuroimage 28: 4–13. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J 1996: Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A 93: 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA 2002: Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp 15: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Battaglini PP 1989: Gaze‐dependent visual neurons in area V3A of monkey prestriate cortex. J Neurosci 9: 1112–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Battaglini PP, Fattori P 1995: Eye position influence on the parieto‐occipital area (V6) of the macaque monkey. Eur J Neurosci 7: 2486–2501. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, LaBar KS, Mesulam MM 2000: Real‐time monitoring of eye movements using infrared video‐oculography during functional magnetic resonance imaging of the frontal eye fields. Neuroimage 11: 58–65. [DOI] [PubMed] [Google Scholar]
- Guo K, Li CY 1997: Eye position‐dependent activation of neurons in striate cortex of macaque. Neuroreport 8: 1405–1409. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J 1996: Attentional resolution and the locus of visual awareness. Nature 383: 334–337. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF 1991: The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol 109: 816–824. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG 2000: Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23: 315–341. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo‐Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA 1999: Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369. [DOI] [PubMed] [Google Scholar]
- Martinez A, DiRusso F, Anllo‐Vento L, Sereno MI, Buxton RB, Hillyard SA 2001: Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Res 41: 1437–1457. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA 1993: Visual pathways to perception and action. Prog Neurobiol 95: 317–337. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Chung HH, Graziano MSA, Gross CG 1999: Dynamic representation of eye position in the parieto‐occipital sulcus. J Neurophysiol 81: 2374–2385. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard S, Woldorff M, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze H 2002: Delayed striate cortical activation during spatial attention. Neuron 35: 575. [DOI] [PubMed] [Google Scholar]
- Oldfield RC 1971: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD 1990: Atlas of the cerebral sulci. Stuttgart: Georg Thieme. [Google Scholar]
- Portin K, Vanni S, Virsu V, Hari R 1999: Stronger occipital cortical activation to lower than upper visual field stimuli. Neuromagnetic recordings. Exp Brain Res 124: 287–294. [DOI] [PubMed] [Google Scholar]
- Pouget A, Fisher SA, Sejnowski TJ 1993: Egocentric spatial representation in early vision. J Cogn Neurosci 5: 150–161. [DOI] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Duhamel JR 2002: A computational perspective on the neural basis of multisensory spatial representations. Nat Rev Neurosci 3: 741–747. [DOI] [PubMed] [Google Scholar]
- Previc FH 1990: Functional specialization in the lower and upper visual fields in humans: its ecological origins and neuropsychological implications. Behav Brain Sci 13: 519–575. [Google Scholar]
- Rubin N, Nakayama K, Shapley R 1996: Enhanced perception of illusory contours in the lower versus upper visual hemifields. Science 271: 651–653. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH 1995: Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Maioli MG 1996: Gaze field properties of eye position neurones in areas MST and 7a of the macaque monkey. Vis Neurosci 13: 385–398. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Maioli MG 1997: Encoding of smooth pursuit direction and eye position by neurons of area MSTd of macaque monkey. J Neurosci 17: 3847–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Hadjikhani NK, Mendola JD, Marrett S, Dale AM 1998: From retinotopy to recognition: fMRI in human visual cortex. Trends Cogn Sci 2: 174–183. [DOI] [PubMed] [Google Scholar]
- Toyama K, Komatsu Y, Shibuki K 1984: Integration of retinal and motor signals of eye movements in striate cortex cells of the alert cat. J Neurophysiol 51: 649–665. [DOI] [PubMed] [Google Scholar]
- Trotter Y, Celebrini S 1999: Gaze direction controls response gain in primary visual‐cortex neurons. Nature 398: 239–242. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Malpeli JG 1993: Responses of neurons in primary visual cortex are modulated by eye position. J Neurophysiol 69: 2258–2260. [DOI] [PubMed] [Google Scholar]


