Abstract
Neuropsychological evidence regarding grammatical category suggests that deficits affecting verbs tend to localize differently from those affecting nouns, but previous functional imaging studies on healthy subjects fail to show consistent results that correspond to the clinical dissociation. In the current imaging study, we addressed this issue by manipulating not only the grammatical category but also the processing mode, using auditory presentation of Hebrew words. Subjects were presented with verbs and nouns and were instructed to make either a semantic decision (“Does the word belong to a given semantic category?”) or a morphological decision (“Is the word inflected in plural?”). The results showed different patterns of activation across distinct regions of interest. With respect to grammatical category effects, we found increased activation for verbs in the posterior portion of the left superior temporal sulcus, left dorsal premotor area, and posterior inferior frontal gyrus. In each of these regions, the effect was sensitive to task. None of the ROIs showed noun advantage. With respect to task effects, we found a semantic advantage in left anterior inferior frontal gyrus, as well as in left posterior middle temporal gyrus. The results suggest that cerebral verb‐noun dissociation is a result of localized and subtle processes that take place in a set of left frontal and temporal regions, and that the cognitive and neural processes involved in analyzing grammatical category depend on the lexical characteristics of the stimuli, as well as on task requirements. The discrepancy between functional imaging and patient data is also discussed. Hum. Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: fMRI, verbs, nouns, neurolinguistics, language processing, Hebrew
INTRODUCTION
During the past 2 decades, ample evidence in the neuropsychological literature has shown that the retrieval of verbs and nouns can be selectively damaged [to mention a few: Miceli et al., 1984, 1988; McCarthy and Warrington, 1985; Zingeser and Berndt, 1988, 1990; Saffran et al., 1989; Caramazza and Hillis, 1991; Damasio and Tranel, 1993; Hillis and Caramazza, 1995; Silveri and Di Betta, 1997]. Commonly, impairment in the retrieval of verbs relative to nouns has been associated with damage in the left frontal lobe, whereas the opposite impairment has been ascribed to lesions in the left temporal lobe or temporoparietal regions. However, exceptions to this, as well as conflicting and inconsistent results, challenged the association of the retrieval of different grammatical categories with different brain areas [Luzzatti et al., 2002; Corina et al., 2005].
Theoretically, the processing origin of verb‐noun dissociation is subject to further controversy [for a recent review, see Crepaldi et al., 2005]. Lexical accounts suggest that this is rooted in either a lexical‐syntactic level [Berndt et al., 1997, 2002] or, more specifically, in a lexicomorphological level [Shapiro et al., 2000, 2001; Shapiro and Caramazza, 2003a]. By contrast, semantic accounts suggest that the dissociation originates in conceptual [McCarthy and Warrington, 1985; Damasio and Tranel, 1993] or featural [Bird et al., 2000, 2003] differences.
Recently, functional brain imaging was used to investigate the neuronal representation of verbs and nouns in healthy brains. This did not yield consistent results and failed to confirm the neuropsychological observations. Specifically, while most studies revealed large networks of brain regions that were activated during lexical processing, no clear association of cortical regions with grammatical category was observed. Early PET studies found greater activation for verbs than for nouns in a large network of regions, including the temporal, parietal, and prefrontal regions, as well as the supplementary motor area (SMA), whereas the opposite contrast elicited more activation in the right prefrontal cortex [Warburton et al., 1996]. A more recent PET study by Perani et al. [1999] employed visual lexical decision for both verbs and nouns in Italian. Here the verb (+)/noun (−) contrast yielded activation in the left dorsolateral prefrontal, left superior parietal, left middle temporal, and occipital cortices. No regions were activated more by nouns than by verbs.
Additional studies in English [Tyler et al., 2003, 2004] and Chinese [Li et al., 2004] failed to replicate the results of Perani et al. [1999] and did not find any neuronal correlates of grammatical category differences. A subsequent study by Tyler et al. [2004] found that inflected verbs evoked significantly greater activations than inflected nouns only in left inferior frontal gyrus (IFG), in support of the claim that differences in processing verbs and nouns were rooted in morphological rather than semantic properties. However, none of the above studies identified brain regions that were activated more by nouns than by verbs. Shapiro et al. [2005] ascribed the latter failure to the inability of the tasks to tap semantics‐independent grammatical knowledge. In their PET study, Shapiro et al. [2005] used a morphological task in which subjects produced singular and plural forms of written nouns (N), verbs (V), pseudonouns, and pseudoverbs. By conjuncting real and pseudoword contrasts, they aimed to neutralize semantics and reveal activations that appertain to grammatical differences as such. For the V > N contrast, the conjunction yielded activation in the left superior frontal gyrus, the left anterior temporal gyrus, the cerebellum, and thalamus. For N > V, the conjunction also yielded activations, mainly in the right superior temporal gyrus (STG), left fusiform, left precentral gyrus, and cerebellum. This result supports the idea that some aspects of processing nouns were functionally distinct from those of processing verbs. However, the cerebral locations that showed this in Shapiro et al. [2005] differed from those implicated in noun impairments following brain damage. Markedly, neither of the conjunction contrasts in the latter study activated the main language regions on the left IFG and left STG (Broca's and Wernicke's area, respectively). It may well be that the choice of a task was crucial here, as one could infer from Shapiro and Carmazza [2003b], Shapiro et al. [2005], and Tyler et al. [2004]. However, the effect of task on the V‐N dissociation has never been tested in a direct comparison within a single experiment.
The present fMRI study was set out to test the processing mode effect directly by comparing the grammatical category effect under two different tasks, semantic and morphological, within the same experiment. We employed comprehension rather than production tasks in order to avoid in‐scanner motion artifacts associated with overt articulation. In the semantic task, subjects judged the association of Hebrew verbs and nouns with a given semantic category. In the morphological task, subjects had to judge the number inflection of these stimuli (number inflection for both verbs and nouns is specified in Hebrew by a morphological suffix). Applying the latter task in a morphologically rich language like Hebrew enabled us to test the role of inflectional morphology in the dissociation, as was suggested by Tyler et al. [2004]. The tasks were identical for verbs and nouns so that grammatical category was an implicit property of the stimuli. To neutralize imageability effects [Bird et al., 2000, 2003], we used only imageable words from both categories. Behavioral data on other semantic features of the words (e.g., association with body movement) were collected separately. The experimental blocks were carefully arranged to fit both tasks. This allowed us to swap the tasks relative to stimuli in half of the subjects for counterbalancing purposes. We performed a region‐of‐interest (ROI) analysis to assess the effect of grammatical category in the classical language regions compared to other regions of the cortical language network.
MATERIALS AND METHODS
Subjects
Fourteen healthy volunteers (5 men and 9 women), aged 21–50 (mean age, 30.5), with no psychiatric or neurological history, participated in the study. All subjects gave written informed consent. The Tel Aviv Sourasky Medical Center and Tel Aviv University ethics committees approved the experimental protocol. All subjects were native speakers of Hebrew, and Hebrew was their sole mother tongue. They were all right‐handed as assessed by the Edinburgh handedness inventory [Oldfield, 1971].
Experimental Design
The experiment included four word conditions in a 2 × 2 factorial block design, created by manipulating grammatical category (verbs/nouns) and task (semantic/morphological). Word stimuli consisted of single words in Hebrew that were presented auditorily. In order to control for task‐stimuli interactions, all blocks were designed for both tasks. Consequently, by swapping the tasks relative to stimuli, we could create two versions that were counterbalanced between subjects (Fig. 1). The tasks consisted of two “yes/no” decisions: semantic, in which the subject had to decide whether the word was related to a given category (food and drinks in version 1 and agriculture in version 2; during each version, the category remained constant for all blocks requiring a semantic decision); and morphological, in which the subject had to decide whether the word was inflected in plural.
Figure 1.
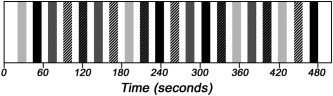
Experimental paradigm, version 1. Each of the 5 conditions repeated 4 times throughout the experiment, creating overall 20 blocks. The blocks lasted 10 s each and were separated by silence intervals of 7.5 s. Each pattern represents a different block type (Verb‐Semantic, Verb‐Morphological, Nouns‐Semantic, Nouns‐Morphological, Reversed Words). Version 2 was created by associating the blocks with the alternative task, thus controlling for potential task‐stimulus interactions.
In addition to the four experimental conditions, we included an auditory control condition that consisted of Hebrew words played backward. This condition created a baseline activation of low‐level speech perception.
Materials
A brief description of Hebrew grammatical system with respect to inflectional morphology of verbs and nouns is given in Appendix A. The crucial grammatical difference here is that, for some inflections, morphological properties fully identify the verb forms. Consequently, unlike in English, the recognition of grammatical category is unambiguous (even with regard to “inflected” nonwords). Only unambiguous verbal inflections were used in the study.
Preliminary tests
Stimuli were selected on the basis of preliminary ratings on 264 nonabstract verbs and nouns made by 37 subjects who did not participate in the imaging experiment. In these, subjects were asked to rate for each word the perceived familiarity (ranging from 1, “I hardly come across the word,” to 5, “I frequently come across the word”), degree of imageability (ranging from 1, “unimaginable,” to 5, “very easily imaginable”), and semantic associations with certain categories (ranging from 1, “not related at all,” to 5, “very much related”). All verbs were transitive with one or two complements and none of the nouns had an argument structure.
Selection of stimuli
Based on the preliminary ratings, 48 verbs and 48 nouns were selected for the imaging experiment according to the following criteria: imageability rate above 3.4; mean rank of semantic association to the given semantic category above 3.5 for the words that matched a “yes” response and no more than 1 for the words that matched a “no” response; verbs were of the three active grammatical patterns (Pa'al, Pi'el, Hif'il; see Appendix A); all nouns were of masculine grammatical gender (Appendix A); and no verb‐noun homophones were included. Verbs were inflected in the past tense, third person masculine form, half in singular (e.g., mazag: he poured) and half in plural (e.g., pizr u: they distributed). Similarly, half of the nouns were inflected in singular (e.g., mazleg: a fork) and the other half in plural (e.g., patish im: hammers). In this way, all other inflections except for the number suffixes of the words were constant throughout the experiment. In order to evaluate the selected words for relevant semantic dimensions, 14 additional subjects judged their visual clarity and the extent to which they associated with body movement (also on 1–5 scales). The results of these assessments are presented in Figures 2. The visual clarity scale (Fig. 2A) established that both verbs and nouns were associated with clear visual shapes (none of the words was rated as having no visual shape). Yet nouns had greater clarity ratings than verbs, which difference coheres with previous reports regarding imageability differences between verbs and nouns [Bird et al., 2003]. This seems to hold even when only highly imageable words from both categories are compared. Results of the movement assessment showed that all verbs, but not all nouns, were associated with body movement, and that 90% of the verbs were rated as “always or usually associated with body movement” as opposed to only 4% of the nouns (Fig. 2B).
Figure 2.
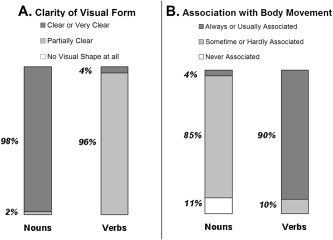
Results of semantic judgments on verbs and nouns: distribution of word classification according to their median scores. A: Results on the visual clarity scale (“To what extent does the word refer to a visually clear form?”). B: Results on the body movement scale (“To what extent is the word associated with body movement?”).
Timing Parameters
Stimuli were presented in a standard block design. Each of the five conditions repeated in four different blocks, six words in each block. The resulting 20 blocks were ordered randomly within the experiment. Among them, there were 16 experimental blocks, 8 of verbs and 8 of nouns. Within each block, both singular and plural forms were included and the number of syllables, as well as imageability and familiarity ratings, were matched as closely as possible (Appendix B). Blocks lasted 10 s, with an interstimulus interval (ISI) of 1,650 ms (the rate of the stimuli was unsynchronized with the TR in order to distribute the timing of data acquisition throughout the peristimulus time, as recommended by Price et al. [1999]). In each block, two or three words matched a “yes” response. Word blocks were separated by silence periods of 7.5 s. Each block was preceded by a prerecorded spoken instruction that determined the task during the block and ended with an auditory end‐of‐block cue.
Procedure
Each experimental session lasted approximately 1–1.5 hr and comprised of both anatomical and functional scans. Anatomical scanning included the acquisition of structural images in axial plane (see also Data Acquisition below) and a 3D spoiled gradient echo (SPGR) scan. In functional scans, subjects were requested to press a yes/no response button after each word with their left pointer finger according to the instructions of each block. During the control condition (reversed words), subjects were asked to press the button at the end of each stimulus. Each functional run lasted 7 min and 15 s. Responses were recorded online. No further information regarding type of stimuli was given to the subjects. Subjects performed a short practice prior to entering the magnet.
Data Acquisition
MRI scans were conducted in a whole body 1.5 Tesla, Signa Horizon, LX, 8.25 GE scanner, located at the Whol Institute for Advanced Imaging in Tel Aviv Sourasky Medical Center. Anatomical images were acquired using a standard T1‐weighted SE pulse sequence (voxel size, 0.8 mm × 1.5 mm × 5 mm). Fourteen slices, 5 mm thick with 1 mm gap, were selected, based on a mid‐sagittal slice covering most of the cerebrum (excluding the dorsal and ventral tips). In addition, a 3D SPGR sequence with high resolution was acquired for each subject in order to allow volume statistical analyses. Functional MRI protocols included T2*‐weighted EPI images (at the same locations as the T1‐weighted anatomical images). A total of 174 volumes were acquired in each functional run, with a field of view of 24 cm2 and matrix size of 80 × 80, time to repeat (TR) = 2,500 ms, TE = 55, and flip angle = 90°.
Data Analysis
fMRI data were processed using BrainVoyager 4.1 software package (http://www.brainvoyager.com) [Goebel et al., 1998]. The first six scans of the time series were discarded. Functional images were superimposed on 2D (T1‐weighted) anatomical images and projected on the 3D data sets through trilinear interpolation. The complete data set was transformed into Talairach space [Talairach and Tournoux, 1988]. Preprocessing of functional scans included head motion estimation, as well as high‐pass and low‐pass temporal frequency filtering. Volume statistical parametric maps were calculated separately for each subject using a general linear model [Friston et al., 1995] by contrasting all word conditions (both verbs and nouns) with the control (reversed words) condition. In this way, we identified brain regions that were activated during the high‐level processing of words without imposing our preliminary hypothesis on the data. Time courses of activated clusters within the predefined ROIs were collected using voxel‐number criterion. The exact threshold of each ROI was set to the point of 150 activated voxels in posterior IFG (pIFG) and anterior IFG (aIFG) and 500 voxels in other regions of interest (see Regions of Interest below). To account for the hemodynamic response delay, a lag of 2.5–5 s was inserted. Shifts were determined individually (per ROI and per subject) in a manner that maximized the correlation between the time course and the “all‐words” predictor. After shifting, the data were transformed into scores of percent signal change (PSC) using the average values of the silent blocks as a baseline. For each ROI, the PSC values of all data points in each condition were inserted into a within‐subject ANOVA, in which grammatical category, task, and repetition were used as within‐subject factors, while the version was used as a between‐subject factor. In order to examine lateralization effects, a second ANOVA was carried out on the activations from pIFG and posterior superior temporal sulcus (pSTS) in the left and right hemispheres, with hemisphere, grammatical category, and task as within subject variables.
Regions of Interest
Six regions of interest were defined in the left hemisphere: three anterior (in the frontal lobe) and three posterior (in the temporal and parietal lobes). The three anterior regions were pIFG (BA 44, pars opercularis), defined in each subject separately by the common markers suggested in the literature [Tomaiuolo et al., 1999], selected for its documented role in syntactic processing [Bookheimer, 2002; Heim et al., 2003]; aIFG (BA 45‐7), selected for its well‐documented involvement with aspects of semantic processing [Bookheimer, 2002]; and premotor, on the dorsal part of the precentral sulcus and gyrus (BA 4‐6), chosen in order to check the relation of motor schemata represented in this region to the processing of verbs [Grezes and Decety, 2001]. The three posterior regions were pSTS (BA 39), considered a subregion within a more wide‐ranging Wernicke's area, which was chosen on the basis of extensive evidence showing its involvement in speech perception [Wise et al., 2001]; posterior middle temporal gyrus (pMTG; BA 37), in the posterior part of the mid‐inferior temporal gyrus, located ventrally to Wernicke's area, chosen for its extensive involvement in semantic processing [Friederici et al., 2000]; and intraparietal sulcus (IPS), in the dorsal part of the parietal lobe, which is known to be involved in general language‐related processes [Cabeza and Nyberg, 2000] and was chosen as a control region.
On the right hemisphere, two additional ROIs were defined by homology with left pIFG and left pSTS in order to examine aspects of language processing that are related to hemispheric dominance. Brain activations were measured within these regions separately, as well as in comparison with the activation in the left hemisphere. Mean Talairach coordinates of the activations in each ROI are given in Table I.
Table I.
Talairach coordinates of regions of interest
| Region | Brodmann area | Mean Talairach coordinates ± SD | ||
|---|---|---|---|---|
| x | y | z | ||
| pIFG (left) | 44 | −47 ± 6 | 7 ± 5 | 14 ± 6 |
| pIFG (right) | 44 | 45 ± 3 | 11 ± 3 | 11 ± 7 |
| aIFG | 45,47 | −43 ± 5 | 33 ± 3 | 11 ± 3 |
| Premotor | 6 | −25 ± 3 | 9 ± 3 | 51 ± 2 |
| pSTS (left) | 39,22 | −54 ± 4 | −36 ± 5 | 3 ± 3 |
| pSTS (right) | 39,22 | 54 ± 6 | −31 ± 6 | 3 ± 5 |
| pMTG | 37,21 | −52 ± 6 | −50 ± 8 | −12 ± 4 |
| IPS | 7 | −36 ± 5 | −41 ± 6 | 38 ± 7 |
Reaction Time Measurements
Reaction time measurements were collected outside of the magnet. Thirteen psychology students aged 19–26 participated in this experiment for course credits. None of them participated in the imaging experiment. Subjects were tested using the same experimental protocol that was used in the scanner. Reaction times (RTs) were measured with homemade software installed on an IBM PC. A repeated‐measures ANOVA was performed on the reaction times using a 2 (grammatical category: verbs or nouns) × 2 (task: semantic or morphological) design. The version was added as a between‐subject‐independent variable to control for stimuli and task interaction.
RESULTS
Reaction Time Measurements
Factorial ANOVA on the behavioral data revealed a main effect of grammatical category. The reactions to verbs (mean RT = 655 ± 191 ms) were slower than to nouns (mean RT = 553 ± 173 ms; F(1,11) = 83.62; P < 0. 00001). A main effect of task was also documented in which the semantic task evoked slower responses (mean RT = 645 ± 203 ms) than the morphological task (mean RT = 562 ± 155 ms; F(1,11) = 31.11; P < 0. 0002). The interaction of category and task was also significant (F(1,11) = 6.22; P < 0. 0298): the task effect was slightly greater in verbs than in nouns.
Imaging Experiment
No effect of version was observed in any of the ROIs, hence the results are presented across versions (i.e., across specific task‐stimulus assignments). Statistical parametric maps, obtained by contrasting all‐words conditions with the reversed‐words condition, are presented in Figure 3. Above‐threshold activation was shown in all 14 subjects in left pIFG, aIFG, premotor, left pSTS, and IPS. Twelve of the 14 subjects showed above‐threshold activations in pMTG and right pIFG, and 10/14 subjects in right pSTS. As none of the regions revealed an interaction of task and category, the results are presented as main and simple effects in each factor separately.
Figure 3.
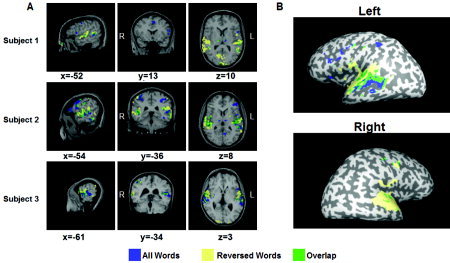
Activation contrast (all words vs. reversed words). Areas activated during real‐words (verbs or nouns) conditions are marked in blue. Areas activated during reversed‐words conditions are marked in yellow. Overlapping areas are marked in green. A: Variance in individual activations is demonstrated in three subjects. The activation is shown on structural anatomy images of each of the subjects. Regions of interest were defined as blue activations in anatomical predefined areas for each subject separately. B: Multisubject activation superimposed on inflated brain images of one of the subjects. General linear model (GLM) for 13 subjects, P < 0.01, uncorrected.
Grammatical Category Effects
A main effect of grammatical category, showing more activation for verbs than for nouns (V > N), was revealed in the left pIFG (F(1,12) = 9.69; P < 0.01), premotor (F(1,12) = 8.95; P < 0.05), and left pSTS (F(1,12) = 36.6; P < 0.01), but not in aIFG, pMTG, IPS, right pIFG, or right pSTS (Fig. 4). No region with the reversed pattern was found. A simple‐effect analysis revealed a V > N effect in left pIFG during the semantic task only (F(1,12) = 7.3; P < 0.05), in the premotor area during the morphological task only (F(1,12) = 8.2; P < 0.05), and in left pSTS during both tasks (semantic: F(1,12) = 21.5, P < 0.01; morphological: F(1,12) = 26.8, P < 0.01; Fig. 5).
Figure 4.
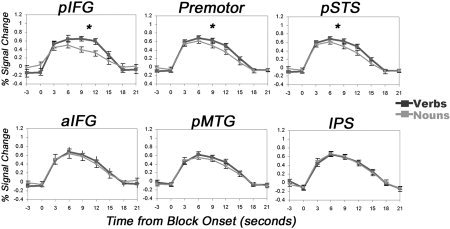
Verb‐noun effects in regions of interest. Mean percent signal change during verb and noun blocks in the six regions of interest on the left hemisphere. Data are averaged across 14 subjects and 8 blocks. Time courses for verbs are denoted in dark gray, nouns in light gray. Error bars show the standard error of the mean (across subjects) in each time point. *Significant (P < 0.05) effect.
Figure 5.
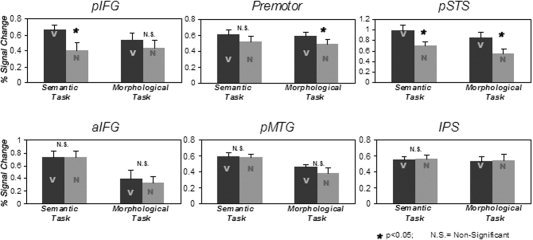
Verb‐noun differences shown for each task separately in the six ROIs on the left hemisphere.
Task Effects
A main effect of task, showing more activation for the semantic than for the morphological task, was revealed in aIFG (F(1,12) = 40.51; P < 0.01), left pSTS (F(1,12) = 11.4; P < 0.01), and pMTG (F(1,10) = 10.0; P < 0.01), but not in left pIFG, premotor, IPS, right pIFG, or right pSTS (Fig. 6). No region with the reversed pattern was found. A simple‐effect analysis in these ROIs revealed more activation for the semantic task than for the morphological task in both the verb and the noun conditions in all three ROIs (in aIFG verbs, F(1,12) = 11.77, P < 0.01; nouns, F(1,12) = 28.5, P < 0.01; in pSTS verbs, F(1,12) = 9.05, P < 0.05; nouns, F(1,12) = 5.2, P < 0.05; in pMTG verbs, F(1,10) = 5.7, P < 0.05; nouns, F(1,10) = 8.16, P < 0.05).
Figure 6.
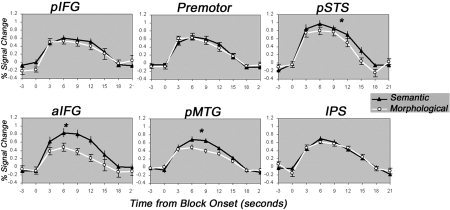
Task effects in regions of interest. Mean percent signal change during blocks of semantic and morphological tasks in the six regions of interest on the left hemisphere. Data are averaged across 14 subjects and 8 blocks. Time courses for the semantic task are marked by black triangles; time courses for the morphological task are marked in white circles. Asterisks mark a significant (P < 0.05) effect.
Hemispheric Differences
In pIFG, an overall lateralization effect was observed (F(1,12) = 5.3; P < 0.05), with greater activation on the left hemisphere than on the right hemisphere across conditions (Fig. 7A). Across hemispheres, there was also a marginal effect of grammatical category, whereby activation for verbs was greater than activation for nouns (F(1,12) = 4.62; P = 0.052; Fig. 7C, upper panel). No other effects were revealed in pIFG.
Figure 7.
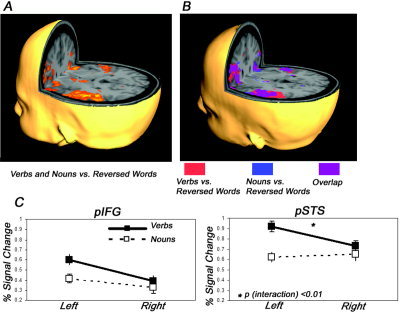
Lateralization of activation in language regions. A: A statistical parametric map of verbs and nouns vs. reversed words (RW) shows activation on left and right hemispheres (n = 13; P < 0.05). Left dominance was statistically significant in pIFG, but not in pSTS. B: A statistical parametric map of areas activated for contrasting verbs vs. RW, nouns vs. RW, and the overlapping areas (n = 13; P < 0.05). C: Analysis of verb‐noun differences in each hemisphere separately reveals similar, but not identical, patterns of results in pIFG and pSTS, and a significant interaction of hemisphere and grammatical category only in pSTS.
In pSTS, there was no overall lateralization in activation; right and left pSTS showed similar levels of activation (F(1,12) = 0.3; P = NS). Across sides, there was a main effect of grammatical category, in which activation for verbs was greater than activation for nouns (F(1,12) = 54.5; P < 0.001). There was also an interaction between hemisphere and grammatical category (F(1,12) = 10.5; P < 0.001), showing that the grammatical category effect was larger in left pSTS than in right pSTS (Fig. 7B and C, lower panel). A task effect showing more activation in the semantic than in the morphological task across hemispheres was also observed (F(1,12) = 8.55; P < 0.05).
DISCUSSION
Our results suggest that verbs and nouns are processed differently in the brain. Three of the ROIs (left pIFG, left pSTS, and left premotor) were activated more by verbs than by nouns during the semantic task, the morphological task, or both (Figs. 4 and 5). This result is consistent with previous neuroimaging studies in which an overall greater activation while processing verbs relative to nouns has been observed in several frontotemporal regions [Warburton et al., 1996; Perani et al., 1999]. With respect to the frontal ROIs, the results also correspond to the verb deficit observed in patients following damage to the left frontal lobe. However, in contrast to the double dissociation suggested by the neuropsychological evidence, but still in line with most previous imaging studies [Warburton et al., 1996; Perani et al., 1999; Tyler et al., 2003], none of our ROIs seem to be activated by nouns more than by verbs (Fig. 4). Moreover, while all previous imaging studies used only reading as the input modality for the stimuli, the present results show the dissociation with auditory inputs as well. This suggests that verbs may generally require more processing resources than nouns and, moreover, that these resources are distributed over several brain regions, rather than localize to a unitary region. Similarly, our results suggest that the observed differences implicate more than one level of processing [Black and Chiat, 2003; Randall and Tyler, 2003]. Below we discuss the results for each of the effects in each ROI.
Verb‐Noun Dissociation in Comprehension
The neuropsychological dissociation between nouns and verbs was observed primarily in brain‐damaged patients performing naming tasks. In order to make a generalization about the representation of grammatical category in the brain, we need to demonstrate dissociations in other tasks (e.g., comprehension or word reading) and other methodologies (e.g., neuroimaging). The results about comprehension in brain‐damaged patients are inconclusive; some suggest that comprehension deficits replicate the noun‐verb dissociation [e.g., Daniele et al., 1994; Silveri and Di Betta, 1997], while others fail to find this [e.g., Hillis et al., 2002; Silveri et al., 2003]. The current study examined this issue in comprehension using fMRI with healthy subjects. Our theoretical motivation originated in several models of lexical access that assumed a unitary lexical representation across modalities [Levelt, 1989; Levelt et al., 1999]. More specifically to verb‐noun dissociation, Shapiro and Caramazza [2003b] suggested that “similar representations (to production) are invoked in comprehension” (p. 201).
Grammatical Category Effects
Left pIFG
Overall, the main effect of grammatical category observed in this region, in which verbs generate more activation than nouns, is compatible with the verb deficits displayed by left frontal aphasic patients. However, this effect was significant only during the semantic task, and not during the morphological task (Fig. 5).
Previous neuroimaging studies in healthy subjects have linked pIFG with phonological processing [Poldrack et al., 1999; Bookheimer, 2002] and syntactic processing [Just et al., 1996; Stromswold et al., 1996]. However, in our experiment, it was during the semantic task that verbs activated this region more than nouns. This might imply that pIFG is also engaged in semantic processing [see also evidence for this in Vigneau et al., 2006], with a preference for verb semantics. Alternatively, the semantic task might have recruited other nonsemantic processes (syntactic, phonological, or both) within pIFG.
The absence of grammatical category effect in pIFG during the morphological task also calls for explanation. In our experiment, this task could have been accomplished by detection of certain phonemes (in nouns and in verbs). The phonological load of such detection is probably equal between nouns and verbs and shows as similar activations in pIFG.
Overall, while the modulation of the verb‐noun effects by the processing mode lends itself to various interpretations, its existence demonstrates the importance of examining grammatical category effects in different tasks.
Left pSTS
The main effect of grammatical category in pSTS is in the same direction as in pIFG, showing greater activation for verbs than for nouns. This pattern is seen here during both the semantic and the morphological tasks (Fig. 5). Similar results have already been observed in previous PET studies with normal subjects [Warburton et al., 1996; Perani et al., 1999], but our study is the first to show this in a morphological task. This result leaves unexplained the noun deficits ascribed to patients with left temporal lesions. Yet there is also evidence that damage to posterior temporoparietal regions can produce selective verb deficits, rather than noun deficits [Daniele et al., 1994; Silveri et al., 2003]. Furthermore, a careful examination of a large sample of patients performed by Luzzatti et al. [2002] suggests that verb‐related deficits are associated with posterior lesions no less than with anterior lesions. These observations are also in line with the view that temporoparietal cortices are in fact part of a cortical network (which includes also frontal regions) involved in processing verbs, whereas noun processing is subserved by more anterior and medial regions in the temporal lobe [Damasio and Tranel, 1993; Tranel et al., 2005]. Our finding in pSTS is consistent with these views.
The possible role of pSTS in processing verbs may relate to the computation of argument structure, that is, the number and type of complements required by all verbs and some nouns [Grimshaw, 1990]. Interestingly, it has recently been suggested that impaired representation of argument structure is necessary in order to account for verb‐noun dissociations beyond imageability effects [Crepaldi et al., 2005]. In patients, Shapiro and Levine [1990] and Shapiro et al. [1993] have shown that Broca's aphasics demonstrate normal sensitivity to argument complexity, whereas Wernicke's aphasics do not. A recent fMRI study of healthy subjects showed greater activation in pSTS when processing sentences with transitive (two‐argument) verbs than when processing matched sentences with intransitive (one‐argument) verbs [Ben‐Shachar et al., 2003]. These results, obtained both in patients and in healthy subjects, also suggest the involvement of pSTS in processing argument structure. According to Shapiro et al. [1989], access to argument structure is automatic so that whenever a verb is processed, all its arguments are activated, even if only one of them is realized. This automatic access may explain the greater pSTS activation for verbs over nouns in the present study during both the semantic and the morphological tasks. Issues of argument structure are currently investigated in greater detail in our imaging laboratory.
Left premotor
A most interesting result here is the grammatical category main effect observed in the premotor area (Fig. 4). This effect cannot be explained by actual motor activity, as button‐pressing was equal in all experimental conditions. It might be explained, however, by the fact that verbs are associated with movement more strongly than nouns (Fig. 2B). Several studies have shown that the dorsal premotor area is activated not only by the execution or planning of motor actions, but also by imagery of movement [Decety et al., 1994]. Verbal processes that relate to action may also activate the left premotor area [e.g., verb generation in Wise et al., 1991; Martin et al., 1995; Warburton et al., 1996]. Indeed, this region was included in a set of cortical areas suggested by Grezes and Decety [2001] to be involved in the processing of motion‐related aspects of diverse cognitive functions. The idea that some of the lexical knowledge that differentiates verbs is represented through semantic attributes is also in line with the sensory‐functional account proposed by Bird et al. [2000]. We suggest, however, that the term “functional” may be too general here, confounding aspects of movement with those of purpose and use. Our results suggest that the association of words with movement may be a crucial determinant of left premotor activation and also suffice for explaining the differences in premotor activation between verbs and nouns. In our study, these differences manifested as a main effect of grammatical category in the premotor area. It is surprising, however, that the V > N effect reached significance in the simple effects only during the morphological task and not during the semantic task (Fig. 5). This may imply that the premotor area is sensitive not only to grammatical category but also to the mode of processing. Further studies are needed in order to clarify the extent of premotor involvement in different lexical tasks and the interaction of task with grammatical category.
Task Effects
In addition to the grammatical category effects discussed so far, we have also observed main task effects in three ROIs: aIFG, pSTS, and pMTG. These regions reveal greater activation during semantic processing than during morphological processing (Fig. 6). The above regions are known to be part of a distributed network of semantic processing that extends over the frontal and temporal lobes [Martin and Chao, 2001; Bookheimer, 2002] and that probably includes additional regions. Indeed, the term “semantics” generally, and in relation to the semantic task used here particularly, probably comprises several distinct aspects of processing, and each aspect may be related to a different area in the semantic network [Martin and Chao, 2001; Bookheimer, 2002].
Left aIFG
Numerous previous studies have reported semantic activity in the inferior frontal gyrus [to name a few: Demonet et al., 1992; Demb et al., 1995; Gabrieli et al., 1996; Poldrack et al., 1999; Buckner et al., 2000; Wagner et al., 2001]. This frontal semantic activity is presumed to reflect associative semantic retrieval [Wagner et al., 2001] or task‐specific general selection processes [Thompson‐Schill et al., 1997, 1999]. The observation of a semantic effect in aIFG, but not in pIFG, is also in line with the previously suggested subdivision of Broca's area into two subareas. The anterior part (aIFG) is probably involved in semantic processing, while the posterior part (pIFG) may concern syntax and phonology [Dapretto and Bookheimer, 1999; Friederici et al., 2000; Vigliocco, 2000; Keller et al., 2001].
Left pSTS and left pMTG
Semantic effects in the left temporal lobe have previously been ascribed to the retrieval of sensory (mainly visual) attributes [Martin and Chao, 2001; Bookheimer, 2002]. Our results indicate that semantic activity in this area is not limited to visual displays, but can also occur in response to auditory stimuli. Moreover, for our auditory inputs, differences in the visual attributes associated with a word (as indicated by the visual clarity scale) may not be sufficient to produce a neuronal effect in this region. It is also interesting to note the functional disparity within lateral temporal regions between the superior temporal sulcus (pSTS) and its ventral neighbor (pMTG). Both regions have demonstrated semantic effects, but pSTS displayed also a preference for verbs over nouns. It is perhaps this latter effect that marks the central role in the processing of language of pSTS (but not of other neighboring temporal regions).
Hemispheric Differences
Our results show a general left lateralization for language tasks (Fig. 7A), in line with the accepted view of cerebral lateralization. However, this effect was mainly due to pIFG (and not pSTS), in agreement with previous imaging results [Zahn et al., 2000; Ben‐Shachar et al., 2004]. Indeed, pSTS showed a more subtle pattern of lateralization, seen in the interaction between hemisphere and grammatical category; greater activation for verbs than for nouns was much more prominent on the left than on the right (Fig. 7B and C). This result may further emphasize the specificity of the activation for verbs in left pSTS. It suggests that despite the apparent homology frequently observed in the activity of the left and right posterior temporal regions, each side may be involved with somewhat different processes.
Conclusion
The presence of grammatical category effects in both pIFG and the premotor area may offer a different perspective for understanding the verb deficits frequently observed in patients suffering left frontal lesions. As suggested above, each of these areas may contribute differentially to the representation and processing of verbs. However, damage to the left frontal lobe frequently encompasses both of these neighboring regions and thus may prevent access to either of the processes that they subserve, resulting in a more frequent impairment of verb processing. By contrast, an extensive damage in the temporal lobe may obscure a relatively localized damage of verb processing in pSTS and may affect only argument‐related aspects of processing, leaving intact sufficient aspects of verb representation to allow successful retrieval.
We remain with the riddle of selective impairments of nouns following temporal brain damage. This need not necessarily originate in deficits that affect noun representation or process. For example, it could originate in damage to the system that processes argument structure, with consequent errors in the assignment of thematic roles and the production of nouns. Alternatively, the processing of nouns could be impaired following temporal damage due to errors in assigning motion‐related features. While for motion verbs such damage may be offset by intact motor representations in the frontal regions, such compensation may not be available for nouns. However, these possibilities are at present highly speculative.
Taken as a whole, our study suggests that cerebral verb‐noun dissociation is a result of localized and subtle processes that take place in the left frontal and temporal lobes. The anatomical differences that subserve the processing of grammatical category may depend on the lexical characteristics of the stimuli, as well as on task requirements.
Acknowledgements
The authors thank two anonymous reviewers for their helpful comments.
APPENDIX A.
Inflectional Morphology of Verbs and Nouns in Hebrew
Hebrew verbs are inflected for gender, person, number, and tense. As in other Semitic languages, verbs are derived from three‐letter roots that remain constant across all inflections (in the following examples: l‐m‐d). The base form of all verbs is the third person masculine singular past active indicative (e.g., lamad). For each person, there are both singular and plural forms. In the past tense, both person and number affect the suffix of the verb, e.g., lamadti (I learned) vs. lamadnu (we learned) in first person; lamadta (you learned, singular) vs. lamadtem (you learned, plural) in second person; and lamad (he learned) vs. lamdu (they learned) in third person.
Verbs are derived from roots in a number of ways, all involving the use of intermediate vowels and prefixes. These patterns are classified into seven basic groups (called binyanim), each of which has a conjugate, which is usually apparent in the binyan's name. There are three active binyanim (Pa'al, Pi'el, Hif'il) and four passive ones (Nif'al, Pu'al, Huf'al, Hitpa'el). In the current experiment, we used verbs of only the three active binyanim, inflected in third person masculine in the past tense, either as singular (as in the base form, e.g., lamad) or as plural (with a suffix, e.g., lamdu). Consequently, half of the verb stimuli had a morphological suffix of /u/.
Hebrew nouns are inflected for gender and number (and sometimes for possession) but not for case. Nouns have an inflectional affinity to verbs (often acting on shared roots), but their derivative morphology is not as systematic, and there are over 100 word patterns of nouns (called mishkalim). Generally, Hebrew distinguishes between singular and plural forms of a noun. Masculine plural forms are usually created by adding the suffix; and feminine plural forms can be usually recognized by the suffix, e.g., yeled (a boy) vs. yeladim (boys), tmuna (picture) vs. tmunot (pictures). In this experiment, we used only regular nouns of masculine grammatical gender. Half of the noun stimuli were inflected in plural and thus had a morphological suffix of /im/.
APPENDIX B.
Familiarity, Imageability, and Number of Syllables of Experimental Stimuli
Familiarity is measured on a 1–5 scale where 1 represented “very rare” and 5 represented “very frequent.” Imageability is measured on a 1–5 scale where 1 represented “unimaginable” and 5 represented “very easily imaginable.” Standard deviations are noted in brackets. Based on preliminary ratings performed by 37 subjects as described in text.
Table .
| Means | Verbs, food | Verbs, agriculture | Nouns, food | Nouns, agriculture |
|---|---|---|---|---|
| Familiarity | 3.68 (0.456) | 3.73 (0.714) | 2.98 (0.573) | 3.22 (0.895) |
| Imageability | 4.08 (0.437) | 4.25 (0.31) | 4.52 (0.488) | 4.49 (0.450) |
| Syllables | 2.16 (0.380) | 2.04 (0.208) | 2.08 (0.408) | 2.08 (0.282) |
REFERENCES
- Ben‐Shachar M, Hendler T, Kahn I, Ben‐Bashat D, Grodzinsky Y (2003): The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychol Sci 14: 433–440. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Palti D, Grodzinsky Y (2004): Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN, Sandson J (1997): Verb retrieval in aphasia: 1, characterizing single word impairments. Brain Lang 56: 68–106. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Haendiges AN, Burton MW, Mitchum CC (2002): Grammatical class and imageability in aphasic word production: their effects are independent. J Neuroling 5: 353–371. [Google Scholar]
- Bird H, Howard D, Franklin S (2000): Why is a verb like an inanimate object? grammatical category and semantic category deficits. Brain Lang 72: 246–309. [DOI] [PubMed] [Google Scholar]
- Bird H, Howard D, Franklin S (2003): Verbs and nouns: the importance of being imageable. J Neuroling 16: 113–149. [Google Scholar]
- Black M, Chiat S (2003): Noun‐verb dissociations: a multi‐faceted phenomenon. J Neuroling 16: 231–250. [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Ann Rev Neurosci 25: 151–188. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR (2000): Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain 123: 620–640. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE (1991): Lexical organization of nouns and verbs in the brain. Nature 349: 788–790. [DOI] [PubMed] [Google Scholar]
- Corina DP, Gibson EK, Martin R, Poliakov A, Brinkley J, Ojemann GA (2005): Dissociation of action and object naming: evidence from cortical stimulation mapping. Hum Brain Mapp 24: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi D, Aggujaro S, Arduino LS, Zonca G, Ghirardi G, Inzaghi MG, Colombo M, Chierchia G, Luzzatti C (2005): Noun‐verb dissociation in aphasia: the role of imageability and functional locus of the lesion. Neuropsychologia 44: 73–89. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D (1993): Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci U S A 90: 4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, Gainotti G (1994): Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia 32: 1325–1341. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY (1999): Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, Mazziotta JC, Fazio F (1994): Mapping motor representations with positron emission tomography. Nature 371: 600–602. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE (1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task‐difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R (1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY (2000): Segregating semantic and syntactic aspects of processing in the human brain: an fMRI investigation of different word types. Cereb Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RSJ, Turner R (1995): Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2: 166–172. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH (1996): Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychol Sci 7: 278–283. [Google Scholar]
- Goebel R, Khorram‐Sefat D, Muckli L, Hacker H, Singer W (1998): The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci 10: 1563–1573. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J (2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw J (1990): Argument structure. Cambridge, MA: MIT Press. [Google Scholar]
- Heim S, Opitz B, Friederici AD (2003): Distributed cortical networks for syntax processing: Broca's area as the common denominator. Brain Lang 85: 402–408. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A (1995): Representation of grammatical categories of words in the brain. J Cogn Neurosci 7: 396–407. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A (2002): Modality‐specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci 14: 1099–1108. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR (1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA (2001): The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex 11: 223–237. [DOI] [PubMed] [Google Scholar]
- Levelt WJM (1989): Speaking: From Intention to Articulation. Cambridge, MA: MIT Press. [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS (1999): A theory of lexical access in speech production. Behav Brain Sci 22: 1–38. [DOI] [PubMed] [Google Scholar]
- Li P, Jin Z, Tan LH (2004): Neural representations of nouns and verbs in Chinese: an fMRI study. Neuroimage 21: 1533–1541. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, Pinna GD (2002): Verb‐noun double dissociation in aphasic lexical impairments: the role of word frequency and imageability. Brain Lang 81: 432–444. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG (1995): Discrete cortical regions associated with knowledge of color and knowledge of action. Science 270: 102–105. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL (2001): Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 11: 194–201. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK (1985): Category specificity in an agrammatic patient: the relative impairment of verb retrieval and comprehension. Neuropsychologia 23: 709–727. [DOI] [PubMed] [Google Scholar]
- Miceli G, Silveri MC, Villa G, Caramazza A (1984): On the basis for the agrammatics difficulty in producing main verbs. Cortex 20: 207–220. [DOI] [PubMed] [Google Scholar]
- Miceli G, Silveri MC, Nocentini U, Caramazza A (1988): Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology 2: 351–358. [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F (1999): The neural correlates of verb and noun processing: a PET study. Brain 122: 2337–2344. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ, Veltman DJ, Ashburner J, Josephs O, Friston KJ (1999): The critical relationship between the timing of stimulus presentation and data acquisition in blocked designs with fMRI. Neuroimage 10: 36–44. [DOI] [PubMed] [Google Scholar]
- Randall B, Tyler LK (2003): The trouble with nouns and verbs. Brain Lang 87: 53–54. [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF (1989): The quantitative‐analysis of agrammatic production: procedure and data. Brain Lang 37: 440–479. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Zurif EB, Grimshaw J (1989): Verb processing during sentence comprehension: contextual impenetrability. J Psycholing Res 18: 223–243. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Levine BA (1990): Verb processing during sentence comprehension in aphasia. Brain Lang 38: 21–47. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Gordon B, Hack N, Killackey J (1993): Verb‐argument structure processing in complex sentences in Broca and wernicke aphasia. Brain Lang 45: 423–447. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Shelton J, Caramazza A (2000): Grammatical class in lexical production and morhpological processing: evidence from a case of fluent aphasia. Cogn Neuropsychol 17: 665–682. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Pascual‐Leone A, Mottaghy FM, Gangitano M, Caramazza A (2001): Grammatical distinctions in the left frontal cortex. J Cogn Neurosci 13: 713–720. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A (2003a): Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia 41: 1189–1198. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A (2003b): The representation of grammatical categories in the brain. Trends Cogn Sci 7: 201–206. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Mottaghy FM, Schiller NO, Poeppel TD, Fluss MO, Muller HW, Caramazza A, Krause BJ (2005): Dissociating neural correlates for nouns and verbs. Neuroimage 24: 1058–1067. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Di Betta AM (1997): Noun‐verb dissociations in brain‐damaged patients: further evidence. Neurocase 3: 477–488. [Google Scholar]
- Silveri MC, Perri R, Cappa A (2003): Grammatical class effects in brain‐damaged patients: functional locus of noun and verb deficit. Brain Lang 85: 49–66. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S (1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Coplanar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme. [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Kan IP (1999): Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 23: 513–522. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Petrides M (1999): Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11: 3033–3046. [DOI] [PubMed] [Google Scholar]
- Tranel D, Martin C, Damasio H, Grabowski TJ, Hichwa R (2005): Effects of noun‐verb homonymy on the neural correlates of naming concrete entities and actions. Brain Lang 92: 288–299. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss H (2003): Objects and their actions: evidence for a neurally distribute semantic system. Neuroimage 18: 542–557. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Bright P, Fletcher P, Stamatakis EA (2004): Neural processing of nouns and verbs: the role of inflectional morphology. Neuropsychologia 42: 512–523. [DOI] [PubMed] [Google Scholar]
- Vigliocco G (2000): Language processing: the anatomy of meaning and syntax. Curr Biol 10: R78–R80. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N (2006): Meta‐analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30: 1414–1432. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare‐Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RSJ (1996): Noun and verb retrieval by normal subjects studies with PET. Brain 119: 159–179. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA (2001): Separate neural subsystems within “Wernicke's area”. Brain 124: 83–95. [DOI] [PubMed] [Google Scholar]
- Zahn R, Huber W, Drews E, Erberich S, Krings T, Willmes K, Schwarz M (2000): Hemispheric lateralization at different levels of human auditory word processing: a functional magnetic resonance imaging study. Neurosci Lett 287: 195–198. [DOI] [PubMed] [Google Scholar]
- Zingeser LB, Berndt RS (1988): Grammatical class and context effects in a case of pure anomia: implications for models of language production. Cogn Neuropsychol 5: 473–516. [Google Scholar]
- Zingeser LB, Berndt RS (1990): Retrieval of nouns and verbs in agrammatism and anomia. Brain Lang 39: 14–32. [DOI] [PubMed] [Google Scholar]


