Abstract
Facial expressions of fear are universally recognized signals of potential threat. Humans may have evolved specialized neural systems for responding to fear in the absence of conscious stimulus detection. We used functional neuroimaging to establish whether the amygdala and the medial prefrontal regions to which it projects are engaged by subliminal fearful faces and whether responses to subliminal fear are distinguished from those to supraliminal fear. We also examined the time course of amygdala‐medial prefrontal responses to supraliminal and subliminal fear. Stimuli were fearful and neutral baseline faces, presented under subliminal (16.7 ms and masked) or supraliminal (500 ms) conditions. Skin conductance responses (SCRs) were recorded simultaneously as an objective index of fear perception. SPM2 was used to undertake search region‐of‐interest (ROI) analyses for the amygdala and medial prefrontal (including anterior cingulate) cortex, and complementary whole‐brain analyses. Time series data were extracted from ROIs to examine activity across early versus late phases of the experiment. SCRs and amygdala activity were enhanced in response to both subliminal and supraliminal fear perception. Time series analysis showed a trend toward greater right amygdala responses to subliminal fear, but left‐sided responses to supraliminal fear. Cortically, subliminal fear was distinguished by right ventral anterior cingulate activity and supraliminal fear by dorsal anterior cingulate and medial prefrontal activity. Although subcortical amygdala activity was relatively persistent for subliminal fear, supraliminal fear showed more sustained cortical activity. The findings suggest that preverbal processing of fear may occur via a direct rostral–ventral amygdala pathway without the need for conscious surveillance, whereas elaboration of consciously attended signals of fear may rely on higher‐order processing within a dorsal cortico–amygdala pathway. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: functional neuroimaging, amygdala, medial prefrontal cortex, anterior cingulate, fear face, backward masking
INTRODUCTION
Facial expressions of fear are universally recognized social signals of potential threat, associated with a distinct physiological action tendency. Given the adaptive survival value of facial signals of fear, humans may have evolved specialized neural systems for giving precedence to these signals without the need for conscious awareness [Williams, 2006]. Such automaticity may be uniquely associated with amygdala processing of fear. It has been proposed that fear recruits the amygdala via two neural streams associated with different degrees of conscious awareness [Le Doux,1996; Zald,2003]. Low‐level sensory input may be transmitted directly from thalamus to amygdala for rapid and automatic responses. The amygdala projects to the ventral portion of the medial prefrontal cortex [Porrino et al.,1981], which has also been implicated in the rapid processing of facial emotion before complete analysis in the visual cortex [Kawasaki et al.,2001]. More detailed, conscious analysis of fear signals may rely on a slower, cortical pathway to the amygdala [Le Doux,1996].
Backward masking is an effective paradigm for examining the neural substrates of fear processing at different levels of awareness. Amygdala modulation has been demonstrated in response to fear‐conditioned face stimuli, presented for 30 ms and immediately masked by a neutral face [Morris et al.,1998,1999]. It has also been observed in response to unconditioned fearful faces of similar duration [Whalen et al.,1998], but not at 30 ms when the masking protocol is designed to control for perceptual priming [Phillips et al.,2004].
The duration of 30 ms may not provide an exhaustive test for amygdala responses to nonconscious perception of fear. At 30 ms, stimulus detection remains possible and masking interferes only with the subsequent ability to report emotional valence [Williams et al.,2004b]. Detection without recognition may cause subject uncertainty, sufficient to engage cortical inhibitory influences on the amygdala. Indeed, amygdala responses are diminished with competing attentional demands [Pessoa et al.,2002] and event‐related potentials (ERPs) are generally suppressed for masked fearful faces at 30 ms [Williams et al.,2004b].
Subliminal stimuli, which prevent both detection as well as recognition, may provide a more explicit probe of the direct amygdala pathway. Seminal studies by Zajonc [1980] and Murphy and Zajonc [1993] demonstrated subliminal signals of emotion have a greater ability to prime responses than do supraliminal signals. Subliminal fear has also been found to enhance the N2 and early P3a ERP components [Liddell et al.,2004], associated previously with generators in the amygdala and anterior cingulate [Halgren and Marinkovic,1995]. In this study, we used functional neuroimaging to establish whether subliminal fear engages the amygdala and medial prefrontal–anterior cingulate regions to which it projects. We compared subliminal (undetected) to supraliminal fear, using psychophysical thresholds for awareness based on signal detection theory. Simultaneous skin conductance provided an independent index of emotional arousal due to fear signals, regardless of awareness. We considered the time course of amygdala–medial prefrontal responses, given that they may vary across experimental phases [Wright et al.,2001], and the relationships between these regions.
SUBJECTS AND METHODS
Subjects
Fifteen healthy controls (mean age = 35.80 years, standard deviation [SD] = 9.06 years; seven males, eight females) were recruited in collaboration with the Brain Resource International Database [http://www.brainresource.com; Gordon,2003]. All subjects were within the normal range of tested intelligence (mean = 105), based on the Spot the Word estimate of IQ [Baddeley et al.,1993]. Exclusion criteria included Axis‐I psychiatric diagnosis, brain injury (via radiological assessment of structural magnetic resonance imaging [MRI] scans), history of loss of consciousness (>10 min), history of other neurological disorder or genetic disorder, and substance abuse.
All participants provided written informed consent to participate in accordance with Medical Health and Research Council guidelines.
Threshold Setting
During scanning, face stimuli were presented under subliminal and supraliminal conditions. We drew on the findings of our initial psychophysics experiment [Williams et al.,2004b] to determine the durations for these stimuli. Using a psychophysics framework [Macmillan,1986], subliminal perception was defined using the “detection threshold,” the duration at which face stimuli can no longer be detected with above‐chance accuracy. In the supraliminal perception condition, we used a stimulus duration at which both detection and discrimination of face stimuli could be consistently reported with significantly above‐chance accuracy.
To establish the detection threshold, fear and neutral stimuli were presented with equal numbers of blank screen stimuli at a series of durations (10, 20, 30, 40 and 50 ms, counterbalanced across subjects), each followed immediately by a neutral mask stimulus. The neutral mask was slightly spatially offset from the preceding face (1 degree in one of the four diagonals, randomly) to control for perceptual priming. Subjects responded (via button press) as to whether they detected the presence of a face versus a blank screen stimulus. Detection accuracy was found to significantly and clearly differ from chance only for durations of 30 ms and above [Williams et al.,2004b], suggesting that a duration of <20 ms is required for subliminal detection.
In a second session of this initial experiment, subjects were asked to identify the facial expression (via forced‐choice button press; fear vs. neutral) for a series of fear–neutral mask and neutral–neutral mask stimuli, presented at 20, 30, 50, 90, 170, and 330 ms, counterbalanced across subjects. Discrimination accuracy reached a significant (P < 0.0001) and high level (>90%) of accuracy at 170 ms, which was consistent at 330 ms [Williams et al.,2004b]. Durations of 500 ms may be sufficient to elicit the subjective experience of emotion [Wild et al,2001].
Behavioral Task
Participants viewed gray‐scale face stimuli from a standardized series [Gur et al.,2002a], consisting of four female and four male individuals depicting fear and neutral facial expressions. Faces were matched for overall luminosity and size. Sequences of supraliminal and subliminal presentations each comprised 240 stimuli (120 fear and 120 neutral) in a pseudorandom sequence of 30 blocks (comprising eight fear or eight neutral stimuli each). From the psychophysics findings outlined above, subliminal stimuli (fear or neutral) were presented for 16.7 ms, followed by a 150‐ms neutral mask, with an interstimulus interval (ISI) between target‐mask pairs of 1,100 ms. Supraliminal stimuli were presented for 500 ms and unmasked, with an ISI of 767 ms, to ensure that the total stimulus duration plus ISI period was equivalent across conditions (1,267 ms). The ISI was jittered by ±500 ms for each condition to ensure that stimulus onset did not coincide with a constant slice position during image acquisition.
Face stimuli were presented via a projector (Sanyo ProX; Multiverse, Tokyo, Japan) and mirror system. Experimental software ensured that stimulus presentation was synchronized with projector refresh cycles (60 Hz). Subjects received standardized and synchronized visual and audio (through headphones) instructions. To ensure active attention to the task, they were asked to actively attend to the face stimuli and to determine whether the faces were male or female and young or old, in preparation for a post‐scan briefing. For the subliminal condition, they were instructed to focus on the first face even though it may be difficult to see. After scanning, subjects were asked to identify the expressions on each of the stimuli they had been shown in the scanner to confirm that they were normally able to discriminate fear and neutral.
Skin Conductance Response Acquisition and Analysis
Skin conductance responses (SCRs) were recorded simultaneously with functional MRI (fMRI) data via a customized system [Williams et al.,2001,2004a], using a pair of silver–silver chloride electrodes with 0.05 M sodium chloride gel placed on the distal phalanges of digits II and III of the left hand. The electrode pairs were supplied by a constant voltage and the current change representing conductance was recorded using the DC amplifier.
The presence of a phasic SCR to each stimulus event was defined by an unambiguous increase (>0.05 μS) with respect to each pretarget baseline and occurring 1–3 s after the event. In the supraliminal condition, an event was defined as a target face/mask pair. Customized software was based on a sigmoid‐exponent mathematical model that allows each SCR to be linked to the individual eliciting stimulus, and potentially overlapping SCRs in short ISI paradigms to be disentangled [Lim et al.,1997]. In both supraliminal and subliminal conditions, there was an average of 16 SCR events. SCR amplitude was analyzed using within‐subject, repeated‐measures analysis of variance (ANOVA), with condition (supraliminal vs. subliminal) and stimulus (fear vs. neutral) as within‐subject factors. Paired t‐tests were used to explore the a priori contrasts of interest.
Image Acquisition and Analysis
Imaging was carried out on a 1.5T scanner (Siemens Vision Plus; Siemens, Munich Germany) using an echo echoplanar protocol. In total, 90 functional T2*‐weighted volumes (three per stimulus block) were acquired, comprising 15 noncontiguous slices parallel to the intercommissural (anterior–posterior commissure [AC–PC]) line, with 6.6 mm thickness, repetition time (TR) = 3.3 s, echo time (TE) = 40 ms, and flip angle = 90 degrees, with field of view (FOV) 24 × 24 cm2 and matrix size 128 × 128. Three initial dummy volumes were acquired to ensure blood oxygen level‐dependent (BOLD) saturation.
The ability of the functional imaging protocol to elicit robust signal change in the amygdala was demonstrated by a calculation of signal‐to‐noise ratio (SNR). Based on Parrish et al. [2000], the minimum SNR value (for an α level of 0.01, β value of 0.95, expected signal change of 1%, and at least 80 images) is 96. The observed SNR values in this study were calculated for each subject on a voxel‐by‐voxel basis by taking the mean signal of the entire smoothed realigned time series for the left amygdala and dividing this mean value by the standard deviation [LaBar et al.,2001]. Observed SNR values far exceeded this minimum SNR (ranging from 129 to 484 for the right amygdala and from 163 to 406 for the left amygdala).
Preprocessing and statistical analysis of fMRI data was conducted using statistical parametric mapping (SPM2, Wellcome Department of Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/spm2.html). Functional scans were realigned (followed by the SPM2 unwarping routine to remove residual movement‐related variance), spatially normalized into standardized Montreal Neurological Institute (MNI) anatomical space, and smoothed using a Gaussian kernel (full‐width half‐maximum [FWHM]: 8 mm) [Penny et al.,2001]. The experimental sequences (subliminal/supraliminal fear vs. neutral) were modeled using an hemodynamic response function (HRF)‐convolved boxcar model with temporal derivative, and a high‐pass filter was applied to remove low‐frequency fluctuations in the BOLD signal.
Search Region‐of‐Interest Analyses
Region‐of‐interest (ROI) masks for the bilateral amygdala, anterior cingulate cortex (ACC), and medial prefrontal cortex (MPFC) were specified according to the anatomical automatic labelling (AAL) masks of Tzourio‐Mazoyer et al. [2002], consistent with Killgore et al. [2004]. We defined the ACC as corresponding to Brodmann area (BA) 24 and BA32, and the MPFC as encompassing the ventral medial to dorsal superior structures, extending to BA8, 9, and 10.
Statistical random‐effects analyses were undertaken for each ROI on the obtained statistical parameter maps (SPMs), in two stages. We first produced single within‐condition SPMs for both the subliminal and supraliminal conditions. In these analyses, activation to fear was examined relative to the neutral baseline. Second, we undertook between‐condition contrasts of subliminal versus supraliminal perception to isolate the unique regions of activation to fear (relative to neutral) in each condition. Consistent with previous studies of masked emotion stimuli [e.g., Killgore et al.,2004], clusters of significant activity were determined according to the threshold of P < 0.05, corrected for small volume effects.
Whole‐Brain Analyses
Although the focus of this study was on the hypothesized ROIs, we undertook whole‐brain analyses for both within‐ and between‐condition contrasts to explore the robustness of ROI findings relative to the whole brain and to determine whether concomitantly greater cortical activity was present for supraliminal fear compared to that for subliminal fear. The statistical threshold for whole‐brain analysis was P < 0.001.
Time Series Analyses
To provide information complementary to image analyses, and in light of evidence for attenuation of amygdala activity over the experimental time course [Phillips et al.,2001; Wright et al.,2001], we also examined the time series data for amygdala and ACC ROIs. To examine both attenuation and laterality, we extracted the time series of signal intensity from both the right and left ROIs using a sphere (8‐mm radius) based on the central coordinate of clusters reported in Table I. Where there was no suprathreshold cluster, we used the coordinates based on the corresponding hemisphere. We followed the procedure of Hariri et al. [2000] in time series, which showed a significant correlation of at least 0.2 (P < 0.05) with the experimental model. For each cluster, percentage signal change (for fear relative to neutral) was calculated for the full experimental time course (Total) for both subliminal and supraliminal conditions. Following Wright et al. [2001], percentage signal change was also determined for the first (early phase) and second (late phase) halves of the experiment for each condition. We first undertook repeated‐measures ANOVAs for total signal change in each ROI cluster with condition (supraliminal vs. subliminal) and laterality as within‐subject factors. To examine changes over the experimental time course, ANOVAs were then undertaken phase (early vs. late) as well as condition and laterality as within‐subjects factors. Contrasts were used to examine each significant effect.
Table I.
Activity in hypothesized regions of interest (threshold, P < 0.05, SVC) in response to fear (versus neutral) for conscious and nonconscious perception conditions
| Condition | Side | MNI coordinates (x, y, z) | Cluster sizea | t |
|---|---|---|---|---|
| Subliminal | ||||
| Amygdala | L | −16, 2, −16 | 12c | 2.71 |
| R | 16, 2, −16 | 6b | 2.64 | |
| Ventral medial prefrontal (BA10/32) extending to ventral anterior cingulate | R | 8, 58, −12 | 12 | 2.27 |
| Supraliminal | ||||
| Amygdala | L | −26, 2, −16 | 37 | 2.41 |
| Dorsal anterior cingulate (BA24/32), extending right | L | −8, 16, 26 | 177d | 3.16 |
| R | 8, 6, 28 | — | 3.36 | |
| Dorsal medial prefrontal (BA8), extending right | L | −10, 42, 46 | 809c | 3.55 |
| R | 8, 40, 42 | — | 4.00 | |
| Supraliminal > subliminal | ||||
| Amygdala | L | −26, −4, −24 | 78c | 3.19 |
| R | 30, −2, −26 | 48c | 3.23 | |
| Dorsal anterior cingulate (BA24) | L | −6, 18, 22 | 1,772d | 3.03 |
| Extending to dorsomedial prefrontal (BA8/9), and | L | −10, 40, 40 | — | 4.55 |
| Extending right | R | 12, 62, 28 | — | 2.08 |
| Subliminal > supraliminal | ||||
| Ventral anterior cingulate, extending to ventral medial prefrontal (BA32) | R | 2, 34, −4 | 38 | 2.15 |
The cluster with the largest number of voxels in each region is reported. Cluster size refers to the number of suprathreshold voxels contributing to the cluster (and each voxel was 2 mm3). Montreal Neurological Institute (MNI) coordinates (x, y, z, in mm) refer to the voxel of maximum signal change in each cluster. BA, Brodmann area. SVC, small volume corrected. Significant at the more stringent levels of
P < 0.01;
P < 0.005;
P < 0.0001.
RESULTS
Behavioral and SCR Data
In post‐scan briefings, subjects were able to discriminate fear (82%) and neutral (77%) with well above chance accuracy, indicating that any differential effects in neural responses were unlikely to be due to visual processing or discrimination difficulties. In this briefing, subjects also confirmed they were unable to detect the first face in subliminal presentations.
For the SCR amplitude, ANOVA revealed a trend toward an interaction (F = 3.74, degrees of freedom [df] = 1,14, P = 0.07) between condition (supraliminal vs. subliminal) and stimulus (fear vs. neutral). Contrasts showed that SCRs elicited by supraliminal fear were significantly greater than were those elicited by supraliminal neutral (t = 4.28, df = 14, P = 0.001), whereas SCRs elicited by subliminal fear showed a trend toward being greater than those for neutral were (t = 1.89, df = 14, P = 0.08; Fig. 1). This pattern of results may account for the highly significant main effect for emotion (F = 16.11, df = 1,14, P = 0.001) and the lack of a main effect for condition (F = 2.51, df = 1,14, P = 0.14).
Figure 1.
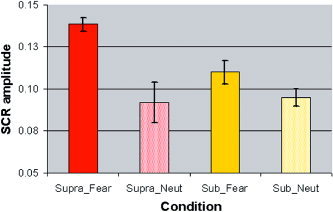
Mean and standard error for the amplitude of skin conductance responses (SCRs) in microsiemens for supraliminal presentations of fear (F‐Supra) and neutral (N‐Supra) and for subliminal presentations of fear (F‐Sub) and neutral (N‐Sub). ANOVA showed that SCRs were higher for fear relative to neutral for both supraliminal and subliminal conditions, although SCRs were generally greater in the supraliminal condition. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effect of Awareness on Amygdala–Medial Prefrontal Activity
Supraliminal fear (relative to neutral) perception elicited significant activity in the left amygdala, and in the right dorsal portion of the ACC and MPFC (Table I). Activation was maximal in the left dorsal ACC/MFPC but extended to the right hemisphere. At the low threshold of P < 0.1, a small region of right amygdala activity was also observed for supraliminal fear, with coordinates in a similarly central location (x = −24, y = 0, z = −16). By contrast, in response to subliminal fear (relative to neutral), there were clusters of significant activity in the bilateral amygdala and ventral portion of the right MPFC, extending into the ventral ACC (Table I; Fig. 2).
Figure 2.
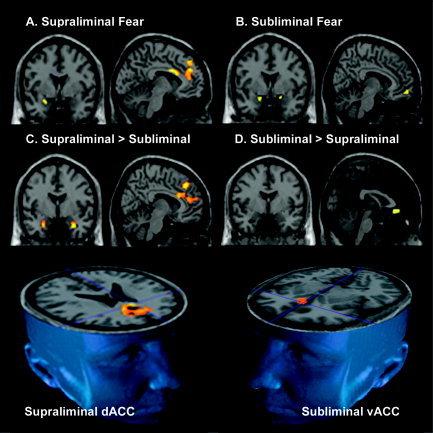
A–D: Statistical parameter maps (SPMs at P < 0.05small volume corrected), overlaid on the canonical T1 images, derived from the Montreal Neurological Institute. Images are in neurological orientation (left hemisphere = left of image). SPMs are for within‐ and between‐condition contrasts of supraliminal and subliminal fear, relative to a neutral baseline, for the regions of interest: amygdala, ventral (vACC) and dorsal anterior cingulate cortex (dACC), and connected ventral (vMPFC) and dorsal (dMPFC) portions of the medial prefrontal cortex. The within‐condition contrast of supraliminal fear relative to neutral elicited significant responses in the left amygdala (A, image on left) and left dACC/dMPFC (A, image on right). The contrast of subliminal fear relative to neutral elicited significant activity in the bilateral amygdala (B, image on left) and vACC (B, image on right). In between‐condition contrasts, supraliminal fear was distinguished by significantly greater responses in the bilateral amygdala, most pronounced in the left amygdala, and in the dACC and dMPFC (C), whereas subliminal fear was distinguished by relatively greater activity in the vACC (D). The 3D images (bottom row) illustrate further the distinction between conditions in prefrontal responses: whereas supraliminal fear was distinguished by greater responses in the dorsal ACC, supraliminal fear was distinguished by greater responses in the ventral ACC. The standardized anatomical coordinates for these regions of activity are presented in Table I.
Direct comparisons between conditions were then undertaken. Supraliminal fear elicited comparatively greater bilateral amygdala activity, which was most prominent in the left amygdala (Table I; Fig. 2). The amygdala coordinates indicated that activity was maximal in an extremely ventral portion of the amygdala not revealed in within‐condition contrasts. Supraliminal fear was also associated with comparatively greater activity in a large region of the dorsal ACC and MPFC, extending from z = 22 to z = 40 in standardized space (Table I; Fig. 2), again most prominent in the left hemisphere. By contrast, subliminal fear elicited significantly greater activity in the ventral portion of the right ACC (Table I; Fig. 2).
Effect of Awareness on Whole‐Brain Activity
Although the focus of this study was on the hypothesized search regions, we undertook a parallel analysis of whole‐brain activity to explore the robustness of ROI findings and to determine whether cortical activity was generally greater for supraliminal fear. Whole‐brain analysis (P < 0.0001) confirmed that responses to supraliminal fear were present in the left amygdala (x = −20, y = −6, z = −20), dorsal ACC (x = −4, y = 6, z = 28; x = 8, y = −6, z = 30) and dorsal MPFC (x = 8, y = 40, z = 42). Additional regions of significant (P < 0.0001) activity were observed in the dorsolateral prefrontal cortex (x = 40, y = 14, z = 30) and visual association cortices (precuneus, x = −12, y = −74, z = 42; x = 4, y = −56, z = 38; fusiform, x = −44, y = −40, z = −26).
For subliminal fear, relative to the neutral baseline, whole‐brain analysis confirmed the pattern of bilateral amygdala (x = −18, y = 2, z = −18; x = 18, y = 2, z = −14) and ventral ACC (x = −18, y = 14, z = −16; x = 22, y = 10, z = −18) activity (at P < 0.005). Additional significant (P < 0.0001) regions of nonhypothesized activity were in the left somatosensory‐related cortex (post‐central gyrus, x = −38, y = −38, z = 45) and right premotor region of the dorsal middle prefrontal cortex (x = 40, y = 14, z = 30).
Between‐condition contrasts confirmed ROI findings that supraliminal fear (relative to the neutral baseline) was distinguished by significantly greater responses relative to subliminal fear in the left amygdala (P < 0.05), left dorsal ACC and MPFC (P < 0.0001), and in the additional dorsolateral prefrontal and visual regions of activity (P < 0.0001). By contrast, subliminal fear elicited comparatively greater responses in the right hypothalamus (P < 0.005) as well as the right ventral ACC (P < 0.05).
Spatiotemporal Dynamics of Supraliminal and Subliminal Responses
We first examined condition by laterality effects for percentage signal change, averaged across the full experiment (total), for each ROI. For the amygdala, ANOVA revealed a weak trend toward an interaction between condition and laterality (F = 3.40, df = 1,14, P = 0.086). Contrasts showed that signal change in the left amygdala was comparatively greater for the supraliminal condition (F = 6.73, df = 1,14, P = 0.02), consistent with image analyses, but greater in the right amygdala for the subliminal condition (F = 5.56, df = 1,14, P = 0.03; Fig. 3A,B). By contrast, there were no differences between right and left amygdala within each condition. The presence of comparatively enhanced right amygdala activity may be due to the use in time series analyses of a relatively dorsal amygdala coordinate (at z = −16), whereas image analyses revealed generally greater activity in the supraliminal condition in an extremely ventral portion of the amygdala (z = −24 and z = −26).
Figure 3.
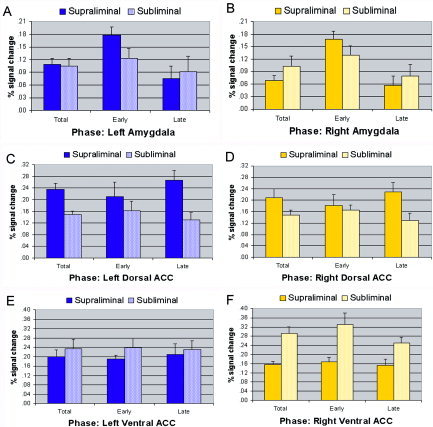
The percentage blood oxygenation level‐dependent (BOLD) signal change and standard error for supraliminal and subliminal fear, relative to a neutral baseline, for the regions of interest: left (A) and right (B) amygdala, and both left (C) and right (D) dorsal portions of the anterior cingulate cortex (ACC) and left (E) and right (F) ventral portions of the ACC. In each graph, bars depict signal change for the full experimental time course (total), and for the early and late phases of the time course. The total percentage signal change is included as a frame of reference to findings from image analyses. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We then examined the time course of amygdala activity across the early and late phases of the experiment (Fig. 3A,B). For the amygdala, there was a significant interaction between condition and phase (early vs. late; F = 5.49, df = 1,14, P = 0.026), which did not vary with hemisphere. Contrasts confirmed that responses to supraliminal fear were generally greater in the early phase and declined in the later phase for both the left (F = 12.84, df = 1,14, P = 0.003) and right (F = 12.42, df = 1,14, P = 0.003) amygdala, whereas there was only a marginally significant reduction across phases for subliminal fear for the left (F = 4.47, df = 1,14, P = 0.053) and right (F = 4.12, df = 1,14, P = 0.062) amygdala (Fig. 3A,B).
Averaged across the time course, (total) signal change in the dorsal ACC did not show an interaction between condition and laterality (F = 0.90, df = 1,14, P = 0.35), but there was a significant main effect for condition (F = 13.46, df = 1,14, P = 0.001) due to the relatively greater responses to supraliminal fear (Fig. 3C,D) revealed in image analyses. There was also no interaction between condition, phase and laterality for responses in the dorsal ACC (F = 0.02, df = 1,14, P = 0.88). These findings suggest that the relatively greater response in the supraliminal condition was relatively sustained for both the left and right dorsal ACC (Fig. 3C,D).
For the total percentage signal change in the ventral ACC, there was a significant condition by laterality interaction (F = 5.00, df = 1,14, P = 0.034). Consistent with image analyses, subliminal fear elicited greater right ventral ACC responses than did supraliminal fear (F = 19.24, df = 1,14, P < 0.0001). Responses were also relatively greater in the right compared to left ventral ACC for subliminal fear at a strong trend level (F = 4.15, df = 1,14, P = 0.06; Fig. 3E,F). When we examined responses across the early and late phases, there was a marginally significant effect for phase (F = 3.90, df = 1,14, P = 0.058), but no interaction effects involving phase. Contrasts showed that there was no significant attenuation in the supraliminal condition for either the left (F = 0.45, df = 1,14, P = 0.52) or right (F = 0.20, df = 1,14, P = 0.66). For subliminal fear, there was also no attenuation for the left ventral ACC (F = 0.73, df = 1,14, P = 0.41), but there was a significant attenuation for the right‐sided ventral ACC (F = 6.66, df = 1,14, P = 0.022), suggesting that it was this latter attenuation for subliminal fear that largely accounted for the main effect (Fig. 3E,F).
DISCUSSION
The findings from this study suggest that facial signals of fear engage dedicated neural systems, even in the absence of conscious detection, consistent with their biological significance [Williams, 2006]. Subliminal and supraliminal fear were subserved by partially separable neural systems, which dissociate early in the processing sequence and along preferential dorsal–ventral and left–right hemisphere dimensions. Supraliminal fear elicited responses in the bilateral dorsal anterior cingulate and relatively dorsal portion of the left amygdala, whereas subliminal fear showed activation in the right‐sided ventral anterior cingulate and right as well as left amygdala. When conditions were compared in both image and time series analyses, supraliminal fear was distinguished by comparatively enhanced and persistent responses in the dorsal anterior cingulate extending to medial prefrontal cortex, whereas subliminal fear elicited greater right ventral anterior cingulate activity, which attenuated over the time course. Supraliminal fear perception was also characterized by comparatively greater activity in an extremely ventral portion of the bilateral amygdala. In a more dorsal portion of the amygdala, time series analysis revealed a tendency toward greater left‐sided amygdala activity for supraliminal fear, but greater right‐sided activity for subliminal fear, with amygdala attenuation most pronounced for supraliminal perception bilaterally. This dissociation of activity by awareness accords with the involvement of a direct route to the amygdala, which supports the course processing of sensory input without the need for conscious detection, and a cortical route to the amygdala, supporting higher‐level cortical elaboration of consciously attended input [Le Doux,1996].
Subliminal presentations of fear may provide a parallel to “blindsight” in the intact brain. Blindsight patients with striate cortex lesions exhibit amygdala responses to both unconditioned and conditioned fear faces, despite being unable to report the presence of these stimuli [de Gelder et al.,1999; Morris et al.,2001]. Amygdala activity has been observed to “unseen” fear in blindsight patient GY [Morris et al.,2001], and both amygdala and ventral (orbitofrontal) prefrontal activity has been reported in response to extinguished fear faces in parietal lesion patients [Vuilleumier et al.,2002]. Our observation of concomitant activation in amygdala and ventral anterior cingulate regions is also consistent with neuroanatomical evidence that these areas are highly connected [Cassell and Wright,1986; Porrino et al.,1981]. Convergent functional neuroimaging evidence comes from the presence of activity in these regions in response to subliminal fear in an independent sample [Liddell et al.,2005].
Our findings provide in vivo evidence from the intact human brain to suggest that a ventral processing stream, with direct projections from the amygdala to ventral anterior cingulate, may support simple responses to signals of biological significance (such as fear) that occur in the absence of conscious awareness [Zajonc,1980]. The comparative enhancement of ventral anterior cingulate responses to subliminal fear perception accords with evidence that this region is centrally involved in the neural mechanisms of automatic orienting to salient and novel stimuli [Berns et al.,1997; Ranganath and Rainer,2003]. At the whole‐brain level, the ventral anterior cingulate was recruited along with responses in the somatosensory postcentral gyrus and middle frontal premotor area. This pattern of activity accords with evidence that a direct pathway through the amygdala may recruit these cortical regions as part of an early alerting system for biologically salient signals, without the need for conscious awareness [Liddell et al.,2005]. Collateral efferents from brainstem structures may provide direct excitation of these cortical regions in the absence of conscious processing via sensory cortices [Aston‐Jones et al.,1996; Liddell et al.,2005].
In addition to receiving direct brainstem input, the amygdala and ventral medial prefrontal region are associated with the automatic triggering of autonomic (“body”) responses to emotion via connections to the hypothalamus and brainstem arousal networks [Damasio et al.,2000]. Consistent with this association, subliminal fear perception elicited greater SCRs than did neutral along with comparatively greater hypothalamus activity in whole‐brain analyses. The ventral anterior cingulate and amygdala have been described as key components of a rostral–ventral limbic system, associated with bodily‐driven affective states [Devinksy et al.,1995]. This system has been associated preferentially with the right hemisphere and with a nonconscious and preverbal mode of processing, described initially by Hughlings‐Jackson [1931] as the “physiological bottom of the mind” [Edelman,1989]. We found evidence of enhanced responses in the right ventral anterior cingulate, and a tendency for enhanced activity in the right amygdala (in time series analyses), for subliminal compared to supraliminal fear. However, within the subliminal condition responses were not enhanced for the right‐ relative to left‐sided amygdala. It is possible that recruitment of right hemisphere regions, but not necessarily preferentially greater activity in these regions, is required for subliminal perception of fear. Right as well as left amygdala and ventral anterior cingulate activation for subliminal fear were observed in our complementary study [Liddell et al.,2005]. Blindsight studies have also reported bilateral amygdala responses, in this case with more pronounced right‐sided activity [Morris et al.,2001].
Conscious elaboration of fear signals may rely on a cortical route to the amygdala and recruitment of the dorsal medial prefrontal regions. The separation of dorsal versus ventral anterior cingulate with conscious versus non‐conscious fear perception, respectively, accords with models of attention and consciousness that propose a functional differentiation of the anterior cingulate in terms of its role in conscious regulation of attention and its interface with emotion [Posner et al.,1998]. Whereas the ventral portion of the anterior cingulate and related medial prefrontal cortex has been associated with body states of emotion, the dorsal portion has been implicated in top‐down regulation of attention and emotional responses [Allman et al.,2001; Devinsky et al.,1995; Drevets and Raichle,1998]. Our findings suggest that the dorsal portion may be preferentially involved in conscious attention to threat‐related signals of emotion. The concomitant engagement of the dorsolateral prefrontal cortex at the whole‐brain level suggests that conscious attention to fear signals engages verbal and semantic elaboration of these signals [Binder et al.,1995], in contrast to the proposed preverbal mode for nonconscious perception.
The concurrently recorded SCR data may provide complementary evidence for a proposed distinction between ventral and dorsal cortical systems for subliminal and supraliminal fear perception, respectively. In the subliminal condition, SCRs showed a trend level enhancement to fear suggesting that, due to a lack of cortical feedback, the direct and nonconscious modulation of autonomic responses may be relatively subtle. In the supraliminal condition, greater attention to fear signals, mediated by dorsal prefrontal and sensory cortices, may provide top‐down feedback that acts to potentiate autonomic responses to these signals. Mutual feedback might also be reflected in the comparatively greater responses to supraliminal fear in the ventral boundary of the amygdala, which receives the strongest projections from prefrontal cortices [Amorapanth et al.,2000]. Consistent with this suggestion, we observed previously that the augmentation in SCRs to supraliminal versus subliminal fear is associated with the 200‐ms poststimulus period in which emotional reactions involving the body are thought to be elicited [Adolphs,2002; Williams et al.,2004b].
Subliminal and supraliminal fear perception were dissociated further in terms of the persistence of subcortical (amygdala) versus cortical (dorsal anterior cingulate) activity. Responses in the amygdala showed a significant reduction in the later phase of the experiment for supraliminal presentations of fear, consistent with previous reports of amygdala attenuation [Phillips et al.,2001; Wright et al.,2001]. By contrast, amygdala responses to subliminal fear showed only a marginal reduction, which suggests that responses may habituate less in the absence of conscious awareness, feasibly due to the lack of top‐down inhibition. Alternatively, the absence of a marked increased in activity during the early phase of nonconscious fear perception may mean that there is only a minimal impact from habituation.
In terms of cortical activity, ventral anterior cingulate responses to subliminal fear were most pronounced in the early phase of the experiment, and attenuated over the later phase, highlighting the importance of temporal dynamics to elucidating these neural systems. Our observation of an early increase in ventral anterior cingulate responses to subliminal fear accords with electrophysiological evidence from both scalp and intracerebral recordings. In healthy subjects, the scalp‐recorded N2 and early P3 ERP components peaking 200–250 ms poststimulus have been shown to increase in response to “unseen” fear [Liddell et al.,2004; Williams et al.,2004b]. These components may involve generators in the amygdala and anterior cingulate, respectively [Halgren and Marinkovic,1995]. Fast‐latency intracerebral potentials to fear have been observed before full analysis in the visual cortex [Kawasaki et al.,2001], and in blindsight subject GY, electrical responses to facial expressions of emotion occurred within the first 200 ms poststimulus [de Gelder et al.,1999]. However, for supraliminal fear, activity in the dorsal anterior cingulate was sustained across the experimental time course. This persistence may subserve the conscious cortical elaboration of fear signals and allow for modulation from cortical feedback.
By using masking, the limitation of our protocol was the need to compare double‐stimulus (target/mask) to single‐stimulus presentations in the contrast of subliminal and supraliminal conditions, respectively. This difference may contribute a perceptual confound to the contrast, but could not account for the differential effect of subliminal fear relative to neutral. We have considered the effect of mask onsets and offsets in our ERP studies [e.g., Williams et al.,2004b], which also suggest that the mask does not interfere with between‐condition contrasts, especially given its immediate onset. We ensured that the ISI was equivalent in each condition to minimize the effect of presentation differences. However, a strength of the backward masking protocol was its control of implicit motion cues (due to the perceptual change from fear to neutral mask, vs. the absence of change for neutral–neutral pairs), by slightly spatially offsetting the mask in relation to the preceding target face. Blindsight studies suggest it is important to control for implicit motion cues, as they may contribute to residual visual processing in the absence of conscious stimulus detection. For instance, accuracy for distinguishing facial expressions was found to be better for moving than for stationary stimuli in patient GY [de Gelder et al.,1999]. Future studies might seek to address this issue directly by including dynamic face stimuli. Moreover, research employing additional expressions of emotion is warranted to examine the specificity of amygdala and prefrontal responses to subliminal fear. Although amygdala responses are robustly elicited by fear, they have been observed in response to other salient expressions [Gur et al.,2002b; Phan et al.,2002].
The findings of this study suggest that subliminal signals of fear preferentially engage a ventral processing stream with direct sensory input to the amygdala, and which may rely on the presence of right‐sided activity. However, supraliminal signals of fear may undergo cortical elaboration within a dorsal stream. These findings have direct implications for understanding the mechanisms of fear reactions following trauma, which are automatic and outside immediate conscious control, such as those observed in posttraumatic stress disorder.
Acknowledgements
This work was funded by the Australian Research Council (DP0345481) and by Pfizer (an independent senior research fellowship to L.W.). We thank the Brain Resource International Database (http://www.brainresource.com) for collaboration and support in regard to subject recruitment and data acquisition.
REFERENCES
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001): The anterior cingulate cortex. The evolution of an interface between cognition and emotion. Ann N Y Acad Sci 935: 107–117. [PubMed] [Google Scholar]
- Adolphs R (2002): Neural systems for recognizing emotion. Curr Opin Neurobiol 12: 169–177. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K (2000): Differential lateral amygdala outputs mediate reactions and actions elicited by a fear‐arousing stimulus. Nat Neurosi 3: 74–79. [DOI] [PubMed] [Google Scholar]
- Aston‐Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT (1996): Role of the locus coeruleus in emotional activation. Progress Brain Res 107: 379–402. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Emslie H, Nimmo‐Smith I (1993): The Spot‐the‐Word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol 32: 55–65. [DOI] [PubMed] [Google Scholar]
- Berns GS, Cohen JD, Mintun MA (1997): Brain regions responsive to novelty in the absence of awareness. Science 276: 1272–1275. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS (1995): Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol 52: 593–601. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ (1986): Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull 17: 321–333. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD (2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Pourtois G, Weiskrantz L (1999): Non‐conscious recognition of affect in the absence of striate cortex. Neuroreport 10: 3759–3763. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BJ (1995): Contributions of anterior cingulate cortex to behavior. Brain 118: 279–208. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME (1998): Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implication for interaction between emotion and cognition. Cogn Emot 12: 353–385. [Google Scholar]
- Edelman G (1989): The remembered present: a biological theory of consciousness. New York: Basic Books. [Google Scholar]
- Gordon E (2003): Integrative neuroscience and psychiatry. Neuropsycopharmacology 28(Suppl): 2–8. [DOI] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE (2002a): A method for obtaining 3‐dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 115: 137–143. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE (2002b): Brain activation during facial emotion processing. Neuroimage 16: 651–662. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. 1995. Neurophysiological networks integrating human emotions In: Gazzaniga M, editor. The cognitive neurosciences. Cambridge: MIT Press; p 1137–1152. [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC (2000): Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11: 43–48. [DOI] [PubMed] [Google Scholar]
- Jackson JH (1931): Selected writings of J.H. Jackson, Vol 1 London: Hodder & Soughton. [Google Scholar]
- Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, Hori T, Howard MA 3rd, Adolphs R (2001): Single‐neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci 4: 15–16. [DOI] [PubMed] [Google Scholar]
- Killgore DS, Yurgelun‐Todd DA (2004): Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage 21: 1215–1223. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB (2001): Impact of signal‐to‐noise on functional MRI of the human amygdala. Neuroimage 12: 3461–3464. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 1996. The emotional brain. London: Weidenfeld Nicolson. [Google Scholar]
- Liddell BJ, Williams LM, Rathjen J, Shevrin H, Gordon E (2004): A temporal dissociation of subliminal versus supraliminal fear perception: an event‐related potential study. J Cogn Neurosci 16: 479–486. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM (2005): A direct brainstem‐amygdala‐cortical “alarm” system for subliminal signals of fear. Neuroimage 24: 235–243. [DOI] [PubMed] [Google Scholar]
- Lim CL, Rennie C, Barry RJ, Bahramali H, Lazzaro I, Manor BR, Gordon E (1997): Decomposing skin conductance into tonic and phasic components. Int J Psychophysiol 25: 97–109. [DOI] [PubMed] [Google Scholar]
- Macmillan N (1986): The psychophysics of subliminal perception. Behav Brain Sci 9: 38–39. [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ (1999): A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA 96: 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ (2001): Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124: 1241–1252. [DOI] [PubMed] [Google Scholar]
- Murphy S, Zajonc R (1993): Affect, cognition, and awareness: affective priming with suboptimal and optimal stimuli. J Pers Soc Psychol 64: 723–739. [DOI] [PubMed] [Google Scholar]
- Parrish TB, Gitelman DR, LaBar KS, Mesulam MM (2000): Impact of signal‐to‐noise on functional MRI. Magn Reson Med 44: 925–932. [DOI] [PubMed] [Google Scholar]
- Penny WD, Ashburner J, Kiebel S, Henson R, Glaser DE, Phillips C, Friston K (2001): Statistical parametric mapping: an annotated bibliography. Available online at: http://www.fil.ion.ucl.ac.uk/spm/course/notes02/misc/bib.pdf
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002): Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA 99: 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ (2001): Time courses of left and right amygdalar responses to fearful facial expressions. Hum Brain Mapp 12: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SC, Bullmore ET, Gray JA, Brammer MJ (2004): Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 21: 1486–1498. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman‐Rakic PS (1981): Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 198: 121–136. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK (1998): Attention, self‐regulation and consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Rainer G (2003): Neural mechanisms for detecting and remembering novel events. Nat Neurosci Rev 4: 193–202. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ (2002): Neural response to emotional faces with and without awareness: event‐related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia 40: 2156–2166. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild B, Erb M, Bartels M (2001): Are emotions contagious? Evoked emotions while viewing emotionally expressive faces: quality, quantity, time course and gender differences. Psychiatry Res 102: 109–124. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Das P, Boucsein W, Sokolov EN, Brammer MJ, Olivieri G, Peduto A, Gordon E (2004a): The dynamics of cortico‐amygdala and autonomic activity over the experimental time course of fear perception. Brain Res Cogn Brain Res 21: 114–123. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, Young A, Gordon E (2004b): Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp 21: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM (2005): An integrative neuroscience model of significance processing. J Integrative Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerret D, Lagopolous J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E (2001): Arousal dissociates amygdala and hippocampal fear responses: Evidence from simultaneous fMRI and skin conductance recording. Neuroimage 14: 1070–1079. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL (2001): Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport 12: 379–383. [DOI] [PubMed] [Google Scholar]
- Zajonc R (1980): Feeling and thinking: preferences need no inferences. Am Psychol 35: 151–75. [Google Scholar]
- Zald DH (2003): The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]


