Abstract
The psychological processes through which humans learn a language have gained considerable interest over the past years. It has been previously suggested that language acquisition partly relies on a rule‐based mechanism that is mediated by the frontal cortex. Interestingly, the actual structure involved within the frontal cortex varies with the kind of rules being processed. By means of functional MRI we investigated the neural underpinnings of rule‐based language processing using an artificial language that allows direct comparisons between local phrase structure dependencies and hierarchically structured long‐distance dependencies. Activation in the left ventral premotor cortex (PMC) was related to the local character of rule change, whereas long‐distance dependencies activated the opercular part of the inferior frontal gyrus (Broca's area (BA) 44). These results suggest that the brain's involvement in syntactic processing is determined by the type of rule used, with BA 44/45 playing an important role during language processing when long‐distance dependencies are processed. In contrast, the ventral PMC seems to subserve the processing of local dependencies. In addition, hippocampal activity was observed for local dependencies, indicating that the processing of such dependencies may be mediated by a second mechanism. Hum Brain Mapp, 2007. © 2007 Wiley‐Liss, Inc.
Keywords: syntactic hierarchies, learning, prefrontal cortex, premotor cortex, hippocampus, fMRI
INTRODUCTION
The psychological representation of grammatical/syntactical knowledge in language has been widely assumed to involve rules [e.g., Chomsky, 1957, Chomsky, 1965]. Rule systems capable of generating an infinite set of outputs (“grammars”) vary in their generative power. The weakest possess only local organizational principles, with regularities limited to neighboring units. Such grammars are a set of continuation relations among symbols that allow the specification of symbol sequences [Chomsky and Miller, 1958], i.e., they can be fully specified by transition probabilities between elements in a sequence. The sequences that comply with the continuation relations of a given grammar are called grammatical, while the ones that do not are called nongrammatical. In contrast, all natural languages minimally require so‐called phrase structure grammars. In addition to concatenating items, a phrase structure grammar (PSG) can embed sequences within other sequences, thus creating complex hierarchical structures and long‐distance dependencies.
In a recent article, Fitch and Hauser [ 2004] demonstrated that cotton‐top tamarins can learn the local dependencies of a so‐called finite state grammar (FSG), but that they cannot learn PSG, whereas humans showed learning for both grammars, easily discriminating grammatical from nongrammatical sequences in both the FSG and the PSG. Based on these results, it has been predicted that in humans the processing of these two grammars are subserved by separable brain structures of different phylogenetic age, with the processing of the FSG being supported by the phylogenetically older structure and the processing of the PSG by a phylogenetically younger structure [Friederici, 2004]. Supporting evidence for this notion with respect to the PSG is provided by recent imaging studies that have investigated rule‐based language learning in natural and artificial languages that meet the universal principles of natural grammars [Musso et al., 2003; Opitz and Friederici, 2003; Tettamanti et al., 2002]. These studies consistently report activation in Broca's area (BA) for the learning of phrase structure rules. This brain region, however, was not activated when a rule that cannot exist in any natural language was to be learnt [Musso et al., 2003].
To our knowledge, there is no brain imaging study in humans so far that has directly compared the processing of these two types of rules. As the determining aspect of natural languages is the processing of hierarchical structure and long‐distance dependencies, an evaluation of the possible differences between local and nonlocal dependencies might provide a first insight in the neural underpinnings of language processing as a function of rule type.
Although language processing depends on a variety of components of grammar (syntax, morphology, phonology, etc.), we were specifically interested in brain structures underlying the processing of sequential and hierarchical syntactic rules. It has been previously argued that the large but poorly established vocabulary of real languages could delay the fast availability of syntactic word category information crucial in building up syntactic structures on‐line [Kersten and Earles, 2001]. Also, the syntactic rules may be very different between the mother tongue and a second language (i.e., L2), causing interference between the two grammar systems [Birdsong and Molis, 2001]. Therefore, a simple and highly controlled artificial grammar, BROCANTO [Friederici et al., 2002], was investigated using functional MRI (fMRI) that allows direct comparisons between local phrase structure dependencies and long‐distance dependencies (i.e., hierarchical rules; Fig. 1), while keeping all other aspects of language processing constant. Participants who were trained on BROCANTO prior to scanning performed a word verification task on grammatical and nongrammatical sentences. We compared behavioral responses and brain activity for violations of local and long‐distance dependencies with correct sentences. Based on previous studies, we hypothesized that the activity in the left frontal operculum (ventral premotor cortex, vPMC) is related to the local character of rule change [Friederici et al., 2003]. Long‐distance dependencies, rather, should activate BA 44.
Figure 1.
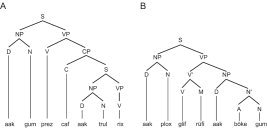
Examples of the hierarchical structure (A) and local dependencies (B) of the grammar system. D, determiner; A, adjective; N, noun; V, verb; M, verb modifier; C, complementizer.
There are several further aspects known to influence the acquisition of L2. It has been previously proposed that any newly learned language in adults is restricted by maturational neural constraints [Birdsong, 1999]. It has been demonstrated previously that not only the age of acquisition but also the degree of proficiency is an influential factor of the neural organization of L2 processing. There is evidence suggesting that with a high degree of language proficiency the brain mechanisms involved in L1 and L2 processing are similar even when the L2 has been learned late [Friederici et al., 2002; Perani et al., 1998; Tatsuno and Sakai, 2005]. Therefore, a participant's degree of proficiency was explicitly modeled as a covariate of interest in the present fMRI data analysis of L2 processing learned under well‐controlled conditions. Crucially, we expect that activation in Broca's area and the vPMC changes as a function of a participant's degree of proficiency.
SUBJECTS AND METHODS
Participants
A total of 24 monolingual participants (13 male; ages 21–31 years, mean age, 25 years) with no history of neurological or psychiatric disorder volunteered for this study. All participants were right‐handed, with normal or corrected‐to‐normal vision, and were paid for participating. Informed consent was obtained before scanning. Data from one participant were discarded because of excessive motion artifacts.
Stimuli
Sentence stimuli were formulated according to a modified version of the grammar system BROCANTO [Friederici et al., 2002; Opitz and Friederici, 2003]. BROCANTO is based on the universal principles of natural languages (i.e., it consists of different syntactic word categories and defined phrase structure rules). As processing of hierarchical structure is the determining aspect of PSG, a complementizer structure (C) was added that allowed the direct comparison of local phrase structure dependencies and long‐distance dependencies (Fig. 1).
A total of 96 correct sentences were formulated, half of them including local dependencies and the other half including long‐distance dependencies. All sentences were divided into two lists of 48 sentences each used during the initial training or during the actual experiment. Another 96 sentences assigned to the two lists contained a severe syntactic violation: a long‐distance violation, a word category repetition, or a local phrase structure violation, both composing local violations (see Table I for examples). The assignment of the lists to either initial training or the actual experiment was counterbalanced across participants.
Table I.
Examples of grammatical and nongrammatical sentences of the modified version of BROCANTO
| Grammatical sentences | Nongrammatical sentences | |
|---|---|---|
| Long distance dependencies | (a) aak gum prez caf aak trul rix | (b) * aak gum prez nöri aak trul rix |
| D N V C D N V | D N V M D N V | |
| Local dependencies | (c) aak plox glif rüfi aak böke gum | (d) * aak plox glif pel aak böke gum |
| D N V M D A N | D N V V D A N |
D, determiner; N, noun; V, verb; M, verb modifier; A, adjective; C, complementizer. Violations that rendered sentences nongrammatical are in italics. The two elements whose dependency is crucial are underlined. Note that the nongrammatical version of the long‐distance dependency condition is ungrammatical as the sequence D‐N‐V at the end of the sentence is only licensed after a C‐element as in its grammatical counterpart. In case of local dependencies ungrammaticality is realized by two successive elements of the same class (V‐elements in the present example), not allowed by the grammar. An example of each of these sentences for English would be the following:
The man wondered whether the boy lied.
The man wondered slowly the boy lied.
The man greeted enthusiastically the young girl.
The man greeted saw the young girl.
Procedure
Two days prior to scanning all participants were trained on a modified version of the miniature artificial grammar system BROCANTO. The training procedure was highly similar to that described in Opitz and Friederici [ 2004]. Participants were given observation training on 16 sentences and were instructed to extract the underlying grammatical rules. After training, participants were asked to judge the correctness of a new set of sentences according to the grammar they learned. This procedure was repeated 12 times with different sentences and ended when a total of 192 sentences were accomplished.
During the actual scanning experiment participants were presented with the second list of sentences (not used during initial training), including both grammatically correct and incorrect sentences. Grammatical judgment tasks usually employed to assess grammatical processing require a strategic decisional component. Thus, such tasks involve executive resources both as part of the sentence comprehension process and as a component of the “off‐line” procedure that supports task performance [Grossman et al., 2002; Price and Grossman, 2005]. To minimize this confound, we administered a word verification procedure. After presentation of a sentence on the screen for 6 s and a blank interval of 1 s, participants were visually probed with a word from the vocabulary of BROCANTO. Participants had to decide whether the word had been part of the preceding sentence or not by pressing a corresponding button as soon as possible. Total trial length was 8 s. Each sentence was presented twice; for one presentation the word was contained in the sentence; for the other presentation it was not. Thus, half of the sentences contained the word that was asked for, and the other half did not.
Data Acquisition
The identical imaging procedure was used as described in Opitz and Friederici [ 2004]. Imaging was performed on a 3T‐Bruker Medspec 30/100 system using a standard birdcage headcoil. Subjects were supine on the scanner bed, with cushions and a stereotactic fixation system used to reduce head motion. In a separate session, high‐resolution whole‐brain images were acquired to assist localization of activation foci using a 3D MDEFT (128 slice sagittal, 1.5‐mm thickness, 256 × 256 voxel [Ugurbil et al., 1993]). For each subject, conventional T1‐weighted anatomic images (MDEFT: data matrix 256 3 256, TR 1.3 s, TE 10 ms [Norris, 2000]) in plane with the echo‐planar images were acquired in order to align the functional images to the 3D images. Finally, functional images sensitive to blood oxygenation level‐dependent (BOLD) contrast were acquired with a gradient‐echo echo‐planar imaging sequence (echo time, 30 ms; flip angle 90°). The repetition time was 2 s. An acquisition volume consisted of 12 slices (5 mm thickness, 2 mm gap) parallel to the plane intersecting the anterior and posterior commissures with an in‐plane resolution of 3 mm2. Slices were positioned individually to cover the medial temporal lobe up to the superior frontal sulcus. Eight discarded volumes were acquired at the beginning of each run to allow stabilization of magnetization. Thus, a total of 772 functional volumes per participant were acquired. Responses were acquired with an MR‐compatible response device.
Data Analysis
The data processing was performed using the software package LIPSIA [Lohmann et al., 2001]. Prior to statistical analyses, motion artifacts were corrected using an affine rotation and translation correction. Second, low‐frequency signal fluctuations were removed on a voxel‐by‐voxel basis. Finally, a spatial smoothing with a Gaussian kernel of 6 mm full‐width at half‐maximum was applied to emphasize spatially coherent activation pattern. For each participant, neural activity was modeled by convolving a stimulus function representing a whole sentence with a canonical hemodynamic response function [Friston et al., 1998]. Thus, the following responses could be modeled separately: grammatical sentences and nongrammatical sentences containing long‐distance and local dependencies. The parameter estimates derived from these linear contrasts were subsequently transformed into the Talairach coordinate space [Talairach and Tournoux, 1988] and entered into a second‐level group analysis treating subjects as a random effect, using a one‐sample t‐test against a contrast value of zero at each voxel [Holmes and Friston, 1998]. As in previous studies [Opitz and Friederici, 2003, Opitz and Friederici, 2004], activations were considered significant when comprised of 10 or more contiguous voxels surviving a threshold of P < 0. 0001, uncorrected. More specifically, we were interested in those brain areas whose activity varied by the participant's proficiency level. Therefore, a participant's performance during the initial training procedure was taken as a covariate of interest in the second‐level analysis. In this analysis a statistical threshold of P < 0.01 corrected for multiple comparisons was used. Subsequently, the violation effects were estimated for the voxels that showed significant activation in this analysis. The mean percent signal change of all activated voxels for the time points corresponding to the expected peak in the hemodynamic response (4–8 s poststimulus) entered into a repeated‐measures analysis of variance (ANOVA) using violation type (local vs. long‐distance violations) and brain region as within‐subject factors. This ANOVA allowed us to look at the differential involvement of different brain regions in processing local and long‐distance dependencies.
RESULTS
Behavioral Results
As is apparent from Figure 2, participants exhibited knowledge of the grammar system of BROCANTO at the end of the initial training by successfully discriminating between grammatical and nongrammatical sentences (80% correct, t22 = 16.63, P < 0. 0001). An ANOVA contrasting the two violation conditions and the six time bins (each representing the performance of 32 successive trials) indicated more proficient knowledge of the local dependencies than of the long‐distance dependencies (F1,22 = 28.7, P < 0.001). Nevertheless, the learning rates for the two conditions were not distinct, as indicated by a nonsignificant time bin by violation type interaction (F < 1). Based on this, we can conclude that participants acquired both local and long‐distance dependencies.
Figure 2.
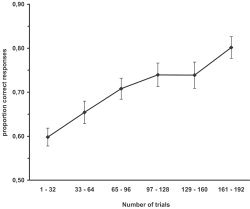
Increase of syntactic proficiency (proportion correct response with SE bars) across participants during initial training 48 h prior to the experiment.
During the scanning experiment participants correctly responded to about 83% of the word probes. No differences between conditions, i.e., type of dependency (local vs. long‐distance dependency) and grammatical status of the sentence were observed. Analyses of reaction times revealed faster responses for correct than for incorrect word detections (F1,20 = 14.77, P < 0.001; because of missing values for incorrect answers, only 21 subjects entered this analysis). No differences between conditions were observed. This homogeneity in probe responses indicated that sentences of the four conditions were equally well processed. In order to increase statistical power the subsequent imaging analysis included all trials irrespective of the correctness of the probe responses.
Brain Imaging Results
A first analysis of the fMRI data contrasted sentences containing local violations or long‐distance violations with correct sentences. Local violations led to increased activity in a number of brain areas (Table II), including bilateral hippocampus (Fig. 3) and the left vPMC. In contrast, long‐distance violations gave rise to increased activity in the right parahippocampal gyrus, the mammillary body, and the right lingual gyrus. No differences in brain activity between long‐distance violations and correct sentences even at a more liberal threshold of P < 0.005 were observed in bilateral hippocampus. This is also reflected in the peak activity for both hippocampi showing larger responses to local than to long‐distance violations in the left hippocampus (Fig. 3).
Table II.
Brain areas exhibiting activation increases for sentences containing local and long‐distance violations as compared to correct sentences
| Cortical region | Peak location | Z‐score | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Local violations vs. correct | |||||
| Left hippocampus | −26 | −21 | −15 | 3.75 | |
| Right hippocampus | 23 | −10 | −15 | 3.66 | |
| Left ventral PMC | BA 6/4 | −56 | −22 | 35 | 3.29 |
| Left inf. parietal lobule | BA 40 | −53 | −37 | 24 | 3.40 |
| Left STG | BA 22 | −62 | −34 | 12 | 3.52 |
| Right STG | BA 22 | 59 | −28 | 15 | 4.42 |
| Right temporal pole | BA 22 | 34 | 8 | −18 | 3.47 |
| Left insula | −35 | −10 | −6 | 3.69 | |
| Right nsula | 38 | −7 | −6 | 3.94 | |
| Right putamen | 26 | 8 | −3 | 3.47 | |
| Long‐distance violations vs. correct | |||||
| Right parahippocampal gyrus | BA 35 | 16 | −25 | −21 | 4.02 |
| Mammillary body | −1 | −10 | −12 | 4.40 | |
| Right lingual gyrus | BA19 | 7 | −79 | 3 | 3.70, |
Figure 3.
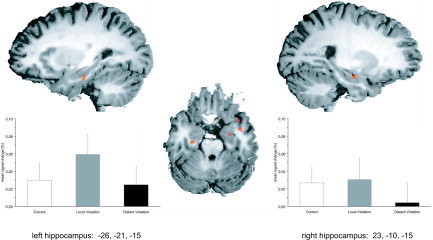
Increase in hippocampal activity for local violations as compared to correct sentences. Below the brain activity is plotted (mean % signal change of the BOLD response of three consecutive time points around the peak ± SE of the mean across participants) for both violation conditions compared to correct sentences. Note that long‐distance violations exhibited hippocampal activity that did not differ from the activity for correct sentences.
The second analysis focused on the influence of a participant's proficiency in the use of the newly learned grammar. Therefore, this analysis included the performance during the initial training procedure as a covariate of interest. By this we sought to reveal brain regions whose activity was larger for participants that showed better discrimination between grammatical and nongrammatical sentences and, as a consequence, acquired both local and long‐distance dependencies to a larger degree. This analysis takes into account interindividual differences in the acquisition process of BROCANTO and, therefore, possible differences in the processing of local and long‐distance dependencies. As is apparent from Figure 4, this analysis revealed only two areas with increased activity (Fig. 4A,4B): the opercular part of the inferior frontal gyrus (oIFG: −47, −12, 24) and the vPMC (BA 4/6: −47, −6, 35), respectively. As predicted, the oIFG (BA 44) exhibited a differential response between violations and correct sentences only for long‐distance dependencies (Fig. 4C). In this brain region no stronger activity for violations of local dependencies as compared to correct sentences could be observed. The opposite response pattern was found in the vPMC (Fig. 4D). Here, violations of local dependencies exhibited greater activity as compared to correct sentences and violations of long‐distance dependencies. This differential response pattern was confirmed by an ANOVA contrasting the percent signal change for both violation types in both regions (oIFG and vPMC). A violation type × region interaction (F1,22 = 5.95, P < 0.05) was obtained. More detailed evaluation of this interaction effect revealed greater activation for long‐distance as compared to local violations in oIFG (F1,22 = 4.34, P < 0.05). The opposite effect, namely, greater activation for local than for long‐distance violations in vPMC, did not reach significance (F1,22 = 2.15, P < 0.13).
Figure 4.
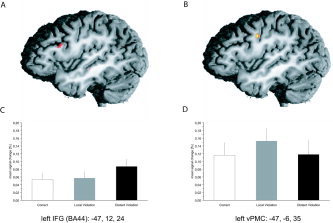
Regions in the frontal cortex demonstrating increased activity to violations as compared to correct sentences. A: The left IFG (BA 44) is selectively more engaged in processing long‐distance violations as compared to local violations or correct sentences. B: The vPMC shows more activity for local as compared to long‐distance violations and correct sentences. C,D: Brain activity (mean % signal change of the BOLD response of three consecutive time points around the peak ± SE of the mean across participants) for both violation conditions compared to correct sentences.
To further explore the influence of proficiency on brain activity we conducted a median split of the sample according to the performance in the initial training. Participants exhibiting a high percentage of correct responses (n = 13, mean percent correct 86%) will be further referred to as the high‐proficiency group, while the other participants with low percentage correct (n = 10, mean: 67%) formed the low‐proficiency group. As is apparent from Figure 5A, high‐proficiency participants activated the oIFG (BA 44) stronger than the low‐proficiency group. Therefore, the differences in peak activity between both violation conditions and the correct sentences were calculated separately for both regions of interest (oIFG and vPMC; Fig. 5) and was subjected to an ANOVA contrasting these differences for both groups. Both groups elicited comparable overall activity in oIFG (BA 44) as indicated by a nonsignificant main effect group (F < 1). However, there was a significant condition by group interaction (F1,21 = 8.56, P < 0.01), suggesting a different engagement of this brain region by both groups. Post‐hoc tests revealed greater involvement of oIFG (BA 44) in the processing of distant violation as compared to local violations in the high‐proficiency group (F1,12 = 8.13, P < 0.05) but not in the low‐proficiency group (F1,9 = 1.94, P < 0.2). Similarly, both groups exhibited comparable activity in the vPMC (Fig. 5B); the main effect group was again not significant (F < 1). Furthermore, the condition by group interaction was also not significant (F < 1), suggesting a similar involvement of the vPMC in the detection of local grammatical violations for both groups.
Figure 5.
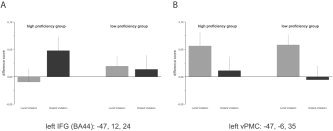
Difference in brain activity for local and distant violations minus correct responses in BA 44 (A) and in the vPMC (B) for both groups of participants. Note that in the vPMC the relative activation pattern of local violations and distant violations was quite similar. BA 44, in contrast, exhibits group differences.
DISCUSSION
The goal of this study was to investigate which brain areas are involved in rule‐based grammar processing using an artificial language that allows direct comparisons between local phrase structure dependencies and long‐distance dependencies (i.e., hierarchical rules). Specifically, we investigated brain regions thought to be important in the proficient use of either dependency. There are a number of critical findings. First, anterior hippocampal activity was only evident in response to violations of local syntactic dependencies. Moreover, this activity did not vary as a function of proficiency. Second, we demonstrated that the processing of such local dependencies involves the left ventral premotor cortex. Lastly, our results show that Broca's area (specifically, BA 44) plays a key role in the efficient processing of long‐distance dependencies and that this involvement depends on the proficiency level.
The increased activity of anterior hippocampus during the processing of local dependencies might be interpreted to reflect the binding requirements needed to establish relationships between words and their possible positions in a sentence, i.e., their specific syntactic category (e.g., noun, verb). As we have previously demonstrated, the repeated occurrence of a particular relationship, as in syntactically correct phrases, facilitates the binding of a word to its syntactic category and, therefore, the binding requirements reduce with increased proficiency [Opitz and Friederici, 2003, Opitz and Friederici, 2004]. Crucially, a local phrase structure violation introduces a new relationship between a particular word and its new syntactic role in a sentence and, therefore, would lead to enhanced activity of the left hippocampal formation as compared to correct sentences because of increased relational processing demands [Cohen et al., 1999; Preston et al., 2004]. This is consistent with our view that the left hippocampal system mediates similarity‐based processing; that is, the hippocampus binds the perceptual characteristics of each word to its possible sentence position [Opitz and Friederici, 2003, Opitz and Friederici, 2004].
In addition in the present experiment, the processing of local dependencies during a probe detection task triggered an increased engagement of the left vPMC. This is in agreement with recent results demonstrating that this brain region was involved in the detection of local ungrammaticalities in naturally existing languages [Friederici et al., 2003; Newman et al., 2003]. Using artificial languages mimicking natural grammars, it has been demonstrated that this brain region in addition to Broca's area is also activated during the acquisition and use of grammatical rules [Tettamanti et al., 2002]. Our previous observation of vPMC involvement in learning an artificial language solely comprised of local phrase structure dependencies [Opitz and Friederici, 2004] also adds evidence to the notion that the vPMC generally supports the processing of local structures irrespective of the actual task demands. This receives further support from recent studies demonstrating the involvement of the vPMC in visual object sequence learning tasks [Schubotz et al., 2004] and motor sequence learning tasks [Sakai et al., 1998; Toni et al., 1998]. Both tasks are characterized by a full specification of the sequence by transitional probabilities, i.e., local dependencies between elements in a sequence.
In contrast, violations of hierarchical rules activated the posterior part of Broca's area, i.e., BA 44. Broca's area was shown to be involved in syntactic processes in a number of neuropsychological studies, in particular in that patients with lesions centered on Broca's area are clearly impaired in processing syntactic rules, including hierarchical rules [Caplan and Waters, 1999; Grodzinsky, 2000]. In addition, brain imaging studies investigating long‐distance dependencies involving syntactic movement and transformations have unequivocally reported Broca's area to be active [Ben‐Shachar et al., 2003; Caplan, 2001; Kuperberg et al., 2000; Röder et al., 2002; Stromswold et al., 1996].
One could argue that greater activation in BA 44 for the long‐distance condition reflects greater difficulty of this condition due to greater syntactic complexity. Increased activity in Broca's area has been previously reported in comparisons involving differences in syntactic complexity (e.g., center‐embedded vs. right‐branching relative clauses [Caplan et al., 2000]). However, in the present study the activation in BA 44 was modulated by proficiency with only high‐proficiency participants exhibiting stronger involvement of BA 44. This might indicate that syntactic complexity per se is not the cause of this activity.
A recent intriguing study further investigated the role of Broca's area in learning natural languages, independently of the linguistic family to which the language belongs [Musso et al., 2003]. In that study, subjects were required to learn some rules of parametrically different languages, such as Italian and Japanese. Broca's area became more active over the course of time as participants became adept with theses rules, irrespective of semantic knowledge, i.e., the fact that participants did not know the translation of the vocabulary. Crucially, comparable presentations of pseudo‐linguistic rules (i.e., rules that are not based on the principles of PSG) using the same vocabulary did not activate Broca's area.
In addition, the present data suggest that the involvement of Broca's area, in particular BA 44, depends on the proficiency level. Crucially, only high‐proficiency participants activated this brain region when processing hierarchical structures. This is in accordance with earlier findings [Perani et al., 1998] showing a similar pattern of brain activity when listening to stories in either their native language or a second language solely for high‐proficiency participants. This supports the view that Broca's area comes into play when successful computations of hierarchical dependencies are necessary for successful language processing, even without semantic knowledge, as in the study by Musso et al. [ 2003] or in the artificial language BROCANTO of the present experiment.
In contrast, independent of the absolute signal change the relative activation pattern of the vPMC for correct sequences, local violations, and distant violations was quite similar between the two proficiency groups, with an activation increase for the local violation condition compared to the two other conditions. This suggests that the vPMC reacts in a qualitatively similar manner to local violations in high‐ and low‐proficient learners and might indicate that local dependencies are much easier to process and that a low proficiency level is sufficient to lead to activity of the vPMC. This is also suggested by previous findings demonstrating that cotton‐top tamarins can learn local dependencies but fail to acquire hierarchical dependencies [Fitch and Hauser, 2004]. It is known from evolutionary neuroanatomy that the precentral gyrus is a phylogentically older structure and also develops earlier in ontogeny than the inferior frontal gyrus [Sanides, 1962]. Thus, it is conceivable that the vPMC subserves the processing of the less complex local phrase structure violations that are limited to neighboring elements of a sentence. In contrast, the evolution of well‐developed hierarchical processing abilities in humans may be a crucial requirement for mastering any human language.
Taken together, the present results are compatible with our view that Broca's area as opposed to the adjacent vPMC is involved in interpreting hierarchical dependencies between related elements. In general, this mechanism is not necessarily tied to the language domain, but might rather support hierarchical operations equally applied in linguistic and nonlinguistic domains, including human imitation and musical syntax [Iacoboni and Woods, 1999; Maess et al., 2001].
REFERENCES
- Ackermann H, Lutzenberger W, Hertrich I ( 1999): Hemispheric lateralization of the neural encoding of temporal speech features. A whole‐head magnetoencephalography study. Cogn Brain Res 7: 511–518. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Hendler T, Kahn I, Ben‐Bashat D, Grodzinsky Y ( 2003): The neural reality of syntactic transformations: evidence from fMRI. Psychol Sci 14: 433–440. [DOI] [PubMed] [Google Scholar]
- Birdsong D. (ed.) ( 1999): Introduction: whys and why nots of the critical period hypothesis for second language acquisition. Mahwah, NJ: Lawrence Erlbaum; p 1–22. [Google Scholar]
- Birdsong D, Molis M ( 2001): On the evidence for maturational constraints in second‐language acquisition. J Mem Lang 44: 235–249. [Google Scholar]
- Caplan D ( 2001): Functional neuroimaging studies of syntactic processing. J Psycholinguist Res 30: 297–320. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters GS ( 1999): Verbal working memory and sentence comprehension. Behav Brain Sci 22: 114–126. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A ( 2000): Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp 9: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N ( 1957): Syntactic structures. The Hague: Mouten. [Google Scholar]
- Chomsky N ( 1965): Aspects of the theory of syntax. Cambridge, MA: MIT Press. [Google Scholar]
- Chomsky N, Miller GA ( 1958): Finite state languages. Inf Control 1: 91–112. [Google Scholar]
- Cohen NJ, Ryan E, Hunt C, Romine L, Wszalek T, Nash C ( 1999): Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus 9: 83–98. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Hauser MD ( 2004): Computational constraints on syntactic processing in a nonhuman primate. Science 303: 377–380. [DOI] [PubMed] [Google Scholar]
- Friederici AD ( 2004): Processing local transitions versus long‐distance syntactic hierarchies. Trends Cogn Sci 8: 245–247. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Steinhauer K, Pfeifer E ( 2002): Brain signatures of second language acquisition: evidence challenging the critical period. Proc Natl Acad Sci U S A 99: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Rüschemeyer S‐A, Hahne A, Fiebach CJ ( 2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex 13: 170–177. [DOI] [PubMed] [Google Scholar]
- Friston K, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y ( 2000): The neurology of syntax: language use without Brocas area. Behav Brain Sci 23: 1–71. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, Hurtig HI ( 2002): Assessing resource demands during sentence processing in Parkinson's disease. Brain Lang 80: 603–616. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ ( 1998): Generalizability, random effects and population inference. Neuroimage 7: 754. [Google Scholar]
- Iacoboni M, Woods RP ( 1999): Cortical mechanisms of human imitation. Science 286: 2526–2529. [DOI] [PubMed] [Google Scholar]
- Kersten AW, Earles JL ( 2001): Less really is more for adults learning a miniature artificial language. J Mem Lang 44: 250–273. [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe‐Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS ( 2000): Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci 12: 321–341. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Mueller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, von Cramon DY ( 2001): A new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph 25: 449–457. [DOI] [PubMed] [Google Scholar]
- Maess B, Koelsch S, Gunter TC, Friederici AD ( 2001): Musical syntax is processed in Broca's area: an MEG study. Nat Neurosci 4: 540–545. [DOI] [PubMed] [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Büchel C, Weiller C ( 2003): Broca's area and the language instinct. Nat Neurosci 6: 774–781. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA, Keller TA, Roth J, Carpenter PA ( 2003): Differential effects of syntactic and semantic processing on the subregions of Broca's area. Cogn Brain Res 16: 297–307. [DOI] [PubMed] [Google Scholar]
- Norris DG ( 2000): Reduced power multi‐slice MDEFT imaging. J Magn Reson Imaging 11: 445–451. [DOI] [PubMed] [Google Scholar]
- Opitz B, Friederici AD ( 2003): Interactions of the hippocampal system and the prefontal cortex in learning language‐like rules. Neuroimage 19: 1730–1737. [DOI] [PubMed] [Google Scholar]
- Opitz B, Friederici AD ( 2004): Brain correlates of language learning: the neuronal dissociation of rule‐based versus similarity‐based learning. J Neurosci 24: 8436–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Paulescu E, Galles N, Dupoux E, Dehaene S, Bettinardi V, Cappa S, Fazio F, Mehler J ( 1998): The bilingual brain — proficiency and age of acquisition of the second language. Brain 121: 1841–1852. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE ( 2004): Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus 14: 148–152. [DOI] [PubMed] [Google Scholar]
- Price CC, Grossman M ( 2005): Verb agreements during on‐line sentence processing in Alzheimer's disease and frontotemporal dementia. Brain Lang 94: 217–232. [DOI] [PubMed] [Google Scholar]
- Röder B, Stock O, Neville H, Bien S, Rösler F ( 2002): Brain activation modulated by the comprehension of normal and pseudo‐word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage 15: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sakaki Y, Pütz B ( 1998): Transition of brain activation from frontal to parietal areas in visuo‐motor sequence learning. J Neurosci 18: 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F ( 1962): Die Architektonik des menschlichen Stirnhirns. Frankfurt: Springer. [Google Scholar]
- Schubotz RI, Sakreida K, Tittgemeyer M, von Cramon DY ( 2004): Motor areas beyond motor performance: deficits in sensory prediction following ventrolateral premotor lesions. Neuropsychology 18: 638–645. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S ( 1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar stereotaxis atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Tatsuno Y, Sakai KL ( 2005): Language‐related activations in the left prefrontal regions are differentially modulated by age, proficiency, and task demands. J Neurosci 25: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Alkadhi H, Moro A, Perani D, Kollias S, Weniger D ( 2002): Neural correlates for the acquisition of natural language syntax. Neuroimage 17: 700–709. [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE ( 1998): The time course of changes during motor sequence learning: a whole‐brain fMRI study. Neuroimage 8: 50–61. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu X, Kim SG, Menon R, Merkle H, Ogawa S ( 1993): Imaging at high magnetic fields: initial experiences at 4T. Magn Reson Q 9: 259–277. [PubMed] [Google Scholar]


