Abstract
Learning a specific skill during childhood may partly determine the functional organization of the adult brain. This hypothesis led us to study brain activation patterns using positron emission tomography (PET), in which we compared word and nonword repetition in 10 right‐handed native English‐speakers (L1) who were proficient in their second language, French (L2), which was learned after the age of 5 years. Regional cerebral blood flow (rCBF) was measured by the H2 15O intravenous bolus method with intersubject averaging and coregistration of magnetic resonance and PET images. A comparison of CBF changes when repeating words in L2 with those seen when repeating words in (L1) demonstrated that the pattern of CBF was similar across the two conditions, with several significant CBF differences in the vicinity of the left insular cortex, ventral premotor region, and in the striatum. We hypothesize that these regions are activated when subjects are required to repeat known words, showing increased activity when there are increased articulatory demands imposed by speaking L2. Comparisons of nonword repetition in L1 and L2 revealed increased activity for L2 in the left ventral premotor region and in the cerebellum; rCBF increases were also observed in these regions in both L1 and L2 with increased number of syllables and increased articulatory complexity, suggesting a role for these regions in the complex motor control needed for the production of novel sequences. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: bilingualism, speech production, word repetition, nonwords, brain imaging
INTRODUCTION
Linguistic abilities are sensitive to the age of exposure to language; people who learn at later ages do not learn a language as well as younger learners [Birdsong,1999; Johnson and Newport,1989]. This finding holds for exposure not only to a first language but also to second and subsequent languages. Many behavioral studies indicate that age of exposure does not affect all language capacities equally. In contrast to lexico‐semantic aspects, syntactic and phonological aspects of a second language are particularly hard to master during late acquisition [De Groot and Kroll,1997; Ritchie and Bhatia,1996]. Neurophysiological studies, though less numerous, provide additional evidence for the view that age at the time of second language acquisition is critical for the mastery of that language and, moreover, for the functional specialization of specific aspects of language in the brain [Neville et al.,1992; Weber et al.,1996].
Within the first year of life, speech‐perception skills of infants change from language‐general to language‐specific as a result of their language experience [Best,1994; Best et al.,1998; Jusczyk,1999; Jusczyk et al.,1993; Kuhl,1998; Kuhl et al.,1992; Polka and Werker,1994; Trehub,1976; Werker and Tees,1984]. Whereas infants exposed to a specific language begin to produce speech patterns characteristic of that language, child and adult learners of a second language (L2) are virtually guaranteed to produce segments in the second language with a nonnative accent [Flege et al.,1999; Johnson and Newport,1989]. This is because the way in which temporal information determines specific aspects of speech varies across languages [Schirmer,2004]. To ensure accurate speech production, speakers must time the production of individual sounds precisely. This necessity arises because speech perception and comprehension are largely determined by temporal order and duration. Age at exposure is the single largest determinant of native‐like speech perception and production skills in L2 learners. Since the age of second‐language acquisition is thought to affect the ultimate phonological skill achievable by a speaker [Lenneberg,1967], it seems reasonable to predict that differences in the recruitment of different brain regions for speech articulation of L1 and L2 will be related to the age when speech motor patterns of a specific language are formed [Kuhl,1998].
In our earlier work using the functional neuroimaging technique of positron emission tomography (PET), we explored the brain regions activated during language processing in English‐speaking (L1) volunteers who had become proficient in French (L2) after the age of 5 years [Klein et al.,1994,1995]. We found that similar brain regions support lexical search and retrieval processes in both L1 and L2, but we observed increased activation in the left basal ganglia, specifically in the putamen, when native English speakers produced an output response in their second language, French. We interpreted this finding as reflecting the role of the left basal ganglia in the complex motor timing involved in speaking a language that had been acquired later in life.
Several studies in aphasic stroke patients have suggested a role for the left basal ganglia in speech production [Aglioti and Fabbro,1993; Blumstein et al.,1987]. Disorders of articulation have been attributed to lesions in the “lenticular zone,” which includes the insula, claustrum, white‐matter fiber tracts passing through the external and internal capsule, the caudate nucleus, and lentiform nucleus (putamen and pallidum) [Dronkers,1996; Marie,1906, 1971]. Typically, patients with subcortical aphasias are nonfluent; they are reluctant to initiate speech and show voice disorders, disrupted articulation often leading to mispronunciation, and occasionally a foreign accent syndrome (FAS). The latter is a rare speech disorder characterized by an inability to make the normal phonetic and phonemic contrasts of one's native dialect, thus conferring on the patient's native language a peculiar quality that is perceived by listeners as a foreign accent [Blumstein et al.,1987; Graff Radford et al.,1986; Gurd et al.,1988]. Present knowledge of the neural basis of FAS is limited, as only a few cases have undergone detailed lesion analyses. Most cases with published lesion information have had left‐hemisphere damage. Localization has not been uniform, but all cases have involved either prerolandic motor cortex (Brodmann's area 4), frontal motor association cortex (BA 6/44), or the striatum [Kurowski et al.,1996].
Using computer‐reconstructed lesions in chronic stroke patients with left‐hemisphere lesions and aphasia, Dronkers [1996] found that all of the cases diagnosed with apraxia of speech had a lesion encompassing the anterior insula. In a separate analysis, Dronkers assessed the lesions of 19 aphasic patients who did not carry the additional diagnosis of apraxia of speech. Their lesions spanned the same distribution of the left hemisphere but completely spared the region of the insula that was affected in the aphasic patients with apraxia of speech. This dissociation was taken to mean that the anterior insula plays some role in the coordination of articulatory movements.
Based on the findings from patients with speech deficits comprising slowed and effortful speech, articulatory imprecision, and disordered speech prosody in the absence of higher language dysfunction, Alexander et al. [1990] suggested a compartmentalization of the anterior speech zone into at least two different functional systems. They hypothesize that, whereas the frontal operculum, the target of limbic projections, participates in the initiation of verbal communication, the lower half of the precentral gyrus may support the organization of articulatory processes. Since apraxia of speech is usually unaccompanied by significant paresis of the orofacial and laryngeal musculature, a higher‐order deficit of speech motor control in terms of disordered “programming” [Darley et al.,1975] or compromised “timing” [Buckingham,1991] of articulatory sequences has been advanced as the underlying patho‐mechanism [see Riecker et al.,2000]. Based on the clinical findings of impaired speech rhythm in motor aphasia and apraxia of speech, it has also been suggested [Riecker et al.,2002] that the language‐dominant hemisphere supports the temporal coordination of the vocal‐tract musculature prerequisite to the adjustment of syllable durations.
Investigation of the three‐generation KE family, half of whose members are affected by a pronounced verbal dyspraxia, has led to the identification of their core deficit as one involving sequential articulation and orofacial praxis [Watkins et al.,2002a]. In functional imaging studies, abnormalities were found in both cortical and subcortical motor areas in the affected family members during both overt and covert word repetition [Liegeois et al.,2003; Vargha‐Khadem et al.,1998]. Quantitative analyses of MRI scans revealed structural abnormalities in several of these same areas, including the caudate nucleus and putamen, which were found to have abnormal amounts of gray matter bilaterally [Watkins et al.,2002b].
Functional imaging studies in healthy subjects have reported that tasks of speech production activate the left posterior pallidum, anterior insula, and lateral premotor cortex [e.g., Riecker et al.,2000; Wise et al.,1999]. Similar activations in the left hemisphere, in the anterior insular and frontal opercular regions, have been observed in a PET study of word retrieval in which a less practiced state was compared with a well‐practiced and hence more automatic state [Petersen et al.,1998]. PET and fMRI investigations have also documented bilateral responses of the central motor system (i.e., motor cortex, supplementary motor area, and cerebellum) during isochronous repetitive syllable productions [Paus et al.,1996; Riecker et al.,2000; Wildgruber et al.,2001].
It has been suggested that three different processing strategies involving different processing pathways may be used by normal subjects when repeating verbal material: the semantic, the lexical, and the phonological. Clearly, there is a cognitive/semantic component to learning new words or structures, but there is also evidence for a separate system termed the “phonological loop.” This is a part of working memory, a limited‐capacity system that supports both the storage and processing of information: it provides temporary storage for incoming verbal information [e.g., Baddeley,1986; Gathercole et al.,1994,1997]. The phonological loop is thought to contain two components, a phonological store and an articulatory rehearsal mechanism, and to provide temporary storage of speech, while the main working memory in the central executive controls “modality‐free” reasoning, or cognition [Baddeley,1986]. It is believed that repetition of novel stimuli (nonword repetition), which requires the temporary storage of an unfamiliar phonological sequence, relies on the phonological loop, and that success in the task depends on the capacity of the short‐term storage area of the phonological loop [Gathercole et al.,1994]. In the psycholinguistic literature, nonsense stimuli have thus been used frequently because these letter strings offer the possibility to bypass reliance on the semantic system (and hence to be uninfluenced by prior semantic knowledge). Such material allows one to investigate directly the accomplishment of acoustic‐to‐phonological conversion and has been useful with clinical populations. In particular, it has often been found in aphasic patients that words can be repeated while nonwords cannot, suggesting a dissociation between a lexical and a nonlexical repetition route [Kay et al.,1992]. Using a nonword repetition test [Gathercole and Baddeley,1990] that varied in syllable length and in the level of complexity of articulation required, Watkins et al. [2002a] have shown significant impairments in aphasic patients that are related to the level of articulatory difficulty of the stimuli.
The first step in the process of repetition of verbal material is primary auditory analysis. This takes place in the superior temporal gyri and occurs when the subject listens to either words or pseudowords [Zatorre et al.,1996]. This analysis is followed by a process of complex pattern recognition [Demonet et al.,1992; Howard et al.,1992; Wise et al.,1991]. If a subject has previous experience of the verbal material, as in real words, the word is recognized and oral production may then be biased towards lexicosemantic processing and implicit phonological processing. Performance on a nonword repetition task involves the encoding, storage, processing, and reproduction of a novel sequence of speech sounds and is biased towards explicit phonological processing [Castro‐Caldas et al.,1998]. Since familiarity with the articulation patterns characteristic of one's native language aids in the production of unfamiliar words in that language, differences may be expected in the activity in brain regions involved when repeating words and nonwords in L1 and L2. Thorn and Gathercole [2001] demonstrated that subjects perform better on phonological short‐term memory tasks in the language in which they are most proficient, and the aim of the present study was to investigate this at the level of neural representation, using a nonword repetition task with PET, which is well‐suited to tasks that require overt articulation.
In the present experiment, we examined word repetition and repetition of phonologically plausible sequences of phonemes lacking in lexico‐semantic representation to explore the brain regions involved in phonological processing relatively independently of lexico‐semantic processing in bilingual subjects. We used the word lists for word repetition from our original study [Klein et al.,1994], but with a new group of English‐French bilingual volunteers, in order to establish whether our original finding of left putaminal activation for L2 production could be replicated. We also examined under what conditions differences related to articulation in L1 and L2 were evident, by differentiating syllable number from other measures of articulatory difficulty in each language, using a nonword repetition test in French and English. Doyon and Ungerleider [2002] propose a model which suggests that cortico‐striatal and cortico‐cerebellar systems contribute differentially to motor sequence learning; this is most apparent in the contrast between the initial learning of a sequence and the effortless performance seen after repeated practice. In this view, one may therefore hypothesize that different brain regions may be recruited for word and nonword repetition both in the L1 and L2, due to differences in the automaticity of the coordination of movements into a specific sequence for known and unknown words in a language. In addition, one should observe increased activity within these regions for L2 relative to L1.
SUBJECTS AND METHODS
Participants
After a proficiency interview and several prescreening tests, 10 right‐handed volunteers (equal sex distribution, mean age 22 years) were chosen, all of whom had learned English as their native language (L1) but had acquired their L2, French, after the age of 5; the mean age at which learning L2 was initiated was 5.9 years. All subjects were of normal hearing and were tested to ensure that they could identify stimuli presented both monaurally and binaurally. The subjects gave informed consent to participate in this study approved by the Montreal Neurological Institute Review Committee on the Use of Human Subjects. The subjects were all using French in their daily lives and had a high level of proficiency in that language, as measured by their score on naming (Boston naming test: mean score = 77%), and on a nonword repetition test (4‐syllable complex articulation: mean score = 82%). An articulatory fluency test was also administered in which they were required to provide a description of a picture in both their L1 and L2 in a specified time‐period in order to obtain a measure of speech fluency. The resulting participants formed a homogeneous group who were all relatively proficient in their production of speech in their L2.
Procedure
During the experiment the subjects underwent 12 separate 60‐s PET scans to measure rCBF during word and nonword repetition. Before each scan a practice example was given. In each condition stimuli were presented binaurally through insert earphones (Eartone 3A) at a level of approximately 78 dBA at a rate of one trial every 4.2 s, and subjects were required to repeat the item aloud. All the subjects were familiar with the tasks of word and nonword repetition, since they had been selected from a pool of subjects who had previously participated in a behavioral study to ensure that they could perform the tasks. The subjects were instructed to repeat words or pseudowords as follows: “You are going to listen to a list of words or word‐like stimuli presented one at a time that you should repeat. Some of the words are known; others you have never heard. You should repeat the stimuli exactly as you hear them.” There were 22 stimuli in each list, presented in a fixed order to each subject. During testing, their performance in repeating words and pseudowords was recorded, with the number of words and nonwords correctly repeated being calculated for each participant. Each task commenced 30 s before scanning and continued until after the scanning period had finished. Subjects kept their eyes closed for the duration of the scan and lights were kept dimmed throughout.
Word repetition
In one condition, subjects were presented with single English words and were required to repeat the words aloud, and in the second condition the subjects heard and repeated French words. Because these conditions formed part of the broader study of nonword repetition, the two‐word‐repetition conditions were always presented either first or last, counterbalancing for order of language presentation. The lists were matched for frequency of occurrence of the words in the language, word length, part‐of‐speech, and imageability. In order to examine replicability of findings from our previous experiment, the lists for words were the same as those used in the original experiment [Klein et al.,1994,1995]. There were 22 words per list and the lists were relatively well matched for syllable number, with a total number of 47 syllables for the English list and 53 syllables for the French list.
Nonword repetition
There were two factors of interest: the number of syllables (two levels, 2 and 4), and articulation difficulty (two levels, simple nonwords containing single consonants and complex consonant clusters requiring more complex articulation; see Table I). The nonwords requiring simple articulatory output contained only single consonants (e.g., rubid); the nonwords requiring more complex articulation contained consonant clusters (e.g., hampent). There were thus four nonword repetition conditions in English and French: simple‐2syllables, complex‐2syllables, simple‐4syllables, and complex‐4syllables. A silent resting baseline was also performed. During the remaining scan, subjects repeated a two‐syllable word, “mama.” The English nonword stimuli were based on the Gathercole and Baddeley [1996] Test of Nonword Repetition. The French nonword stimuli were constructed by a linguist, Renée Béland (University of Montreal), and were designed to be a parallel version to the English nonword repetition test. The nonwords in both sets conformed to the phonotactic rules and the dominant stress patterns of the language. Pronunciation of the nonwords was adjusted to conform to the phonology of Canadian English and Québequois French. Nonwords were explained as spoken words similar to comprehensible words, but which did not have any meaning, and which the subjects had not previously heard.
Table I.
Nonword repetition conditions
| Scanning conditions | Response | % Correct |
|---|---|---|
| English 2‐syllable, simple | Honish | 95.5 |
| English 2 syllable, complex | Hondest | 95.5 |
| English 4 syllable, simple | Conpantupate | 95.9 |
| English 4 syllable, complex | Conprantuplate | 90.9 |
| French 2 syllable, simple | Guissone | 97.7 |
| French 2 syllable, complex | Glistone | 93.1 |
| French 4 syllable, simple | Pitaturade | 95.5 |
| French 4 syllable, complex | Plitaturade | 82.3 |
PET Scanning
PET scans were obtained with the Siemens Exact HR+ tomograph operating in three‐dimensional mode, using the water‐bolus method and 60‐s scanning periods. T 1‐weighted structural MRI scans (160 1‐mm slices) were also obtained for each subject with a 1.5 T Phillips ACS system to provide anatomical detail. CBF images were reconstructed using an 8.11 mm Hanning filter, normalized for differences in global CBF, and further smoothed such that the resulting FWHM was 14 mm in x,y,z and coregistered with the individual MRI data [Evans et al.,1992]. Each MRI/PET dataset was then linearly resampled into the standardized stereotaxic coordinate system of Talairach and Tournoux [1988] via an automated feature‐matching algorithm [Collins et al.,1994]. The eight scans that formed the nonword repetition experiment were analyzed using analysis of variance (ANOVA) with three main effects: language (English vs. French), articulation difficulty (simple vs. complex), and syllable number (two vs. four). For the word‐repetition scans, comparisons were made between repetition in English and French and between each of these conditions and the baseline scan. PET images were averaged across subjects for each condition, and the mean change image‐volume was obtained for each comparison; this volume was then converted to a t‐statistic map and the significance of focal CBF changes was assessed by a method based on 3D Gaussian random‐field theory [Worsley et al.,1992,1996]. The threshold for reporting a peak as statistically significant was set at 3.53 (P < 0.0002, one‐tailed, uncorrected). Correcting for multiple comparisons, a t‐value of 3.53 yields a false‐positive rate of 0.58, in a search volume of 182 resolution elements (each of which has dimensions 14 × 14 × 14 mm), if the volume of brain gray matter is 500 cm3.
RESULTS
Word Repetition
Subjects were 100% accurate at repeating words in both languages. As expected, the CBF increases were observed bilaterally along the superior temporal gyrus and in the lateral cerebellum, and were also observed unilaterally in the paracingulate gyrus (Table II). The PET results demonstrated that the pattern of CBF was similar across the two languages when compared with a silent baseline (see Table II), except that activity was observed only in the left caudate, putamen, and thalamus for the L2, French, but not for the L1, English. Comparison of CBF when repeating words in L2 (French) with repeating words in L1 (English) revealed greater activity for L2 in several left‐hemisphere speech‐related structures (see Table III, Fig. 1): ventral striatum, caudate nucleus, anterior insula, and ventral premotor cortex.
Table II.
Word repetition minus silent baseline
| Area | L1‐SB (English) | L2‐SB (French) | ||
|---|---|---|---|---|
| x, y, z | t | x, y, z | t | |
| Left hemisphere | ||||
| Paracingulate gyrus (BA 24/32) | −4, 51, −20 | 4.02 | −5, 51, −18 | 3.38 |
| Caudate nucleus (head) | −15, 13, 6 | 2.68 | ||
| Putamen | −28, 13, −8 | 2.61 | ||
| Anterior superior temporal gyrus (BA 22) | −48, 15, −18 | 3.25 | ||
| Precentral gyrus (BA 4) | −52, −9, 26 | 3.57 | ||
| Thalamus | −5, −14, 6 | 3.06 | ||
| Posterior superior temporal gyrus (planum temporale) (BA 42) | −58, −19, 6 | 7.64 | −58, −25, 8 | 8.02 |
| Lateral cerebellum (lobule V) | −16, −59, −20 | 4.03 | −11, −61, −17 | 3.63 |
| Right hemisphere | ||||
| Anterior superior temporal gyrus (BA 22) | 56, 3, −8 | 4.66 | ||
| Posterior superior temporal gyrus (planum temporale) (BA 42) | 55, −19, 6 | 7.29 | 55, −21, 6 | 8.05 |
| Lateral cerebellum (lobule V/VI) | 17, −61, −18 | 3.52 | 20, −57, −20 | 4.39 |
Activation foci in these tables represent peaks of statistically significant increases in normalized CBF for each subtraction of a silent baseline from the word repetition task. x, medial‐lateral distance relative to the midline (positive = right); y, anterior‐posterior distance relative to the anterior commissure (positive = anterior); z is the superior‐inferior distance relative to the anterior commissure line (posterior = superior). Note: The anatomical region reported refers to the position of the peak based on the merged registration image of the PET and MRI. Brodmann's areas based on the atlas of Talairach and Tournoux [1988], and categorization of the cerebellum is based on the atlas of Schmahmann et al. [1999]
Table III.
Word repetition: comparison of L1 and L2
| L2>L1 | x, y, z | t |
|---|---|---|
| Left hemisphere | ||
| Ventral striatum | −11, 5, −6 | 3.8 |
| Insula | −32, 12, 3 | 3.5 |
| Caudate nucleus (head) | −13, 13, 8 | 3.4 |
| Ventral premotor (BA 6) | −50, −1, 27 | 3.1 |
| Hippocampus (head) | −27, −13, −20 | 3.8 |
| L1>L2 | ||
| Left hemisphere | ||
| Temporal pole (BA 22/21) | −36, 1, −27 | 4.8 |
| Mid‐superior temporal sulcus (BA 22/21) | −47, −11, −20 | 5.7 |
| Right hemisphere | ||
| Posterior superior frontal sulcus (BA 6/8) | 31, −2, 53 | 4.2 |
Figure 1.
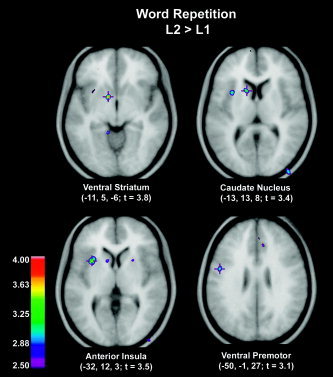
Word repetition. Positron emission tomography (PET) subtraction images showing mean average cerebral blood flow (CBF) increases for the direct comparison of L2 vs. L1 word repetition in the group English‐French bilinguals superimposed on the mean average magnetic resonance image (MRI). For all figures, the range of t‐values for the PET data is coded by the color scale, and the left hemisphere is on the left side in all horizontal sections. Visible in Figure 1 are ventral striatum, caudate nucleus, insular cortex, premotor region.
Nonword Repetition
Nonword repetition accuracy was near ceiling in most conditions. Performance was worse for higher syllable number and for more complex patterns of articulation. Performance was similar across languages (see Table I).
The main effect of language (L2 > L1) was increased activation of the cerebellum (lateral cerebellum and vermal zone, lobule V), the central opercular cortex bilaterally (probably primary motor representation of the tongue and other articulators), and left ventral premotor cortex (Table IV, Fig. 2). No significant increases were observed in the basal ganglia during nonword repetition in L2 as compared to L1. The main effect of articulatory complexity (complex > simple) was increased activation of the cerebellum (vermal zone, lobule III; lobules V/VI) and left thalamus (Table V, Fig. 3). The main effect of syllable number (4 > 2) was increased activation of superior temporal cortex bilaterally, cerebellum bilaterally (vermal zone, lobules V/VI), and left ventral premotor cortex extending to the fronto‐central operculum (posterior to the frontal operculum and at the inferior extent of the central sulcus) (Table VI, Fig. 4).
Table IV.
Nonword repetition: comparison of L1 and L2 (L2>L1) main effect of language
| x, y, z | t | |
|---|---|---|
| Left hemisphere | ||
| Ventral premotor (BA6) | −55, −4, 26 | 4.4 |
| Central operculum (BA 43/4/6) | −51, −4, 11 | 4.5 |
| Cerebellar (vermal zone, lobule V) | −1, −59, −14 | 5.7 |
| Lateral Cerebellum | −16, −59, −20 | 5.2 |
| Right hemisphere | ||
| Supplementary motor area (BA 6) | 1, −6, 62 | 3.8 |
| Central operculum (BA43/4/6) | 47, −7, 11 | 4.5 |
| Posterior superior temporal gyrus (planum temporale) (BA 42) | 56, −28, 9 | 4.0 |
Figure 2.

Nonword repetition. CBF changes for ANOVA comparison of language (L1 English vs. French). Regions significantly more activated in L2 French relative to English included the cerebellum, left ventral premotor cortex, and central operculum bilaterally.
Table V.
Nonword repetition: main effect of articulation difficulty
| x, y, z | t | |
|---|---|---|
| Left hemisphere | ||
| Thalamus | −15, −11, 9 | 3.7 |
| Cerebellum (vermal zone, lobule III) | −3, −50, −21 | 4.4 |
| Right hemisphere | ||
| Cerebellum (lobule V/VI) | 28, −52, −21 | 3.9 |
Figure 3.

Nonword repetition. CBF changes for ANOVA comparison of articulation difficulty (simple vs. complex). Regions significantly more activated in complex articulation relative to simple included the cerebellum and left thalamus.
Table VI.
Nonword repetition: main effect of syllable number
| x, y, z | t | |
|---|---|---|
| Left hemisphere | ||
| Ventral premotor (BA6) | −56, −2, 18 | 3.5 |
| Central operculum (BA43/4/6) | −56, −4, 3 | 4.4 |
| Precentral gyrus (BA4) | −48, −13, 51 | 3.6 |
| Posterior superior temporal gyrus (planum temporale) (BA42) | −55, −21, 6 | 5.7 |
| Parietal operculum (medial) (BA 40) | −43, −33, 21 | 3.7 |
| Cerebellum (lobule V) | −11, −56, −12 | 4.1 |
| Right hemisphere | ||
| Anterior insula/frontal operculum | 39, 17, 8 | 4.6 |
| Lateral posterior superior temporal gyrus (planum temporale) (BA42) | 62, −14, 3 | 5.8 |
| Medial posterior superior temporal gyrus (planum temporale) (BA42) | 47, −26, 8 | 4.2 |
| Cerebellum (lobule V/VI) | 16, −61, −20 | 4.1 |
Figure 4.
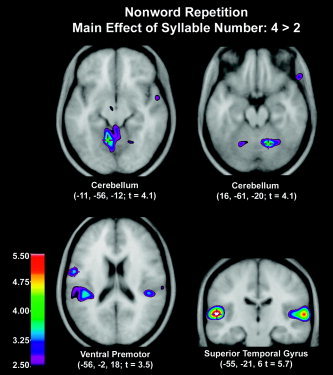
Nonword repetition. CBF changes for ANOVA comparison of number of syllables. Regions significantly more activated in four syllables relative to two syllables included the superior temporal cortex bilaterally, cerebellum, and left ventral premotor cortex.
DISCUSSION
We explored the neural substrates involved in the production of L2 learned after the age of 5 as compared with those brain regions involved in the repetition of a native language. In the present study, speakers who learned an L2 after age 5 activated the left ventral premotor area in both word and nonword repetition in the L2 to a greater extent than in the L1. Activation of the premotor cortex (lateral BA6) was observed in similar locations during both word and nonword repetition. The consistency of the ventral premotor cortex activation supports the hypothesis [Alexander et al.,1990] that this region plays an important role in the programming of articulatory processes and is in accordance with clinical observations of deficient articulatory performance after damage to the premotor cortex [Alexander et al.,1989].
Comparison of repeating words in L2 relative to L1 revealed several regions of significant CBF change, especially in the left insula and striatum. These results partially replicate the original findings, supporting the idea that the increased articulatory demands imposed when speaking the L2 (French) may require more complex motor control for speech production in L2, and therefore involve subcortical motor structures (although the peaks in the basal ganglia are somewhat distant from the peak in the left putamen observed in the original study in response to repeating words in the L2). The asymmetry of activation at the level of the basal ganglia towards the left putamen is in accordance with clinical observations of articulatory impairment after left‐sided subcortical infarction or hemorrhage [Alexander et al.,1987], as well as with neuroimaging studies investigating repetition of single words [Wise et al.,1999; Wildgruber et al.,2000].
To examine the brain regions involved in articulation in L1 and L2, removing the lexical component and manipulating articulatory complexity, the same subjects performed nonword repetition tasks. It is of interest that repeating nonwords showed involvement of a cerebellar‐cortical network in response to increasing demands (in L2 relative to L1, with increasing articulatory difficulty, or with increasing number of syllables). The degree to which different brain regions are activated may be influenced by the meaningfulness of the stimuli to be articulated or may be due to the greater articulatory challenge of repeating novel patterns of sounds. In line with previous work [Wildgruber et al.,2001; Riecker et al.,2002], we observed bilateral cerebellar activation with increasing articulatory complexity and with increasing number of syllables (Figs. 3, 4). Possibly, in this instance, the L2 (French) has more complex articulatory requirements than the L1 (English), posing more demands on articulatory control or on timing. The left and the right motor cortex and the cerebellar hemispheres are interconnected by crossed pathways. Clinical data indicate that the interaction of the anterior language zone and the cerebellum might support the specification of durational parameters of speech utterances [Ackermann and Hertrich,1997; Riecker et al.,2002]. The results also support Doyon et al.'s model [2003] of distinct cortico‐striatal and cortico‐cerebellar systems each associated with motor learning, which can be extended here to learning the articulatory patterns of a language. The consistency of the activation of the cerebellum in all these nonword conditions points to a specific cerebellar contribution to speech motor control, depending on task difficulty and stimulus novelty. The findings suggest that increased articulatory demands imposed when producing novel sequences activate a cerebellar network, and to a greater extent in L2, which requires more complex motor control for speech production. In addition, the increased activity in the fronto‐striatal regions in response to repeating words in the L2 indicates that additional neural processes within these regions are required for production in L2 as compared to L1. Future studies with bilingual subjects who range more widely in proficiency could correlate functional activations with the speakers' language proficiency and daily usage in L1 and L2.
The findings of this study are in general agreement with the hypothesis that experience through learning partly determines the development and organization of the human brain and, in particular, that language experience influences the functional organization of language‐relevant systems [Neville et al.,1998; Snowling et al.,1997]. One aspect of young children's ability to acquire new languages with ease is that they can quickly become proficient in the accent of the new language [Long,1990], and children display a remarkable capacity to adjust to the segmental and nonsegmental features of different accents.
This interpretation is supported in the present study in our later‐acquired L2 English‐French bilinguals inasmuch as several regions of significant bloodflow change were observed in the cortico‐striatal network, most specifically when these subjects were speaking French words, their L2. However, these differences could plausibly be related to language‐specific differences even for language pairs that are structurally more rather than less similar. In this, and in our earlier study [Klein et al.,1994], the increased activation in the left lenticular region for L2 production has been interpreted in terms of a greater role of the left basal ganglia in the coordination of speech articulation, and that in order to guarantee sufficiently precise timing, these brain structures might be recruited more in the L2 than in the L1. However, differences between L1 and L2 in these studies could reduce to language‐specific effects, as this finding was not replicated in another of our studies in Chinese‐English speakers, even though these subjects had strong foreign accents in their L2 [Klein et al.,1999]. Other studies also point to language‐specific variables even when the languages in question are structurally not too dissimilar, e.g., German‐English [Price et al.,1999], Catalan‐Spanish [Perani et al.,1996]. To tease apart the confounding effects of language‐specific and language‐acquisitional variables, future studies will need to examine real‐word repetition and nonword repetition in English‐French and in French‐English bilingual subjects.
Acknowledgements
Renée Béland developed the French nonword stimuli. Sylvain Milot provided technical assistance. Jen‐Kai Chen assisted with image preparation.
REFERENCES
- Ackermann H, Hertrich I (1997): Voice onset time in ataxic dysarthria. Brain Lang 56: 321–333. [DOI] [PubMed] [Google Scholar]
- Aglioti F, Fabbro S (1993): Paradoxical selective recovery in a bilingual aphasic following subcortical lesions. Neuroreport 30: 1359–1362. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Naeser MA, Palumbo CL (1987): Correlation of subcortical CT lesion sites and aphasia profiles. Brain 110: 961–991. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Benson DF, Stuss DT (1989): Frontal lobes and language. Brain Lang 37: 656–691. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Naeser MA, Palumbo C (1990): Broca's area aphasias: aphasia after lesions including the frontal operculum. Neurology 40: 353–362. [DOI] [PubMed] [Google Scholar]
- Baddeley AD (1986): Working memory. Oxford: Oxford University Press. [Google Scholar]
- Best CT (1994): The emergence of native‐language phonological influences in infants: a perceptual assimilation model In: Goodman JC, Nusbaum HC, editors. The development of speech perception: the transition from speech sounds to spoken words. Cambridge, MA: MIT Press; p 167–224. [Google Scholar]
- Best CT, McRoberts GW, Sithole NM (1998): Examination of perceptual reorganization for nonnative speech contrast: Zulu click discrimination by English‐speaking adults and infants. J Exp Psychol Hum Percept Perform 14: 345–360. [DOI] [PubMed] [Google Scholar]
- Birdsong D (1999): Second language acquisition and the critical period hypothesis. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Blumstein SE, Alexander MP, Ryalls JH, Katz W, Dworetzky B. (1987): On the nature of the foreign accent syndrome: a case study. Brain Lang 31: 215–244. [DOI] [PubMed] [Google Scholar]
- Buckingham HW Jr (1991): Explanations for the concept of apraxia of speech In: Sarno T, editor. Acquired aphasia, 2nd ed. New York: Academic Press; p 271–312. [Google Scholar]
- Castro‐Caldas A, Petersson KM, Reis A, Stone‐Elander S, Ingvar M (1998): The illiterate brain. Learning to read and write during childhood influences the functional organization of the adult brain. Brain 121: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairac space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR (1975): Motor speech disorders. Philadelphia: WB Saunders. [Google Scholar]
- De Groot AMB, Kroll JF. (eds.) (1997): Tutorials in bilingualism: psycholinguistic perspectives. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Démonet JF, Chollet F, Ramsay S et al. (1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- Doyon J, Ungerleider LG (2002): Functional anatomy of motor skill learning In: Squire LR, Schacter DL, editors. Neuropsychology of memory. New York: Guilford Press. [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41: 252–262. [DOI] [PubMed] [Google Scholar]
- Dronkers NF (1996): A new brain region for coordinating speech articulation. Nature 384: 159–161. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bab D (1992): Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage 1: 43–53. [DOI] [PubMed] [Google Scholar]
- Flege JE, Yeni‐Komshian GH, Liu S (1999): Age constraints on second‐language acquisition. J Mem Lang 41: 78–104. [Google Scholar]
- Gathercole SE, Baddeley AD (1990): Phonological memory deficits in language disordered children: is there a causal connection? J Mem Lang 29: 336–360. [Google Scholar]
- Gathercole SE, Baddeley AD (1996): The children's test of nonword repetition. London: Psychological Corp. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Willis CS, Baddeley AD, Emslie H (1994): The children's test of nonword repetition: a test of phonological working memory. Memory 2: 103–127. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Hitch GJ, Service E, Martin AJ (1997): Phonological short‐term memory and new word learning in children. Dev Psychol 33: 966–979. [DOI] [PubMed] [Google Scholar]
- Graff‐Radford NR, Cooper WE, Colsher PL, Damasio AR (1986): An unlearned foreign “accent” in a patient with aphasia. Brain Lang 28: 86–94. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Bessell NJ, Bladon RA, Bamford JM (1988): A case of foreign accent syndrome, with follow‐up clinical, neuropsychological and phonetic descriptions. Neuropsychologia 26: 237–251. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R et al. (1992): The cortical localization of the lexicons: positron emission tomography evidence. Brain 115: 1769–1782. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL (1989): Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cognit Psychol 21: 60–99. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW (1999): How infants begin to extract words from speech. Trends Cogn Sci 3: 323–327. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Friederici AD, Wessels JMI, Svenkerud VY, Jusczyk AM (1993): Infants' sensitivity to the sound patterns of native language words. J Mem Lang 32: 402–420. [Google Scholar]
- Kay J, Lesser R, Coltheart M (1992): Palpa (psycholinguistic assessments of language processing in aphasia). LEA England. [Google Scholar]
- Klein D, Zatorre RJ, Milner B, Meyer E, Evans AC (1994): Left putaminal activation when speaking a second language: evidence from PET. Neuroreport 5: 2295–2297. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC (1995): The neural substrates underlying word generation: a bilingual functional‐imaging study. Proc Natl Acad Sci U S A 92: 2899–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Zhao V, Nikelski J (1999): Cerebral organization in bilinguals: a PET study of Chinese‐English verb generation. Neuroreport 10: 2841–2846. [DOI] [PubMed] [Google Scholar]
- Kuhl PK (1998): The development of speech and language In: Carew TC, Menzel R, Shatz CJ, editors. Mechanistic relationships between development and learning. New York: John Wiley & Sons; p 53–73. [Google Scholar]
- Kuhl, PK , Williams KA, Lacerda F, Stevens KN, Lindblom B (1992): Linguistic experience alters phonetic perception in infants by six months of age. Science 255: 606–608. [DOI] [PubMed] [Google Scholar]
- Kurowski KM, Blumstein SE, Alexander M (1996): The foreign accent syndrome: a reconsideration. Brain Lang 54: 1–25. [DOI] [PubMed] [Google Scholar]
- Lenneberg EH (1967): Biological foundations of language. New York: John Wiley & Sons. [Google Scholar]
- Liegeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha‐Khadem F (2003): Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci 6: 1230–1237. [DOI] [PubMed] [Google Scholar]
- Long M (1990): Maturational constraints on language development. Stud Sec Lang Acquisit 12: 251–285. [Google Scholar]
- Marie P (1906, 1971): The third left frontal convolution pays no special role in the function of language. Semaine Méd 26: 241–247. Reprinted in Pierre Marie's Papers on Speech Disorders. Cole MF, Cole M, editors. New York: Hafner. [Google Scholar]
- Neville HJ, Mills DL, Lawson DS (1992): Fractionating language: different neural subsystems with different sensitive periods. Cereb Cortex 2: 244–258. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Bavelier D, Corina D, Rauschecker J, Karni A, Lalwani A, Braun A, Clark V, Jezzard P, Turner R (1998): Cerebral organization for language in deaf and hearing subjects: biological constraints and effects of experience. Proc Natl Acad Sci U S A 95: 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Perry DW, Zatorre RJ, Worsley KJ, Evans AC (1996): Modulation of cerebral blood flow in the human auditory cortex during speech: role of motor‐to‐sensory discharges. Eur J Neurosci 8: 2236–2246. [DOI] [PubMed] [Google Scholar]
- Perani D, Dehaene S, Grassi F, Cohen L, Cappa SF, Dupoux E, Fazio F, Mehler J (1996): Brain processing of native and foreign languages. Neuroreport 7: 2439–2444. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Polka L, Werker JF (1994): Developmental changes in perception of nonnative vowel contrasts. J Exp Psychol Hum Percept Perform 20: 421–435. [DOI] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R (1999): A functional imaging study of translation and language switching. Brain 122: 2221–2235. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W (2000): Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11: 1997–2000. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Dogil G, Grodd W, Ackermann H (2002): Hemispheric lateralization effects of rhythm implementation during syllable repetitions: an fMRI study. Neuroimage 16: 169–176. [DOI] [PubMed] [Google Scholar]
- Ritchie WC, Bhatia TK. (eds.) (1996): Handbook of second language acquisition. San Diego: Academic Press. [Google Scholar]
- Schirmer A (2004): Timing speech: a review of lesion and neuroimaging findings. Cogn Brain Res 21: 269–87. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M (1999): Three‐dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10: 233–60. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Hulme C, Nation K (1997): A connectionist perspective on the development of reading skills in children. Trends Cogn Sci 1: 88–91. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Thorn ASC, Gathercole SE (2001): Language differences in verbal short‐term memory do not exclusively originate in the process of subvocal rehearsal. Psychon Bull Rev 8: 357–364. [DOI] [PubMed] [Google Scholar]
- Trehub SE (1976): The discrimination of foreign speech contrasts by infants and adults. Child Dev 47: 466–472. [Google Scholar]
- Vargha‐Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE (1998): Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A 95: 12695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha‐Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RS, Mishkin M, Gadian DG (2002b): MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain 125: 465–478. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha‐Khadem F (2002a): Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125: 451–63. [DOI] [PubMed] [Google Scholar]
- Weber‐Fox CM, Neville HJ (1996): Maturational constraints on functional specializations for language processing: ERP and behavioral evidence in bilingual speakers. J Cogn Neurosci 8: 231–256. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC (1984): Cross‐language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behav Dev 7: 49–63. [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W (2001): Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. Neuroimage 13: 101–109. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wise R, Greene J, Buchel C, Scott S (1999): Brain regions involved in articulation. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelan P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zatorre R, Meyer E, Gjedde A, Evans A (1996): PET studies of phonetic processing of speech: review, replication and reanalysis. Cereb Cortex 6: 21–30. [DOI] [PubMed] [Google Scholar]


