Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the progressive and simultaneous degeneration of upper and lower motor neurons. The pathological process associated to ALS, albeit more pronounced in the motor/premotor cortices and along the corticospinal tracts (CST), does not spare extra‐motor brain gray (GM) and white (WM) matter structures. However, it remains unclear whether such extra‐motor cerebral abnormalities occur with mildly disabling disease, and how irreversible tissue loss and intrinsic tissue damage are interrelated. To this end, we used an optimized version of voxel‐based morphometry (VBM) analysis to investigate the patterns of regional GM density changes and to quantify GM and WM diffusivity alterations of the entire brain from mildly disabled patients with ALS. A high‐resolution T1‐weighted 3D magnetization‐prepared rapid acquisition gradient echo and a pulsed gradient spin–echo single shot echo–planar sequence of the brain were acquired from 25 mildly disabled patients with ALS and 18 matched healthy controls. An analysis of covariance was used to compare volumetry and diffusivity measurements between patients and controls. Compared with controls, ALS patients had significant clusters of locally reduced GM density (P < 0.001) in the right premotor cortex, left inferior frontal gyrus (IFG), and superior temporal gyrus (STG), bilaterally. In ALS patients contrasted to controls, we also found significant clusters of locally increased MD (P < 0.001) in the splenium of the corpus callosum and in the WM adjacent to the IFG, STG, and middle temporal gyrus (MTG) of the right hemisphere, and in the WM adjacent to the MTG and lingual gyrus in the left hemisphere. Compared with controls, ALS patients also had significant clusters of locally decreased FA values (P < 0.001) in the CST in the midbrain and corpus callosum, bilaterally. This study supports the notion that ALS is a multisystem disorder and suggests that extra‐motor involvement may be an early feature of the disease. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: amyotrophic lateral sclerosis, diffusion tensor MRI, atrophy, voxel‐based morphometry, neurodegenerative disorders
INTRODUCTION
In agreement with the “classical” pathological description of amyotrophic lateral sclerosis (ALS) as a neurodegenerative disorder characterized by the progressive and simultaneous degeneration of upper and lower motor neurons [Hughes, 1982], several studies, using a variety of conventional magnetic resonance imaging (cMRI) sequences, have shown signal intensity changes along the corticospinal tracts (CST) [Abe et al., 1997a; Cheung et al., 1995; Goodin et al., 1988; Hecht et al., 2001, 2002; Ishikawa et al., 1993; Mirowitz et al., 1989; Oba et al., 1993; Thorpe et al., 1996; Waragai, 1997] and in the precentral gyrus [Cheung et al., 1995; Hecht et al., 2002; Ishikawa et al., 1993; Oba et al., 1993; Thorpe et al., 1996; Waragai, 1997]. This view has also been confirmed by the application of modern quantitative MR techniques for the assessment of tissue damage in patients with ALS. Proton MR spectroscopy showed a reduction of the N‐acetylaspartate peak in the motor cortex [Bowen et al., 2000; Ellis et al., 1998, 2001], whereas magnetization transfer (MT) MRI [Kato et al., 1997; Tanabe et al., 1998] and diffusion tensor (DT) MRI [Abe et al., 2004; Ciccarelli et al., 2006; Cosottini et al., 2005; Ellis et al., 1999; Graham et al., 2004; Sach et al., 2004; Toosy et al., 2003] confirmed the presence of intrinsic tissue damage to the CST of these patients.
Recently, several pieces of evidence from neuropsychological [Lomen‐Hoerth et al., 2003; Massman et al., 1996; Ringholz et al., 2005; Strong et al., 2003; Wilson et al., 2001; Yoshida, 2004] and neuroimaging [Abe et al., 1997b, 2001; Abrahams et al., 1996, 1997, 2000, 2004, 2005; Chang et al., 2005; Ellis et al., 2001; Kato et al., 1993; Kiernan and Hudson, 1994; Mantovan et al., 2003; Rule et al., 2004] studies indicate that the pathological process associated to ALS, albeit more pronounced in the motor/premotor cortices and along the CST, does not spare other brain gray (GM) and white (WM) matter structures, especially in the frontal lobes [Abrahams et al., 1996, 1997, 2000, 2004, 2005; Kiernan and Hudson, 1994]. While this suggests that ALS is a multisystem disorder, it remains unclear whether such extra‐motor cerebral abnormalities occur with mildly disabling disease and how irreversible tissue loss and intrinsic tissue damage are interrelated. Against this background, we used voxel‐based morphometry (VBM) analysis, a fully automated and unbiased method which allows to obtain a comprehensive characterization of GM and WM changes on a voxel by voxel basis [Ashburner and Friston, 2000; Good et al., 2001], to investigate the patterns of regional GM density changes and to quantify GM and WM diffusivity alterations from the entire brain of mildly disabled patients with ALS.
PATIENTS AND METHODS
Patients
We recruited 25 patients [14 men and 11 women, mean age = 54.1 years (range = 27–75 years), mean disease duration = 39 months (range = 6–58 months)] with probable or definite ALS, according to the El Escorial criteria [Brooks, 1994], and mild disability, defined as a score equal to or greater than 20 at the ALS Functional Rating Scale (ALSFRS) [The Amyotrophic Lateral Sclerosis Functional Rating Scale, 1996]. Six patients had a bulbar‐onset and 19 patients had a limb‐onset disease. None of the patients had clinical evidence of frontotemporal dementia. Eighteen sex‐ and age‐matched healthy subjects [11 men and 7 women, mean age = 52.2 years (range = 27–72 years)] served as controls. A single experienced neurologist, unaware of the MRI results, administered to all patients the ALSFRS questionnaire [The Amyotrophic Lateral Sclerosis Functional Rating Scale, 1996] within 48 h from acquisition of the MR images. Mean ALSFRS score was 29 (range = 21–38, SD = 4.3). Local Ethical Committee approval and written informed consent from all subjects were obtained prior to study initiation.
MRI Acquisition
Using a magnet operating at 1.5 Tesla (Magnetom Avanto, Siemens, Erlangen, Germany), the following sequences were obtained from the brain of all subjects: (a) dual‐echo (DE) turbo spin echo (TSE) [TR = 3,460, TE = 27/109, echo train length (ETL) = 5, field of view (FOV) = 250 × 250 mm2, matrix size = 512 × 512, 35 contiguous, 4‐mm thick, axial slices); (b) T2‐weighted TSE (TR = 3,460, TE = 109, ETL = 13, number of averages = 2, FOV = 240 × 180 mm2, matrix size = 240 × 320, 24 coronal, 4‐mm thick slices with a distance factor of 30%); (c) sagittal 3D‐T1‐weighted magnetization prepared rapid acquisition gradient echo (MP‐RAGE) (TR = 2,000, TE = 3.93, flip angle (α) = 12°, FOV = 270 × 270 mm2, matrix size = 256 × 256, voxel size = 0.9 × 0.5 × 0.5 mm3, slab thickness = 187.2 mm); (d) pulsed gradient SE single shot echo–planar (PGSE‐SS‐EPI) (TR = 2,900, TE = 84, α = 90°, FOV = 240 × 240 mm2, matrix size = 128 × 128, nominal pixel size = 1.87 × 1.87 mm2, inter‐echo spacing = 0.77 ms, 18 contiguous, 4‐mm thick, axial slices), with diffusion‐encoding gradients applied in 12 non‐collinear directions, coded as default in the scanner. The maximum b factor in each direction was set to 900 s/mm2 and only two b factors were used (b 1 = 0, b 2 = 900 s/mm2). The maximum amplitude of the diffusion gradients was 33 mT/m and a multiple channels head coil was used for signal reception. Two averages were acquired to improve SNR, with no parallel acquisition. The central slice of this sequence was positioned to match exactly the central slice of the DE set.
MRI Post‐Processing
All the structural MRI analysis was performed by two experienced observers by consensus, unaware of subjects' identity. Axial DE and coronal T2‐weighted images were analyzed to assess the presence and location of areas with increased signal intensity. Volumetry measurements were performed using an optimized VBM approach, as described by Ashburner and Friston [2000] and Good et al. [2001], on the 3D‐T1‐weighted MP‐RAGE images, using the statistical parametric mapping (SPM2) software [Friston et al., 1995]. Full details of the steps involved in the optimized method of VBM analysis have been presented extensively elsewhere [Ashburner and Friston, 2000; Good et al., 2001]. Briefly, a customized T1 template, together with the corresponding probability maps of GM, WM, and cerebrospinal fluid (CSF), were first created using MP‐RAGE scans of both healthy controls and patients. This procedure involved spatial normalization of the original images to the standard SPM T1 template, segmentation into WM and GM, averaging of the images, and smoothing with an 8‐mm FWHM Gaussian kernel. Then, the same MP‐RAGE data were segmented and normalized to the customized template using the GM tissue maps driving this transformation. After a new segmentation step in the SPM steoreotaxic space, the GM normalized maps obtained were modulated to incorporate the point‐wise volume expansion/contraction induced by the transformation [Ashburner and Friston, 2000] and smoothed with a 12 mm3 FWHM Gaussian kernel.
PGSE‐SS‐EPI images were corrected for distortion induced by eddy currents and mean diffusivity (MD) and fractional anisotropy (FA) derived for every pixel, as previously described [Rovaris et al., 2005]. MD and FA maps were then normalized into a standard space. Using SPM2, a rigid transformation was calculated between the nondiffusion weighted images and the T2‐weighted images. Then, the T2‐weighted images were normalized to the SPM T2‐weighted atlas. Finally, these two transformations were applied to MD and FA maps. This procedure was applied because of the better brain coverage of the DE scan, which allows a more accurate estimation of the transformation. A customized FA atlas was obtained by averaging the transformed FA maps of both healthy controls and patients. Then, using SPM2, a nonlinear transformation was calculated between the customized FA atlas and the FA maps and applied to MD maps as well. Finally, the normalized FA and MD maps were smoothed with a 12‐mm3 FWHM Gaussian kernel. Average MD and FA were also calculated in those WM areas of the original maps from patients and controls, using regions of interest (ROIs) of 17 mm2 in size, where VBM analysis showed significant clusters of locally abnormal MD and FA values in ALS patients.
Statistical Analysis
An analysis of covariance (ANCOVA) was used to compare volumetry and diffusivity measurements between patients and controls and between patients with and without hyperintense lesions on cMRI scans. Age and intracranial volume were included as nuisance covariates for the GM volume comparisons, whereas age only was used as a nuisance covariate when comparing DT MRI metrics. According to previous reports [Abe et al., 2004; Kassubek et al., 2005] and to a priori hypothesis based on available studies [Chang et al., 2005; Sach et al., 2004], the significance threshold for these comparisons was set at P < 0.001 (uncorrected for multiple comparisons). Since such an uncorrected threshold might result in false positive differences, for those areas which passed this threshold, a small volume correction (SVC) for multiple comparisons was then applied, setting the cut off value for significance at P < 0.05 and using a 10‐mm radius. Only those areas that passed this additional correction are reported. A two‐tailed Student's t test for not‐paired data was used to compare the average MD and FA values of WM ROIs between patients and controls. To assess the correlations between volumetry and diffusivity abnormalities with clinical findings (ALSFRS and disease duration), the corresponding metrics were entered into the SPM design matrix, using basic models and linear regression analysis.
RESULTS
No abnormalities were seen on conventional MRI scans obtained from all control subjects. On the DE scans of the brain from ALS patients, hyperintesities of the CST were detected bilaterally in 14 of them (56%). The regional distribution of such abnormalities was as follows: subcortical precentral gyrus, 3 patients; centrum semiovale, 10 patients; posterior limb of internal capsule, 12 patients; cerebral peduncles, 14 patients; pons, 3 patients; and pyramids, 3 patients.
When ALS patients were compared with controls, significant clusters of locally reduced GM density were found in the right precentral gyrus (SPM coordinates: 45, −12, 38), left inferior frontal gyrus (IFG) (SPM coordinates: −54, 21, 4), and superior temporal gyrus (STG), bilaterally (SPM coordinates: 31, 19, −25, on the right; −26, 23, −34, on the left) (Table I) (Fig. 1). No difference in GM density was found between ALS patients with and without areas of increased signal intensity on cMRI scans (data not shown).
Table I.
Regions of significantly reduced GM density in ALS patients compared to healthy controls
| Anatomical regions | Side | Brodmann areas | SPM space coordinates (x, y, z) | T values |
|---|---|---|---|---|
| Precentral gyrus | Right | 6 | 45, −12, 38 | 4.06 |
| Superior temporal gyrus | Right | 38 | 31, 19, −25 | 3.69 |
| Inferior frontal gyrus | Left | 47 | −54, 21, 4 | 4.12 |
| Superior temporal gyrus | Left | 38 | −26, 23, −34 | 3.45 |
P < 0.001, uncorrected for multiple comparisons; P < 0.05, after small volume correction. See the text for further details.
Figure 1.
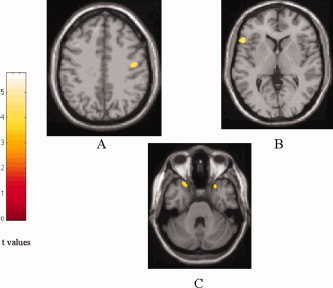
SPM regions with decreased GM density in patients with ALS contrasted to healthy controls (P < 0.001, uncorrected for multiple comparisons; P < 0.05, after SVC): (A) right precentral gyrus, (B) left IFG, and (C) bilateral STG. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Compared with controls, ALS patients had significant clusters of locally increased MD in the splenium of the corpus callosum (SPM coordinates: 16, −26, 32), in the WM adjacent to the IFG (SPM coordinates: 42, 4, 26), STG and middle temporal gyrus (MTG) (SPM coordinates: 54, −16, −4, and 48, −2, −22, respectively) of the right hemisphere, and in the WM adjacent to the MTG (SPM coordinates: −52, −46, 8, and −34, 2, −26) and lingual gyrus (SPM coordinates: −14, −98, −12) of the left hemisphere (Table II) (Fig. 2). No difference in MD was found between ALS patients with and without areas of increased signal intensity on cMRI scans (data not shown).
Table II.
White matter regions with significantly increased mean diffusivity in ALS patients compared to healthy controls
| Anatomical regions | Side | SPM space coordinates (x, y, z) | t values |
|---|---|---|---|
| Corpus callosum (splenium) | Right | 16, −26, 32 | 3.86 |
| Inferior frontal gyrus | Right | 42, 4, 26 | 3.60 |
| Superior temporal gyrus | Right | 54, −16, −4 | 3.83 |
| Middle temporal gyrus | Right | 48, −2, −22 | 4.11 |
| Middle temporal gyrus | Left | −52, −46, 8 | 3.43 |
| Middle temporal gyrus | Left | −34, 2, −26 | 3.32 |
| Lingual gyrus | Left | −14, −98, −12 | 3.72 |
P < 0.001, uncorrected for multiple comparisons; P < 0.05, after small volume correction.
Figure 2.
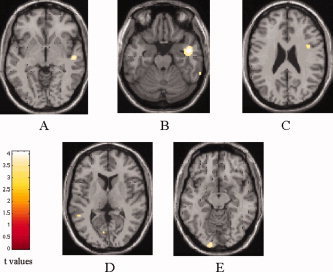
SPM regions with increased mean diffusivity (MD) in patients with ALS contrasted to healthy controls (P < 0.001, uncorrected for multiple comparisons; P < 0.05, after SVC): right STG (A), right (B) and left (D) medial temporal gyrus, right IFG (C) and left lingual gyrus (E). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Compared with controls, ALS patients also had significant clusters of locally decreased FA values in the midbrain portion of the CST (SPM coordinates: 14, −12, −10 [right] and −12, −20, −14 [left]) and in the body of the corpus callosum, bilaterally (SPM coordinates: 20, −20, 26 [right] and −18, −22, 28 [left]) (Table III) (Fig. 3). When ALS patients with hyperintense lesions on brain DE scans were compared with ALS patients without, significant clusters of locally decreased FA values were found in the posterior limb of the internal capsule, bilaterally (SPM coordinates: 10, 6, −2 [right] and −16, 8, −6 [left]; t values: 4.46 [right] and 3.52 [left]).
Table III.
White matter regions with significantly decreased fractional anisotropy in ALS patients compared to healthy controls
| Anatomical regions | Side | SPM space coordinates (x, y, z) | t values |
|---|---|---|---|
| Corticospinal tract in the midbrain | Right | 14, −12, −10 | 3.47 |
| Corticospinal tract in the midbrain | Left | −12, −20, −14 | 4.49 |
| Corpus callosum (corpus) | Right | 20, −20, 26 | 4.59 |
| Corpus callosum (corpus) | Left | −18, −22, 28 | 3.61 |
P < 0.001, uncorrected for multiple comparisons; P < 0.05, after small volume correction. See the text for further details.
Figure 3.
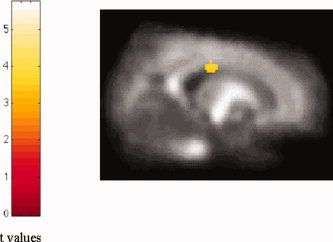
Voxels with decreased fractional anisotropy (FA) in the corpus callosum from in patients with ALS contrasted to healthy controls (P < 0.001, uncorrected for multiple comparisons; P < 0.05, after SVC). Note that, in this case, the region has been superimposed on a customized FA atlas, normalized into standard SPM space. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Compared to controls, ALS patients had significantly increased MD and significantly decreased FA values in all ROIs located in those WM areas where VBM analysis showed diffusivity changes between the two groups (Table IV). No significant correlations were found between regions of decreased GM density or GM and WM diffusivity changes and ALSFRS and disease duration.
Table IV.
Mean diffusivity and fractional anisotropy values of regions of interest located in those white matter areas where VBM analysis showed diffusivity changes between ALS patients and controls
| Side | Controls | ALS patients | P values* | |
|---|---|---|---|---|
| Corpus callosum (splenium) MD (SD) (10−3 mm2 s−1) | Right | 0.71 (0.05) | 0.75 (0.07) | 0.023 |
| Inferior frontal gyrus MD (SD) (10−3 mm2 s−1) | Right | 0.66 (0.04) | 0.72 (0.04) | <0.001 |
| Superior temporal gyrus MD (SD) (10−3 mm2 s−1) | Right | 0.72 (0.03) | 0.76 (0.06) | 0.006 |
| Middle temporal gyrus MD (SD) (10−3 mm2 s−1) | Right | 0.73 (0.05) | 0.83 (0.07) | <0.001 |
| Middle temporal gyrus MD (SD) (10−3 mm2 s−1) | Left | 0.72 (0.04) | 0.75 (0.05) | 0.026 |
| Lingual gyrus MD (SD) (10−3 mm2 s−1) | Left | 0.77 (0.03) | 0.81 (0.06) | 0.001 |
| Corticospinal tract in the midbrain FA (SD) | Right | 0.65 (0.07) | 0.60 (0.08) | 0.037 |
| Corticospinal tract in the midbrain FA (SD) | Left | 0.66 (0.03) | 0.61 (0.07) | 0.004 |
| Corpus callosum (corpus) FA (SD) | Right | 0.75 (0.07) | 0.69 (0.09) | 0.016 |
| Corpus callosum (corpus) FA (SD) | Left | 0.77 (0.05) | 0.68 (0.08) | <0.001 |
ALS, amyotrophic lateral sclerosis; FA, fractional anisotropy; MD, mean diffusivity; SD, standard deviation.
Two‐tailed Student's t test for not‐paired data. See the text for further details.
DISCUSSION
This study shows that patients with mildly disabling ALS have a significant reduction of GM density in the right precentral gyrus. This fits with the notion that one of the pathologic hallmarks in ALS is the loss of cortical pyramidal neurons in the motor and premotor cortices [Hughes, 1982]. Loss of neurons should also result in an increased MD, which, however, was not the case in the present study. Such a dissociation between tissue loss and intrinsic tissue damage might be explained by a shrinkage in size of the surviving motor neurons, which may lead to a reduced GM density without affecting diffusivity to a degree which can be detected in‐vivo using 1.5 T MRI scanners. Disorders of axonal transport are indeed thought to play a role in this condition and a “dying back” model of axon degeneration has been proposed as part of the natural history of ALS [Cavanagh, 1979; Ince, 2000]. Our finding of an asymmetric interhemispheric GM decrease of premotor cortex also agrees with the “right hemi‐aging model”, i.e., the right hemisphere is more vulnerable to age‐related decline than the left hemisphere [Dolcos et al., 2002], as well as with other MRI studies showing that an asymmetric distribution of tissue damage is not uncommon both in ALS [Kassubek et al., 2005] and in other neurodegenerative disorders [Thompson et al., 2003; Whitwell et al., 2005]. However, previous MRI studies did not reach firm conclusions regarding the presence of motor and premotor cortex atrophy in ALS, since this was found by some authors [Chang et al., 2005; Kassubek et al., 2005; Kato et al., 1993], but not by others [Abe et al., 1997a; Abrahams et al., 2005; Cheung et al., 1995; Ellis et al., 2001; Ishikawa et al., 1993; Kiernan and Hudson, 1994]. There are two possible explanations for such a discrepancy. First, the intrinsic heterogeneity of the pathologic process should be considered [Ince, 2000]. Second, the different clinical characteristics of the cohorts of patients studied may be responsible for the conflicting results. Since we studied patients with a mildly disabling ALS and a reduced GM density was also found in ALS patients with much more severe disability [Chang et al., 2005], one tempting speculation is to suggest that reactive gliosis might not have occurred yet in our sample at a degree enough to “mask” tissue loss, as it might be the case in more advanced, but still not very disabled, patients. The progressive loss of tissue might then counterbalance the “pseudo‐normalizing” effect of gliosis in the extremely disabled cases [Chang et al., 2005].
This study also showed a decreased fiber integrity in the midbrain portion of the CST. Since a FA decrease reflects a loss of fiber bundle directionality [Pierpaoli et al., 1996], this finding indicates the presence of distortion of corticospinal tract tissue geometry. This agrees with previous pathological [Cavanagh, 1979; Ince, 2000] and MRI [Abe et al., 2004; Ciccarelli et al., 2006; Cosottini et al., 2005; Ellis et al., 1999; Graham et al., 2004; Sach et al., 2004; Toosy et al., 2003] studies as well as with the presence of precentral gyrus atrophy in these patients cohort, since about 40% of corticospinal tract axons originate from the precentral motor cortex (Brodmann area 6) and the parietal lobe [Davidoff, 1990]. This finding (FA decrease in the midbrain portion of the CST, which was not found more cranially) also confirms the notion that in ALS there might be a downward trend of tissue damage along the CST [Ince, 2000; Toosy et al., 2003]. We did not find a corresponding increase of MD along the CST. Since tissue loss should lead to both an increased MD and a decreased FA (loss of restricting barriers to water molecular motion should indeed affect in concert both water diffusivity and anisotropy), this mismatch between MD and FA might result from glial proliferation along the CST, a common finding in ALS [Ince, 2000; Murray et al., 2006]. Reactive gliosis secondary to tissue loss would lead to a “pseudo‐normalization” of MD values, but which would reduce FA, since glial cells do not have the same anisotropic morphology as the tissue they replace. The presence of cell debris resulting from partially degenerated or disintegrated nerve fibers along the CST [Chou, 1979] is also likely to contribute to a “pseudo‐normalization” of MD and to a reduction of FA.
Another intriguing finding of this study was to demonstrate the presence of an extra‐motor involvement in mildly disabled ALS patients, thus suggesting that motor and extra‐motor involvement in ALS is not dissociated in time. This is consistent with previous pathological [Mackenzie and Feldman, 2003; Okamoto et al., 1991; Piao et al., 2003; Tsuchiya et al., 2002] and neuroimaging [Abe et al., 1997b, 2001; Abrahams et al., 1996, 1997, 2000, 2004, 2005; Chang et al., 2005; Ellis et al., 2001; Kassubek et al., 2005; Kato et al., 1993; Kiernan et al., 1994; Mantovan et al., 2003; Rule et al., 2004] findings. Pathologically, ubiquitin‐positive intraneuronal inclusions and neuronal loss have been found to extend beyond the motor system in ALS patients with or without cognitive impairment [Mackenzie et al., 2003; Okamoto et al., 1991; Tsuchiya et al., 2002]. Structural and functional neuroimaging studies have provided evidence for the presence of atrophy and hypometabolism in extra‐motor frontal and temporal cortices in patients with ALS [Abe et al., 1997b; Abrahams et al., 1996, 1997, 2000, 2004, 2005; Chang et al., 2005; Ellis et al., 2001; Kassubek et al., 2005; Kato et al., 1993; Kiernan et al., 1994; Mantovan et al., 2003; Rule et al., 2004].
The regional pattern of extra‐motor changes seen in our cohort of ALS patients is also of interest. We found that the IFG was not spared by the pathological process and the IFG is known to play a critical role in movement execution and imagination [Binkofski et al., 1999; Harrington et al., 2000; Haslinger et al., 2002; Stephan et al., 1995]. The IFG is also thought to influence finger movements either through a direct action on the motor neurons of the spinal cord [Dum and Strick, 1996; He et al., 1993] or, indirectly, via corticocortical connections to the corticospinal neurons of the motor cortex [Tokuno and Tanji, 1993]. Furthermore, the left IFG (Broca's area) is known to be associated with speech production and word retrieval [Paulesu et al., 1993]. Damage of left IFG may, therefore, be the substrate of the verbal fluency dysfunction described in some nondemented ALS patients [Abe et al., 1997b; Abrahams et al., 1996, 1997, 2000, 2004; Frank et al., 1997; Gallassi et al., 1989; Kew et al., 1993; Lomen‐Hoerth et al., 2003; Ludolph et al., 1992; Massman et al., 1996]. The pattern of regional abnormalities we found in temporal lobe structures (reduced GM density in the STG and increased MD in the STG and MTG) is also in agreement with other volumetric studies [Abrahams et al., 2005; Chang et al., 2005] of more disabled patients and might contribute to explain part of the cognitive symptoms observed in ALS patients [Abe et al., 1997b; Abrahams et al., 1996, 2000, 2004; Frank et al., 1997; Gallassi et al., 1989; Kew et al., 1993; Lomen‐Hoerth et al., 2003; Ludolph et al., 1992; Mantovan et al., 2003; Massman et al., 1996; Ringholz et al., 2005; Strong et al., 2003; Wilson et al., 2001]. The observed MD and FA changes in the corpus callosum are in accordance with the results obtained by Sach et al. [2004] and confirm neuropathological findings of degeneration in regions outside the motor tracts sensu stricto [Brownell et al., 1970; Murray et al., 2006]. It is also worth noting that FA changes were affecting the central part of the corpus callosum which harbors interhemispheric fibers connecting the two motor cortices. Thus, such a transcallosal fiber damage might yet represent an additional mechanism responsible for the motor manifestations of the disease. Admittedly, volumetry and diffusivity changes were not correlated with the clinical manifestations of the disease in this patients' cohort. However, the absence of such a correlation might be due to the fact that the subtle MRI abnormalities we found may preceded the appearance of clinical symptoms and signs. Longitudinal studies are now warranted to confirm this hypothesis and to assess whether the extent and severity of the MRI‐detectable extra‐motor damage is predictive of subsequent development of cognitive impairment, known to occur in patients with ALS [Abe et al., 1997b; Abrahams et al., 1996, 2000, 2004; Frank et al., 1997; Gallassi et al., 1989; Kew et al., 1993; Lomen‐Hoerth et al., 2003; Ludolph et al., 1992; Mantovan et al., 2003; Massman et al., 1996; Ringholz et al., 2005; Strong et al., 2003; Wilson et al., 2001].
REFERENCES
- Abe K,Fujimura H,Kobayashi Y,Fujita N,Yanagihara T ( 1997a): Degeneration of the corticospinal tracts in patients with amyotrophic lateral sclerosis. A premortem and postmortem magnetic resonance imaging study. J Neuroimaging 7: 208–212. [DOI] [PubMed] [Google Scholar]
- Abe K,Fujimura H,Toyooka K,Sakoda S,Yorifuji S,Yanagihara T ( 1997b): Cognitive function in amyotrophic lateral sclerosis. J Neurol Sci 148: 95–100. [DOI] [PubMed] [Google Scholar]
- Abe K,Takanashi M,Watanabe Y,Tanaka H,Fujita N,Hirabuki N,Yanagihara T ( 2001): Decrease in N‐acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology 43: 537–541. [DOI] [PubMed] [Google Scholar]
- Abe O,Yamada H,Masutani Y,Aoki S,Kunimatsu A,Yamasue H,Yamasue H,Fukuda R,Kasai K,Hayashi N,Masumoto T,Mori H,Soma T,Ohtomo K ( 2004): Amyotrophic lateral sclerosis: Diffusion tensor tractography and voxel‐based analysis. NMR Biomed 17: 411–416. [DOI] [PubMed] [Google Scholar]
- Abrahams S,Goldstein LH,Kew JJ,Brooks DJ,Lloyd CM,Frith CD,Leigh PN ( 1996): Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain 119: 2105–2120. [DOI] [PubMed] [Google Scholar]
- Abrahams S,Goldstein LH,Al‐Chalabi A,Pickering A,Morris RG,Passingham RE,Brooks DJ,Leigh PN ( 1997): Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 62: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams S,Leigh PN,Harvey A,Vythelingum GN,Grise D,Goldstein LH ( 2000): Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38: 734–747. [DOI] [PubMed] [Google Scholar]
- Abrahams S,Goldstein LH,Simmons A,Brammer M,Williams SCR,Giampietro V,Leigh PN ( 2004): Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain 127: 1507–1517. [DOI] [PubMed] [Google Scholar]
- Abrahams S,Goldstein LH,Suckling J,Ng V,Simmons A,Chitnis X,Atkins L,Williams SC,Leigh PN ( 2005): Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 252: 321–331. [DOI] [PubMed] [Google Scholar]
- Ashburner J,Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Binkofski F,Buccino G,Posse S,Seitz RJ,Rizzolatti G,Freund H ( 1999): A fronto–parietal circuit for object manipulation in man: Evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Bowen BC,Pattany PM,Bradley WG,Murdoch JB,Rotta F,Younis AA,Duncan RC,Quencer RM ( 2000): MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 21: 647–658. [PMC free article] [PubMed] [Google Scholar]
- Brooks BR ( 1994): El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World Federation of Neurology Research Group on neuromuscular diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 124: S96–S107. [DOI] [PubMed] [Google Scholar]
- Brownell B,Oppenheimer DR,Hughes JT ( 1970): The central nervous system in motor neuron disease. J Neurol Neurosurg Psychiatry 33: 357–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JB ( 1979): The 'dying back' process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med 103: 659–664. [PubMed] [Google Scholar]
- Chang JL,Lomen‐Hoerth C,Murphy J,Henry RG,Kramer JH,Miller BL,Gorno‐Tempini ML ( 2005): A voxel‐based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 65: 75–80. [DOI] [PubMed] [Google Scholar]
- Cheung G,Gawel MJ,Cooper PW,Farb RI,Ang LC,Gawal MJ ( 1995): Amyotrophic lateral sclerosis: Correlation of clinical and MR imaging findings. Radiology 194: 263–270. [DOI] [PubMed] [Google Scholar]
- Chou SM ( 1979): Pathognomy of intraneuronal inclusions in ALS In: Tsubaki T,Toyokura Y, editors. Amyotrophic Lateral Sclerosis. Tokyo: University of Tokyo Press; pp 135–176. [Google Scholar]
- Ciccarelli O,Behrens TE,Altmann DR,Orrell RW,Howard RS,Johansen‐Berg H,Miller DH,Matthews PM,Thompson AJ ( 2006): Probabilistic diffusion tractography: A potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain 129: 1859–1871. [DOI] [PubMed] [Google Scholar]
- Cosottini M,Giannelli M,Siciliano G,Lazzaretti G,Nichelassi MC,Del Corona A,Bartolozzi C,Murri L ( 2005): Diffusion‐tensor MR imaging of cortico‐spinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology 237: 258–264. [DOI] [PubMed] [Google Scholar]
- Davidoff RA ( 1990): The corticospinal tract. Neurology 40: 332–339. [DOI] [PubMed] [Google Scholar]
- Dolcos F,Rice HJ,Cabeza R ( 2002): Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev 26: 819–825. [DOI] [PubMed] [Google Scholar]
- Dum RP,Strick PL ( 1996): Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 16: 6513–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CM,Simmons A,Andrews C,Dawson JM,Williams SC,Leigh PM ( 1998): A proton magnetic resonance spectroscopic study in ALS: Correlation with clinical findings. Neurology 51: 1104–1109. [DOI] [PubMed] [Google Scholar]
- Ellis CM,Simmons A,Jones DK,Bland J,Dawson JM,Horsfield MA,Williams SC,Leigh PN ( 1999): Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53: 1051–1058. [DOI] [PubMed] [Google Scholar]
- Ellis CM,Suckling J,Amaro E Jr,Bullmore ET,Simmons A,Williams SC,Leigh PN ( 2001): Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 57: 1571–1578. [DOI] [PubMed] [Google Scholar]
- Frank B,Haas J,Heinze HJ,Stark E,Munte TF ( 1997): Relation of neuropsychological and magnetic resonance findings in amyotrophic lateral sclerosis: Evidence for subgroups. Clin Neurol Neurosurg 99: 79–86. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Poline JB,Grasby PJ,Williams SC,Frackowiak RS,Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Gallassi R,Montagna P,Morreale A,Lorusso S,Tinuper P,Daidone R,Lugaresi E ( 1989): Neuropsychological, electroencephalogram and brain computed tomography findings in motor neuron disease. Eur Neurol 29: 115–120. [DOI] [PubMed] [Google Scholar]
- Good CD,Johnsrude IS,Ashburner J,Henson RN,Friston KJ,Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Goodin DS,Rowley HA,Olney RK ( 1988): Magnetic resonance imaging in amyotrophic lateral sclerosis. Ann Neurol 23: 418–420. [DOI] [PubMed] [Google Scholar]
- Graham JM,Papadakis N,Evans J,Widjaja E,Romanowski CA,Paley MN,Wallis LI,Wilkinson ID,Shaw PJ,Griffiths PD ( 2004): Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63: 2111–2119. [DOI] [PubMed] [Google Scholar]
- Harrington DL,Rao SM,Haaland KY,Bobholz JA,Mayer AR,Binderx JR,Cox RW ( 2000): Specialized neural systems underlying representations of sequential movements. J Cogn Neurosci 12: 56–77. [DOI] [PubMed] [Google Scholar]
- Haslinger B,Erhard P,Weilke F,Ceballos‐Baumann AO,Bartenstein P,Grafin von Einsiedel H,Schwaiger M,Conrad B,Boecker H ( 2002): The role of lateral premotor‐cerebellar‐parietal circuits in motor sequence control: A parametric fMRI study. Brain Res Cogn Brain Res 13: 159–168. [DOI] [PubMed] [Google Scholar]
- He SQ,Dum RP,Strick PL ( 1993): Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht MJ,Fellner F,Fellner C,Hilz MJ,Heuss D,Neundorfer B ( 2001): MRI‐FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2‐,T1‐ and proton‐density‐weighted images. J Neurol Sci 186: 37–44. [DOI] [PubMed] [Google Scholar]
- Hecht MJ,Fellner F,Fellner C,Hilz MJ,Heuss D,Neundorfer B ( 2002): Hyperintense and hypointense MRI signals of the precentral gyrus and corticospinal tract in ALS: A follow‐up examination including FLAIR images. J Neurol Sci 199: 59–65. [DOI] [PubMed] [Google Scholar]
- Hughes JT ( 1982): Pathology of amyotrophic lateral sclerosis. Adv Neurol 36: 61–74. [PubMed] [Google Scholar]
- Ince PG ( 2000): Neuropathology In: Brown RH,Meininger V,Swash M, editors. Amyotrophic Lateral Sclerosis. London: Martin Dunitz; pp 83–112. [Google Scholar]
- Ishikawa K,Nagura H,Yokota T,Yamanouchi H ( 1993): Signal loss in the motor cortex on magnetic resonance images in amyotrophic lateral sclerosis. Ann Neurol 33: 218–222. [DOI] [PubMed] [Google Scholar]
- Kassubek J,Unrath A,Huppertz HJ,Lule D,Ethofer T,Sperfeld AD,Ludolph AC ( 2005): Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel‐based morphometry of 3D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord 6: 213–220. [DOI] [PubMed] [Google Scholar]
- Kato S,Hayashi H,Yagishita A ( 1993): Involvement of the frontotemporal lobe and limbic system in amyotrophic lateral sclerosis: As assessed by serial computed tomography and magnetic resonance imaging. J Neurol Sci 116: 52–58. [DOI] [PubMed] [Google Scholar]
- Kato Y,Matsumura K,Kinosada Y,Narita Y,Kuzuhara S,Nakagawa T ( 1997): Detection of corticospinal tract lesions in amyotrophic lateral sclerosis with magnetization‐transfer measurements. AJNR Am J Neuroradiol 18: 1541–1547. [PMC free article] [PubMed] [Google Scholar]
- Kew JJM,Goldstein LH,Leigh PN,Abrahams S,Cosgrave N,Passingham RE,Frackowiak RS,Brooks DJ ( 1993): The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis: A neuropsychological and positron emission tomography study. Brain 116: 1399–1423. [DOI] [PubMed] [Google Scholar]
- Kiernan JA,Hudson AJ ( 1994): Frontal lobe atrophy in motor neuron diseases. Brain 117: 747–757. [DOI] [PubMed] [Google Scholar]
- Lomen‐Hoerth C,Murphy J,Langmore S,Kramer JH,Olney RK,Miller B ( 2003): Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60: 1094–1097. [DOI] [PubMed] [Google Scholar]
- Ludolph AC,Langen KJ,Regard M,Herzog H,Kemper B,Kuwert T,Bottger IG,Feinendegen L ( 1992): Frontal lobe function in amyotrophic lateral sclerosis: A neuropsychologic and positron emission tomography study. Acta Neurol Scand 85: 81–89. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR,Feldman H ( 2003): The relationship between extramotor ubiquitin‐immunoreactive neuronal inclusions and dementia in motor neuron disease. Acta Neuropathol (Berl) 105: 98–102. [DOI] [PubMed] [Google Scholar]
- Mantovan MC,Baggio L,Dalla Barba G,Smith P,Pegoraro E,Soraru G,Bonometto P,Angelini C ( 2003): Memory deficits and retrieval processes in ALS. Eur J Neurol 10: 221–227. [DOI] [PubMed] [Google Scholar]
- Massman PJ,Sims J,Cooke N,Haverkamp LJ,Appel V,Appel SH ( 1996): Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 61: 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowitz S,Sartor K,Gado M,Torack R ( 1989): Focal signal‐intensity variations in the posterior internal capsule: Normal MR findings and distinction from pathologic findings. Radiology 172: 535–539. [DOI] [PubMed] [Google Scholar]
- Murray C,Viehman A,Lippa CF ( 2006): The corpus callosum in Pick's disease, Alzheimer's disease, and amyotrophic lateral sclerosis: Gliosis implies possible clinical consequence. Am J Alzheimers Dis Other Demen 21: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba H,Araki T,Ohtoma K,Monzawa S,Uchiyama G,Koizumi K,Nogata Y,Kachi K,Shiozawa Z,Kobayashi M ( 1993): Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189: 843–846. [DOI] [PubMed] [Google Scholar]
- Okamoto K,Hirai S,Yamazaki T,Sun XY,Nakazato Y ( 1991): New ubiquitin‐positive intraneuronal inclusions in the extra‐motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129: 233–236. [DOI] [PubMed] [Google Scholar]
- Paulesu E,Frith CD,Frackowiak RS ( 1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Piao YS,Wakabayashi K,Kakita A,Yamada M,Hayashi S,Morita T,Ikuta F,Oyanagi K,Takahashi H ( 2003): Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 Autopsy cases examined between 1962 and 2000. Brain Pathol 13: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C,Jezzard P,Basser P,Barnett A,Di Chiro G ( 1996): Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Ringholz GM,Appel SH,Bradshaw M,Cooke NA,Mosnik DM,Schulz PE ( 2005): Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65: 586–590. [DOI] [PubMed] [Google Scholar]
- Rovaris M,Gallo A,Valsasina P,Benedetti B,Caputo D,Ghezzi A,Montanari E,Soriani MP,Bertolotto A,Mancardi G,Bergamaschi R,Martinelli V,Comi G,Filippi M ( 2005): Short‐term accrual of gray matter pathology in patients with progressive multiple sclerosis: An in vivo study using diffusion tensor MRI. Neuroimage 24: 1139–1146. [DOI] [PubMed] [Google Scholar]
- Rule RR,Suhy J,Schuff N,Gelinas DF,Miller RG,Weiner MW ( 2004): Reduced NAA in motor and non‐motor brain regions in amyotrophic lateral sclerosis: A cross‐sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord 5: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sach M,Winkler G,Glauche V,Liepert J,Heimbach B,Koch MA,Buchel C,Weiller C ( 2004): Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127: 340–350. [DOI] [PubMed] [Google Scholar]
- Stephan KM,Fink GR,Passingham RE,Silbersweig D,Ceballos‐Baumann AO,Frith CD,Frackowiak RS ( 1995): Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386. [DOI] [PubMed] [Google Scholar]
- Strong MJ,Lomen‐Hoerth C,Caselli RJ,Bigio EH,Yang W ( 2003): Cognitive impairment, frontotemporal dementia, and the motor neuron diseases. Ann Neurol 54: S20–S23. [DOI] [PubMed] [Google Scholar]
- Talbot K ( 2002): Motor neuron disease. Postgrad Med J 78: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe JL,Vermathen M,Miller R,Gelinas D,Weiner MW,Rooney WD ( 1998): Reduced MTR in the corticospinal tract and normal T2 in amyotrophic lateral sclerosis. Magn Reson Imaging 16: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ALS CNTF treatment study (ACTS) phase I‐II study group ( 1996): The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. Arch Neurol 53: 141–147. [PubMed] [Google Scholar]
- Thompson SA,Patterson K,Hodges JR ( 2003): Left/right asymmetry of atrophy in semantic dementia: Behavioral‐cognitive implications. Neurology 61: 1196–1203. [DOI] [PubMed] [Google Scholar]
- Thorpe JW,Moseley IF,Hawkes CH,MacManus DG,McDonald WI,Miller DH ( 1996): Brain and spinal cord MRI in motor neuron disease. J Neurol Neurosurg Psychiatry 61: 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H,Tanji J ( 1993): Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: A retrograde double labeling study. J Comp Neurol 333: 199–209. [DOI] [PubMed] [Google Scholar]
- Toosy AT,Werring DJ,Orrell RW,Howard RS,King MD,Barker GJ,Miller DH,Thompson AJ ( 2003): Diffusion tensor imaging detects cortico‐spinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 74: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K,Takahashi M,Shiotsu H,Akiyama H,Haga C,Watabiki S,Taki K,Nakano I,Ikeda K ( 2002): Sporadic amyotrophic lateral sclerosis with circumscribed temporal atrophy: A report of an autopsy case without dementia and with ubiquitinated intraneuronal inclusions. Neuropathology 22: 308–316. [DOI] [PubMed] [Google Scholar]
- Waragai M ( 1997): MRI and clinical features in amyotrophic lateral sclerosis. Neuroradiology 39: 847–851. [DOI] [PubMed] [Google Scholar]
- Whitwell JL,Sampson EL,Watt HC,Harvey RJ,Rossor MN,Fox NC ( 2005): A volumetric magnetic resonance imaging study of the amygdala in frontotemporal lobar degeneration and Alzheimer's disease. Dement Geriatr Cogn Disord 20: 238–244. [DOI] [PubMed] [Google Scholar]
- Wilson CM,Grace GM,Munoz DG,He BP,Strong MJ ( 2001): Cognitive impairment in sporadic ALS: A pathologic continuum underlying a multisystem disorder. Neurology 57: 651–657. [DOI] [PubMed] [Google Scholar]
- Yoshida M ( 2004): Amyotrophic lateral sclerosis with dementia: The clinicopathological spectrum. Neuropathology 24: 87–102. [DOI] [PubMed] [Google Scholar]


