Abstract
The aim of this study was to test the validity of mirror neuron activity in humans through analysis of electroencephalographic (EEG) functional connectivity during an action not directed towards an object. We investigated changes in EEG interchannel synchronization prior to and during action execution and also prior to and during observation of the same action. Twelve participants observed a simple finger movement sequence. In a second testing session they physically executed the movement. EEGs were recorded from 19 active sites across the cortex. Activity was considered in four frequency bands (7–10 Hz, 10–13 Hz, 13–20 Hz, and 20–30 Hz) using a new measure: synchronization likelihood. This technique considers rapid changes in signal synchronization and spatiotemporal patterns of coherence. The results revealed no statistically significant difference in synchronization likelihood between the observation and execution data. We found an increase in synchronization over a broad frequency range during task processing and suggest that this may reflect interregional cortical coupling of intricately and hierarchically interconnected networks that are active in a similar way during both observation and execution of a movement. While EEG may be insensitive to differences present during the observation and execution of a movement, the results of the present study shed some light on the general mechanisms of cognitive integration. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: perception, imitative behavior, movement, electroencephalography, cortical synchronization, nonlinear dynamics
INTRODUCTION
In all aspects of life, humans spend a considerable amount of time observing others in order to understand their behavior [Decety et al.,2002] and, in some cases, to imitate and learn from that behavior [Demeris and Hayes,1996; Meltzoff and Moore,1977,1997]. The copying or imitation by an observer of a feature of the body movement of a model [Heyes,2001] represents a fundamental part of human behavior used to acquire new skills [Demeris and Hayes,1996]. Since little is known about the mechanisms that underlie these processes [Buccino et al.,2004a,b; Calderon and Hu,2003; Iacoboni et al.,1999; Wohlschlager and Bekkering,2002], the study of these phenomena would seem important for understanding the neurological basis of the processes of observation and imitation.
The neurological foundations seem to lie within “mirror neurons” [e.g., Billard and Arbib,2002; Iacoboni et al.,1999; Rizzolatti and Craighero,2004]. These neurons were first discovered in the ventral premotor cortex of the macaque monkey with single neuron recording [Rizzolatti et al.,1988]. These mirror neurons fired when the monkey executed a goal‐directed hand movement [Rizzolatti et al.,1988] and also when it observed this same action executed by another monkey or by a human [Gallese et al.,1996; Rizzolati et al.,1996]. These findings support a proposal for an observation/execution matching system that maps the observed action onto an internal motor representation of the action [Iacoboni et al.,1999; Rizzolatti et al.,2001].
The research that has attempted to investigate the existence of mirror neurons can be discussed as indirect and direct evidence. A claim cannot be made for direct evidence for the existence of a mirror system unless cortical areas are shown to be active during both the execution and observation of an action. If they are, they can be considered to have mirror properties [Rizzolatti et al.,2001].
Indirect Evidence for the Mirror System
EEG research has provided indirect evidence for mirror neuron activity [e.g., Cochin et al.,1998]. In this study, healthy participants were tested only under observation conditions. The central “mu” rhythm was desynchronized during action observation in a similar way to that observed during actual movement [e.g., Chatrian,1976].
Brain imaging studies have also shown indirect evidence for mirror neuron activity [e.g., Decety et al.,1997; Grèzes et al.,1998]. Unfortunately, participants in these studies only observed hand or arm actions. As discussed above, the involvement of these motor system areas (e.g., dorsolateral prefrontal cortex, presupplementary motor area) during action observation alone is not synonymous with these areas possessing mirror properties [Rizzolatti et al.,2001,2002].
Direct Evidence for the Mirror System
Relatively few studies have provided direct evidence for the existence of an observation/execution matching system in humans. Fadiga et al. [1995] found that the muscular response pattern generated by a transcranial magnetic stimulus during the observation of an action sequence was the same as that recorded while the participants physically executed the same action. Similarly, a number of other groups have provided evidence for the direct matching hypothesis: Cochin et al. [1999] and Muthukumaraswamy and Johnson [2004]; and Muthukumaraswamy et al. [2004] with EEG; Hari et al. [1998]; and Nishitani and Hari [2000] with neuromagnetic recordings; Iacoboni et al. [1999] with functional magnetic resonance imaging; and Flanagan and Johansson [2003] with an infrared eye tracking system.
In these studies participants were directly assessed performing hand movements in an observation condition and in an action condition with various purposes: to observe the movement with the purpose of later imitation [Fadiga et al.,1995; Iacoboni et al.,1999]; to observe the movement with the purpose of recognizing it [Fadiga et al.,1995]; or to observe the movement with no specific goal [Cochin et al.,1999; Hari et al.,1998; Iacoboni et al.,1999; Muthukumaraswamy and Johnson,2004; Muthukumaraswamy et al.,2004; Nishitani and Hari,2000]. The nature of the task is important, as reported by Decety et al. [1997] and Grèzes et al. [1998]. They showed that cortical areas involved in the process of observation were dependent on the instructions given to the participants. For example, Decety et al. [1997] found that the dorsolateral prefrontal cortex and the presupplementary motor area were activated when participants were provided with instructions to observe a movement with the later requirement to imitate it. In contrast, the right parahippocampal gyrus was activated in a situation where there was a requirement to recognize the movement after its observation. In addition, Grèzes et al. [1998] showed that observation of meaningful actions with no goal elicited activity in the ventral pathway. However, observation of meaningless action with no goal activated the dorsal pathway. Observation, with the aim to replicate the action at a later stage, involved the dorsal pathway for both meaningful and meaningless actions. Therefore, the nature of the instructions given to the participants seems to be important and is differentiated by neural activity.
The research that has considered mirror neurons has studied brain activity during the execution and the observation of an action and done so through a variety of different techniques. To our knowledge, only Kilner et al. [2004] examined the existence of mirror neurons by focusing on electrocortical activity prior to the observation of a predicted movement. They showed that a readiness potential, an electrophysiological marker of motor preparation, was also present prior to the observation of an action performed by another.
The precise role of mirror neuron activity during observation of action has only recently been considered [e.g., Buccino et al.,2004a,b; Rizzolatti and Craighero,2004]. Buccino et al. [2004a,b] proposed that the process of observing an action to replicate it later requires a transformation of a visual action into a corresponding motor action and that this process relies on the mirror neuron system. Mirror neurons fire while an individual observes an elementary motor act, and the observed action is then divided into simpler parts and coded motorically. Where the observed action matches or is similar to an existing action already in the mirror neuron system, corresponding motor representations are activated and the action can be replicated [Buccino et al.,2004a,b]. Therefore, cognitive processes such as visual perception, visual and kinesthetic imagery, and working memory are proposed to be involved in the observation process where imitation is required at a later stage.
The analysis techniques used in most of the studies discussed above have been single‐cell recordings from monkeys and functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) for humans. While fMRI and PET show excellent spatial resolution, they provide relatively poor temporal information [Pfurtscheller and Lopes da Silva,1999; Servos,2000]. To allow the examination of movement‐related changes in cortical activation, a technique is required with millisecond temporal resolution. EEG addresses this methodological concern and has been shown to be effective in the analysis of preparation, execution, and recovery of a movement [e.g., Stancak et al.,2000] and observation of an action [e.g., Cochin et al.,1999].
Integration and coordination of different brain regions has been recognized as an important component of information processing [e.g., Stam et al.,1996; van Putten and Stam,2001]. However, the techniques generally used to examine these interactions have some limitations [Stam and van Dijk,2002]. Using power change as an indicator of event‐related desynchronization [e.g., Pfurtscheller,1988; Pfurtscheller and Aranibar,1977; Salmelin and Hari,1994] has been argued to reveal only part of the relevant information since it can only be used as an index of local cortical engagement [Stam et al.,2002]. Estimating the similarity between time series of electrical potential via linear techniques such as coherence includes a number of limitations. These include the inability to characterize nonstationary data with rapidly changing interdependencies and identification of nonlinear interdependencies between the underlying dynamical system [see Stam et al.,2002; Stam and van Dijk,2002, for further details of these concerns]. Friston [2000] has argued that nonlinear interactions between brain regions and the rapid changes in synchronization are important considerations for EEG research. To detect these nonlinear interactions between brain regions, Rulkov et al. [1995] and Schiff et al. [1996] published similar approaches. Unfortunately, they were unable to give a normalized estimate of the interdependencies between time series [e.g., Pereda et al.,2001]. More recently, Stam and van Dijk [2002] provided a direct estimate of the dynamical linear and nonlinear interdependencies between simultaneously recorded EEG time series.
Since there have been few studies that provided direct evidence for the existence of mirror neurons, several issues require further investigation. First, it is important to consider actions not directed towards an object. Actions not oriented towards a goal are of interest since they have been proposed as a more rigorous analysis of the kinematic criteria of the movement than have object‐oriented actions [Babiloni et al.,2002]. Second, consideration of the temporal course of electrocortical activation during a simple motor task completed under observation and execution is also important. Third, measurement of functional integration during the distinct stages of an action performed under different conditions via the synchronization likelihood measure may reveal information relating to mirror neuron activity.
The aim of the present study was to test the validity of mirror neuron activity through the analysis of EEG activity with the synchronization likelihood technique. It was hypothesized that the neurophysiological mechanisms underlying the observation of a sequential finger movement, in particular changes in between‐area coupling, would show functional similarities to those identified in the physical execution of the same movement in frequency bands lying within a range of 7–30 Hz. The premovement and actual movement phase was considered for both conditions.
SUBJECTS AND METHODS
Participants
Twelve individuals (mean age = 23.92 years, SD = 3.17) with no neurological or psychiatric problems participated in the study after they provided written informed consent and the study was approved by the local ethics committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale, CCPPRB). All participants were assessed as right‐handed by the Edinburgh Handedness Inventory [Oldfield,1971]. Participants were not informed of the goals of the study.
Task
The participants were asked to perform a finger movement sequence. This consisted of a flexion followed by an extension of the right forefinger to create an angle of 90° between the distal and medial phalange while the hand remained in a supine position (Fig. 1). This simple sequential finger movement requires the execution of a central motor program with particular temporal and spatial sequences. This movement was chosen because it was executed at a distance from the scalp, preventing mechanographic artifacts in the EEG [Derambure et al.,1999], and because it was not object‐oriented.
Figure 1.

The finger movement sequence.
Experimental Procedure
The participants were examined in two conditions: first an action observation condition and second an action execution condition. The ordering was the same for the 12 participants since we required them to observe the movement and replicate it at a later stage in order to investigate the role of mirror neurons in imitation [viz., Rizzolatti and Craighero,2004]. Multichannel EEG was recorded in both conditions while participants sat in a darkened room with their arms lying on armrests. To reduce artifacts throughout the EEG data collection, participants were asked to fix their attention on a target point placed on a screen situated 1.30 m in front of them, to keep their jaw relaxed, and to avoid blinking. These instructions were provided before each set of trials.
A metronome, set to 2 Hz, was used to impose constant timing of the movement pattern and ensure a similar number of finger contacts per trial across the conditions [Debaere et al.,2003] and the participants [Gerloff et al.,1997]. This consistency also ensured similar experimental circumstances in the execution and observation conditions [Manganotti et al.,1998].
Action observation condition
Each participant performed 40 trials for the observation task described above. Each trial comprised three stages that were presented to the participant via a video display. Instructions were provided to the participants requiring them to observe the movement with the intention of repeating it at a later stage.
The first stage of each trial, lasting 4 s, presented the participant with an amber monitor screen. This screen warned the participant about the imminent movement requirement. During the second stage, lasting 2 s, participants observed a video displaying a live model executing the finger movement sequence at 2Hz (Table I). In the third, 6 s stage, a red background appeared and the participant stopped viewing the movement and relaxed. The time interval between the beginning of viewing the movement and the onset of the next was 12 s.
Table I.
Temporal course of a trial in action observation condition
| Premovement (Stage 1) | Movement observation(Stage 2) | Postmovement (Stage 3) |
|---|---|---|
| 4 s | 2 s | 6 s |
| Amber | Video | Red |
Action execution condition
Each participant performed 40 trials for the task described above and followed a similar procedure to the observation trials. The first stage warned the participant about the imminent movement requirement. In the second stage, a black background was presented to the participant. They performed the metronome‐controlled finger movement at a rate of 2 Hz. In the third stage, the red background prompted the participant to stop the movement and relax. The procedure is represented in Table II. The time interval between the beginning of one movement and the onset of the next was 12 s.
Table II.
Temporal course of a trial in action execution condition
| Premovement (Stage 1) | Movement action (Stage 2) | Postmovement (Stage 3) |
|---|---|---|
| 4 s | 2 s | 6 s |
| Amber | Black | Red |
The metronome provided an audible presence throughout each stage of both conditions. All trials were triggered using a specially designed interface based on a photoresistive diode that responded to the screen color change. Two 8‐min blocks of 40 trials were performed. Each block was separated by a 5‐min rest period. The first block was the action observation block and the second the action execution block.
Data Acquisition and Recording
Electrical brain activity was recorded from 19 Ag/AgCl pad electrodes held on the head with a rubber cap (Fp1, Fp2, Fz, F7, F8, F3, F4, Cz, C3, C4, PZ, P3, P4, T3, T4, T5, T6, O1, and O2) and placed in accordance with the international 10‐20 system [Jasper,1958]. Mastoids were used for the reference electrodes and the ground electrode was located on the forehead. Electro‐oculograms (EOGs) were also registered from the canthi of both eyes (horizontal EOG) and the supra‐ and infraorbital of the right eye (vertical EOG). Electrode impedance was kept homogeneously below 5 kΩ throughout the experimentation and was checked systematically between the two blocks of trials. Amplifier bandwidth was set between 0.15 and 114 Hz using a computer‐based EEG recorder (Coherence, Deltamed, Paris, France). Baseline‐corrected activity was sampled at 256 Hz. AD resolution was 16 bit.
Synchronization Likelihood
Synchronization likelihood [Stam and van Dijk,2002] is an EEG analysis technique that can be used to determine time‐dependent correlations between EEG channels. The method provides a normalized estimate of the dynamical interdependencies between a time series (e.g., an EEG channel) and one or more other time series. Synchronization likelihood is a measure that describes how strongly a channel is synchronized to all the other channels. Values of synchronization likelihood (SL) ranging from 1 to 0.1 indicate maximal synchronization and, in the case of only random correlations, SL tends to be 0 [Stam et al.,2002; Stam and van Dijk,2002]. Consideration of all the possible pair combinations of N electrodes (i.e., [N*N–1]/2) can lead to local and lateralized effects. However, we chose to examine SL across all electrodes to provide a meaningful data reduction and to make analysis manageable in the early stages of the use of this new EEG analysis technique. The technique, used in this way, has successfully studied rapid changes in synchronization and spatiotemporal patterns of coherency. More details concerning the technique can be found in the Appendix.
Data Processing
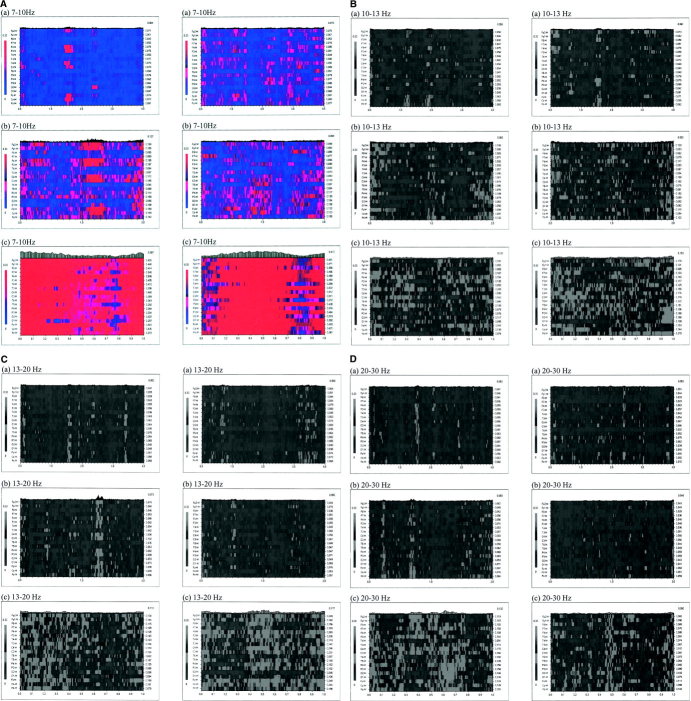
EEG data were analyzed off‐line. Forty trials were completed during the 8‐min condition. Each trial was subdivided into three stage epochs. For the action observation condition, the epochs were 4 s prior to the onset of the observation of movement until the onset of the observation, the 2 s of the observation of movement, and the 6 s after the movement. The same principle was applied to the action condition. Analysis of the third epoch revealed that eye movement artifacts contaminated the data in the final 3 s of some trials. Consequently, this epoch was reduced to 3 s in length and the last second of this 3‐s epoch served as the baseline reading (Fig. 2). This period was chosen as an appropriate reference state, since there was no meaningful stimulus input. Participants were instructed to relax and to rest passively while viewing the simple visual stimuli of a red screen. The reference state was compared with premovement and movement stages during execution and observation to increase the validity of the experimental process (Fig. 2). EEG data were analyzed in four frequency bands: 7–10 Hz, 10–13 Hz, 13–20 Hz, and 20–30 Hz. The digital filter decomposed the data without phase distortion. It did not interfere with any nonlinear component occurring within the frequencies of interest. It applied a Fourier transform to the data, setting all frequencies outside the passband to zero, and then it applied an inverse Fourier transform. In the present study, we used SL as a general measure of functional coupling between EEG signals with the added advantage of a high time resolution. Furthermore, we had a priori reasons to consider specific frequency bands with known physiological properties. Synchronization likelihood was computed for each frequency band, each electrode site, and each stage of the movement (i.e., baseline, premovement, and movement stages) during execution and observation. For each frequency band and for each stage of the movement during execution and observation, the mean SL of the 19 electrodes was also computed.
Figure 2.

Schema for one trial to be analyzed off‐line irrespective of condition. Shaded sections were used for EEG analysis.
The first artifact‐free trial was selected for analysis. Parameters for the computation of the synchronization likelihood were: 1 sample for the lag; 10 for the embedding dimension; 10 for the Theiler correction (w1); 1 for w2; 0.010 for pref; and 1 for the speed. The speed controls how many calculations the software implementation skips. When speed is set to 1, SL is computed for all the time points.
Statistical Analysis
Mean synchronization likelihood and synchronization likelihood for electrode sites were considered successively.
Mean synchronization likelihood
Mean synchronization likelihood data were analyzed for each of the four frequency bands with four separate 3(stages) × 2(conditions) ANOVAs. There were two within‐participant factors: stage (three levels: premovement, movement, and baseline) and condition (two levels: observation and execution). These ANOVAs were computed to determine, more specifically, whether: baseline SL values were significantly different from movement and premovement values; premovement SL values were significantly different from movement SL values; and SL values during observation were significantly different from those during actual execution. Post‐hoc comparisons were calculated using Tukey's HSD test when ANOVA results were significant. Tukey's HSD compares means in order to determine where the significant difference lies.
Synchronization likelihood for electrode sites
SL for each electrode was analyzed for each of the four frequency bands with a 2(conditions) × 19(electrode sites) ANOVA. There were two within‐participant factors: condition (two levels: observation and execution) and electrode (19 levels corresponding to the 19 EEG channels). Movement stages were analyzed separately. Post‐hoc comparisons were calculated using Tukey's HSD test.
A similar procedure to the one employed by Cochin et al. [1998] was used for the post‐hoc tests for the electrode main effect. This consisted of comparing each electrode with all of the others for each frequency band irrespective of condition and grouping electrodes according to the value of their SL and to statistically significant results. The cut‐off values for including a channel in a functional grouping were determined by the significance of the result obtained in the statistical tests. For example, in the 7–10 Hz frequency band for the premovement (stage 1), SL values for FZ, P4, and P3 were 0.0795, 0.0639, and 0.0624, respectively, independent of condition. These electrodes could be ranked in the following decreasing order: FZ, P4, and P3. Tukey's HSD test revealed that FZ was not statistically different from P4. P4 was not significantly different from P3, but FZ was statistically different from P3. Two groupings were made according to the SL values' statistical equality (Fig. 3). The first grouping included FZ and P4, and the second grouping comprised P4 and P3. These two groups were nonhomogeneous, since P4 belonged to two different groupings. This way of proceeding allows a quick and clear view of significant differences between electrodes. All statistical analyses were performed using Statistica (1997).
Figure 3.

Electrode classification in groups for each frequency band and each stage of the movement.
RESULTS
Mean Synchronization Likelihood
Four separate 3(stages) × 2(conditions) ANOVAs were computed (see Table III).
Table III.
Summary of the 3(stages) × 2(conditions) ANOVAs for each frequency band
| 7–10Hz | 10–13Hz | 13–20Hz | 20–30Hz | |||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| S | 331.25 | * | 114.62 | * | 149.48 | * | 152.02 | * |
| C | 0.006 | 0.94 | 0.366 | 0.56 | 0.038 | 0.85 | 1.699 | 0.22 |
| S × C | 0.252 | 0.78 | 0.583 | 0.57 | 0.699 | 0.51 | 0.440 | 0.65 |
P < 0.000001.
S, stage; C, condition.
No interaction and no main effect for the conditions factor were found at any frequency band (see Table III). However, ANOVAs yielded significant main effects for the stages factor for the 7–10 Hz band, F(2,22) = 331.25, P < 0.000001; the 10–13 Hz band, F(2,22) = 114.62, P < 0.000001; the 13–20 Hz band, F(2,22) = 149.48, P < 0.000001; and the 20–30 Hz band, F(2,22) = 152.02, P < 0.000001 (see Table III).
Tukey's HSD post‐hoc tests were computed, since ANOVAs displayed main effects for the stages factor. Irrespective of observation or execution, four results were found. For the 10–13 Hz, 13–20 Hz, and 20–30 Hz bands, synchronization for the premovement stage was significantly lower than the movement stage (P = 0.000216; P = 0.000558; P = 0.016553). For the 7–10 Hz band, there was no change in synchronization from the premovement to the movement stage (Fig. 4). For all the frequency bands, synchronization was significantly higher in the baseline than the premovement phase (P = 0.000136, 7–10 Hz band; P = 0.000136, 10–13 Hz; P = 0.000136, 13–20 Hz; P = 0.000136, 20–30 Hz). For all the frequency bands, synchronization was significantly higher in the baseline than in the movement phase (P = 0.000136, 7–10 Hz band; P = 0.000136, 10–13 Hz; P = 0.000136, 13–20 Hz; P = 0.000136, 20–30 Hz) (see Figs. 4, 5 for more detailed information).
Figure 4.
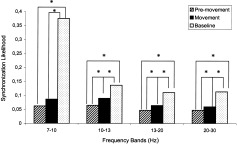
Mean synchronization likelihood in each of the four frequency bands irrespective of condition.
Figure 5.
Example of the synchronization likelihood (SL) variability over time in the (A) 7–10 Hz, (B) 10–13 Hz, (C) 13–20 Hz, and (D) 20–30 Hz frequency bands for a single participant during the observation condition (column 1) and the execution condition (column 2): (a) pre‐movement stage; (b) movement stage; (c) Baseline stage. The abscissa represents time (in seconds), the ordinate indicates the EEG channels. The value of the SL for each channel and each time point is indicated through a grey scale; darker shades correspond to higher levels of synchronization. The numbers on the right scale indicate the average synchronization values for each of the channels. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
Synchronization Likelihood for Electrode Sites
Twelve separate 2(conditions) × 19(electrode sites) ANOVAs were computed (Table IV). No main effect was found for the condition factor in any frequency band at any stage of the movement (i.e., baseline, premovement, and movement stages). However, ANOVAs revealed a significant main effect for the electrode site factor across the frequency bands and for all the stages of the movement (Table IV).
Table IV.
Summary of the 2(conditions) × 19(electrode sites) ANOVAs for each frequency band and for each stage presented
| 7–10Hz | 10–13Hz | 13–20Hz | 20–30Hz | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Pre‐mvt | Mvt | Baseline | Pre‐mvt | Mvt | Baseline | Pre‐mvt | Mvt | Baseline | Pre‐mvt | Mvt | |||||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| C | 0.024 | 0.88 | 2.442 | 0.15 | 2.067 | 0.18 | 0.146 | 0.71 | 0.764 | 0.40 | 2.344 | 0.15 | 0.043 | 0.84 | 1.373 | 0.27 | 0.172 | 0.69 | 1.344 | 0.27 | 1.669 | 0.22 | 1.177 | 0.30 |
| ES | 5.128 | *** | 11.87 | ***, * | 8.652 | *** | 4.005 | *** | 4.771 | *** | 3.846 | ** | 12.25 | *** | 10.27 | *** | 11.75 | *** | 6.802 | *** | 9.594 | *** | 9.158 | *** |
| CxES | 0.833 | 0.66 | 0.793 | 0.71 | 2.166 | * | 0.587 | 0.91 | 1.331 | 0.17 | 0.718 | 0.79 | 0.277 | 0.99 | 0.514 | 0.95 | 1.258 | 0.22 | 0.787 | 0.71 | 0.455 | 0.97 | 0.263 | 0.99 |
P < 0.006,
P < 0.00001,
P < 0.000001.
Mvt, movement; C, condition; ES, electrode site.
Tukey's HSD test completed on the main electrode sites effects allowed the classification of electrodes into groups. Electrode sites were grouped by similar SLs. Up to seven electrode groups were identified, depending on the movement stage and the frequency band (Fig. 3). None of the groups were homogeneous. Regardless of the movement stage and the conditions, electrode classification can be summarized thus: for the 7–10 Hz and 13–20 Hz bands, SL values of F3, F4, FZ, C3, C4, CZ, and P3 were significantly higher than those of F7, F8, O1, O2, and T6; for the 10–13 Hz band, FZ was significantly higher than O1, O2, and F8. For the 20–30 Hz, SL values of FZ, C3, C4, CZ, and PZ were significantly higher than those of F7, F8, T3, T4, T5, T6, FP2, O1, and O2 (Fig. 3).
Significant condition‐electrode site interaction was only observed for the 7–10 Hz band in the movement stage, F(18,198) = 2.166, P < 0.006 (Table IV). Tukey's HSD post‐hoc test analysis showed no significant differences for any given electrode under the conditions of observation and execution (Fig. 6).
Figure 6.
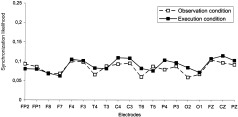
Synchronization likelihood for each of the 19 electrodes in the conditions of observation and execution in the 7–10 Hz band.
DISCUSSION
The aim of this study was to compare directly the EEG activity prior to and during the physical execution of a self‐paced finger movement with that prior to and during the observation of the same task in frequency bands 7–30 Hz.
The discussion is organized into two sections. The first discusses the existence of an execution/observation matching system in humans. The second considers the variability of EEG synchronization in the four frequency bands.
While there was evidence for local synchronization and global coupling, interpretation of these results should be made cautiously. The data analysis technique of SL does not allow for absolute comparison with research that has not used this approach. SL considers more global integration and synchronization of brain activity, whereas measures of event‐related synchronization and desynchronization (ERS/ERD) power assess local changes in activation. In addition, SL measurement does not depend on amplitude. Therefore, a local decrease in power may coincide with a global increase in coupling. For example, Gerloff et al. [1998] have shown that changes in regional EEG power and EEG coherence describe different aspects of cortical activity and thus act independently.
Execution/Observation Matching System
There was no significant difference in SL between the observation and execution data. Higher SL values were found during the baseline condition in comparison to those obtained during the premovement and movement stages irrespective of conditions. This finding suggests that interactions between brain areas are more extensive during a resting state than during an active state. This point is supported by Mazoyer et al. [2001], Shulman et al. [1997], and Wicker et al. [2003]. In their meta‐analysis, they have shown that groups of brain areas were active at rest (i.e., in a state where the eyes are closed, during visual fixation, during the passive observation of a visual stimuli, or in situations in which relaxing and thinking of nothing was proposed) and that this activity decreased during the completion of diversified goal‐directed actions such as visual tasks [Corbetta et al.,1995], cognitive tasks [e.g., Greicius et al.,2003], and tasks in which the participants had to focus on external stimuli [e.g., Brunet et al.,2000]. The interpretation of these findings has been that a resting state is far from being an inactive state [e.g., Binder et al.,1999]. Sustained information processing occurs during this state and may be attenuated when individuals are involved in a task [Gusnard and Raichle,2001]. More specifically, when a person is not actively committed to a behavior, mental activities occur spontaneously, such as daydreams [e.g., Stark and Squire,2001], free association [e.g., Mazoyer et al.,2001], autobiographic episodes [e.g., Mazoyer et al.,2001], and inner speech and imagery [e.g., Mazoyer et al.,2001]. Ingvar [1985] and Gusnard and Raichle [2001] suggested that these unpremeditated activities do not express noise but are a simulation of behaviors, an inner rehearsal or optimization of cognitive and behavioral serial programs.
Wicker et al. [2003] has suggested that the choice of an appropriate baseline is particularly problematic for cognitive tasks involving integrated brain areas. The baseline values may display variations both within and across participants. Bearing this in mind, the function of the baseline measure in this study was to reference a state of “zero activity” [Stark and Squire,2001] to be compared with the experimental states examining the processes of action and observation of action.
The data from this study suggest a close functional equivalence, in terms of neuronal synchronization, between the observation and execution conditions. No significant difference in SL was found between the two conditions irrespective of the stage of the sequential finger movement (i.e., prior to and during the movement). This finding offers support to Kilner et al. [2004], who showed the presence of a readiness potential prior to observation of an action. The data are also consistent with Cochin et al. [1999], Babiloni et al. [2002], Muthukumaraswamy and Johnson [2004], and Muthukumaraswamy et al. [2004].
Cochin et al. [1999] showed equivalence in EEG data and especially in lower alpha‐band power over posterior frontal cortex, motor cortex, posterior temporal cortex, and centroparietal cortex, during the observation and execution of finger movements. Within the same frequency band, we also found the greatest SL in these regions irrespective of condition. Similarly, Muthukumaraswamy et al. [2004] reported an attenuation of the mu rhythm (an 8–13 Hz rhythm generated by the sensorimotor cortex) during both observation and execution of a precision grip. Babiloni et al. [2002] also found comparable alpha and beta synchronization and desynchronization values in central regions overlying premotor and primary sensorimotor cortex during observation and action of an aimless middle‐finger extension task. Therefore, the nature of the task, whether it is goal‐directed or aimless, seems to have had little effect on the responsivity of the observation/execution matching system and supports the findings of Fadiga et al. [1995], Maeda et al.[2002], and Iacoboni et al. [1999]. However, certain caution should be exercised when considering such an assertion. Muthukumaraswamy and Johnson [2004] and Muthukumaraswamy et al. [2004] recently reported the sensitivity of the mu rhythm to different forms of observed motor behaviors. Mu rhythms were desynchronized during the observation of effector‐object interaction and, to a lesser extent, the observation of motorically equivalent but nonobject‐directed movement.
Our results offer some support for the existence of a mirror neuron system in humans. However, it is possible that, as Muthukumaraswamy and Johnson [2004] asserted, the results show only a tenuous link with “true” mirror neuron activity since it is improbable that surface EEG can directly record the activity of such neurons. EEG may also be insensitive to “real” differences that are present between the observation and execution of a movement.
There is strong evidence that cortical motor areas are active during observation and execution of actions, [e.g., Fadiga et al.,1995] even though during the observation of an action no discernible muscle activity is present [Baldissera et al.,2001; Gallese et al.,1996; Muthukumaraswamy and Johnson,2004; Rizzolatti and Craighero,2004]. The activation of the motor system and the absence of electromyographic (EMG) activity during observation have been explained by motor output being blocked through an inhibitory mechanism at the level of the spinal cord [Jeannerod,2001; Rizzolati and Craighero,2004]. Therefore, EEG measures may not be able to account for EMG differences between observation and execution of an action, since the inhibitory mechanism, which is at the origin of these differences, operates outside of the scalp region. It may also be possible that differences across observation and execution are related to functional localization and not to functional integration. This suggestion is consistent with the findings of Grèzes and Decety [2001]. In their meta‐analysis, which was limited to PET and fMRI studies, they showed that the brain areas involved during action observation did not completely overlap those which were identified during the execution of the same action. Although some common cortical areas were activated in both situations (e.g., supramarginal gyrus and dorsal premotor cortex), some were only involved in execution (e.g., primary motor cortex, sensorimotor cortex, opercular premotor cortex), whereas others were exclusively seen in observation (e.g., inferior, superior, and middle temporal gyrus). EEG, which is arguably a weaker technique for the study of functional localization, may not be as effective in locating regions of the brain that are directly responsible for motor, sensory, or cognitive functions. Finally, a difference between execution and observation could lie in deep motor structures, such as basal ganglia, whose activity is not present in scalp EEG.
A further methodological issue requiring consideration relates to the instructions provided to the participants. In this study, the participants were instructed to observe the movements with the intention of repeating it at a later stage. This requirement led to a greater attention to the task and a subsequent retention phase. Both these behaviors have been found to influence the nature of cortical activity. For example, Decety et al. [1997] and Grèzes et al. [1998] have shown the involvement of the dorsolateral prefrontal cortex, the presupplementary motor area, and the dorsal pathway when participants were provided with the instructions to observe a movement with the later requirement to imitate it. The results of the present study are therefore consistent with those of Decety et al. [1997] since, in all frequency bands, the greatest synchronization likelihood was seen in the frontal areas (F3, Fz, F4), motor areas (C3, Cz, C4), and parietal area (P3, Pz, P4) (Fig. 3). This finding emphasizes the importance of clear, well‐understood, and consistent instruction provided to the participants in this type of research in order to reduce instructional content as a possible confounding variable within the datasets.
Frequency Bands
Since there was no statistically significant difference between the execution and observation data in the present study, the results have been compared to research that has independently studied perception and observation and that which considered motor tasks such as self‐paced voluntary movements.
In all the frequency bands except for 7–10 Hz, there was a significant increase in synchronization from the premovement to the movement phase irrespective of condition. This finding is similar to studies that have described local cortical activity during movement preparation and execution, in that changes in brain oscillations are connected with the different stages of the movement. General findings indicate that upper alpha band desynchronization (10–13 Hz) starts about 2.5 s before a movement starts. After the movement onset, alpha returns to its initial level of synchronization within a few seconds [e.g., Stancak and Pfurtscheller,1996; Toro et al.,1994]. In contrast, beta desynchronization (14–30 Hz) begins around 1.5 s before the movement onset [e.g., Derambure et al.,1993], reaches its maximum level before the end of the movement, and is followed by synchronization reaching a maximum after the movement execution [Pfurtscheller et al.,1996; Stancak and Pfurtscheller,1995]. It is important to note that beta synchronization occurs while the upper alpha rhythm still displays a desynchronization.
As discussed above, interpretation of the results should be made cautiously, since local and global activity may depict different aspects of cortical activity. We would support further consideration of this relationship during physical execution and observation of a movement. However, we have compared our results with those of two studies that considered coherence estimates [Manganotti et al.,1998; Serrien et al.,2003]. While this indicator is not entirely suitable to consider nonstationary data with rapidly changing interdependencies [Stam and van Dijk,2002], alpha band and beta band activity was contrasted.
The profile of the upper and lower alpha band activity revealed coupling in the frontal, central, and parietal regions during observation and action across the premovement and the movement stages. These findings are consistent with those of Serrien et al. [2003]. They found a stronger coherence in the contralateral hemisphere than in the ipsilateral hemisphere for a right‐hand visual‐manual reaction time task. EEG coherence increased between the left sensorimotor area and the frontal (C3–F3, C3–FC3) and parietal (C3–P3) regions during movement preparation in the 8–12 Hz frequency band. Stam et al. [2002] have shown lower alpha band decrease in synchronization during the retention phase of a working memory task. They interpreted this as reflecting attentional processes. This finding supports the point of view of Klimesch [1996] and Klimesch et al. [1996]. Although the authors characterized synchronization in terms of local ERD/ERS effects, they suggested that local desynchronization in the lower alpha band is associated with attentional processes and upper alpha band desynchronization with semantic memory. The functional meaning of long‐distance coupling in the alpha band is less clear [Stam et al.,2003] but may provide support for an intentional focus during the finger movement during both observation and action [see Wertheim,1974,1981].
In the case of beta activity (13–20 Hz and 20–30 Hz), the greatest synchronization likelihood was seen in the frontal areas (F3, Fz, F4), motor areas (C3, Cz, C4), and parietal areas (P3, Pz, P4). This result supports Manganotti et al.'s [1998] findings. They examined coherence changes during sequential finger movements in the lower beta frequency band. They showed that task‐related coherence increases were more important in electrode pairs located in the frontal, central, and parietal areas. Task‐related coherence decreases were detected for electrode pairs situated in the temporal, occipital, and prefrontal areas. Beta band coherence changes were also smaller than those of alpha (8–12 Hz), which was also the case in the present study.
CONCLUSION
This study provides support for similar EEG synchronization patterns in the frequency band 7–30 Hz for execution and observation of an action not directed towards an object. The findings also revealed a common cortical profile for the premovement stages under the conditions of observation and execution.
The new assessment technique of synchronization likelihood provided a normalized estimate of the dynamical interdependencies between the simultaneously recorded EEG time series. The increase in SL may reflect interregional cortical coupling of intricately and hierarchically interconnected networks [Classen et al.,1998]. The largest synchronization likelihood values, found during resting state, suggest that sustained information processing is held during this state and attenuated when individuals perform a motor task [Gusnard and Raichle,2001]. This may reflect a special role for resting states that are not yet fully understood and that warrant further investigation.
Finally, while we cannot conclusively provide direct support for the existence of the mirror system in humans, these results shed some light on the mechanisms of cognitive integration in observation and execution conditions.
Acknowledgements
We thank the participants in this study. We also thank D. Jelobaieff and D. Keil for sharing their EEG knowledge, A. Brossier and C. Debouzy for their knowledge in computer science and electronics, and M. Paisley for the video footage editing.
Mathematical Background of Synchronization Likelihood
Synchronization likelihood (SL) is a measure of the generalized synchronization between two dynamical systems X and Y [Stam and van Dijk,2002]. Generalized synchronization [Rulkov et al.,1995] exists between X and Y of the state of the response system is a function of the driver system: Y = F(X). The first step in the computation of SL is to convert the time series xi and yi recorded from X and Y as a series of state space vectors using the method of time delay embedding [Takens,1981]:
| (1) |
where L is the time lag and m is the embedding dimension. From a time series of N samples, N‐(m × L) vectors can be reconstructed. State space vectors Yi are reconstructed in the same way.
SL is defined as the conditional likelihood that the distance between Yi and Yj will be smaller than a cutoff distance ry, given that the distance between Xi and Xj is smaller than a cutoff distance rx. In the case of maximal synchronization this likelihood is 1; in the case of independent systems it is a small but nonzero number, namely Pref. This small number is the likelihood that two randomly chosen vectors Y (or X) will be closer than the cut‐off distance r. In practice, the cut‐off distance is chosen such that the likelihood of random vectors being close is fixed at Pref, which is chosen the same for X and for Y. To understand how Pref is used to fix rx and ry, we first consider the correlation integral:
| (2) |
Here the correlation integral Cr is the likelihood that two randomly chosen vectors X will be closer than r. The vertical bars represent the Euclidean distance between the vectors. N is the number of vectors, w is the Theiler correction for autocorrelation [Theiler,1986], and θ is the Heaviside function: θ(X) = 0 if X > = 0 and θ(X) = 1 if X < 0. Now, rx is chosen such that Crx = Pref and ry is chosen such that Cry = Pref. The SL between X and Y can now be formally defined as:
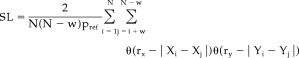 |
(3) |
SL is a symmetric measure of the strength of synchronization between X and Y (SLXY = SLYX). In Eq. 3 the averaging is done over all i and j; by doing the averaging only over j, SL can be computed as a function of time i. From Eq. 3 it can be seen that in the case of complete synchronization SL = 1 and in the case of complete independence SL = Pref. In the case of intermediate levels of synchronization Pref < SL < 1.
REFERENCES
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Del Percio C, Vito Moretti D, Rossini PM (2002): Quantitative EEG: modeling time, space, and phase of brain oscillatory activity In: Reisin RC, Nuwer MR, Hallett M, Medina C, editors. Advances in clinical neurophysiology. Amsterdam: Elsevier Science; p 284–288. [Google Scholar]
- Baldissera F, Cavallari P, Craigero L, Fadiga L (2001): Modulation of spinal excitability during observation of hand actions in humans. Eur J Neurosci 13: 190–194. [DOI] [PubMed] [Google Scholar]
- Billard A, Arbib M (2002): Mirror neurons and the neural basis for learning by imitation: computational modelling In: Stamenov MI, Gallese V, editors. Mirror neurons and the evolution of brain and language. Amsterdam: John Benjamins; p 343–353. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy‐Bayle MC, Decety J (2000): A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage 11: 157–166. [DOI] [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi N, Porro CA, Rizzolatti, G (2004a): Neural circuits involved in the recognition of actions performed by nonspecifics: an fMRI study. J Cogn Neurosci 16: 114–126. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund H‐J, Rizzolatti G (2004b): Neural circuits underlying imitation learning of hand actions: an event‐related fMRI study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Calderon CAA, Hu H (2003): Robotic societies: elements of learning by imitation. Proc 21st IASTED Int Conf Appl Informatics, Innsbruck, Austria. p 315–320.
- Chatrian GE (1976): The mu rhythms In: Remond A, editor. Handbook of electroencephalography. Amsterdam: Elsevier; p 104–114. [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M (1998): Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol 79: 1567–1573. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J (1998): Perception of motion and qEEG activity in human adults. Electroenceph Clin Neurophysiol 107: 287–295. [DOI] [PubMed] [Google Scholar]
- Cochin S, Bathelemy C, Roux S, Martineau J (1999): Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci 11: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shuman GL, Miezin FM, Petersen SE (1995): Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270: 802–805. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2003): Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 19: 764–776. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F (1997): Brain activity during observation of actions. Influence of action content and subject's strategy. Brain 120: 1763–1777. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T, Grèzes J, Meltzoff AN (2002): A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage 15: 265–272. [DOI] [PubMed] [Google Scholar]
- Demeris J, Hayes G (1996): Imitative learning mechanisms in robots and humans. In: Proc 5th Eur Workshop Learn Robots, Bari, Italy. p 9–16.
- Derambure P, Dujardin K, Defebvre L, Bourriez JL, Jacquesson JM, Guieu JD (1993): Etude spatiotemporelle des désynchronisations liées à l'événement au cours d'une activité motrice autocommandée [Spatiotemporal study of event‐related desynchronization during self‐paced movement]. Neurophysiol Clin 23: 337–351. [DOI] [PubMed] [Google Scholar]
- Derambure P, Defebvre L, Bourriez JL, Cassim F, Guieu JD (1999): Désynchronisation et synchronisation liées à l'événement. Etude de la réactivité des rythmes électrocorticaux en relation avec la planification et l'exécution du mouvement volontaire [Event‐related desynchronisation and synchronization. Reactivity of cortical electroencephalographic rhythms related to planning and performance of voluntary movement]. Neurophysiol Clin 29: 53–70. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (1995): Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73: 2608–2611. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS (2003): Action plans used in action observation. Nature 424: 769–771. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2000): The labile brain. I. Neuronal transients and nonlinear coupling. Philos Trans R Soc Lond B Biol Sci 355: 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996): Action recognition in the premotor cortex. Brain 119: 593–609. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen L (1997): Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 120: 1587–1602. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M (1998): Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531. [DOI] [PubMed] [Google Scholar]
- Greicius M, Krasnow B, Reiss A, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Decety J (2001): Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta‐analysis. Hum Brain Mapp 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Costes N, Decety J (1998): Top‐down effect of strategy on the perception of human biological motion: a PET investigation. Cogn Neuropsychol 15: 553–582. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G (1998): Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C (2001): Causes and consequences of imitation. Trends Cogn Sci 5: 253–261. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC (1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Ingvar DH (1985): Memory of the future: an essay on the temporal organization of conscious awareness. Hum Neurobiol 4: 127–136. [PubMed] [Google Scholar]
- Jasper HH (1958): Report of the committee on methods of clinical examination in electroencephalography. Electroenceph Clin Neurophysiol 10: 370–375. [Google Scholar]
- Jeannerod M (2001): Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14: S103–S109. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore S‐J, Sirigu A (2004): Motor activation prior to observation of a predicted movement. Nat Neurosci 7: 1299–1301. [DOI] [PubMed] [Google Scholar]
- Klimesch W (1996): Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol 24: 61–100. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Doppelmayer M, Ripper B, Schwaiger J, Pfurtscheller G (1996): Event‐related desynchronization (ERD) and the Dm effect: does alpha desynchronization during encoding predict later recall performance? Int J Psychophysiol 24: 47–60. [DOI] [PubMed] [Google Scholar]
- Maeda F, Kleiner‐Fisman G, Pascual‐Leone A (2002): Motor facilitation while observing hand actions: specificity of the effect and role of observer's orientation. J Neurophysiol 87: 1329–1335. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M (1998): Task‐related coherence and task‐related spectral power changes during sequential finger movements. Electroenceph Clin Neurophysiol 109: 50–62. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK (1977): Imitation of facial and manual gestures by human neonates. Science 198: 75–78. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK (1997): Explaining facial imitation: a theoretical model. Early Dev Parent 6: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW (2004): Changes in Rolandic mu rhythm during observation of a precision grip. Psychophysiology 41: 152–156. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair, NA (2004): Mu rhythm modulation during observation of an object‐directed grasp. Cogn Brain Res 19: 195–201. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R (2000): Temporal dynamics of cortical representation for action. Neurobiology 97: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pereda E, Rial R, Gamundi A, Gonzalez J (2001): Assessment of changing interdependencies between human electroencephalograms using non‐linear methods. Physica D 148: 147–158. [Google Scholar]
- Pfurtscheller G (1988): Mapping of event‐related desynchronization and type of derivation. Electroenceph Clin Neurophysiol 70: 190–193. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A (1977): Event‐related cortical desynchronization detected by power measurement of scalp EEG. Electroenceph Clin Neurophysiol 42: 817–826. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Neuper C (1996): Post‐movement beta synchronization. A correlate of an idling motor area? Electroenceph Clin Neurophysiol 98: 281–293. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes Da Silva FH (1999): Event‐related desynchronization. Handbook of Electroencephalography and clinical neurophysiology revised series. Amsterdam: Elsevier. [Google Scholar]
- Rizzolatti G, Craighero L (2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Carmada R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988): Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71: 491–507. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996): Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V (2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L, Fadiga L (2002): The mirror system in humans In: Stamenov MI, Gallese V, editors. Mirror neurons and the evolution of brain and language. Amsterdam: John Benjamins; p 37–63. [Google Scholar]
- Rulkov NF, Sushchik MM, Ysimring LS, Abarbanel HDI (1995): Generalized synchronization of chaos in directionally coupled chaotic systems. Phys Rev E 51: 980–994. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R (1994): Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550. [DOI] [PubMed] [Google Scholar]
- Schiff SJ, So P, Chang T, Burke RE, Sauer T (1996): Detecting dynamical interdependence and generalized synchrony through mutual prediction in a neural ensemble. Phys Rev E 54: 6708–6724. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Fisher RJ, Brown P (2003): Transient increases of synchronized neural activity during movement preparation: influence of cognitive constraints. Exp Brain Res 153: 27–34. [DOI] [PubMed] [Google Scholar]
- Servos P (2000): Functional neuroimaging of mental chronometry. Brain Cogn 42: 72–74. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin F, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks. II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Dijk BW (2002): Synchronization likehood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D 163: 236–251. [Google Scholar]
- Stam CJ, van Woerkom TCAM, Pritchard WS (1996): Use of non‐linear EEG measures to characterize EEG changes during mental activity. Electroenceph Clin Neurophysiol 99: 214–224. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Micheloyannis S (2002): Variability of EEG synchronization during a working memory task in healthy subjects. Int J Psychophysiol 46: 53–66. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Breakspear M, van Cappellen van Walsum AM, van Dijk, BW (2003): Non linear synchronization in EEG and whole‐head MEG recordings of healthy subjects. Hum Brain Mapp 19: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancak A, Pfurtscheller G (1995): Desynchronization and recovery of beta rhythms during brisk and slow self‐paced finger movements in man. Neurosci Lett 196: 21–24. [DOI] [PubMed] [Google Scholar]
- Stancak A, Pfurtscheller G (1996): The effects of handedness and type of movement on the contralateral preponderance of μ‐rhythm desynchronisation. Electroenceph Clin Neurophysiol 99: 174–182. [DOI] [PubMed] [Google Scholar]
- Stancak A, Feige B, Lucking CH, Kristeva‐Feige R (2000): Oscillatory cortical activity and movement‐related potentials in proximal and distal movements. Clin Neurophysiol 111: 636–650. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Squire LR (2001): When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A 98: 12760–12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takens F (1981): Detecting strange attractors in turbulence. Lecture Notes Math 898: 366–381. [Google Scholar]
- Theiler J (1986): Spurious dimension from correlation algorithms applied to time‐series data. Phys Rev A 34: 2427–2432. [DOI] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M (1994): Event‐related desynchronization and movement‐related cortical potentials on the EcoG and EEG. Electroenceph Clin Neurophysiol 93: 380–389. [DOI] [PubMed] [Google Scholar]
- Van Putten MJAM, Stam CJ (2001): Application of a neural complexity measure to multichannel EEG. Phys Lett A 281: 131–141. [Google Scholar]
- Wertheim AH (1974): Oculomotor control and occipital alpha activity: a review and a hypothesis. Acta Psychol 38: 235–256. [DOI] [PubMed] [Google Scholar]
- Wertheim AH (1981): Occipital alpha activity as a measure of retinal involvement in oculomotor control. Psychophysiology 18: 432–439. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet J‐P, Fonlupt P (2003): A relation between rest and the self in the brain? Brain Res Rev 43: 224–230. [DOI] [PubMed] [Google Scholar]
- Wohlschlager A, Bekkering H (2002): Is human imitation based on mirror‐neuron system? Some behavioural evidence. Exp Brain Res 143: 335–341. [DOI] [PubMed] [Google Scholar]