Abstract
The left hemispheric dominance for complex motor behavior is undisputed. Clinical observations of complex motor deficits in patients with right hemispheric lesions, however, suggest an additional contribution of the right hemisphere to higher motor control. We assessed, using functional MRI (fMRI), which brain regions are implicated in processing the spatial aspects of complex, object‐related actions. Using a blocked, factorial design, 17 healthy volunteers were asked to detect either spatial or sequential errors (factor ERROR) in complex activities of daily living, presented as video sequences with the appropriate object(s) or as pantomimes (factor STIMULUS). Observing complex actions (irrespective of stimulus type) activated a bilateral frontoparietal network. Observing actions with objects (relative to pantomimes) differentially increased neural activity in the fusiform gyrus and inferior occipital cortex bilaterally. Observing pantomimes, i.e., the same actions but without any object, differentially activated right prefrontal cortex, anterior cingulate cortex, the precuneus, and left cerebellum. The left cingulate cortex was differentially activated when subjects assessed the sequencing of actions. By contrast, assessing the spatial configuration of complex actions differentially increased neural activity in right posterior parietal cortex. A significant interaction of ERROR and STIMULUS was revealed for the right inferior parietal cortex only. These findings suggest a specific role of the right hemisphere, especially of right posterior parietal cortex, in processing spatial aspects of complex actions and thus provide a physiological basis for the observed apraxic motor deficits in patients with right hemispheric damage. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: visuospatial attention, apraxia, object use, pantomime, tool use
INTRODUCTION
Since Liepmann's seminal work [Liepmann,1905], the left‐hemisphere dominance for praxis has been confirmed by many lesion studies [e.g., Haaland et al.,2000; Kimura and Archibald,1974]. However, patients with right hemispheric lesions may also show action performance errors that go beyond their sensorimotor deficit [De Renzi et al.,1980; Goldenberg,1996; Haaland and Flaherty,1984]. For example, De Renzi et al. [1980] investigated patients with left‐ or right‐hemisphere damage performing movement imitation and pantomimed object‐use. They found that both patient groups made more errors than control subjects. However, the errors of right hemisphere‐damaged patients were less frequent and less severe than those made by left hemisphere‐damaged patients. Analyzing the error types in patients with unilateral brain damage when imitating gestures or pantomiming object use, Haaland and Flaherty [1984] confirmed and extended these findings. With regard to the imitation tasks, left and right hemisphere‐damaged patients made similar errors, like hand position, arm position, or omission errors. In contrast, left hemisphere‐damaged patients were more impaired in the pantomime task, which also revealed differential error patterns for the two patient groups: left hemisphere‐damaged patients showed more arm‐position and “classical” body‐part‐as‐object (BPO) errors (i.e., a body part substitutes for an object while the hand/finger configuration resembles the object shape, e.g., an index finger touches the teeth as a toothbrush), whereas right hemisphere‐damaged patients showed more spatial BPO errors (a body part substitutes for an object while the hand/finger configuration is crude and incomplete, e.g., a fist touches the teeth as toothbrush). Taken together, the observed differences in error type, severity, and frequency between left‐ and right‐hemisphere–damaged patients during movement imitation and object use pantomime imply a specific contribution of each hemisphere to higher motor control.
Goldenberg [1996] tried to further elucidate the specific contributions of the two hemispheres by investigating the imitation of meaningless gestures (hand positions and finger configurations) in patients with either left or right brain damage. He hypothesized that imitation of hand positions would require a specific knowledge of the human body as a complex tool [Goldenberg,1995] and would, therefore, be more vulnerable to damage of the left hemisphere. In contrast, imitation of finger configurations might rely on an exact visual‐spatial analysis of the presented gesture that might be disturbed by right hemisphere lesions. Supporting his hypothesis, Goldenberg [1999] found differential deficits in imitating hand positions and finger configurations in left‐ and right‐hemisphere‐damaged patients, respectively.
Goldenberg's hypothesis and data are, of course, consistent with neuropsychological and functional imaging evidence implicating the right hemisphere and especially the right parietal cortex in visual‐spatial processing [for review, see, e.g., Fink and Heide,2004; Halligan et al.,2003; Marshall and Fink,2001]. However, the specific contribution of the right hemisphere to processes of (higher) motor control remains to be established. Taking into account the specific role of the right posterior parietal cortex in visual‐spatial processes such as visual‐spatial judgments and orienting as well as reorienting of spatially directed attention [Corbetta and Shulman,2002; Fink et al.,2000,2001,2003; Thiel et al.,2004], it can be hypothesized that the right hemisphere may be engaged when attention to the spatial aspects of an action is required, e.g., to the exact spatial interplay between a tool and its corresponding object, or to the spatial layout when exactly imitating finger positions.
To test this hypothesis we conducted a functional imaging experiment, in which healthy subjects were asked to detect errors of either the spatial organization or the sequential structure of complex actions (activities of daily living; factor ERROR). As studies of patients with higher‐order motor deficits have revealed that some patients show a specific impairment in object use [De Renzi and Lucchelli,1988], while other patients are mainly impaired in pantomime [Rothi et al.,1985], two different types of stimuli were used in our experiment. In half of the experimental trials the stimuli were actions executed with the appropriate object(s); in the other half of trials the same actions were pantomimed, i.e., no object was present (factor STIMULUS). This constituted a two‐factorial design that not only enabled us to analyze the main effect of error analysis (i.e., spatial vs. temporal/sequential) and stimulus type (i.e., processing of actions with objects vs. pantomimed actions), but also possible interactions between these factors.
SUBJECTS AND METHODS
Subjects
Seventeen healthy, right‐handed male volunteers (mean age, 23; range, 21–25), with no current or past neurological or psychiatric disease, gave informed consent. Only male participants were studied in order to reduce intersubject variation in brain size and shape. In turn, however, including only male participants compromises the generalizability of our study since we cannot comment on possible sex differences in praxis processing [Kimura,1982,1983]. All subjects reported strong right‐hand preference as measured by the Edinburgh Handedness Inventory [Oldfield,1971]. The study was approved by the local ethics committee of the University Hospital of the RWTH Aachen, and complied to the Code of Ethics of the World Medical Association (Declaration of Helsinki; revised version, October, 2000).
Experimental Stimuli and Setup
Thirty different action sequences performed with both hands were selected, e.g., affixing a stamp on a letter, filling a glass with water and drinking the water, lighting a candle using matches, cleaning glasses, etc. Every action sequence was recorded on video and later presented in six different ways, yielding a total of 180 action sequences: actions with the appropriate object(s) without an error, with a sequential error, or with a spatial error; pantomimed actions without an error, with a sequential error, or with a spatial error. For example, the sequence of filling a glass (i.e., filling a glass with water and then drinking the water) was recorded twice (with or without the corresponding objects) without error, twice with the water being poured next to the glass (as a spatial error, once with the objects, once as a pantomime) and twice showing the actor drinking, before the glass was filled (as a sequential error, again once with the objects, once as a pantomime). Each video sequence had a fixed duration of 6 s, showing the action sequences performed half by a black‐dressed actor and half by a black‐dressed actress sitting centrally at a table with a black cover. Video sequences were recorded from a fixed perspective with a digital video camera (Sony DCR‐PC100E, Tokyo, Japan). The field of view was centered on the upper part of the actor's body, including the actor's arms, and the table, on which the actions took place. The other aspects of the scene, i.e., the background, were kept constant throughout all sequences. Since in all trials both hands were used for action performance, the movements occupied both the right and left side of space/visual field to a similar extent. The digital videos were processed with the help of an image editing software (Adobe Premiere 6.0, San Jose, CA) in order to adjust the sequence duration and to change the software‐specific format into avi‐format (24 bit, 25 frames/sec, 524 × 400 pixel) for further presentation. Microsoft Powerpoint 2000 was used for visual stimulus presentation. The videotaped action sequences were projected by a shielded LCD‐beamer onto a stimulus display (diameter 29 cm, horizontal visual angle 60°, and vertical visual angle 30°) which was viewed by the subjects from a distance of 25 cm (14 cm screen to mirror, 11 cm mirror to subjects' eyes; the mirror reflected the stimulus display in correct orientation).
The videotaped action sequences were used to create experimental trials that all had the same structure. First, a short German description of the next action sequence was presented for 1 s. This description consisted of two parts, e.g., “Flasche öffnen – Glas füllen” (“Opening the bottle – Filling the glass”), depicting the following action sequence. This description was presented prior to each trial, since prescanning testing of the action sequences revealed that actions performed with the appropriate objects were recognized more easily than their pantomimed counterparts. The preceding description thus minimized performance differences due to the differential difficulty in recognizing pantomimed action sequences. The description was then followed by a videotaped action sequence lasting 6 s. Finally, a further display following the action sequence appeared for 1 s, showing the German words “Bitte antworten!” (“Please respond!”) prompting subjects to respond. Responses were made by button presses: a right index finger button press indicated “yes, the action sequence contained a spatial (or sequential) error” (depending on the respective task) and a right middle finger button press indicated “no, the action sequence did not contain a spatial (or sequential) error.” Subjects' responses were recorded using a tapping apparatus in which the button press interrupted an optic fiber light beam. Given the length of the action sequences displayed (6 s) and the variable onset of errors (in error trials), we considered reaction times to be noninformative and hence acquired only error rates.
Experimental Design
A blocked, 2 × 2 factorial design with the factors ERROR (spatial vs. sequential) and STIMULUS (actions with appropriate object(s) vs. pantomimed actions) was adopted. For the factor ERROR, subjects were asked to carefully watch the videotaped action sequences and to detect either spatial or sequential errors. It should be noted that across conditions a third of the actions contained an error in the relevant dimension and the remaining two‐thirds of trials were either correctly performed actions or actions with an error of the irrelevant dimension, e.g., in the spatial error conditions actions with a sequential/temporal error but correct spatial configuration were shown and had to be judged as correct. For the factor STIMULUS, either actions with objects or pantomimed actions were presented. This design yielded the following four experimental conditions: Detection of spatial errors in actions with objects (OS), detection of temporal/sequential errors in actions with objects (OT), detection of spatial errors in pantomimed actions (PS), and detection of temporal/sequential errors in pantomimed actions (PT). Conditions appeared in alteration with a low‐level baseline instructing the subject for the subsequent block. Apart from silently reading the instructions, no other task was performed during the baseline. Besides the presentation of task instructions, the baseline prevented an overlap of neural activity between conditions and therefore allowed us to separate the cerebral hemodynamic responses specific to the different experimental conditions.
Eye Movement Measurement and Analysis
All tasks were performed under free vision to avoid any interaction of covert attention with the tasks of interest [Fink et al.,1997]. Eye movements were measured to assess whether differential eye movements occurred across conditions. Due to a computer failure, eye movement data acquired during the scanning could not be used for further analysis. Therefore, additional eye measurements were performed outside the scanner replicating the experimental setup. Twelve of the 17 subjects took part in this additional eye movement study. For monitoring the eye positions relative to the screen and the stimuli thereon, an infrared device (iView system; Sensomotoric Instruments, Teltow, Germany) was used.
First, the data were analyzed for contamination by eye blinks. Thereafter, data were analyzed using the normalized x and y coordinates of the subjects' gaze on the screen. Pupil diameter changes were assessed by measuring the horizontal pupil diameter in arbitrary units. For each experimental condition, the data were analyzed for the length of the total scan path and the mean pupil diameter. The mean values of the experimental conditions were compared using a two‐way analysis of variance (ANOVA) with the factors ERROR (spatial vs. sequential) and STIMULUS (actions with object(s) vs. pantomimed actions).
fMRI Hardware and Procedures
Functional magnetic resonance images were acquired on a Siemens Vision (Erlangen, Germany) 1.5 T whole‐body scanner with echoplanar imaging (EPI) capability and using the standard radiofrequency headcoil for transmit and receive. Sequences with the following parameters were used: gradient‐echo EPI; TE = 66 ms; TR = 4.2 s; flip angle = 90°; 31 axial slices of 4.0 mm thickness; field of view (FOV) = 200 × 200 mm; matrix size = 64 × 64; pixel size = 3.125 × 3.125 mm; interslice gap = 0.4 mm. Using a midsagittal scout image, the slices were oriented along the anterior–posterior commissure (AC‐PC). Since the EPI sequence triggered data acquisition for each slice individually, the data were scaled to ensure interslice, intravolume comparability of the raw signal intensities. In addition, high‐resolution anatomical images were acquired for all subjects using the 3D MP‐RAGE (magnetization‐prepared, rapid acquisition gradient echo) sequence with the following parameters: TE = 4.4 ms, TR = 11.4 ms, flip angle = 15°; 1 excitation; matrix = 200 × 256; FOV = 230 mm, 128 sagittal slices of 1.41 mm slice thickness.
The functional MRI (fMRI) paradigm consisted of a baseline of 29.4 s (7 × TR) followed by 12 repetitions of a cycle with a 42‐s (10 × TR) activation period (containing five trials of 8‐s duration) and a 16.8‐s (4 × TR) baseline period. The order of conditions was counterbalanced across time series. Furthermore, time series were counterbalanced across subjects.
Image Processing
All calculations and image manipulations were performed on Sun Ultra 60 workstations (SUN Microsystems Computers, Palo Alta, CA) using Matlab 5.3 (MathWorks, Natick, MA) and SPM99 (Statistical Parametric Mapping software, SPM; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk). SPM was used for image realignment, image normalization, smoothing, and to create statistical maps of significant regional BOLD (blood oxygen level‐dependent) response changes [Friston et al.,1995a,c].
The first three images of each time series (175 images), during which the MRI signal reaches a steady state, were discarded. The remaining 172 volume images of each time series were automatically realigned to the first image (corresponding to the fourth acquired image of the time series) to correct for head movement between scans. Image sets of the conditions and baselines were then coregistered to the 3D anatomical dataset using SPM. The AC (anterior commissure) and PC (posterior commissure) points were identified and transformed into a standard stereotaxic space using the intracommissural line as a plane for transformation. This spatial transformation uses linear proportions and a nonlinear sampling algorithm and a representative brain from the Montreal Neurological Institute (MNI) series as a template. Data were thereafter expressed in terms of MNI stereotactic coordinates in the x, y, and z axes (as defined for Tables I and II). The resulting voxel size in standard stereotactic space was 2 × 2 × 2 mm3. Transformed functional datasets from each subject were smoothed with a Gaussian kernel of 10 mm (full‐width at half‐maximum, FWHM) to meet the statistical requirements of the theory of Gaussian fields presupposed by the General Linear Model (GLM) employed in SPM and to compensate for normal variation in individual brain size, shape, and sulcal/gyral anatomy across subjects. Voxels that had values greater than 0.8 of the volume mean in all the images were selected to restrict analysis to intracranial regions.
Table I.
All conditions vs. baseline (OS + OT + PS + PT > BL)
| Brain area | Side | Coordinates: x, y, z | Tmax |
|---|---|---|---|
| Occipitotemporal junction (V5) | R | 54, −64, 4 | 13.79 |
| L | −52, −68, 0 | 11.84 | |
| Superior temporal cortex | R | 56, −42, 20 | 9.22 |
| L | −58, −40, 18 | 8.52 | |
| Superior parietal cortex | R | 30, −52, 70 | 8.73 |
| L | −24, −56, 66 | 6.83 | |
| Superior occipital cortex | R | 26, −82, 30 | 10.19 |
| L | −26, −78, 36 | 8.37 | |
| Dorsolateral prefrontal cortex | R | 46, 28, 17 | 9.48 |
| L | −46, 30, 18 | 7.75 | |
| Ventrolateral prefrontal cortex | L | −52, 20, −8 | 6.86 |
| R | 50, 26, −12 | 6.25 | |
| Dorsal premotor cortex | R | 40, −2, 58 | 8.98 |
| L | −40, −2, 52 | 11.42 | |
| Ventral premotor cortex | R | 56, 15, 12 | 7.50 |
| L | −56, 16, 28 | 9.04 | |
| Anterior cingulate cortex | L | −4, 14, 44 | 8.01 |
| Fusiform gyrus | R | 42, −60, −16 | 12.31 |
| L | −46, −50, −18 | 9.55 | |
| Cerebellar hemisphere | R | 32, −68, −30 | 7.54 |
| L | −32, −66, −30 | 7.26 | |
| Cerebellar vermis | L | −12, −78, −30 | 8.13 |
| Insula | R | 36, 24, −6 | 8.83 |
| L | −30, 26, −4 | 7.93 | |
| Thalamus | L | −10, −16, 6 | 6.03 |
All activations are P ≤ 0.05 (corrected for multiple comparisons within the whole brain volume). Brain regions showing relative BOLD signal increases associated with the contrast all conditions versus baseline. For each region of activation, the coordinates in MNI stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest T‐value. x, distance (mm) to right (+) or left (−) of the midsagittal plane; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; z, distance above (+) or below (−) the inter‐commissural (AC‐PC) plane.
BL, baseline; OS, spatial analysis of actions with objects; OT, temporal/sequential analysis of actions with objects; PS, spatial analysis of pantomimed actions; PT, temporal/sequential analysis of pantomimed actions.
Table II.
Main effects and interaction
| Brain area | Side | Coordinates: x, y, z | k | Tmax |
|---|---|---|---|---|
| Pantomimed actions vs. actions with objects [(PS+PT) > (OS+OT)] | ||||
| Prefrontal cortex | R | 26, 50, 0 | 1006 | 5.54 |
| R | 38, 42, 16 | 344 | 5.08 | |
| Precuneus | R | 6, −68, 50 | 859 | 7.25 |
| R | 6, −70, 50 | 170 | 7.23 | |
| Anterior cingulate cortex | R | 10, 36, 32 | 400 | 5.83 |
| R | 8, 36, 34 | 241 | 5.58 | |
| Cerebellar hemisphere | L | −28, −66, −32 | 558 | 5.85 |
| L | −28, −66, −32 | 523 | 5.85 | |
| Cerebellar vermis | 0, −58, −22 | 1428 | 5.30 | |
| 2, −64, −20 | 324 | 4.93 | ||
| Insula | R | 34, 20, −6 | 507 | 6.81 |
| R | 34, 20, −6 | 430 | 6.81 | |
| Actions with objects vs. pantomimed actions [(OS+OT) > (PS+PT)] | ||||
| Inferior occipital cortex | R | 28, −96, 4 | 3172 | 11.91 |
| R | 28, −96, 4 | 2135 | 11.91 | |
| Fusiform gyrus | L | −26, −48, −16 | 2086 | 9.26 |
| L | −26, −48, −16 | 743 | 9.26 | |
| Spatial analysis vs. temporal analysis [(OS+PS) > (OT+PT)] | ||||
| Posterior parietal cortex | R | 40, −68, 40 | 553 | 4.83 |
| R | 26, −68, 48 | 217 | 4.38 | |
| Temporal analysis vs. spatial analysis [(OT+PT) > (OS+PS)] | ||||
| Cingulate cortex | L | −6, −12, 42 | 412 | 5.07 |
| L | −8, −8, 46 | 317 | 4.63 | |
| Interaction [(PS > PT) > (OS > OT)] | ||||
| Inferior parietal cortex | R | 54, −44, 30 | 288 | 4.78 |
Activations reported in Table II are P ≤ 0.05 (corrected for multiple comparisons at the cluster level). Brain regions showing relative BOLD signal increases associated with each comparison of interest. In addition to the coordinates of the maximally activated voxel within each cluster, the corresponding coordinates are given for each activation cluster (in italics) after the contrasts of interest were masked with the positive term of the corresponding main effect compared to baseline, which ensures that the reported BOLD signal changes reflect increases of neural activity in the relevant conditions rather than deactivations in the contrasted conditions, e.g. [(PS+PT) > (OS+OT) masked by (PS + PT > BL)]. For further definitions, see Table I.
Statistical Analysis
Following stereotactic normalization and smoothing, statistical analysis was performed. Low‐frequency cosine waves modeled and removed subject‐specific low‐frequency drifts in signal (using a highpass filter of 128 s) and the global means were normalized by proportional scaling. Each condition (i.e., each block of trials) was convolved with the hemodynamic response function [Friston et al.,1995b] in the context of the GLM employed by SPM. Specific effects were tested by applying appropriate linear contrasts to the parameter estimates for the experimental conditions, resulting in a t‐statistic for each and every voxel. These t‐statistics were subsequently interpreted by referring to the probabilistic behavior of Gaussian random fields. Activations were identified as significant only if the corresponding cluster of activated voxels passed a height threshold of P < 0.001, uncorrected, and a threshold of P ≤ 0.05, corrected for multiple comparisons at the cluster level, adopting a random effects model.
The neural activity of all four experimental conditions combined was contrasted with the neural activity during the baseline (OS + OT + PS + PT > BL). This analysis reveals those brain areas that are activated in one or more of the experimental conditions, i.e., it reflects observation of complex actions, general aspects of error detection, and response implementation. In addition, data were analyzed for the main effect of ERROR (i.e., [OS + PS] > [OT + PT], and vice versa), and for the main effect of STIMULUS (i.e., [OS + OT] > [PS + PT], and vice versa). Furthermore, the interaction terms were analyzed ([PS > PT] > [OS > OT], and vice versa).
Localization of Activations
The stereotactic coordinates of the pixels of local maximum significant activation were determined within areas of significant relative activity change associated with the different conditions. The anatomical localization of these local maxima was assessed by reference to a standard stereotactic atlas [Talairach and Tournoux,1988], and validation of this method of localization was obtained by superimposition of the SPM maps on the group mean MR image after each individual's MR image had been stereotactically transformed into the same standard stereotactic space [Friston et al.,1995a].
RESULTS
Neural Activations
All conditions vs. baseline
The contrast of all experimental conditions of interest vs. baseline (OS + OT + PS + PT > BL) revealed increased neural activity (P < 0.05, corrected for whole brain volume) in a network concerned with visual perception of objects and actions (superior temporal cortex bilaterally, ventral and dorsal premotor cortex bilaterally, (superior) parietal cortex bilaterally, occipito‐temporal junction bilaterally (V5), superior occipital cortex bilaterally, and fusiform gyrus bilaterally) as well as working memory and decision making (dorsolateral and ventrolateral prefrontal cortex bilaterally and anterior cingulate cortex). There were additional activations in the insula bilaterally and the left thalamus. Finally, the cerebellum (both cerebellar hemispheres and vermis) was activated (Table I, Fig. 1A).
Figure 1.
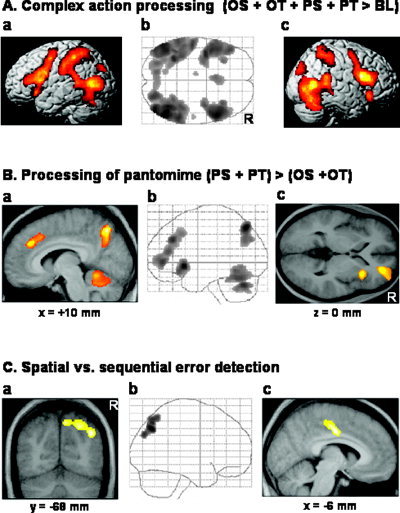
Changes of neural activity associated with the main effects. A: Relative increases in neural activity associated with all experimental conditions vs. baseline (OS + OT + PS + PT > BL, P ≤ 0.05, corrected for multiple comparisons for whole brain volume). In the middle (b), the axial SPM{Z} map is shown as a through‐projection onto a representation of MNI stereotactic space (Evans et al.,1994). On the left (a) and on the right (c), the SPM{Z} map is superimposed onto the left (a) and right (b) hemisphere of a rendered single‐subject brain. The exact coordinates of the clusters of activation and their t‐statistics are given in Table I. OS = spatial analysis of object use actions, OT = temporal/sequential analysis of object use actions, PS = spatial analysis of pantomime actions, PT = temporal/sequential analysis of pantomime actions, and BL = baseline. R, right. Axial, view from above; coronal, view from the back; sagittal, view from the right. B: Relative increases in neural activity (P ≤ 0.05, corrected for multiple comparisons at cluster level) associated with the processing of pantomimed action sequences (PS + PT) > (OS + OT). The middle panel (b) shows the sagittal SPM{Z} map as through‐projections onto representations of MNI stereotactic space [Evans et al.,1994]. On the left (a) and on the right (c), the SPM{Z} map is superimposed on a sagittal (a) and an axial (c) section of the group mean MR image that had been spatially normalized into the same stereotactic space [Evans et al.,1994] showing the activation clusters in the anterior cingulate cortex, precuneus, and cerebellar vermis (a), as well as the activation cluster in the right insula and right prefrontal cortex (c). The level of the anatomical sections was selected to show the local maxima within the activated brain areas and their relationship to the underlying structural anatomy. The exact coordinates of the clusters of activation and their t‐statistics are given in Table II. C: Relative increases in neural activity (P ≤ 0.05, corrected for multiple comparisons at cluster level) associated with (a, b) the spatial analysis of action sequences relative to the sequential analysis of the same action sequences irrespective of action type and (c) the reverse contrast, i.e., the sequential vs. the spatial analysis of action sequences. In the middle, the sagittal SPM{Z} map of spatial error detection [(OS + PS) > (OT + PT)] is shown as through‐projections onto representations of MNI stereotactic space [Evans et al.,1994]. The left panel (a) shows the SPM{Z} map of spatial error detection superimposed on a coronal (a) section of the group mean MR image. The right panel (c) shows the SPM{Z} map of sequential error detection [(OT + PT) > (OS + PS)] superimposed on a sagittal (c) section of the group mean MR image. The exact coordinates of the clusters of activation and their t‐statistics are given in Table II.
Main effect of STIMULUS
Differentially increased neural activity associated with the observation of pantomimed action sequences relative to action sequences performed with objects (PS + PT > OS + OT) was observed in the right precuneus, the right prefrontal cortex (medial and inferior frontal gyrus), the right anterior cingulate cortex, the cerebellar vermis, the left cerebellar hemisphere, and the right insula (Table II, Fig. 1B). Table II summarizes the regions with increased neural activity associated with observation of actions performed with objects relative to pantomimed action sequences (OS + OT > PS + PT). This contrast revealed differential neural activations bilaterally in the inferior occipital gyrus and the fusiform gyrus. To ensure that the reported neural activations reflect increases of neural activity in the relevant conditions rather than deactivations in the contrasted conditions, each contrast of interest was masked with the positive term of the corresponding main effect compared to baseline (Table II, clusters in italics).
Main effect of ERROR
The detection of spatial vs. sequential errors in action sequences independent of stimulus type (i.e., OS + PS > OT + PT) differentially activated the right posterior parietal cortex only (Table II, Fig. 1C). The detection of sequential vs. spatial errors in action sequences independent of stimulus type (i.e., OT + PT > OS + PS) differentially activated the left cingulate cortex only (Table II, Fig. 1C).
To further explore the missing differential activity in left parietal cortex during the detection of sequential vs. spatial errors in action sequences, we performed region of interest (ROI) analyses using the coordinates of left parietal cortex activations reported in Assmus et al. [2003] (−60, −36, +34), Assmus et al. [2005] (−60, −42, +30), Coull and Nobre [1998] (−42, −48, +48 and −44, −44, +38), and Lux et al. [2003] (−64, −36, +26). The extent of the spherical ROIs was 10 mm, corresponding to the Gaussian kernel used for smoothing. However, these ROI analyses failed to reveal any significant differential neural activity in left parietal cortex, even at a liberal threshold of P < 0.001, uncorrected.
Interaction
A significant interaction between the two factors STIMULUS (actions performed with objects vs. pantomimed actions) and ERROR (spatial vs. sequential) was observed in the right inferior parietal cortex only (Table II, Fig. 2). This interaction was predominantly caused by a significant augmentation of neural activity when the spatial configuration of pantomimed action sequences was assessed, as depicted by the analysis of the averaged BOLD signal changes of all voxels within the right inferior parietal cortex cluster (Fig. 2B).
Figure 2.
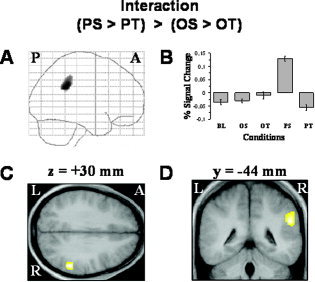
Relative increases in neural activity (P ≤ 0.05, corrected for multiple comparisons at cluster level) associated with the interaction term (PS > PT) > (OS > OT) are found in the right inferior parietal cortex only. In the left upper row (A), the sagittal SPM{Z} map is shown as a through‐projection onto a representation of standard stereotactic space [Evans et al.,1994]. At the upper right (B), the relative signal changes for the baseline (BL) and the four experimental conditions (OS, OT, PS, and PT) are displayed for the maximally activated voxel within the right inferior parietal cortex. The lower row shows the SPM{Z} maps superimposed on an axial (C) and a coronal (D) section of the group mean MR image, which has been spatially normalized into the same stereotactic space [Evans et al.,1994]. The level of the anatomical sections was selected to show the local maximum within the right inferior parietal cortex and its relationship to underlying structural anatomy. The exact coordinates of the activation cluster and the t‐statistics are given in Table II. For further explanations and abbreviations, see the legend to Figure 1. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Error Rates and Eye Movements
Error rates were compared between the four experimental conditions by a two‐way ANOVA (Sigma Stat 2.03, SPSS, Chicago, IL) with STIMULUS (actions with objects vs. pantomimed actions) and ERROR (spatial vs. sequential) as factors (Table III). There were significantly (F(1,64) = 57.38, P ≤ 0.001) higher error rates in the pantomimed action conditions (mean, 6.6%; standard deviation [SD], 5.3%) than in the actions with objects conditions (mean [SD], 1.53% [2.1%]). Subjects also performed significantly (F(1,64) = 41.01, P ≤ 0.001) worse during conditions that required detection of spatial errors (mean [SD] error rate, 6.2% [5.5%]) than during conditions that required the detection of an error of the sequential organization of the action (mean [SD] error rate, 1.9% [2.4%]). Finally, a significant interaction (F(1,64) = 35, P ≤ 0.001) between action type and error modality was observed, showing an augmentation of error rates associated with analysis of the spatial aspects of pantomimed actions.
Table III.
Behavioral results
| Actions with objects | Pantomimes | Stimulus (actions with objects vs. pantomimes) | Error (spatial vs. sequential) | |||||
|---|---|---|---|---|---|---|---|---|
| Spatial error | Sequential error | Spatial error | Sequential error | |||||
| Errors (%) | 1.53 ± 2.1 | 1.7 ± 2.26 | 1.37 ± 1.98 | 6.61 ± 5.29 | 10.73 ± 3.74 | 2.48 ± 2.74 | F(1,64) = 57.38P < 0.001 | F(1,64) = 41.01 P < 0.001 |
| Scanpath | 13,680 ± 457 | 13843 ± 680 | 13516 ± 681 | 14,009 ± 455 | 14038 ± 681 | 13979 ± 681 | n.s. | n.s. |
| Pupil diameter | 56.7 ± 0.69 | 56.5 ± 1.01 | 56.9 ± 1.03 | 58.1 ± 0.69 | 58.3 ± 1.03 | 57.9 ± 1.03 | n.s. | n.s. |
Behavioral results. While scanpath and pupil diameter were not influenced by the experimental factors, subjects exhibited significantly higher errors rates for pantomimes compared to actions with objects (irrespective of task) and for the spatial analysis compared to the sequential analysis of the actions. Given are means with SD. n.s., not significant.
Analysis of the eye movement data taken outside the MR scanner during the eye movement study showed no significant differences in the mean scanpath or the horizontal pupil diameter across conditions (Table III).
DISCUSSION
Neural Activations Associated with the Observation of Complex Actions
Recent functional imaging studies on the neural mechanisms of action observation have yielded evidence for a motor observation/execution matching system (i.e., the mirror neuron system) in humans [Buccino et al.,2004; Iacoboni et al.,1999; Schürmann et al.,2005]. The main components of this mirror neuron system are the superior temporal cortex [Iacoboni et al.,2001], the (ventral) premotor cortex, and the (inferior) parietal cortex [Buccino et al.,2001]. While electrophysiological studies in monkeys show that the mirror neuron system is especially activated by observation of object‐related actions [for a recent review, see Rizzolatti and Craighero,2004], functional imaging studies in humans have focused on the observation or imitation of rather simple movements (e.g., finger movements). Only recently have object‐related movements been examined [Rumiati et al.,2004]. In the current study the complexity of the examined actions was further increased, since our subjects observed (and assessed) sequential, object‐related actions some of which included multiple objects. As expected, when all action observation conditions were compared to the baseline, the premotor and parietal cortices as well as the superior temporal cortex were activated bilaterally (besides other movement‐related areas, e.g., V5/MT). Therefore, components of the mirror neuron system were activated throughout the four experimental conditions. Unfortunately, the blocked design of our study did not allow us to examine whether this neural activity reflects the observation of complex (i.e., sequential) actions involving multiple objects, or also—at least in part—an activation of action representations by reading the description of the action sequences during the experimental conditions [Tettamanti et al.,2005] or even the preparation and execution of the (relatively rare) response button presses.
As in other cognitive domains, e.g., memory [Piefke et al.,2005], there is neuropsychological evidence that higher praxis functions may be differently organized in women and men [Kimura,1982,1983]. Since we enrolled only male volunteers in our study, we cannot assess possible sex differences in praxis processing in the current study. This remains an interesting topic for further research.
Neural Processing of Complex Actions Performed with or without Objects
By analyzing the differential activations associated with the factor STIMULUS, we determined which brain areas are involved in processing the same actions in the presence vs. the absence of objects (i.e., pantomimed actions). The fact that subjects were given a description of the following action sequence prior to each trial ensured that all subjects correctly recognized the following action. Therefore, any differential neural activations observed as an effect of the factor STIMULUS cannot be ascribed to increased demands on the action recognition system per se. Rather, the observed differences in neural activity result from the specific processing demands of the experimental conditions despite correct identification of the action: In the actions with objects conditions, subjects put particular emphasis on processing the (movement‐relevant) properties of the involved objects [Rumiati et al.,2004]. In contrast, the pantomime conditions put additional demands on working memory [Bartolo et al.,2003] and monitoring‐related processes. In accordance with our previous study [Rumiati et al.,2004], we found bilateral activations of the fusiform gyrus and the (ventral) occipital cortex when subjects observed actions performed with objects compared to pantomimed actions. These areas are part of the so‐called ventral visual processing stream, known to be involved in the processing of object properties for the purpose of object recognition [Goodale and Milner,1992; Ungerleider and Mishkin,1982]. Thus, the data may suggest that the ventral stream becomes active when day‐to‐day actions with objects are observed (relative to the corresponding pantomimes). Alternatively, one might argue that the ventral stream activation in the current study simply results from the presence of objects. To test these differential hypotheses, one would have to include another condition, in which only objects were presented, and then contrast the difference between action with objects and pantomime with the object‐only condition. The latter, however, would be confounded by occipital‐temporal activations resulting from the objects being moved in space in the actions with objects condition.
Observing pantomimed actions vs. observing the performance of the same actions with objects led to an activation of a predominantly right hemispheric network. The relative lack of left hemisphere activation in this comparison (e.g., in contrast to the study of Moll et al. [2000]) results from the fact that the contrasting conditions (actions with objects) involved the left hemisphere to a similar extent as the pantomiming conditions (as evidenced in the contrast of all conditions vs. baseline; see Statistical Analysis, Fig. 1A, and Table I). In the pantomime conditions, however, the important task‐relevant clues are missing (e.g., spatial cues that can be derived from the object), leading to increased monitoring demands when subjects have to assess complex actions [Goldenberg et al.,2003]. This increased difficulty led to activation of the anterior cingulate cortex (ACC) and the right dorsolateral prefrontal cortex (DLPFC), areas known to be involved in cognitive control and monitoring [MacDonald et al.,2000; Stephan et al.,2003], with the latter being additionally implicated in the supervisory attentional systems [Fink et al.,1999; Shallice and Burgess,1996]. The additional activation of the right precuneus when observing pantomimes might reflect memory‐related imagery [Fletcher et al.,1995] eliciting in front of the “mind's eye” the template of the action sequence that then can be compared to the presented pantomime.
Role of the Cingulate Cortex in the Sequential Analysis of Action Sequences
The involvement of the cingulate cortex in the analysis of the sequential aspects, i.e., the temporal organization, of complex object‐related actions is consistent with other imaging studies showing activation of the cingulate cortex in tasks involving processing of temporal information [Coull et al.,2004; Fink et al.,1999; Maquet et al.,1996; Picard and Strick,2003]. More specifically, the cingulate cortex is also known to be implicated in selection‐for‐action [Botvinick et al.,1999]. In our study, subjects assessed the correct selection of action segments within an action sequence. This observation is consistent with the fact that medial motor areas, including the cingulate motor areas, are also involved in the timing of movements required for bimanual coordination [Stephan et al.,1999], which is often a prerequisite of proper object use. Furthermore, the cingulate cortex was recently implicated in the appropriate selection of motor schemata in the context of grasping familiar objects [Sugio et al.,2003].
Nonetheless, it has to be acknowledged that, a priori, one would have expected left parietal cortex involvement in the sequential analysis of action sequences, since Liepmann [1920] suggested that left parietal cortex is concerned with the space‐time engrams of actions. Consistent with Liepmann's conjecture, we have previously shown that left inferior parietal cortex is specifically involved in binding temporal and spatial information [Assmus et al.,2003,2005]. The lack of significant differential activation of left parietal cortex in the current study (even in the ROI analyses) suggests that the left parietal cortex is similarly activated in analyzing the sequential and spatial aspects of complex action sequences. This notion is supported by the contrast of all experimental conditions vs. baseline (Fig. 1A), which shows left parietal cortex activation.
Role of Right Parietal Cortex in the Spatial Analysis of Action Sequences
The current study provides direct evidence for the involvement of the right hemisphere (especially the right posterior parietal cortex, see above, and inferior parietal cortex, as indicated by the interaction) in the spatial analysis of object‐related action sequences, indicating a distinct contribution of the right hemisphere to the observation and processing of complex motor acts. The right parietal cortex is well known to be involved in visual‐spatial functions and has an important role in spatial cognition [for recent reviews, see Fink and Heide,2004; Halligan et al.,2003]. This functional specialization of the right parietal cortex also explains why it was especially driven by the most difficult condition, PS, in which we observed the highest error rates. In this condition, the lack of objects puts special demands on spatial processing since the spatial configuration of an object‐related action is strongly influenced by the concrete properties of the objects used (e.g., size, shape), which were not visible in the condition PS. In contrast, the sequential organization of an action is influenced rather by the identity than by the properties of the objects involved. Since the stimuli were identical across conditions (a third correct actions, a third actions with spatial errors, and the remaining third actions with temporal errors), but depending on the experimental condition our subjects had to focus on, either the spatial or temporal aspects of the complex actions, our findings could also be interpreted as suggesting a role of right parietal cortex in spatial motor attention. Therefore, our results support and extend previous data by pointing to the relevance of right parietal cortex spatial functions in processing the spatial configuration of sequential, object‐related actions. Furthermore, these findings are in line with previous neuropsychological observations in patients with right parietal lesions performing meaningless movement sequences [Weiss et al.,2001]. Finally, the current results suggest an important role of right parietal cortex in spatial processing for perception as well as for action [Weiss et al.,2000,2003] implicating right parietal cortex in spatial cognition in a very broad sense.
Clinical Implications
Finally, our finding of a right‐hemispheric involvement in the processing of complex motor acts in healthy subjects is in good accordance with lesion studies comparing motor deficits in patients with unilateral lesions of either hemisphere. These clinical studies revealed apraxic errors also for right hemisphere‐damaged patients [De Renzi et al.,1980; Goldenberg,1996; Haaland and Flaherty,1984], which suggests that certain components of complex actions are processed in the right hemisphere or are, at least in part, represented bilaterally. The results of our study specify this conjecture in showing that processing of the spatial configuration of an action specifically draws upon right posterior parietal cortex. This proposed function of right posterior parietal cortex may well explain the observed apraxic errors in patients with right hemisphere damage and gives credit to Liepmann, who stated in 1920: “It can be expected that lesions of the right parietal lobe won't be without any effect on the praxis of the left hand.”
Acknowledgements
The authors thank their volunteers and colleagues from the MRI and Cognitive Neurology groups.
REFERENCES
- Assmus A, Marshall JC, Ritzl A, Zilles K, Noth J, Fink GR (2003): Left inferior parietal cortex integrates time and space during collision judgements. Neuroimage 20: S82–S88. [DOI] [PubMed] [Google Scholar]
- Assmus A, Marshall JC, Noth J, Zilles K, Fink GR (2005): Difficulty of perceptual spatiotemporal integration modulates the neural activity of left inferior parietal cortex. Neuroscience 132: 923–927. [DOI] [PubMed] [Google Scholar]
- Bartolo A, Cubelli R, Della Sala S, Drei S (2003): Pantomimes are special gestures which rely on working memory. Brain Cogn 53: 483–494. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund H‐J (2001): Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund H‐J, Rizzolatti G (2004): Neural circuits underlying imitation learning of hand actions: an event related fMRI study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC (1998): Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18: 7426–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F (2004): Functional anatomy of the attentional modulation of time estimation. Science 303: 1506–1508. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Lucchelli F (1988): Ideational apraxia. Brain 111: 1173–1185. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Motti F, Nichelli P (1980): Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol 37: 6–10. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D (1994): An MRI‐based probabilistic atlas of neuroanatomy In: Shorvon S, Fish D, Andermann F, Bydder GM, Stefan H, editors. Magnetic resonance scanning and epilepsy. New York: Plenum Press; p 263–274. [Google Scholar]
- Fink GR, Heide W (2004): Räumlicher Neglekt. Nervenarzt 75: 389–410. [DOI] [PubMed] [Google Scholar]
- Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD (1997): Space‐based and object‐based visual attention: shared and specific neural domains. Brain 120: 2013–2028. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, Frith CD, Driver J, Frackowiak RS, Dolan RJ (1999): The neural consequences of conflict between intention and the senses. Brain 122: 497–512. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse‐Ruyken M, Ziemons K, Zilles K, Freund H‐J (2000): Line bisection judgements implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54: 1324–1331. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K (2001): The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. Neuroimage 14: S59–S67. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, Zilles K, Dieterich M (2003): Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage 20: 1505–1517. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RSJ, Dolan RJ (1995): The mind's eye—precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J‐B, Heather JD, Frackowiak RSJ (1995a): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RSJ (1995b): Characterising evoked hemodynamics with fMRI. Neuroimage 2: 157–165. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ (1995c): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Goldenberg G (1995): Imitating gestures and manipulating a mannikin — the representation of the human body in ideomotor apraxia. Neuropsychologia 33: 63–72. [DOI] [PubMed] [Google Scholar]
- Goldenberg G (1996): Defective imitation of gestures in patients with damage in the left or right hemispheres. J Neurol Neurosurg Psychiatry 61: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G (1999): Matching and imitation of hand and finger postures in patients with damage in the left or right hemisphere. Neuropsychologia 37: 559–566. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hartmann K, Schlott I (2003): Defective pantomime of object use in left brain damage: apraxia or asymbolia? Neuropsychologia 41: 1565–1573. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD (1992): Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Flaherty D (1984): The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn 3: 370–384. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT (2000): Neural representation of skilled movement. Brain 123: 2306–2313. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Fink GR, Marshall JC, Vallar G (2003): Spatial cognition: evidence from visual neglect. Trends Cogn Sci 7: 125–133. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999): Cortical mechanisms of human imitation. Science 286: 2526–2528. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski L, Brass M, Bekkering H, Woods RP, Dubeau M‐C, Mazziotta JC, Rizzolatti G (2001): Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci U S A 98: 13995–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D (1982): Left‐hemisphere control of oral and brachial movements and their relation to communication. Philos Trans R Soc Lond B 298: 135–149. [DOI] [PubMed] [Google Scholar]
- Kimura D (1983): Sex differences in cerebral organization for speech and praxic functions. Can J Psychol 37: 19–35. [DOI] [PubMed] [Google Scholar]
- Kimura D, Archibald Y (1974): Motor functions of the left hemisphere. Brain 97: 337–350. [DOI] [PubMed] [Google Scholar]
- Liepmann H (1905): Die linke Hemisphäre und das Handeln. Münchener Medizinische Wochenschrift 52: 232. [Google Scholar]
- Liepmann H (1920): Apraxie. Ergebnisse der gesamten Medizin 1: 516–543. [Google Scholar]
- Lux S, Marshall JC, Ritzl A, Zilles K, Fink GR (2003): Neural mechanisms associated with attention to temporal synchrony versus spatial orientation: an fMRI study. Neuroimage 20: S58–S65. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit‐Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D (1996): Brain activation induced by estimation of duration: a PET study. Neuroimage 3: 119–126. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Fink GR (2001): Spatial cognition: where we were and where we are. Neuroimage 14: S2–S7. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Passman LJ, Cunha FC, Souza‐Lima F, Andreiuolo PA (2000): Functional MRI correlates of real and imagined tool‐use pantomimes. Neurology 54: 1331–1336. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2003): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR (2005): Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp 24: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L (2004): The mirror‐neuron system. Annu Rev Neurosci 27: 169–192. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Heilman KM, Watson RT (1985): Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry 48: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, Fink GR (2004): Neural basis of pantomiming the use of visually presented objects. Neuroimage 21: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Hesse MD, Stephan KE, Saarela M, Zilles K, Hari R, Fink GR (2005): Yearning to yawn: the neural basis of contagious yawning. Neuroimage 24: 1260–1264. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P (1996): The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B 351: 1405–1412. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse P, Herzog H, Sturm V, Zilles K, Seitz RJ, Freund H‐J (1999): The role of ventral medial wall motor areas in bimanual co‐ordination. A combined lesion and activation study. Brain 122: 351–368. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR (2003): Lateralized cognitive processes and lateralized task control in the human brain. Science 301: 384–386. [DOI] [PubMed] [Google Scholar]
- Sugio T, Ogawa K, Inui T (2003): Neural correlates of semantic effects on grasping familiar objects. Neuroreport 14: 2297–2301. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotactic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa S, Perani D (2005): Listening to action‐related sentences activates fronto‐parietal motor circuits. J Cogn Neurosci 17: 273–281. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR (2004): Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event‐related fMRI study. Neuroimage 21: 318–328. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982): Two cortical visual systems In: Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; p 549–586. [Google Scholar]
- Weiss PH, Marshall JC, Wunderlich G, Tellmann L, Halligan PW, Freund H‐J, Zilles K, Fink GR (2000): Neural consequences of acting in near versus far space: a physiological basis for clinical dissociations. Brain 123: 2531–2541. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Dohle C, Binkofski F, Schnitzler A, Freund H, Hefter H (2001): Motor impairment in patients with parietal lesions: disturbances of meaningless arm movement sequences. Neuropsychologia 39: 397–405. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Zilles K, Fink GR (2003): Are action and perception in near and far space additive or interactive factors? Neuroimage 18: 837–846. [DOI] [PubMed] [Google Scholar]


