Abstract
The role of oscillatory alpha activity (8–13 Hz) in cognitive processing remains an open question. It has been debated whether alpha activity plays a direct role in the neuronal processing required for a given task or whether it reflects idling and/or functional inhibition. Recent electroencephalography (EEG) studies have demonstrated that alpha activity increases parametrically with load during retention in working memory paradigms. While it is known that the parieto‐occipital cortex is involved in the generation of the spontaneous alpha oscillations, it remains unknown where the sources of the memory‐dependent alpha activity are located. We recorded brain activity using magnetoencephalography (MEG) from human subjects performing a Sternberg memory task where faces were used as stimuli. Spectral analysis revealed a parametric increase in alpha activity with memory load over posterior brain areas. We then applied a source reconstruction technique that allowed us to map the parametric increase in alpha activity to the anatomical magnetic resonance (MR) images of the subject. The primary sources of the memory‐dependent alpha activity were in the vicinity of the parieto‐occipital sulcus. This region is not directly involved in working memory maintenance of faces. Our findings are consistent with the notion that alpha activity reflects disengagement or inhibition of the visual dorsal stream. We propose that the disengagement reflected in alpha power serves to suppress visual input in order to devote resources to structures responsible for working memory maintenance. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: electroencephalography, EEG, magnetoencephalography, MEG, oscillations, attention, encoding, recall
INTRODUCTION
In the late 1920s the first electroencephalographic scalp recordings in humans were performed by Hans Berger [1929]. The dominating feature in the EEG was oscillatory activity in the alpha band (8–13 Hz). Even though alpha band activity has been the subject of multiple EEG and MEG studies, its actual role in cognitive processing remains unclear. It has been proposed that oscillatory alpha activity reflects a state in which the brain is idle but ready to be engaged [Adrian and Matthews, 1934; Pfurtscheller et al., 1996]. However, the notion of idling has recently been challenged by working memory studies. Alpha activity was found to be enhanced with working memory demands [Jensen et al., 2002; Klimesch et al., 1999]. In particular, Jensen et al. [2002] showed that alpha activity increased systematically with memory load in a modified version of the Sternberg task. This increase was sustained during most of the 3‐s retention interval.
The working memory load‐dependent alpha activity is open to several interpretations. One interpretation is that alpha activity reflects disengagement or inhibition of posterior brain areas [Cooper et al., 2003; Jensen et al., 2002; Klimesch et al., 2000; Ray and Cole, 1985; Vanni et al., 1997]. As the demands to the working memory system increase, areas not necessary for the task are disengaged. The function of this disengagement could be to reduce interfering sensory inputs to areas involved in working memory maintenance. The higher the working memory load, the stronger the need for disengagement, and thus the stronger the alpha rhythm. A second interpretation is that alpha activity reflects activity from brain regions performing the neuronal processing required for working memory maintenance. The more items in working memory, the stronger the alpha power becomes. Indeed, it has been suggested that rhythmic alpha activity could reflect neuronal processing required for attention and memory operations [Kolev et al., 2001; Maltseva et al., 2000; Sewards and Sewards, 1999]. Specifically, Sauseng et al. [2002] proposed that synchronization in the upper alpha band reflects information transfer between working and long‐term memory areas. It has also been suggested that long‐range coherence in the alpha band reflects perceptual and cross‐modal binding [Hummel and Gerloff, 2005; Mima et al., 2001].
The aim of our study was to identify the sources accounting for the increase in alpha activity with working memory load using MEG. We hypothesized that if the neuronal sources of the memory‐dependent alpha activity are found in areas known to be required for working memory, this would speak in favor of an active role of alpha activity in memory processing. However, if the sources are found in other areas not directly associated with working memory maintenance, this would speak in favor of the alpha inhibition hypothesis.
SUBJECTS AND METHODS
Subjects
Five right‐handed male subjects, age 23–26 years participated in the experiment. All subjects had corrected to normal vision and reported having no neurological impairments. After an explanation of the paradigm, informed consent was obtained from the subjects. The studies were approved by the local ethics committee.
MEG Acquisition
Brain activity was recorded using a whole‐head MEG system (CTF/VSM MedTech, Vancouver, Canada) with 151 first‐order axial gradiometer sensors. The vertical electro‐oculogram (EOG) was simultaneously recorded. The MEG signals were lowpass‐filtered at 200 Hz and sampled at 600 Hz. To measure the position of the head with respect to the sensor array, three coils were placed at anatomical landmarks (left, right ear canal, and the bridge of the nose). The positions of the coils were determined from the magnetic signals produced by the coils when currents were passed through them before and after the experiment. MRIs were obtained using a 1.5 T Siemens Sonata scanner (Erlangen, Germany) and aligned to the MEG data according to the coils and anatomical landmarks. Visual stimuli were presented to the subjects using an LCD projector and nonmagnetic buttons were used for behavioral responses.
Experimental Procedure
A modified Sternberg task using pictures of faces as mnemonic items was applied (Fig. 1). Each trial started with the word “Blink,” encouraging the subjects to make eye blinks in order to reduce artifacts later in the trial. After 2.5 s, memory lists of 1–4 faces were sequentially presented. Each face was presented for 0.3 s with 1.25‐s intervals between the items. Following a 2.7‐s delay period, a probe face was presented for 0.3 s. Subjects were instructed to indicate whether the probe matched an item in the memory list (“positive probes”) or did not (“negative probes”). The responses were given by pressing one of two buttons, one by the right and the other by the left index finger. Feedback on correct and incorrect responses was presented at the end of each trial. After 2 s the next trial started. In the control condition three crosses were shown instead of the faces. After these three crosses, following a 2.7‐s delay period, another cross was presented as a probe and the subjects were instructed to press the right button. The experiment consisted of six blocks of 60 trials presented randomly. The face database was provided by the Max‐Planck Institute for Biological Cybernetics (Tübingen, Germany). Subjects were trained on the task for 15–30 min prior to the recordings.
Figure 1.

The Sternberg task using faces as stimuli. Each trial started with a blink period lasting 2.5 s. A list of 1–4 faces were then presented sequentially at a rate of 1.25 s per item. After a delay period of 2.7 s, a probe was shown. Subjects had to respond to whether the probe face was in the list or not. Feedback was given after every response.
Data Analysis
Incorrect trials and trials contaminated by artifacts caused by eye movements, SQUID artifacts, and muscle activity were excluded from the analysis. The sensor level analysis was performed on data that was numerically transformed to a representation of the planar field gradient [Bastiaansen and Knosche, 2000]. The horizontal and vertical components of the planar field gradient were estimated at each sensor location using the signals from the neighboring sensors. The planar field gradient computed in this way approximates the signals measured by physical planar gradiometers (e.g., as in Neuromag systems, Elekta Neuromag Oy, Stockholm, Sweden). Power representations calculated for the horizontal and vertical gradient for a given sensor location were subsequently summed. This procedure simplifies the sensor‐level analysis of the MEG signals in the frequency domain, since the strongest power usually is situated directly above the neural source [Hämäläinen et al., 1993].
Time‐frequency representations (TFRs) of power were characterized using a spectrogram computed from short sliding time‐windows [Percival and Walden, 1993]. The data in each time window were multiplied with a Slepian taper (k = 1). The Fourier transforms and power of the tapered time windows were then calculated. The power estimates were subsequently averaged over multiple trials for a given memory load. We applied a 0.4‐s time window and 2‐Hz frequency smoothing. The absolute change in power of the TFRs was then calculated by subtracting the baseline power from a 0.5‐s period prior to the presentation of the first memory item. Since the TFRs of power were calculated for the individual trials and then averaged, oscillatory activity that is not phase‐locked to the stimuli can be detected. To examine parametric changes in power of the TFRs with respect to load, L, we fitted power to the function P = α+β·L. β is the regression coefficient (or the slope):
| (1) |
and α the intercept. The test statistic following Student's t‐distribution with n−2 degrees of freedom is:
| (2) |
where:
| (3) |
| (4) |
and n the number of trials.
To test the statistical significance of the regression coefficient, a nonparametric randomization procedure was applied that controls for multiple comparisons over sensors [Maris, 2004; Maris and Oostenveld, submitted]. This method first identifies sensors for which the t‐statistic of the regression coefficient is below a threshold (P < 0.05). Clusters of spatially contiguous sensors exceeding the threshold are then identified. The cluster‐level statistic is defined as the sum of the t‐statistics of the sensors in a cluster. The Type I error rate for the complete set of 151 sensors is controlled by evaluating the cluster‐level test statistic under the randomization null distribution of the maximum cluster‐level test statistic. The randomization null distribution was obtained by randomly distributing the trials over the four memory loads within every participant. For every randomization the regression coefficients and t‐statistics were recomputed, the sensors were thresholded, clusters were identified, cluster‐level statistics were calculated, and their maximum was taken. The randomization distribution was approximated by performing 1,000 randomizations and calculating the maximum cluster‐level statistic for each of them. The P‐value was approximated by the proportion of these 1,000 random permutation in which the maximum cluster‐level statistic exceeded the observed maximum cluster‐level statistic.
A beamforming technique called dynamical imaging of coherent sources (DICS) was applied to identify the sources of the memory‐dependent alpha power (10.5 Hz). The technique uses adaptive spatial filters to localize power in the brain [Gross et al., 2001; Liljestrom et al., 2005]. The brain volumes for individual subjects were discretized to a grid with 0.5 cm resolution. Using head‐shapes identified from the MRIs of the individual subjects, we constructed a forward model for each grid point using a multiple sphere approximation [Huang and Mosher, 1997]. From the forward models and cross‐spectral densities at the frequency of interest, spatial filters were constructed for each grid point. This resulted in power estimates for each grid point. Note that the DICS estimates were calculated from the planar sensor data directly, not from the synthetic planar gradient. The output of the DICS calculations (“source power”) for the four memory loads was fitted to P = α+β·L. This yielded a volume of regression coefficients, β, which then was colocalized on the MRI of each subject.
All the analyses were done using Matlab (MathWorks, Natick, MA) and the FieldTrip toolbox developed at the F.C. Donders Centre for Cognitive Neuroimaging (Nijmegen, The Netherlands, http://www.ru.nl/fcdonders/fieldtrip/).
RESULTS
The behavioral data were characterized in terms of reaction time and amount of errors for the different memory loads. The grand average showed a systematic increase in reaction time (40 ms/item) and errors with increasing memory load for both positive and negative probes (Fig. 2). The increase for both positive and negative probes combined was significant (P < 0.034; t‐test of regression analysis); for positive probes the increase was significant (P < 0.005) and for negative probes there was a trend (P < 0.115). This demonstrates that the basic finding of the original Sternberg task was reproduced, namely, a parametric increase in reaction time with memory load [Sternberg, 1966].
Figure 2.
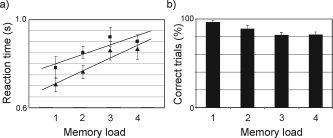
The behavioral data from the modified Sternberg task. a: Reaction times for both positive and negative probes increase systematically with memory load (slope 40 ms/item). b: The response errors as a function of memory load. Error bars indicate SEM (n = 5).
To characterize the alpha activity at the sensor level, we first analyzed the spectral components of the brain activity during the retention interval. Trials with artifacts and wrong answers were rejected (≈25%). Figure 3a shows a TFR of the power for all memory loads and the five subjects combined for a single sensor. The sensor that showed the strongest alpha power for the averaged conditions was selected in each subject. Note that we calculated the spectral representations for the individual trials prior to averaging in order to be able to study oscillatory activity not necessarily phase‐locked to the stimuli. Strong alpha activity (8–12 Hz) emerged about 1 s after the presentation of the last items in the list. The relative increase was about 2‐fold with respect to baseline and highly significant. The activity was sustained during the retention interval until the probe was presented. Changes in power with respect to memory load were quantified using the regression coefficient β and averaged over the five subjects. As seen in Figure 3b, we observed a load‐dependent increase in the alpha band. The load dependence with respect to time and frequency followed the profile of the alpha activity (Fig. 3a). Figure 3c shows the alpha activity (8–12 Hz) for the four memory loads and the control condition. Note the systematic increase in alpha power as the memory load increases. The alpha power of the control condition was not statistically different from the power of memory load S = 1. Nevertheless, the alpha power was significantly different for load S = 2, 3, and 4 when individually compared to the control condition. When comparing the control condition (three items; no memory requirement) to the load three memory condition, the alpha power of the memory condition is higher (see Fig. 3c). This argues that the alpha increase is related to working memory maintenance rather than task‐related effects such as timing. As seen in Figure 3a, some beta power (18–22 Hz) was also present during the retention interval. This beta activity might be explained by harmonics of the alpha activity; however, it did increase significantly with memory load.
Figure 3.
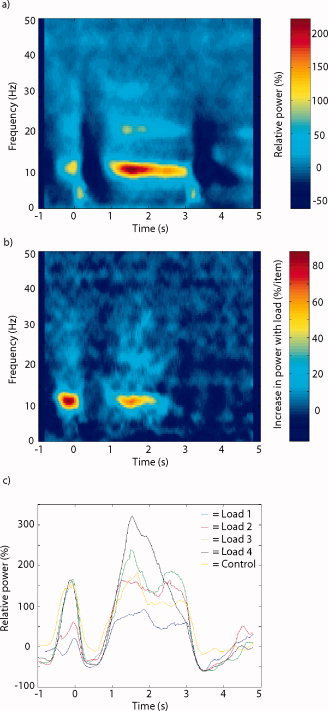
The time‐frequency representations (TFRs) of the posterior alpha activity for occipital sensors averaged over the five subjects. The last item was presented at t = 0 s and the probe item at t = 3 s. a: The TFRs of all four memory loads averaged. Strong alpha activity (8–12 Hz) is clearly visible. b: The TFR of the regression coefficient β relating power to memory load. c: The temporal development of alpha power (8–12 Hz) with respect to the four memory loads. Alpha increased systematically with memory load.
The spatial extent of the memory‐dependent alpha activity at the sensor level was characterized by calculating the regression coefficient of alpha activity with memory load (t = 1.5–2.5 s; f = 10.5 Hz) for the planar field gradient. The topographical distribution of the regression coefficients is shown in Figure 4a. In all subjects we identified at least one cluster of sensors with a significant memory‐dependent alpha increase (uncorrected; see Fig. 4a). To control for multiple comparisons over sensors we used a clustering‐based randomization test (see Subjects and Methods). In four of the five subjects the increase was significant when controlling for multiple comparisons over sensors. The power spectra of the sensors in the clusters with the largest t‐value (“most significant cluster”) are displayed in Figure 4b. The power spectra were calculated by averaging the spectral time‐frequency representations from 1.5 to 2.5 s. The power spectra are dominated by alpha activity that is load‐dependent. Finally, we performed a source reconstruction by means of DICS (see Subjects and Methods) to identify the sources of the load‐dependent alpha activity. Figure 4c shows regression coefficients for the increase in alpha activity (8–12 Hz) with memory load projected on the brain surface. In four subjects the dominant source of the load‐dependent alpha activity was found around the parieto‐occipital sulcus (marked by a green line in Fig. 4c). In the fourth subject the alpha increase was centrally located but strongest in occipital cortex. This difference might be explained by a lower signal‐to‐noise of the alpha, resulting in a slight mislocalization (see Fig. 4b). It should be noted that even though both alpha power and response times increased with memory load for this subject, the response times were in general slower compared to the other subjects.
Figure 4.
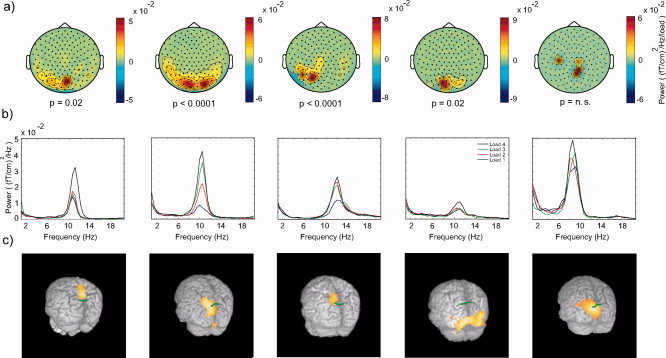
Characterizing the memory‐dependent alpha activity in individual subjects. a: The topographical maps illustrate the regression coefficient β of the alpha activity with respect to memory load. Only statistically significant clusters of positive regressions coefficient are shown (see Subjects and Methods). A significant increase was found in four subjects; the increase in the fifth subject showed a trend but it was not significant. b: The power spectra for the cluster of sensors with significant increase in alpha activity with memory load. c: Maps of the regression coefficient β of the memory‐dependent alpha activity estimated in source space and colocalized on the individual subjects' MRIs. The coefficients in the 3D volume are projected to the brain surface. The green lines indicate the parieto‐occipital sulcus. The maps are thresholded for each subject with respect to 50% of the maximal correlation coefficient.
DISCUSSION
In this study we have reproduced previous findings demonstrating a parametric increase in alpha activity with load during working memory retention. Whereas in a previous study the memory items were consonants [Jensen et al., 2002], we now used faces. We were able to localize the neuronal sources of the memory‐dependent alpha activity to brain regions in the vicinity of the parieto‐occipital sulcus. This location is consistent with the sources accounting for the “classical alpha activity” emerging when subjects rest with their eyes closed identified in previous studies [Salenius et al., 1995; Salmelin and Hari, 1994]. We conclude that the working memory‐dependent alpha activity is most likely produced by sources that also are responsible for the resting alpha activity.
There are several novel elements in the data analysis that we have applied. The use of the planar gradient has previously proven useful in systems with physical planar gradiometers [Hämäläinen et al., 1993]. We used a simple algorithm to estimate the planar gradient from axial gradiometer signals. This is particularly advantageous when analyzing power of oscillatory signals: in the axial gradient, dipolar fields produce two regions of power adjacent to the source. The power estimate in the synthetic planar gradient produces primarily one region of power directly above the source. As a result, the planar gradient provides a more spatially focal power estimate compared to axial gradiometers [Bastiaansen and Knosche, 2000]. The application of a cluster randomization technique for analyzing the significant effects at the sensor level provides a powerful approach to control for multiple comparisons with respect to sensors (Maris and Oostenveld, submitted). Finally, we made use of a beamforming technique to estimate parametric changes in power of the measured signals. The approach allowed us to colocalize the regression coefficients of parametric changes on the subjects MRIs. This effectively deals with the problem of noise bias with respect to depth as discussed in Van Veen et al. [1997].
In a previous study we also identified an increase in alpha power with working memory load; however, source localization was not attempted since the recordings were based on 32 channels EEG and anatomical information was not acquired [Jensen et al., 2002]. The EEG study not only reported on a memory‐dependent alpha increase in posterior sensors, but an alpha increase was also observed in sensors over the central band. This might suggest the involvement of somato‐motor areas in producing memory‐dependent alpha activity. Nevertheless, in the current MEG study we did not find evidence for the engagement of somato‐motor sources. It should also be emphasized that EEG topographies are highly spatially smeared due to volume conduction and cannot be directly compared to MEG topographies.
What is the functional role of the memory‐dependent alpha activity? One possibility is that neuronal rhythmic activity synchronized at ∼10 Hz is required for the active maintenance of working memory. Another possibility is that the alpha activity reflects modulation and/or inhibition of areas not required for the memory maintenance. Recently, Druzgal et al. [2003] used functional MRI (fMRI) to study the brain activation in a Sternberg task that was similar to ours. They identified a parametric increase the blood oxygenation level‐dependent (BOLD) signal with memory load in prefrontal and inferior temporal areas. These areas have been associated with working memory maintenance and face processing, respectively [Curtis and D'Esposito, 2003; Kanwisher et al., 1997]. The MRI‐studies did not report on load‐dependent activity around the parieto‐occipital sulcus. Thus, we conclude that the memory‐ dependent alpha activity is not likely to reflect neuronal processing directly required for working memory maintenance. Often, brain activity related to working memory maintenance has been shown to be lateralized. We do not find a lateralization in the memory‐dependent alpha activity, which is consistent with the idea that alpha reflects inhibition of the dorsal visual stream. We would like to emphasize that our findings are not compatible with the idling hypothesis, given that the notion of idling cannot explain the increase in alpha power with memory load [Pfurtscheller et al., 1996].
Is the increase in alpha activity due to a rebound following visual stimulation? The rebound effect is observed as a decrease in alpha power after visual stimulation followed by a transient increase. In the case of the memory task, one could hypothesize that the more items presented, the stronger the rebound. However, several findings are not consistent with the rebound hypothesis. First, the degree of initial alpha suppression (0.2–0.7 s) with respect to baseline does not increase with memory load. Second, the alpha power is sustained during most of the retention interval (see Fig. 3b and Jensen et al. [2002, fig. 3D]), whereas an alpha rebound would be transient. Third, note that the alpha increase was much stronger during the retention period compared to the recall period. It should be noted that the stimulation protocol was different in the previous EEG study in the sense that the items were presented simultaneously rather than sequentially. Despite these differences the time‐course and load dependence of the alpha activity were similar.
In line with other studies, we propose that the load‐dependent alpha activity reflects inhibition or disengagement of the dorsal visual stream [Cooper et al., 2003; Jensen et al., 2002; Jung‐Beeman et al., 2004; Ray and Cole, 1985; Vanni et al., 1997]. This inhibition might serve to suppress visual inputs in order to prevent interfering signals to brain areas involved in actual working memory maintenance. The inhibition hypothesis is consistent with EEG findings demonstrating that directed visual attention suppresses alpha activity in posterior areas contralateral to the hemifield of attention [Worden et al., 2000]. Why does alpha activity result in functional inhibition? One simple hypothesis is that if neurons are strongly entrained by a 10‐Hz rhythm, they will not fire more often than every 100 ms. This will effectively prevent the neurons from participating in information processing. Thus, in the case of working memory retention, the alpha activity in the parieto‐occipital sulcus will block the dorsal information flow from visual areas.
Which areas provide the signal that results in an increase of alpha power with memory load? One possibility is that frontal areas engaged in executive control required for the memory task provide a top‐down drive, which increases with working memory load. This top‐down drive could either be phasic or constant. A phasic drive could be measured as coherence in the alpha band between frontal and posterior areas during working memory maintenance. This is in line with work by Von Stein et al. [2000], who propose that top‐down processing can be studied by measures of synchronization in the lower frequency bands (theta and alpha). Indeed, several EEG studies have identified fronto‐posterior coherence in the alpha band in working memory tasks [Sauseng et al., 2005; Schack et al., 2005]. If the posterior alpha activity during memory maintenance is due to a top‐down drive, measures of causal interactions [e.g., Brovelli et al., 2004] should reveal that the posterior alpha activity is controlled by frontal activity. Further investigations of fronto‐temporal coherence in the alpha band and measures of directionality as a function of memory load would help to elucidate whether the posterior alpha activity is a consequence of phasic top‐down inhibition.
REFERENCES
- Adrian ED,Matthews BH ( 1934): The Berger rhythm: potential changes from the occipital lobes in man. Brain 57: 355–385. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC,Knosche TR ( 2000): Tangential derivative mapping of axial MEG applied to event‐related desynchronization research. Clin Neurophysiol 111: 1300–1305. [DOI] [PubMed] [Google Scholar]
- Berger H ( 1929): Über das Elektroenkephalogramm des Menschen. Arch Psychiatr Nervenkr 87: 527–570. [Google Scholar]
- Brovelli A,Ding M,Ledberg A,Chen Y,Nakamura R,Bressler SL ( 2004): Beta oscillations in a large‐scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A 101: 9849–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR,Croft RJ,Dominey SJ,Burgess AP,Gruzelier JH ( 2003): Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47: 65–74. [DOI] [PubMed] [Google Scholar]
- Curtis CE,D'Esposito M ( 2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ,D'Esposito M ( 2003): Dissecting contributions of prefrontal cortex and fusiform face area to face working memory. J Cogn Neurosci 15: 771–784. [DOI] [PubMed] [Google Scholar]
- Gross J,Kujala J,Hämäläinen M,Timmermann L,Schnitzler A,Salmelin R ( 2001): Dynamic imaging of coherent sources: studying neural interaction in the human brain. Proc Natl Acad Sci U S A 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M,Hari R,Ilmoniemi RJ,Knuutila J,Lounasmaa OV ( 1993): Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65: 413–497. [Google Scholar]
- Huang M,Mosher JC ( 1997): A novel head model for the MEG forward problem: BEM accuracy with only spherical model complexity. Neuroimage Suppl Proceedings of Third International Conference on Functional Mapping of the Human Brain, May 19–23 1997, Copenhagen, Denmark 5: S441.
- Hummel F,Gerloff C ( 2005): Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cereb Cortex 15: 670–678. [DOI] [PubMed] [Google Scholar]
- Jensen O,Gelfand J,Kounios J,Lisman JE ( 2002): Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 12: 877–882. [DOI] [PubMed] [Google Scholar]
- Jung‐Beeman M,Bowden EM,Haberman J,Frymiare JL,Arambel‐Liu S,Greenblatt R,Reber PJ,Kounios J ( 2004): Neural activity when people solve verbal problems with insight. PLoS Biol 2: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Schwaiger J,Auinger P,Winkler T ( 1999): ‘Paradoxical’ alpha synchronization in a memory task. Brain Res Cogn Brain Res 7: 493–501. [DOI] [PubMed] [Google Scholar]
- Klimesch W,Doppelmayr M,Rohm D,Pollhuber D,Stadler W ( 2000): Simultaneous desynchronization and synchronization of different alpha responses in the human electroencephalograph: a neglected paradox? Neurosci Lett 284: 97–100. [DOI] [PubMed] [Google Scholar]
- Kolev V,Yordanova J,Schurmann M,Basar E ( 2001): Increased frontal phase‐locking of event‐related alpha oscillations during task processing. Int J Psychophysiol 39: 159–165. [DOI] [PubMed] [Google Scholar]
- Liljestrom M,Kujala J,Jensen O,Salmelin R ( 2005): Neuromagnetic localization of rhythmic activity in the human brain: a comparison of three methods. Neuroimage 25: 734–745. [DOI] [PubMed] [Google Scholar]
- Maltseva I,Geissler HG,Basar E ( 2000): Alpha oscillations as an indicator of dynamic memory operations—anticipation of omitted stimuli. Int J Psychophysiol 36: 185–197. [DOI] [PubMed] [Google Scholar]
- Maris E ( 2004): Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology 41: 142–151. [DOI] [PubMed] [Google Scholar]
- Mima T,Oluwatimilehin T,Hiraoka T,Hallett M ( 2001): Transient interhemispheric neuronal synchrony correlates with object recognition. J Neurosci 21: 3942–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival DB,Walden AT ( 1993): Spectral analysis for physical applications: multitaper and conventional univariate techniques. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Pfurtscheller G,Stancak A,Neuper C ( 1996): Event‐related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol 24: 39–46. [DOI] [PubMed] [Google Scholar]
- Ray WJ,Cole HW ( 1985): EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 228: 750–752. [DOI] [PubMed] [Google Scholar]
- Salenius S,Kajola M,Thompson WL,Kosslyn S,Hari R ( 1995): Reactivity of magnetic parieto‐occipital alpha rhythm during visual imagery. Electroencephalogr Clin Neurophysiol 95: 453–462. [DOI] [PubMed] [Google Scholar]
- Salmelin R,Hari R ( 1994): Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr Clin Neurophysiol 91: 237–248. [DOI] [PubMed] [Google Scholar]
- Sauseng P,Klimesch W,Gruber W,Doppelmayr M,Stadler W,Schabus M ( 2002): The interplay between theta and alpha oscillations in the human electroencephalogram reflects the transfer of information between memory systems. Neurosci Lett 324: 121–124. [DOI] [PubMed] [Google Scholar]
- Sauseng P,Klimesch W,Schabus M,Doppelmayr M ( 2005): Fronto‐parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol 57: 97–103. [DOI] [PubMed] [Google Scholar]
- Schack B,Klimesch W,Sauseng P ( 2005): Phase synchronization between theta and upper alpha oscillations in a working memory task. Int J Psychophysiol 57: 105–114. [DOI] [PubMed] [Google Scholar]
- Sewards TV,Sewards MA ( 1999): Alpha‐band oscillations in visual cortex: part of the neural correlate of visual awareness? Int J Psychophysiol 32: 35–45. [DOI] [PubMed] [Google Scholar]
- Sternberg S ( 1966): High‐speed scanning in human memory. Science 153: 652–654. [DOI] [PubMed] [Google Scholar]
- van Veen BD,van Drongelen W,Yuchtman M,Suzuki A ( 1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880. [DOI] [PubMed] [Google Scholar]
- Vanni S,Revonsuo A,Hari R ( 1997): Modulation of the parieto‐occipital alpha rhythm during object detection. J Neurosci 17: 7141–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stein A,Chiang C,Konig P ( 2000): Top‐down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A 97: 14748–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden MS,Foxe JJ,Wang N,Simpson GV ( 2000): Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha‐band electroencephalography increases over occipital cortex. J Neurosci 20: RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]


