Abstract
Purpose
We investigated whether daily consumption of Spirulina, an antioxidant generating cyanobacterial nutritional supplement, would suppress photostress-induced retinal damage and prevent vision loss in mice.
Methods
Six-week-old male BALB/cAJcl mice were allowed constant access to either a standard or Spirulina-supplemented diet (20% Spirulina) that included the antioxidants, β-carotene and zeaxanthin, and proteins for 4 weeks. Following dark adaptation, mice were exposed to 3000-lux white light for 1 hour and returned to their cages. Visual function was analyzed by electroretinogram, and retinal histology by hematoxylin and eosin staining, terminal deoxynucleotidyl transferase-mediated, deoxyuridine triphosphate nick-end labeling (TUNEL) assay, and immunohistochemistry. Retinal expression of proteins, reactive oxygen species (ROS), and mRNAs were measured using immunoblot analysis, enzyme-linked immunosorbent assay (ELISA), 2′,7′-dichlorofluorescein-diacetate, or ROS Brite 700 Dyes, and real-time reverse-transcription polymerase chain reaction, respectively.
Results
Light-induced visual function impairment was suppressed by constant Spirulina intake. Thinning of the photoreceptor layer and outer segments, photoreceptor cell death, decreased rhodopsin protein, and induction of glial fibrillary acidic protein were ameliorated in the Spirulina-intake group. Increased retinal ROS levels after light exposure were reduced by Spirulina supplementation. Light-induced superoxide dismutase 2 and heme oxygenase-1 mRNAs in the retina, and Nrf2 activation in the photoreceptor cells, were preserved with Spirulina supplementation, despite reduced ROS levels, suggesting two pathways for suppressing ROS, scavenging and induction of endogenous antioxidative enzymes. Light-induced MCP-1 retinal mRNA and proteins were also suppressed by Spirulina.
Conclusions
Spirulina ingestion protected retinal photoreceptors from photostress in the retina.
Translational Relevance
Spirulina has potential as a nutrient supplement to prevent vision loss related to oxidative damage in the future.
Keywords: retina, neurodegeneration, light exposure, photoreceptor, oxidative stress, antioxidative enzyme
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide.1–3 As neural tissue, the retina cannot self-repair once the disease progresses; therefore, the possibility that ingestion of antioxidant supplements might prevent AMD progression has gained global attention.4 Vitamin C, vitamin E, and lutein are the main micronutrient supplements recommended for the prevention of AMD by two large clinical studies, the Age-Related Eye Disease Study (AREDS)5 and AREDS2.6 These recommendations are also followed in clinical practice.7 The biological activity and mechanisms have been elucidated using animal model and cell culture studies.8–15 The prevention of AMD requires daily administration of these compounds, and combination tablets have been developed for this purpose. However, the requirement for long-term use has led to research into alternative choices that would be valuable for patients with difficulties in swallowing or other age- and disease-related problems with tablet use. Moreover, the antioxidant supplements proposed in the AREDS5/AREDS26 did not produce an effect that was sufficient to prevent AMD progression in all patients. These studies identified nonresponders to the formula who require the administration of alternative nutrients.
In this study, we focused on Spirulina (Arthrospira platensis), a prokaryotic cyanobacterium (a group formerly called blue–green algae) with a helical shape (width, 5–8 μm; length, 300–500 μm)16 that appeared in the fossil record approximately 3 billion years ago. Spirulina is cultivated worldwide and used as a dietary supplement and whole food.17–21 It contains proteins, vitamins, minerals, polysaccharides (dietary fiber), and phytochemicals. In addition to the orange–yellow carotenoids, β-carotene and zeaxanthin, it contains blue phycocyanin and green chlorophyll pigments; therefore, it has antioxidative properties.18 Human studies have shown that Spirulina can supply protein, vitamins, and minerals to resolve nutritional deficiencies18,19 and has an antioxidative effect in exercise-induced oxidative stress.22,23 In addition, Spirulina has been used in clinical studies of patients with chronic obstructive pulmonary disease (COPD) and skeletal muscle disorders.18,22,23 Its antioxidant activity exhibits antipathologic effects in allergic rhinitis24 and obesity.25 Further, neuroprotective effects in the brain have been observed in animal models of Parkinson's disease26 and a neurotoxic model generated using tributyltin chloride.27 However, effects on the retina and vision have not been reported in animal experiments or clinical studies.
One of the risk factors for AMD is light exposure,28 which causes local oxidative stress in the retina.9,14,29–31 Thus, protecting retinal tissue from photostress may also prevent AMD progression. To that end, we analyzed the biological effects of Spirulina ingestion on the retinas of mice exposed to light at an intensity known to promote retinal degeneration. The results provide a proof of concept for use of Spirulina as a retina- and vision-protective nutritional supplement.
Methods
Preparation of the Spirulina-Supplemented Diet
Spirulina was provided by DIC LIFETEC CO., Ltd. (Tokyo, Japan), and was processed into the Spirulina-supplemented solid diet used here by CLEA Japan (Tokyo, Japan). Diet compositions were measured by Japan Food Research Laboratories (Tokyo, Japan) (Table).
Table.
Contents of Experimental Diets
| Spirulina Supplemented |
Control (CE-2) |
|
| Moisture, % | 9.8 | 9.1 |
| Crude protein, % | 33.6 | 25.4 |
| Crude fat, % | 5.0 | 5.0 |
| Crude fiber, % | 3.3 | 4.0 |
| Crude ash, % | 6.8 | 6.8 |
| Total carotenoids, mg/100 g | 79.0 | 0.97 |
| β-carotene, mg/100 g | 32.5 | 0.07 |
| Zeaxanthin, mg/100 g | 17.7 | 0.16 |
| Retinol, mg/100 g | 0.88 | 0.89 |
| c-phycocyanin, mg/100 g | 860.0 | <0.0001 |
| Vitamin A, IU/100 g | 11,960 | 3010 |
| Vitamin C (total ascorbic acid), mg/100 g | 4.0 | 24.0 |
| Vitamin E (total tocopherol), mg/100 g | 6.8 | 7.8 |
| α-tocopherol, mg/100 g | 5.4 | 5.2 |
| β-tocopherol, mg/100 g | 0.6 | 1.0 |
| γ-tocopherol, mg/100 g | 0.7 | 1.4 |
| δ-tocopherol, mg/100 g | 0.1 | 0.2 |
The measurement methods were as follows: moisture, atmospheric-pressure heat-drying; protein, Kjeldahl method; lipid, diethyl ether extraction; fiber, standing method; ash, direct ashing; phycocyanin, absorptiometry; β-carotene and zeaxanthin, retinol and vitamins C and E, high-performance liquid chromotography; vitamin A, estimated from the values for β carotene and retinol. Data were obtained at the Japan Food Research Laboratories.
Animals
Six-week-old male BALB/cAJcl mice (CLEA Japan) were maintained for 4 weeks with unlimited access to either a standard CE-2 solid diet (CLEA Japan) or Spirulina-supplemented solid diet, which contained 20% Spirulina (Table). The mean mice weights in the control diet–fed and nonlight-exposed, control diet–fed and light-exposed, and Spirulina-supplemented diet-fed and light-exposed mice were 29.2 ± 9.8, 28.9 ± 8.8, and 27.9 ± 10.2 g, respectively, at the time of the experiment. There were no significant differences in weight between groups (data not shown). No adverse events were observed. All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Animal Care Committee of Keio University. The animal experimental protocols were approved by the Animal Care Committee of Keio University (approval No. 08002).
Light Exposure
Mice were exposed to light as previously described.9,14,29–33 Briefly, mice were dark-adapted in complete darkness for 12 hours before light exposure. Their pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P; Santen Pharmaceutical, Osaka, Japan) just before light exposure. Mice were exposed to a white fluorescent lamp (FHD100ECW; Panasonic, Osaka, Japan) at 3000 lux for 1 hour (starting at 9 AM) in a dedicated exposure box maintained at 22 ± 2°C and containing stainless steel mirrors on each wall and the floor (Tinker N, Kyoto, Japan). After light exposure, the mice were returned to their cages and maintained under dim cyclic light (5 lux, 12-hours on/off) until they were euthanized with 70 mg/kg sodium pentobarbital at the end of each experiment. Unexposed control mice were also maintained under dim cyclic lighting and euthanized with 70 mg/kg sodium pentobarbital at the end of each experiment.
Electroretinogram (ERG)
Mice were dark-adapted for at least 12 hours, then placed under dim red illumination before the start of ERG. Mice were anesthetized with 0.1 mL/10 g of mixed anesthetic (0.75 mg/kg medetomidine, 4.0 mg/kg midazolam, and 5.0 mg/kg butorphanol), and warmed on a heating pad throughout the experiment. Before recording, the pupils of the mice were dilated using Mydrin-P as described in the previous section. Recording used the PuREC system (Mayo Corp, Inazawa, Japan). Ground and reference electrodes were placed on the tail and head, respectively, and the active gold-wire electrodes were placed on the cornea. Full-field, scotopic ERGs were obtained in response to a flash at intensities ranging from 0.01 to 30 cd·s/m2. Stimuli were delivered using an LED Visual Stimulator (LS-100; Mayo Corp) and a Hemisphere Stimulator (Mayo Corp). Data were differentially amplified and filtered through a 300-Hz digital lowpass filter. The a-wave amplitude was measured from baseline to the trough, while the b-wave amplitude was measured from the trough of the a-wave to the peak of the b-wave. The implicit times of the a- and b-waves were measured from the stimulus onset to the wave peaks. The peak points were automatically indicated by the system and confirmed by the examiner.
Histologic Analysis
Eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C, then embedded in paraffin (Sakura Finetek, Tokyo, Japan). Sections (thickness, 8–10 μm), including the portion from the optic nerve head to the most peripheral region of the retina, were deparaffinized by passing them through a graded solvent series with three 5-minute exposures to each of the following stages: xylene, 1:1 xylene-alcohol, 100% ethanol, 90% ethanol, 70% ethanol, and 50% ethanol. The remaining ethanol was removed by rinsing with distilled water.
To measure the lengths and thicknesses of retinal components, sections were stained with hematoxylin and eosin. Measurements were performed using ImageJ (National Institutes of Health, Bethesda, MD, USA, at http://rsb.info.nih.gov/ij/index.html), and averaged as described previously.34–37
The terminal deoxynucleotidyl transferase-mediated, deoxyuridine triphosphate nick-end labeling (TUNEL) assay was performed using a commercial ApopTag Red apoptosis detection kit (Millipore, Bedford, MA) according to the manufacturer's protocol. Nuclei were stained with Cellstain (4′,6-diamidino-2-phenylindole [DAPI]) solution (12 μg/mL; Dojindo Molecular Technologies, Kumamoto, Japan). TUNEL-positive cells in each section were counted and averaged as described previously.34–37
For immunostaining, sections were incubated with blocking buffer followed by the primary antibody against glial fibrillary acidic protein (GFAP; 1:1,000; Dako, Carpinteria, CA), or rhodopsin (1:10,000; Thermo Fisher Scientific Inc., Waltham, MA) and Nrf2 (1:100; Abcam, Cambridge, FL) overnight at 4°C. Signals were detected by Alexa 488- (1:500; Thermo Fisher Scientific, Inc.) or Alexa 555-conjugated secondary (1:500; Thermo Fisher Scientific, Inc.) antibodies. Nuclei were stained with Cellstain solution (2 μg/mL). The Nrf2 staining procedure included heating the sections in Immunosaver solution (1:200; Wako, Tokyo, Japan) for 45 minutes at 90°C for antigen retrieval before blocking. Nrf2-positive cells in each section were counted and averaged in each group.
All sections were examined under a fluorescent microscope equipped with a digital camera (Olympus Co., Tokyo, Japan).
Immunoblot Analysis
Eyes were enucleated, and each retina was isolated and placed in lysis buffer that included protease inhibitor cocktail (Complete, EDTA-free; Roche, Mannheim, Germany) and phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich, St. Louis, MO), and sonicated on ice using a Handy Sonic UR-20 (Tomy Seiko, Tokyo, Japan). The iced suspension was incubated for 30 minutes, centrifuged at 20,400 g for 15 minutes at 4°C, and the supernatant transferred to a new tube. The lysate was treated with Laemmli sample buffer and separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore) in a Trans-Blot SD Cell (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked with 5% skim milk in Tris Buffered Saline with Tween (TBS-T) or Tris-NaCl (TNB) blocking buffer (0.1 M Tris-HCl, pH 7.5; 0.15 M NaCl; and 0.5% TSA blocking reagent; Perkin-Elmer Life Sciences, Waltham, MA), and incubated overnight at 4°C with a rabbit anti-rhodopsin antibody (1:100,000; LSL, Osaka, Japan). Next, membranes were incubated with horseradish peroxidase-conjugated secondary antibody. Signals were detected using an enhanced chemiluminescence system (ECL Blotting Analysis System; Amersham, Arlington Heights, IL), and measured with the ImageJ program. The same membrane was incubated with mouse anti-α-tubulin (1:100,000; Sigma-Aldrich), then with a horseradish peroxidase-conjugated secondary antibody to detect the signal in the same manner as rhodopsin. The value for rhodopsin was normalized to α-tubulin.
Real-Time, Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from mouse retinas with TRIzol reagent (Life Technologies, Carlsbad, CA). RNA concentration was measured using a NanoDrop 1000 (Thermo Fisher Scientific, Inc.), and 1 μg of RNA was reverse-transcribed using SuperScript VILO master mix (Life Technologies) according to the manufacturer's instructions. For real-time PCR, the SYBR system31 was used. The forward and reverse primer sequences were as follows: superoxide dismutase 2 (SOD2),9 forward: 5′-CTGGACAAACCTGAGCCCTA-3′, reverse: 5′-GAACCTTGGACTCCCACAGA-3′; and heme oxygenase-1 (HO-1), forward: 5′-GCTCTATCGTGCTCGCATGA-3′, reverse: 5′-AGTGCCTGCAGCTCCTCAA-3′. The mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences for GAPDH31 were as follows: forward: 5′-AACTTCGGCCCCATCTTCA-3′ and reverse: 5′-GATGACCCTTTTGGCTCCAC-3′. For Monocyte Chemoattractant Perotein-1 (MCP-1), the TaqMan gene expression assay (TaqMan probe assay ID; Mm00441242_m1; Life Technologies) was used. Real-time PCR was performed using the StepOnePlus PCR system (Applied Biosystems, Foster City, CA) and gene expression was quantified using the ΔΔCT method.
ROS Measurement
Mouse eyes were enucleated and the retinas were immediately frozen and homogenized in phosphate buffered saline (PBS) using a Mixer Mill homogenizer (MM 300; Qiagen Inc., Chatsworth, CA) according to the manufacturer's protocol and previous reports, to maintain cellular conditions at the time of sampling and avoid cellular changes in response to the ROS released from homogenized cells. Homogenates were incubated with 2′, 7′-dichlorofluorescein-diacetate (DCFH; 50 μM; Sigma-Aldrich) or ROS Brite 700 Dyes (50 μM; AAT Bioquest, Inc., Sunnyvale, CA) at 37°C in the dark for 1 hour. DCFH detects peroxides, peroxyl radicals, and Nitrogen Oxide (NO), and ROS Brite 700 Dyes detect hydroxyl radicals and superoxide anions. Samples were centrifuged for 5 minutes and 20,400 g at 4°C. Pellets were washed with cold PBS twice and resuspended in cold PBS or N-2-hydroxyethyl-1-piperazine-N′-2-ethanesulfonic acid (HEPES)-buffered saline containing 100 μg/mL heparin. DCFH and ROS Brite 700 Dye fluorescence was measured using a Synergy4 system (BioTek Instruments, Inc., Winooski, VT).
Enzyme-Linked Immunosorbent Assay (ELISA)
Total protein was isolated from mouse retinas. The MCP-1 cytokine content was measured using ELISA kits (R&D Systems, Minneapolis, MN), and a spectrometer (Wallac ARVO SX 1420 Multilabel Counter; Perkin-Elmer). All procedures were performed according to the manufacturers' instructions.
Statistical Analysis
Results are expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) with Tukey post-hoc tests were used to assess statistical significance between the groups using commercially available software (IBM SPSS Ver 23; IBM Corp, Armonk, NY). Results were considered significant if P < 0.05.
Results
Spirulina Protects Against Light-Induced Visual Impairment
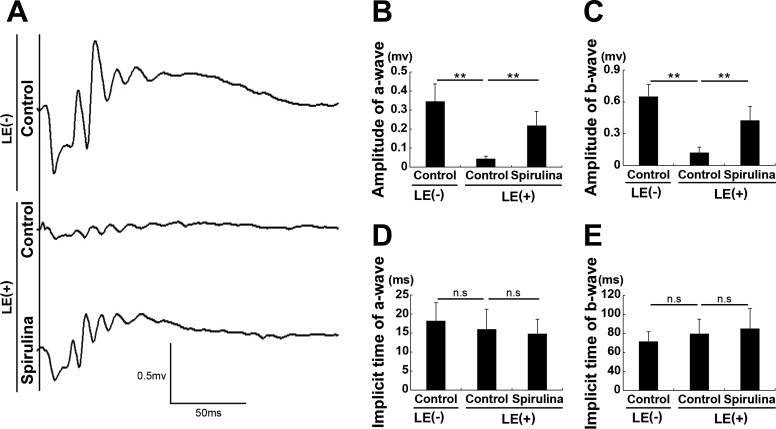
In the control-diet group, ERG demonstrated reduction in amplitudes of both a- and b-waves 4 days after light exposure compared with control diet–fed and nonlight-exposed mouse group (Figs. 1A–E). These data indicated photoreceptor function, represented by the a-wave, was impaired by the light exposure at this time point, likely with a subsequent reduction in the electrical responses in the retina (represented by the b-wave). This finding is consistent with our previous studies.30,31,38,39 However, in mice from the Spirulina-supplementation group, light-induced amplitude reductions in both a- and b-waves were suppressed compared with the control-diet group at the same time point after light exposure.
Figure 1.
Spirulina protected against light-induced impairment of visual function. (A) Representative waveforms from an individual mouse recorded by ERG 4 days after light exposure. (B, C) The amplitudes of both the a- and b-waves were reduced after light exposure, which were attenuated by dietary Spirulina supplementation. (D, E) The implicit times of the a- and b-waves were not changed. LE, light exposure. LE (–) + control diet; LE (+) + control diet; and LE (+) + Spirulina-supplemented diet. n = 5 in each group. **P < 0.01.
Spirulina Prevents Light-Induced Histologic Changes in the Retina
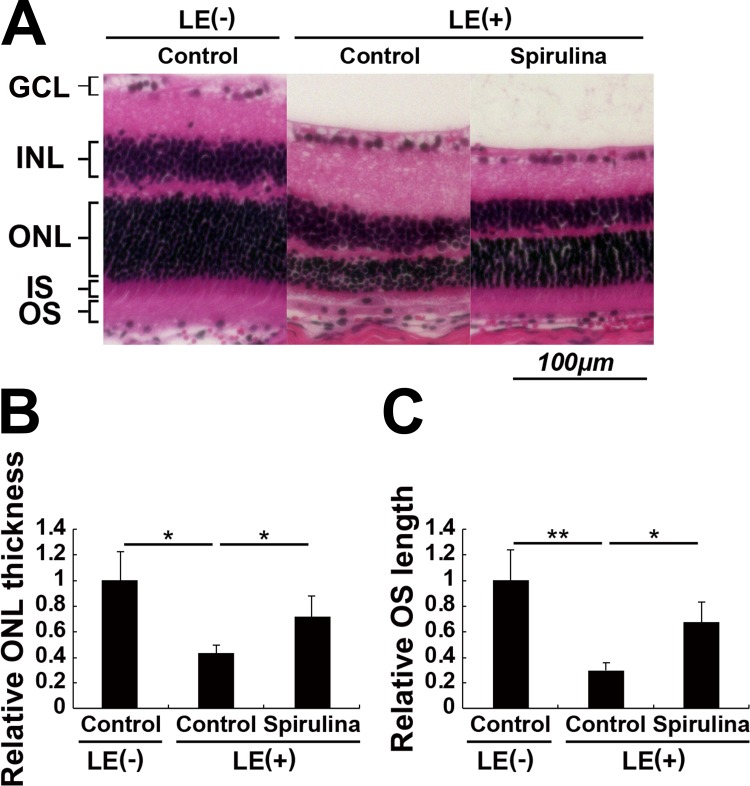
We analyzed the retinas for histologic changes at the time point when ERG was recorded (Figs. 2A–C). The thickness of the photoreceptor layer, outer nuclear layer (ONL), and length of outer segments (OSs) where visual pigments are distributed in the photoreceptor cells were reduced. These changes were significantly attenuated in the Spirulina supplementation group, indicating that Spirulina preserved photoreceptor cell number, and OS length in the remaining photoreceptor cells against photostress. There were no significant changes in the inner retinal layers, suggesting that the b-wave change in the ERG was most likely because of the difference in the input from the photoreceptor cells.
Figure 2.
Spirulina suppressed light-induced histological changes in the retina. (A) The hematoxylin and eosin–stained retinal sections 4 days after light exposure. (B, C) The thickness of the ONL and the length of the OSs of the photoreceptor cells were reduced after light exposure, which were significantly attenuated by Spirulina supplementation. GCL, ganglion cell layer; INL, inner nuclear layer; IS, inner segment. n = 4 in each group. *P < 0.05, **P < 0.01. Scale bar, 100 μm.
Spirulina Suppresses Light-Induced Photoreceptor Apoptosis and Degeneration
Next, we quantified apoptotic cells using the TUNEL assay. TUNEL-positive, apoptotic cells appeared in the ONL 2 days after light exposure (Figs. 3A, 3B). This is consistent with previous reports.34–37 In the Spirulina-supplementation group, the number of apoptotic cells was significantly lower than those in the light-exposed control-diet group at the same timepoint.
Figure 3.
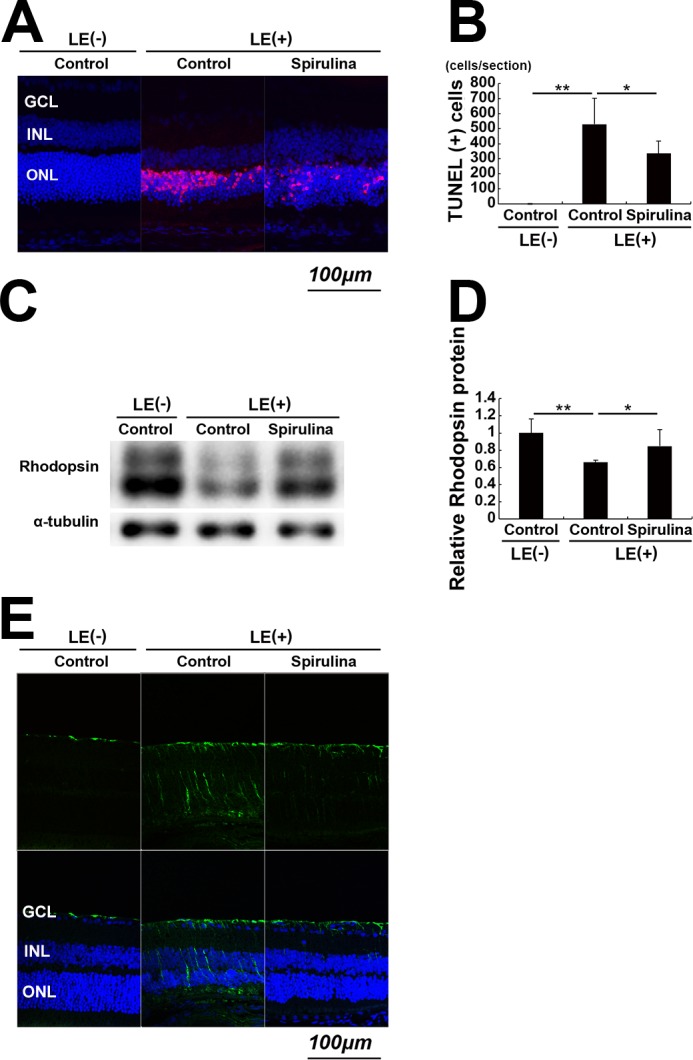
Spirulina suppressed light-induced photoreceptor apoptosis and degeneration. (A, B) The TUNEL assay, which was performed 2 days after light exposure. TUNEL-positive cells (red) appeared in the ONL after light exposure. Apoptotic cells were significantly reduced by Spirulina supplementation. (C, D) Immunoblot analysis. Rhodopsin protein in the retina was reduced 2 days after light exposure, which was attenuated by Spirulina supplementation. Rhodopsin and α-tubulin were measured sequentially in the same membrane, and rhodopsin monomer bands and α-tubulin bands are shown separately. (E) Staining for GFAP was performed 4 days after light exposure. Light-induced increases in GFAP expression were attenuated by Spirulina supplementation. n = 4 in each group. *P < 0.05, **P < 0.01. Scale bar, 100 μm.
Then, we quantified the expression of retinal rhodopsin protein at the same 2-day timepoint (Figs. 3C, 3D). Rhodopsin protein expression was significantly decreased by light exposure, indicating a loss and degeneration of photoreceptor cells. This reduction was significantly attenuated in the Spirulina-supplementation group, indicating a protective effect of Spirulina supplementation against cell degeneration and death.
In addition, we analyzed the expression of GFAP, a marker for glial reactivation during retinal degeneration, 4 days after light exposure. We found that light exposure upregulated GFAP expression, which was attenuated by Spirulina (Fig. 3E). This result suggested that Spirulina suppressed Müller glial cell reactivation, which may be related to photoreceptor degeneration.
Spirulina Suppresses Light-Induced ROS Accumulation in the Retina
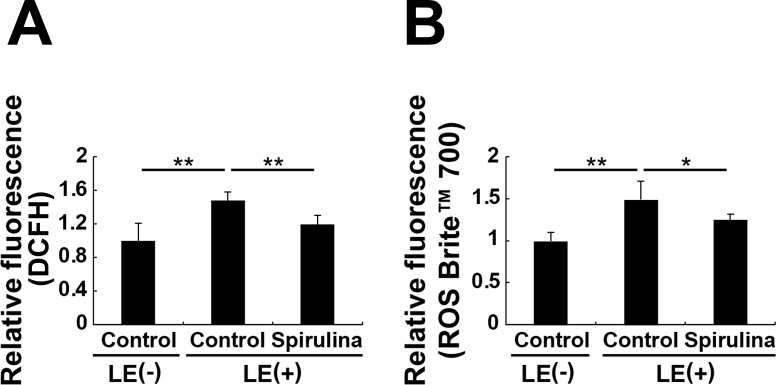
Although Spirulina supplementation includes antioxidant compounds by its nature (Table), it was unclear whether this affected levels of retinal ROS. We found that light exposure significantly increased levels of retinal ROS at 6 hours after exposure, as measured by DCFH and ROS Brite 700 assays (Figs. 4A, 4B). Further, this increase was significantly reduced by Spirulina supplementation.
Figure 4.
Spirulina suppressed accumulation of light-induced ROS. (A, B) ROS levels in the retina 6 hours after light exposure. Measurement of ROS accumulation was performed using DCFH (A) and ROS Brite 700 (B). Spirulina suppressed the accumulation of light-induced ROS. n = 5 in each group; *P < 0.05, **P < 0.01.
Spirulina Preserves Expression of Antioxidative Molecules in the Retina
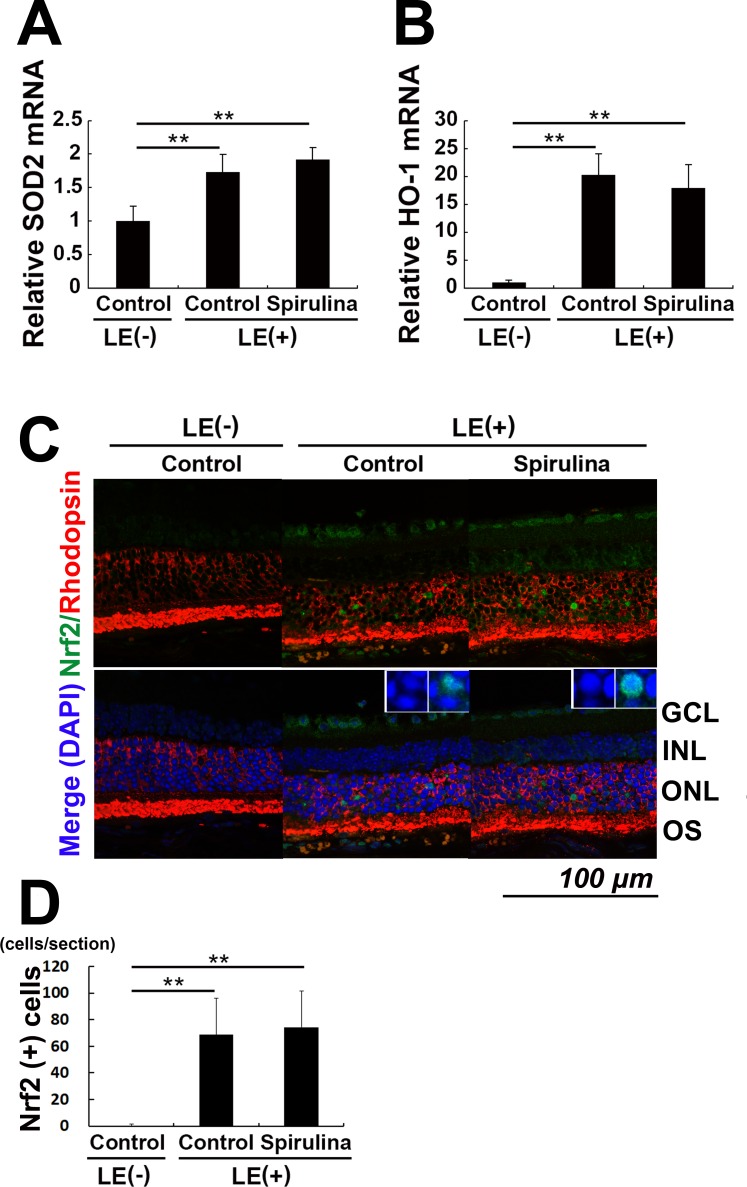
We further measured the levels of antioxidative enzymes. Light exposure induced both SOD2 and HO-1 mRNA expression in the retina at 6 hours, and this expression was preserved in the Spirulina-supplementation group (Figs. 5A, 5B). The light-induced upregulation of these molecules was consistent with the fact that these mRNAs are upregulated in response to ROS, which stabilizes and promotes the nuclear transfer of the transcription factor Nrf2.40 Immunostaining against Nrf2 in the retinal section obtained 24 hours after light exposure revealed an induction of nuclear Nrf2 transported and activated by light exposure in rhodopsin-positive neurons, classified as photoreceptor cells (Fig. 5C). In addition, we found that the nuclear Nrf2 was preserved after Spirulina administration (Figs. 5C, 5D), suggesting that Spirulina administration stabilized Nrf2 in the photoreceptor cells to induce antioxidative enzymes irrespective of ROS level.
Figure 5.
Spirulina preserved expression of antioxidative molecules in the retina. (A, B) The mRNA levels of SOD2 (A) and HO-1 (B) in the retina were increased 6 hours after light exposure, both with and without Spirulina supplementation. (C) Immunohistochemistry for rhodopsin and Nrf2, a transcription factor upstream of SOD2 and HO-1, with nuclear counter staining with DAPI 24 hours after light exposure. Insets show magnified regions. (C, D) Nrf2 was expressed in the photoreceptor nuclei after light exposure, a condition preserved after Spirulina administration. n = 5 in each group; **P < 0.01. Scale bar, 100 μm.
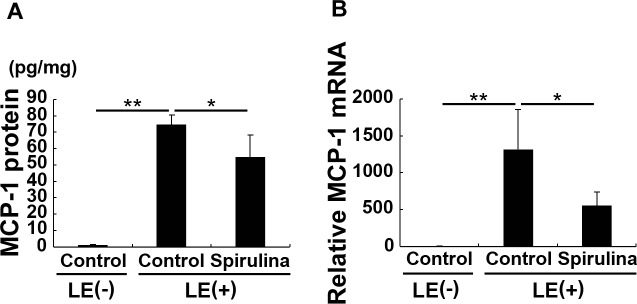
Spirulina Reduces Light-Induced Expression of MCP-1 in the Retina
MCP-1, a chemokine that modulates inflammation and cell–cell adhesion,41 was previously shown to have a critical role in light-induced retinal degeneration.42,43 Here, the mRNA and protein levels of MCP-1 were significantly increased in the retina 24 hours after light exposure; however, both changes were significantly attenuated by Spirulina supplementation (Figs. 6A, 6B).
Figure 6.
Spirulina reduced light-induced MCP-1 expression in the retina. (A) MRNA and (B) protein levels of retinal MCP-1 24 hours after light exposure. Light exposure increased the levels of MCP-1 at both mRNA and protein levels. However, these increases were significantly attenuated by Spirulina supplementation. n = 5 in each group (A) and n = 6 in each group (B). *P < 0.05; **P < 0.01.
Discussion
The light-induced impairment of visual function due to retinal damage was demonstrated by ERG, retinal histologic changes, increased apoptosis, decreased rhodopsin levels, and GFAP induction, in association with increased ROS accumulation. These factors were prevented or attenuated by continuous dietary Spirulina supplementation. In addition, Spirulina preserved antioxidative enzyme expression, most likely by preserving the nuclear Nrf2 expression in the photoreceptor cells. Spirulina also suppressed photostress-mediated MCP-1 expression.
The concept that Spirulina and light damage both act primarily at the photoreceptor level is supported by the fact that the light-induced photoreceptor dysfunction seen on ERG is consistent with light-induced tissue damage that we identified histologically, such as thinning of the ONL and photoreceptor cell layer and shortening of photoreceptor cell outer segments (OSs). Notably, these histopathologic changes were clearly attenuated by Spirulina supplementation. Furthermore, the light-induced reduction in ONL thickness was observed in association with a restriction of TUNEL-positive, apoptotic cells to photoreceptor cells. This observation is consistent with previous studies.14,30,31,33,38 In the present study, Spirulina supplementation decreased the number of apoptotic photoreceptor cells, which explained the preservation of ONL thickness found in the Spirulina-supplementation group. Taken together, these data indicated that Spirulina increased photoreceptor survival under conditions of intense photostress.
Rhodopsin, the pigment that receives photons and converts them into electrical activity, is concentrated in the OS.44 Rhodopsin knockout mice demonstrated reduced OS length.45 Reduction in rhodopsin protein after light exposure in the current study may have induced OS thinning and degeneration of the photoreceptor cells that remained after light exposure. Thus, the postlight-exposure preservation of rhodopsin levels by Spirulina supplementation may explain the prevention of light-induced OS thinning.
Furthermore, we observed an increase in GFAP expression following light stimulation that corresponded with reactivation and pathologic changes in Müller glial cells. This may have been caused by photoreceptor cell degeneration and caused photoreceptor cell degeneration via glial-neuronal interactions. Therefore, the suppressive effect of Spirulina on this reactivation may have also contributed to the photoreceptor protection that we observed.
ROS have well-documented pathologic effects in the retina; they negatively affect rhodopsin levels13,30,31,33,46,47 and increase apoptosis.14,30,33,38,48 Lipopolysaccharide-induced uveitis and retinitis models have not reported apoptotic photoreceptors; however, rhodopsin protein expression is reduced posttranscriptionally,47,49 which is ameliorated by antioxidants.14,46 Light-induced ROS accumulation in the retina causes photoreceptor cell apoptosis14,30,31,33; however, blocking light stimuli,32,33 administering antioxidants,14,31 and inhibiting the inflammatory mediator angiotensin II30 decreases ROS and attenuates light-induced photoreceptor apoptosis. Moreover, enhancing ROS accumulation by removing antioxidants from the culture medium induces retinal neuronal cell death in vitro,48 which indicated that there is a direct apoptosis-inducing effect of ROS. In the present study, reduced rhodopsin protein expression and apoptosis were attenuated by Spirulina supplementation, most likely due to the suppression of retinal ROS accumulation.
The components of the carotenoids, Spirulina, β-catenin, and zeaxanthin, are lipophilic, exist in the cellular membrane, and can scavenge ROS.2,50–53 Additionally, another component of Spirulina, c-phycocyanin, is a water-soluble, pigment-protein complex that can be transferred intracellularly with antioxidant and radical-scavenging activity.54 These factors may have scavenged retinal ROS in the current study. Interestingly, zeaxanthin, a xanthophyll, may have other relevant actions. Another xanthophyll, lutein, induces expression of phase-II antioxidative enzymes, such as SOD2 and HO-1, irrespective of ROS levels.9,10 In this study, Spirulina preserved the expression of Nrf2, which was degraded under the control condition, and antioxidative enzyme despite a reduction of ROS accumulation; these results are consistent with those of previous studies in fish.18,55 Therefore, Spirulina may preserve Nrf2 expression independently of ROS levels via the effects of these components. Alternatively, Spirulina involves various proteins, which could have biological activities18; protein contents may have induced Nrf2 stabilization and antioxidative enzymes. MCP-1, which is involved in AMD pathogensis56 and disrupts cell–cell junctions41 to make the tissue vulnerable to damage, was suppressed by Spirulina under the photostressed condition; this may also provide protective effects for the retina. Spirulina contains multiple phytochemicals, including the vitamins and carotenoids in the AREDS formula,5,6 and proteins that could act through a variety of pathways to protect against oxidative damage. Moreover, it can lower the risk of AMD by reducing body mass index25,28 and actually reduce oxidative markers in the serum of COPD patients.18 These effects may be advantageous for its use as an antioxidant nutritional supplement.
In summary, light-induced photoreceptor death, retinal degeneration, and subsequent visual impairment were suppressed by dietary Spirulina supplementation in mice. Although further studies are required, Spirulina could be useful in suppressing retinal damage and the progression of diseases, such as AMD.
Acknowledgments
We thank all the members of the Laboratory of Retinal Cell Biology (RCB Lab).
Spirulina was provided by DIC LIFETEC CO., Ltd.
Disclosure: T. Okamoto, None; H. Kawashima, None; H. Osada, None; E. Toda, None; K. Homma, None; N. Nagai, None; Y. Imai, DIC LIFETEC CO., Ltd (E); K. Tsubota, None; Y. Ozawa, DIC LIFETEC CO., Ltd (F)
References
- 1.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016;54:64–102. doi: 10.1016/j.preteyeres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 5.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew EY, SanGiovanni JP, Ferris FL, et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131:843–850. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M, Shinoda H, Koto T, Uchida A, Tsubota K, Ozawa Y. Use of micronutrient supplement for preventing advanced age-related macular degeneration in Japan. Arch Ophthalmol. 2012;130:254–255. doi: 10.1001/archopthalmol.2011.1368. [DOI] [PubMed] [Google Scholar]
- 8.Izumi-Nagai K, Nagai N, Ohgami K, et al. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2007;27:2555–2562. doi: 10.1161/ATVBAHA.107.151431. [DOI] [PubMed] [Google Scholar]
- 9.Kamoshita M, Toda E, Osada H, et al. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep. 2016;6:30226. doi: 10.1038/srep30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake S, Kobayashi S, Tsubota K, Ozawa Y. Phase II enzyme induction by a carotenoid, lutein, in a PC12D neuronal cell line. Biochem Biophys Res Commun. 2014;446:535–540. doi: 10.1016/j.bbrc.2014.02.135. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 2012;18:51–56. doi: 10.2174/138161212798919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki M, Ozawa Y, Kurihara T, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki M, Ozawa Y, Kurihara T, et al. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M, Yuki K, Kurihara T, et al. Biological role of lutein in the light-induced retinal degeneration. J Nutr Biochem. 2012;23:423–429. doi: 10.1016/j.jnutbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Ozawa Y, Kurihara T, Sasaki M, et al. Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp Diabetes Res. 2011;2011:108328. doi: 10.1155/2011/108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamata K, Piao Z, Suzuki S, et al. Spirulina-templated metal microcoils with controlled helical structures for THz electromagnetic responses. Sci Rep. 2014;4:4919. doi: 10.1038/srep04919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoletti M. Microalgae nutraceuticals. Foods. 2016. 5. [DOI] [PMC free article] [PubMed]
- 18.Wu Q, Liu L, Miron A, Klimova B, Wan D, Kuca K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez-Salmean G, Fabila-Castillo L, Chamorro-Cevallos G. Nutritional and toxicological aspects of spirulina (Arthrospira) Nutr Hosp. 2015;32:34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- 20.Marles RJ, Barrett ML, Barnes J, et al. United States pharmacopeia safety evaluation of Spirulina. Crit Rev Food Sci Nutr. 2011;51:593–604. doi: 10.1080/10408391003721719. [DOI] [PubMed] [Google Scholar]
- 21.Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. doi: 10.1093/ecam/nen058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail M, Hossain MF, Tanu AR, Shekhar HU. Effect of Spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. Biomed Res Int. 2015;2015:486120. doi: 10.1155/2015/486120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalafati M, Jamurtas AZ, Nikolaidis MG, et al. Ergogenic and antioxidant effects of Spirulina supplementation in humans. Med Sci Sports Exerc. 2010;42:142–151. doi: 10.1249/MSS.0b013e3181ac7a45. [DOI] [PubMed] [Google Scholar]
- 24.Cingi C, Conk-Dalay M, Cakli H, Bal C. The effects of Spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;265:1219–1223. doi: 10.1007/s00405-008-0642-8. [DOI] [PubMed] [Google Scholar]
- 25.Miczke A, Szulinska M, Hansdorfer-Korzon R, et al. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: a double-blind, placebo-controlled, randomized trial. Eur Rev Med Pharmacol Sci. 2016;20:150–156. [PubMed] [Google Scholar]
- 26.Pabon MM, Jernberg JN, Morganti J, et al. A Spirulina-enhanced diet provides neuroprotection in an alpha-synuclein model of Parkinson's disease. PLoS One. 2012;7:e45256. doi: 10.1371/journal.pone.0045256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra S, Siddiqui WA, Khandelwal S. C-Phycocyanin protects against acute tributyltin chloride neurotoxicity by modulating glial cell activity along with its anti-oxidant and anti-inflammatory property: a comparative efficacy evaluation with N-acetyl cysteine in adult rat brain. Chem Biol Interact. 2015;238:138–150. doi: 10.1016/j.cbi.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL., III Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narimatsu T, Ozawa Y, Miyake S, et al. Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Invest Ophthalmol Vis Sci. 2013;54:4555–4562. doi: 10.1167/iovs.12-11572. [DOI] [PubMed] [Google Scholar]
- 30.Narimatsu T, Ozawa Y, Miyake S, Nagai N, Tsubota K. Angiotensin II type 1 receptor blockade suppresses light-induced neural damage in the mouse retina. Free Radic Biol Med. 2014;71:176–185. doi: 10.1016/j.freeradbiomed.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Osada H, Okamoto T, Kawashima H, et al. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS One. 2017;12:e0178627. doi: 10.1371/journal.pone.0178627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narimatsu T, Negishi K, Miyake S, et al. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp Eye Res. 2015;132:48–51. doi: 10.1016/j.exer.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Narimatsu T, Ozawa Y, Miyake S, et al. Biological effects of blocking blue and other visible light on the mouse retina. Clin Exp Ophthalmol. 2014;42:555–563. doi: 10.1111/ceo.12253. [DOI] [PubMed] [Google Scholar]
- 34.Grimm C, Reme CE. Light damage as a model of retinal degeneration. Methods Mol Biol. 2013;935:87–97. doi: 10.1007/978-1-62703-080-9_6. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel A, Grimm C, Seeliger MW, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- 36.Reme CE, Grimm C, Hafezi F, Marti A, Wenzel A. Apoptotic cell death in retinal degenerations. Prog Retin Eye Res. 1998;17:443–464. doi: 10.1016/s1350-9462(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 37.Hafezi F, Steinbach JP, Marti A, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med. 1997;3:346–349. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 38.Kubota S, Kurihara T, Ebinuma M, et al. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. Am J Pathol. 2010;177:1725–1731. doi: 10.2353/ajpath.2010.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narimatsu T, Ozawa Y, Miyake S, et al. Biological effects of blocking blue and other visible light on the mouse retina. Clin Exp Ophthalmol. 2014;42:555–563. doi: 10.1111/ceo.12253. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284:19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutar M, Natoli R, Provis JM. Small interfering RNA-mediated suppression of Ccl2 in Müller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J Neuroinflammation. 2012;9:221. doi: 10.1186/1742-2094-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutar M, Natoli R, Valter K, Provis JM. Early focal expression of the chemokine Ccl2 by Müller cells during exposure to damage-inducing bright continuous light. Invest Ophthalmol Vis Sci. 2011;52:2379–2388. doi: 10.1167/iovs.10-6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baylor DA. Photoreceptor signals and vision. Proctor lecture. Invest Ophthalmol Vis Sci. 1987;28:34–49. [PubMed] [Google Scholar]
- 45.Lem J, Krasnoperova NV, Calvert PD, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyake S, Takahashi N, Sasaki M, Kobayashi S, Tsubota K, Ozawa Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Lab Invest. 2012;92:102–109. doi: 10.1038/labinvest.2011.132. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto T, Ozawa Y, Kamoshita M, et al. The neuroprotective effect of rapamycin as a modulator of the mTOR-NF-kappaB axis during retinal inflammation. PLoS One. 2016;11:e0146517. doi: 10.1371/journal.pone.0146517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozawa Y, Yuki K, Yamagishi R, Tsubota K, Aihara M. Renin-angiotensin system involvement in the oxidative stress-induced neurodegeneration of cultured retinal ganglion cells. Jpn J Ophthalmol. 2013;57:126–132. doi: 10.1007/s10384-012-0204-x. [DOI] [PubMed] [Google Scholar]
- 49.Ozawa Y, Nakao K, Kurihara T, et al. Roles of STAT3/SOCS3 pathway in regulating the visual function and ubiquitin-proteasome-dependent degradation of rhodopsin during retinal inflammation. J Biol Chem. 2008;283:24561–24570. doi: 10.1074/jbc.M802238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison EH. Mechanisms of transport and delivery of vitamin A and carotenoids to the retinal pigment epithelium. Mol Nutr Food Res. 2019. e1801046. [DOI] [PubMed]
- 51.Lima VC, Rosen RB, Farah M. Macular pigment in retinal health and disease. Int J Retina Vitreous. 2016;2:19. doi: 10.1186/s40942-016-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. 2007;51:107–115. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- 53.El-Beshbishy HA, Hassan MH, Aly HA, Doghish AS, Alghaithy AA. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf. 2012;83:47–54. doi: 10.1016/j.ecoenv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Sonani RR, Patel S, Bhastana B, et al. Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresour Technol. 2017;245:325–331. doi: 10.1016/j.biortech.2017.08.129. [DOI] [PubMed] [Google Scholar]
- 55.Abdelkhalek NK, Ghazy EW, Abdel-Daim MM. Pharmacodynamic interaction of SSpirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res Int. 2015;22:3023–3031. doi: 10.1007/s11356-014-3578-0. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M, Tsujikawa M, Itabe H, et al. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J Cell Sci. 2012;125:2407–2415. doi: 10.1242/jcs.097683. [DOI] [PMC free article] [PubMed] [Google Scholar]