Abstract
Purpose:
Intensity-modulated radiation therapy (IMRT) has been used to spare organs at risk (OARs) in the definitive treatment of anal cancer. However, treatment continues to result in significant hematologic toxicity. In a cooperative trial assessing IMRT (RTOG 0529), the rate of grade 2+ and grade 3+ hematologic toxicity was 73% and 58%, respectively. Intensity-modulated proton therapy (IMPT) has the potential to decrease the integral bone marrow dose and dose to other OARs compared with photon therapy.
Patients and Methods:
Computed tomography datasets of 9 patients with anal cancer previously treated with IMRT, volumetric arc therapy (VMAT), or tomotherapy at our institution were used for comparison. Both VMAT and IMPT plans were created for each patient. The IMPT plans were created using a multi-field optimized, split-target technique. The dose to OARs, including bone marrow, bladder, small bowel, large bowel, femoral heads, and genitalia, were compared using a paired t test.
Results:
The mean bone marrow dose was 17.42 Gy with IMPT plans and 30.76 Gy with VMAT plans (P < .0001). The absolute volume of bone marrow spared 10 and 20 Gy was significantly less with the proton plans. IMPT also showed significant sparing of other OARs, including the small and large bowel, femoral heads, and genitalia. The mean planning target volume receiving at least 95% of the prescribed dose (V95) was similar with IMPT and VMAT plans, 99% and 98%, respectively.
Conclusion:
IMPT can decrease the mean bone marrow dose compared with VMAT plans by minimizing the low dose spill associated with standard photon treatment. Prospective studies assessing proton therapy for anal cancer are ongoing to evaluate the potential for improvement in hematologic toxicity and the acute tolerance of therapy.
Keywords: intensity-modulated proton therapy, anal cancer, volumetric arc therapy, hematologic toxicity
Introduction
Although anal cancer is relatively rare, there were an estimated 8080 new cases of anal cancer diagnosed in the United States in 2016 [1]. Definitive radiation with concurrent 5-FU and mitomycin-C has become the standard of care for patients with localized anal cancer. While this treatment results in long-term disease-free survival and anal sphincter preservation, there is often significant acute toxicity associated with the treatment, particularly dermatologic, gastrointestinal, genitourinary, and hematologic toxicity. Intensity-modulated radiation therapy (IMRT) has been increasingly used over conventional radiation therapy to spare organs at risk (OARs) in the treatment of anal cancer. IMRT has been demonstrated to decrease the risk of gastrointestinal and dermatologic toxicity compared with conventional radiation therapy. However, treatment continues to result in significant hematologic toxicity [2]. In a cooperative trial assessing IMRT (RTOG 0529), the rate of grade 2+ and grade 3+ hematologic toxicity was 73% and 58%, respectively [2]. During IMRT planning, sparing of the bone marrow compartment is often sacrificed in favor of small-bowel sparing or planning target volume (PTV) coverage. Intensity-modulated proton therapy (IMPT) has the potential to decrease integral bone marrow dose and dose to other OARs while still maintaining coverage of the primary tumor.
Materials and Methods
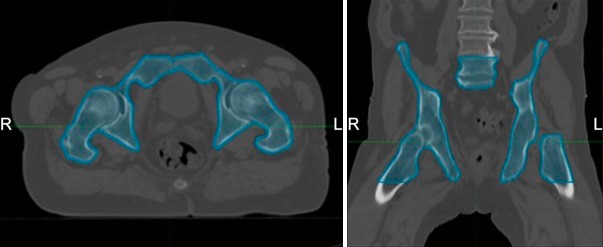
Computed tomography datasets of 9 patients with anal cancer who were previously treated with IMRT, volumetric arc therapy (VMAT), or tomotherapy at our institution were identified and used for comparison after obtaining institutional review board approval. Pelvic bone marrow was delineated using the external surface of bone as described by Mell et al [3] and included (1) iliac bone extending from the iliac crests to the superior border of the femoral head; (2) lower pelvis, including the pubes, ischia, acetabula, and proximal femora extending from the superior border of the femoral heads to the inferior border of the ischial tuberosities; and (3) lumbosacral spine extending from the superior border of the L5 vertebral body to the coccyx but not extending below the superior border of the femoral head. The 3 subsites were included in 1 contour (Figure 1). The bony contour was used as a surrogate for bone marrow. Our patient population consisted of 5 females and 4 males with clinical stages ranging stage II to IIIB disease, based on the American Joint Committee on Cancer's AJCC Cancer Staging Manual (7th edition) [4] (Table 1). Dose prescriptions were the same as those used in Radiation Therapy Oncology Group (RTOG) 0529 [2], except patient No. 1 who was cT2N0 and received 45 Gy to elective lymph nodes based on the guidelines of the Australian Gastrointestinal Trials Group [5].
Figure 1.
Bone marrow delineation, including iliac bone, lower pelvis, and lumbosacral spine.
Table 1.
Patient characteristics.
|
Patient |
Age (y) |
Sex |
Stage |
Primary dose (Gy) |
Elective intermediate dose (Gy) |
Elective low dose (Gy) |
No. of fractions |
PTV V95 IMPT (%) |
PTV V95 VMAT (%) |
| 1 | 76 | M | T2N0 | 50.4 | 45 | 28 | 99.96 | 99.14 | |
| 2 | 48 | F | T3N3 | 54 | 50.4 | 45 | 30 | 95.26 | 91.95 |
| 3 | 84 | F | T3N2 | 54 | 50.4 | 45 | 30 | 97.97 | 98.59 |
| 4 | 62 | M | T2N0 | 50.4 | 42 | 28 | 99.58 | 99.01 | |
| 5 | 50 | F | T3N0 | 54 | 45 | 30 | 100 | 100 | |
| 6 | 57 | M | T4N1 | 54 | 45 | 30 | 99.45 | 98.24 | |
| 7 | 56 | M | T3N2 | 54 | 50.4 | 45 | 30 | 99.6 | 97.29 |
| 8 | 60 | F | T3N1 | 54 | 50.4 | 45 | 30 | 100 | 99.24 |
| 9 | 70 | F | T2N0 | 50.4 | 42 | 28 | 100 | 99.53 |
Abbreviations: PTV, planning target volume; IMPT, intensity-modulated proton therapy; VMAT, volumetric arc therapy; M, male; F, female.
Both VMAT and IMPT plans were created for each patient using the Eclipse treatment planning system (Varian Medical Systems, Inc., North Charleston, SC). The IMPT plans were created using a 3-field multi-field optimized split target technique [6]. A posterior field was used to cover the primary tumor and pelvic lymph nodes and a right and left anterior oblique field was used to cover the right and left inguinal lymph nodes (Figures 2 and 3B). All VMAT plans were accomplished using a 2-arc technique (716 total arc degrees) with complementary collimator angles of 30° and 330° and a 6-MV energy photon mode (Figure 3A). During VMAT optimization, dose conformity and fall-off were controlled using dose-limiting structures (ring) and Eclipse's Normal Tissue Objective. The OAR constraints, per RTOG 0529, and the bone marrow constraints, including mean bone marrow < 22.5 Gy, V10 < 90%, and V40 < 37%, were used for treatment planning (Table 2). The dose to OARs, including bone marrow, bladder, small bowel, large bowel, femoral heads, and genitalia, were compared with a paired t test.
Figure 2.
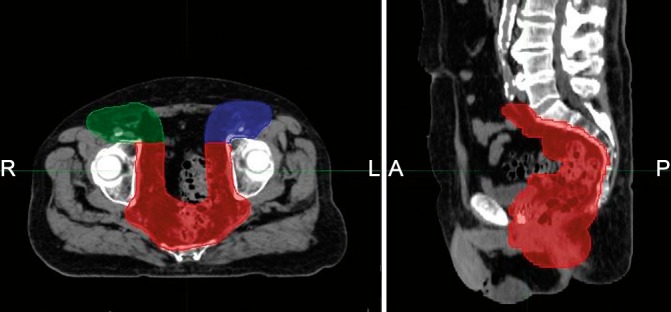
The 3-field, split-target multi-field optimization (MFO) and intensity-modulated proton therapy (IMPT) beam arrangement. One posterior field covers the primary tumor and pelvic lymph nodes (MFO_PA, red) and 2 anterior oblique fields cover the inguinal lymph nodes (MFO_LAO, blue; MFO_RAO, green).
Figure 3.
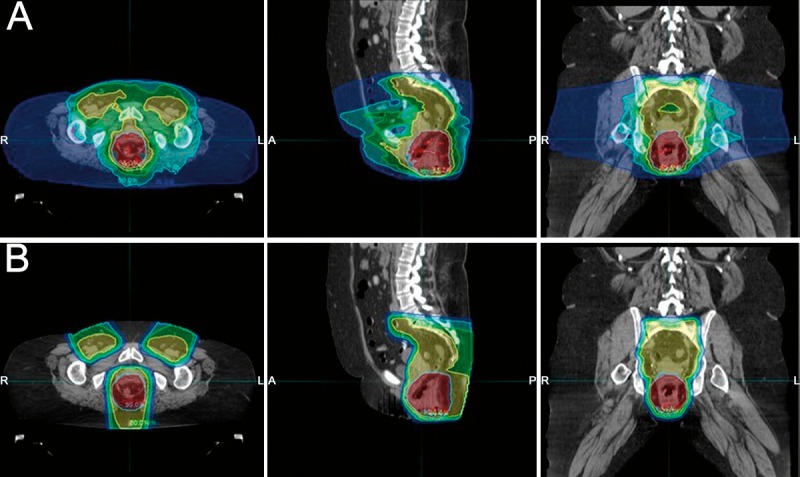
(A) Volumetric arc therapy versus (B) intensity-modulated proton therapy plans for a patient with cT3N0 anal cancer. Isodoses lines are displayed in color wash and represent the following doses: 56.7 Gy (pink), 54 Gy (red), 51.3 Gy (light blue), 43.2 Gy (yellow), 35.2 Gy (green), 27 Gy (teal), 10.8 Gy (dark blue).
Table 2.
Organ at risk constraints.
|
Organ at risk |
Constraint |
| Bone marrow | Mean<22.5 Gy |
| V10<90% | |
| V40<37% | |
| Bladder | V35<50% |
| V40<35% | |
| V50<5% | |
| Small and large bowel | V30<200 cm3 |
| V35<150 cm3 | |
| V45<20 cm3 | |
| Femoral heads | V40<35% |
| V44<5% | |
| Genitalia | V20<50% |
| V30<35% | |
| V40<5% |
Previously published Lyman-Kutcher-Burman normal tissue complication probability modeling suggests that the mean bone marrow dose can predict for grade 3+ hematologic toxicity in patients with anal cancer treated with definitive chemoradiation. Bazan et al [7] found that a mean pelvic bone marrow dose < 22.5 Gy was associated with a 5% risk of hematologic toxicity. Normal tissue complication probability modeling for patients with cervical cancer has also demonstrated an association between the volume of pelvic bone marrow receiving low-dose radiation and acute hematologic toxicity. These patients were similarly treated with concurrent chemotherapy with pelvic radiation. If the volume of bone marrow receiving 10 Gy (V10) was kept less than 90%, there was decreased rate of grade 2 leukopenia [8, 9]. RTOG 0418, a phase II study evaluating the use of IMRT for postoperative gynecologic cancers, also found that the volume of bone marrow receiving 40 Gy was predictive for hematologic toxicity. Among patients with cervical cancer with V40 > 37%, 75% had grade 2 or higher hematologic toxicity compared with 40% of patients with V40 < 37% [10]. As a result of these previously published studies, we selected mean bone marrow dose < 22.5 Gy, V10 < 90%, and V40 < 37% as bone marrow constraints for our comparative plans. In addition, recent data suggest that bone marrow toxicity may be more accurately measured as a volume-based parameter [11]. The absolute volume of bone marrow spared 10, 20, and 40 Gy was therefore also assessed for IMPT and VMAT plans.
For IMPT and VMAT plans, coverage was assessed to confirm that at least 95% of the PTV was receiving at least 95% of the prescribed dose. PTV expansions were the same for both the IMPT and VMAT plans. Additionally, coverage for the IMPT plans was evaluated using a ±3% range uncertainty criteria.
Results
The mean bone marrow dose with IMPT and VMAT plans was 17.42 and 30.76 Gy, respectively (P < .0001). The mean volume of bone marrow receiving 10 Gy (V10) was significantly less with the IMPT plans, 48.97% compared with 92.56% for the VMAT plans (P < .0001). In addition, the absolute volume of bone marrow spared 10 and 20 Gy was higher in the IMPT plans compared with the VMAT plans (10 Gy, 814 cm3 versus 111 cm3 P < .0001; 20 Gy, 932 cm3 versus 246 cm3 P < .0001). There was no significant difference in the volume of bone marrow receiving 40 Gy in the IMPT plans compared with the VMAT plans (23.67% and 20.82%, respectively; P = .346) and the absolute volume of bone marrow spared 40 Gy (1254 cm3 versus 1194 cm3, P = .379).
The IMPT plans provided similar PTV coverage as the VMAT plans (primary PTV V95 was 99% versus 98%). The PTV coverage for each individual computed tomography dataset is listed in Table 1. IMPT also showed significant sparing of the OARs, including the small and large bowel, femoral heads, and genitalia (Table 3). This was more apparent for the lower isodose levels. For example, the mean volume of bladder receiving 35 Gy was 44.2% for IMPT compared with 72.96% for VMAT (P = .0122). In contrast, the mean volume of bladder receiving 50 Gy was not statistically significant (6.69% IMPT versus 7.02% VMAT; P = .8856).
Table 3.
Mean results of organs at risk.
|
Constraint |
Intensity-modulated proton therapy |
Volumetric arc therapy |
P
value |
| Bone marrow | |||
| Mean dose | 17.42 Gy | 30.76 Gy | <.0001 |
| V10 | 48.97% | 92.56% | <.0001 |
| V40 | 23.67% | 20.82% | .3460 |
| Bladder | |||
| V35 | 44.20% | 72.96% | .0122 |
| V40 | 38.94% | 52.35% | .1243 |
| V50 | 6.69% | 7.02% | .8856 |
| Small bowel | |||
| V30 | 176.64 cc | 400 cc | .0009 |
| V35 | 151.12 cc | 291.91 cc | .0051 |
| V45 | 50.93 cc | 48.8 cc | .8076 |
| Large bowel | |||
| V30 | 79.10 cc | 131.68 cc | .0071 |
| V35 | 70.37 cc | 114.23 cc | .0136 |
| V45 | 27.18 cc | 24.95 cc | .7926 |
| Femoral heads | |||
| V40 | 2.80% | 21.94% | .0160 |
| V44 | 1.30% | 4.24% | .2982 |
| Genitalia | |||
| V20 | 2.55% | 63.68% | .0006 |
| V30 | 1.24% | 41.67% | .0164 |
| V40 | 0.53% | 20.47% | .1210 |
Discussion
Treatment for anal cancer has evolved from abdominoperineal resection to organ preservation with concurrent chemoradiation [12]. While chemoradiation has resulted in long-term disease-free survival and sphincter preservation, acute toxicity remains high and can result in treatment breaks. RTOG 0529 demonstrated that IMRT for localized anal cancer can decrease gastrointestinal and dermatologic acute toxicity compared with historical controls treated with 3-dimensional conformal radiation therapy enrolled on RTOG 9811 [2]. However, the rate of grade 3 or higher toxicity events remain high, even with IMRT. In RTOG 0529, 37% of patients had grade 3 or higher gastrointestinal or genitourinary toxicity, and 49% of patients required a treatment break. In addition, grade 3 or higher hematologic toxicity was observed in 58% of patients [2].
The use of mitomycin-C–based chemotherapy has often been implicated in the high rates of hematologic toxicity in the definitive treatment of patients with anal cancer. However, concomitant radiation therapy to the pelvic bones can further increase the risk of hematologic toxicity due to the high radiosensitivity of hematopoietic stem cells in the active bone marrow [13], and pelvic bones may contain up to 40% of the total functional bone marrow. A dose-response relationship of radiation dose to the pelvic bone marrow and rates of grade 3 or higher toxicity have been demonstrated by Bazan et al [7], even in the setting of all patients receiving mitomycin and 5-FU based chemotherapy. This suggests that radiation to the pelvic bone marrow contributes to hematologic toxicity and indicates that, with more conformal avoidance of the bone marrow as an OAR, a reduction in hematologic toxicity may be possible.
Integral bone marrow dose can increase due to the low-dose bath effect associated with IMRT [14]. Historically, pelvic bone marrow has not been considered an avoidance structure in treatment planning. Therefore, low dose received by the bone marrow can increase in an attempt to cover the PTV while decreasing dose to other surrounding bowel and other OARs. Leukopenia and neutropenia can put patients at increased risk for infection and hospitalization, which can compromise treatment efficacy by prolonging the overall treatment time. Multiple institutions have demonstrated that the volume of bone marrow receiving a low dose of radiation, V10 and V20, strongly correlates with cytopenias [8, 9, 15, 16]. In our study, we found that the mean absolute volume of bone marrow spared 10 Gy was significantly higher with IMPT plans, 814 cm3 compared with VMAT plans, 111 cm3 (P < .0001); and the volume of bone marrow spared 20 Gy was also significantly higher (932 cm3 versus 246 cm3, P < .0001).
Since bone marrow is considered a parallel organ, the mean bone marrow dose is a good predictor for hematologic toxicity. In our study, the mean bone marrow dose was significantly lower with IMPT plans compared with VMAT plans, 17.42 and 30.76 Gy, respectively. Based on the Lyman-Kutcher-Burman normal tissue complication probability model developed by Bazan et al [7], the expected rate of grade 3+ hematologic events would be 40% for VMAT plans and <10% for IMPT plans. Despite the heterogeneity of dosimetric parameters assessed for hematologic toxicity in prior studies, there appears to be a consistent association with volume of bone marrow receiving low doses of radiation. Radiation planning and delivery using scanning proton beam therapy offers a reduction in dose to the bone marrow space compared with VMAT. This is similar to the finding by Anand et al, who also demonstrated that scanning proton beam therapy can reduce to dose to bone marrow by >50% compared with IMRT [6].
Given the large volume of the total pelvic bone marrow, investigators have attempted to identify which subsites may contribute most to the hematologic toxicity and therefore allow physicians to spare certain subsites instead of the total pelvic bone marrow. The lumbosacral spine has been found to have the highest region of active bone marrow within the pelvis [17], and the V40 of the lumbosacral spine has been shown to be strong predictor of grade 3+ hematologic toxicity [15]. However, Rose et al [18] has demonstrated that radiation doses to the total pelvic bone marrow, lumbosacral bone marrow, and iliac bone marrow are all individually associated with hematologic toxicity and therefore sparing just a portion of the pelvic bone marrow may be insufficient to decrease bone marrow suppression. Due to this finding, we did not find it necessary to evaluate the dose to each of the 3 subsites of the pelvic bone marrow and instead focused on dose to the total pelvic bone marrow.
One limitation of our study was the use of total bone volume as a surrogate for the bone marrow contour. There is increasing interest in sparing the metabolically active subset of the bone marrow given the inherent difficulty of maintaining adequate tumor coverage while simultaneously sparing pelvic OARs and bone marrow. Computed tomography lacks the ability to delineate active bone marrow composed of hematopoietic cells and inactive bone marrow composed primarily of fat cells. 18F-FDG-PET has been used to identify areas of metabolically active bone marrow. Franco et al [17] demonstrated that areas of active bone marrow significantly correlate with white blood cell count, absolute neutrophil count, and platelet nadirs. Further work is needed to define the optimal SUV parameters to segregate an active marrow subsite and it is uncertain whether this is a better dosimetric predictor of hematologic toxicity compared with total bone. Several investigators have concluded that dose to the total bone contour is still a good surrogate for determining hematologic toxicity. Rose et al [19] demonstrated that FDG-based active bone marrow models failed to improve the ability to predict hematologic toxicity compared with total bone marrow models in patients with anal cancer who were undergoing chemoradiation. For the purposes of this study, the total bone was used a surrogate for bone marrow in comparing the different modalities.
Conclusion
We created IMPT plans using a multi-field optimized, split-target technique and demonstrated that IMPT can decrease the mean dose to the bone marrow in addition to other OARs, including the small and large bowel, femoral heads, and genitalia. IMPT can minimize low dose spill to the bone marrow compared with VMAT plans using standard photon therapy. Prospective studies assessing proton therapy for anal cancer are ongoing to evaluate the potential for improvement in hematologic toxicity and the acute tolerance of therapy.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts to disclose.
Acknowledgments: The abstract of this article was presented as a poster at the 58th Annual Meeting of the American Society for Radiation Oncology in Boston, Massachusetts, during September 25-28, 2016.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H, Willett CG. RTOG. 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, Jani AB, Kindler HL, Mundt AJ, Roeske JC, Chmura SJ. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1431–7. doi: 10.1016/j.ijrobp.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 4.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual Seventh Edition. New York, NY: Springer; 2010. [Google Scholar]
- 5.Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, Wiltshire K, Ngan S, Kachnic L. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1455–62. doi: 10.1016/j.ijrobp.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 6.Anand A, Bues M, Rule WG, Keole SR, Beltran CJ, Yin J, Haddock MG, Hallemeier CL, Miller RC, Ashman JB. Scanning proton beam therapy reduces normal tissue exposure in pelvic radiotherapy for anal cancer. Radiother Oncol. 2015;117:505–8. doi: 10.1016/j.radonc.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Bazan JG, Luxton G, Mok EC, Koong AC, Chang DT. Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84:700–6. doi: 10.1016/j.ijrobp.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 8.Rose BS, Aydogan B, Liang Y, Yeginer M, Hasselle MD, Dandekar V, Bafana R, Yashar CM, Mundt AJ, Roeske JC, Mell LK. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:800–7. doi: 10.1016/j.ijrobp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, Hurteau JA, Collins YC, Lengyel E, Mundt AJ. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–65. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Klopp AH, Moughan J, Portelance L, Miller BE, Salehpour MR, Hildebrandt E, Nuanjing J, D'Souza D, Souhami L, Small W, Jr, Gaur R, Jhingran A. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90. doi: 10.1016/j.ijrobp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AY, Golden DW, Bazan JG, Kopec M, Pelizzari CA, Aggarwal S, Chang DT, Liauw SL. Hematologic nadirs during chemoradiation for anal cancer: temporal characterization and dosimetric predictors. Int J Radiat Oncol Biol Phys. 2017;97:306–12. doi: 10.1016/j.ijrobp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Nigro ND. An evaluation of combined therapy for squamous cell cancer of the anal canal. Dis Colon Rectum. 1984;27:763–6. doi: 10.1007/BF02553933. [DOI] [PubMed] [Google Scholar]
- 13.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist 7th ed. Philadelphia, PA: Lippincottt Williams & Wilkins;; 2011. [Google Scholar]
- 14.Robinson M, Sabbagh A, Muirhead R, Durrant L, Van den Heuvel F, Hawkins M. Modeling early haematologic adverse events in conformal and intensity-modulated pelvic radiotherapy in anal cancer. Radiother Oncol. 2015;117:246–51. doi: 10.1016/j.radonc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco P, Ragona R, Arcadipane F, Mistrangelo M, Cassoni P, Rondi N, Morino M, Racca P, Ricardi U. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Transl Oncol. 2017;19:67–75. doi: 10.1007/s12094-016-1504-2. [DOI] [PubMed] [Google Scholar]
- 16.Albuquerque K, Giangreco D, Morrison C, Siddiqui M, Sinacore J, Potkul R, Roeske J. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79:1043–7. doi: 10.1016/j.ijrobp.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Franco P, Arcadipane F, Ragona R, Lesca A, Gallio E, Mistrangelo M, Cassoni P, Arena V, Bustreo S, Faletti R, Rondi N, Morino M, Ricardi U. Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med Oncol. 2016;33:72. doi: 10.1007/s12032-016-0789-x. [DOI] [PubMed] [Google Scholar]
- 18.Rose B, Mitra D, Hong TS, Jee KW, Niemierko A, Drapek LN, Blaszkowsky LS, Allen JN, Murphy JE, Clark JW, Ryan DP, Wo JY. Irradiation of anatomically defined pelvic subsites and acute hematologic toxicity in anal cancer patients undergoing chemoradiation. Pract Radiat Oncol. 2017;7:e291–7. doi: 10.1016/j.prro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Rose BS, Jee KW, Niemierko A, Murphy JE, Blaszkowsky LS, Allen JN, Lee LK, Wang Y, Drapek LC, Hong TS, Wo JY. Irradiation of FDG-PET-defined active bone marrow subregions and acute hematologic toxicity in anal cancer patients undergoing chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:747–54. doi: 10.1016/j.ijrobp.2015.12.006. [DOI] [PubMed] [Google Scholar]