Abstract
Purpose:
We assessed clinical and radiologic outcomes in adults and children with low-grade glioma (LGG) of the brain treated with pencil-beam scanning (PBS) proton therapy (PT).
Materials and Methods:
Between 1997 and 2014, 28 patients were treated with PBS PT, 20 (71%) of whom were younger than 18 years. Median age at start of PT was 12.3 years (range, 2.2-53.0 years). Nine patients (32%) underwent at least a subtotal resection; 12 (43%) underwent biopsy; and 7 (25%) were diagnosed radiographically. Twelve patients (43%) had grade II and 9 (32%) had grade I gliomas. Eleven patients (39%) received chemotherapy before PT. A median dose of 54 Gy (relative biologic effectiveness) was administered. Radiologic response to PT was determined using the Response Evaluation Criteria in Solid Tumors (RECIST). Eight domains of quality of life (QoL) for 16 pediatric patients were assessed prospectively by patients' parents using the pediatric QoL proxy questionnaire. Progression-free survival and overall survival (OS) were estimated by the Kaplan-Meier method. Median follow-up was 42.1 months for living patients.
Results:
Ten patients (36%) developed local, clinical failure. Three patients (11%) died, all of tumor progression. Radiographic tumor response by RECIST was evaluable in 11 patients: 9 (82%) with stable disease, 1 (9%) with partial response, and 1 (9%) with complete response to PT. Three-year OS and progression-free survival were 83.4% and 56.0%, respectively. No ≥ grade III acute toxicities were observed. Grade III, late radiation necrosis developed in 1 patient (4%). No appreciable change in pediatric QoL proxy scores in children was noted in any of the 8 domains at any time point.
Conclusion:
Treatment with PBS PT is effective for LGG, with minimal acute toxicity and, in children, no appreciable decline in QoL. More patients and longer follow-up are needed to determine the long-term efficacy and toxicity of PT for LGG.
Keywords: proton therapy, low-grade glioma, pencil-beam scanning, pediatric, quality of life
Introduction
Low-grade gliomas (LGGs) are a heterogeneous group of tumors that account for up to 60% of all primary brain tumors in children and 10% in adults [1, 2]. Pilocytic astrocytomas are the most common variety of LGG in children and are classified as World Health Organization (WHO) grade I tumors. Nonpilocytic or diffusely infiltrating gliomas are classified as WHO grade II tumors and are most commonly seen in young adults. The prognosis of patients with LGG varies depending on tumor grade, histology, tumor genetics, and extent of disease but is typically favorable after treatment in adults, with median overall survival (OS) ranging from 4.5 to 13.3 years [3, 4] and is quite favorable in children, with a 20-year OS of 87% [5].
Because of the favorable prognosis and young age of many patients with LGG, treatment is tailored to provide local tumor control while minimizing long-term toxicities. Maximal safe surgical resection is the first-line therapy and provides valuable histologic confirmation of, and prognostic information about, the disease. In some cases, however, biopsy or surgical resection or both are not possible because the tumor arises from critical structures, such as optical pathways. Postoperative radiation therapy has been shown to improve progression-free survival (PFS) and decrease the frequency of symptoms such as seizures [6]. Chemotherapy has been shown to improve outcomes in adults with certain subtypes of WHO grade II LGGs when given with radiation therapy [3, 7] and is frequently used in children to postpone the use of radiation therapy [8].
Proton therapy (PT) is a type of radiation treatment with advantageous dose-deposition properties resulting in a decrease in the volume of healthy tissues irradiated compared with photon irradiation [9]. In patients with expected long survivals, such as those with LGG, that decrease in volume of healthy tissue irradiated has the potential to result in clinically meaningful reductions in long-term toxicities, which may translate into a better-sustained quality of life (QoL). The PT has been delivered in the past with a passive system (passive-scattering PT), but a more-conformal and highly modulated beam can be created with actively scanned proton beamlets (pencil-beam scanning [PBS]) overlaid on the target volume [10]. When compared with passive-scattering PT, PBS PT allows for greater conformality at the proximal end of the target volume and for the delivery of intensity-modulated PT (IMPT), which further enhances the ability to conform the radiation dose to minimize exposure to organs at risk. In addition, PBS PT decreases the neutron production that is present when proton beams pass through materials in passive-scattering systems [11, 12]. These advantages may potentially result in a lower risk of radiation-induced second malignancies in patients with LGG.
Data are limited on long-term outcomes, including QoL, for patients with LGG treated with PT and are even more limited for patients treated with PBS PT [13–16]. Also, to our knowledge, the radiologic response of LGG to PBS PT has not been described in the literature. The aim of this study was to assess radiologic and clinical outcomes, including QoL and toxicity, in children and adults with LGG treated with PBS PT.
Materials and Methods
Patient Characteristics
From 1997 to 2014, 32 patients with radiologically or histologically diagnosed LGG who were treated with PBS PT with curative intent at the Paul Scherrer Institute (PSI; Villigen, Switzerland) were identified in the institutional database. Patients with <4-month follow-up and those who had previously received radiation therapy were excluded. Twenty-eight patients met criteria for inclusion. Patient and tumor characteristics are listed in Table 1. Median age at start of PT was 12.3 years (range, 2.2–53.0 years). Twenty patients (71%) were younger than 18 years. Eleven patients (39%) had optic gliomas, and 4 (14%) had gliomas involving the brainstem. No patients were diagnosed with diffuse, intrinsic pontine glioma. Twenty-two patients (79%) were treated with PBS PT at recurrence or progression and 6 (21%) at first diagnosis. Of the 20 children, 18 (90%) were treated at recurrence or progression, including all 7 children younger than 8 years.
Table 1.
Patient and tumor characteristics (N = 28).
|
Characteristic |
Patients |
| Female patients, No. (%) | 14 (50) |
| Median age at proton therapy, y (range) | 12.3 (2.2-53.0) |
| Age, at start of proton therapy <18 y, No. (%) | 20 (71) |
| Age, >40 y at diagnosis, No. (%) | 2 (7) |
| Grade I, No. (%) | 9 (32) |
| Grade II, No. (%) | 12 (43) |
| Never biopsied, No. (%) | 7 (25) |
| Optic nerve/chiasm glioma, No. (%) | 11 (39) |
| Brainstem glioma, No. (%) | 4 (14) |
| Thalamus/midbrain glioma, No. (%) | 3 (11) |
| Other sites, No. (%) | 10 (36) |
| Tumor crossing midline, No. (%) | 16 (57) |
| Tumor >5 cm, No. (%) | 8 (29) |
Gross total resection was defined as gross macroscopic removal of the visible tumor, as defined by the surgeon's operative notes, and absence of tumor on postoperative imaging. One patient (4%) underwent a gross total resection, 8 (29%) underwent subtotal resection, 12 (43%) underwent biopsy, and 7 (25%) were diagnosed radiographically only. Of patients with histologic confirmation, 12 were diagnosed with grade II gliomas and 9 with grade I pilocytic astrocytomas. Eleven patients (39%) received chemotherapy before PT, including 1 patient who also received chemotherapy concurrent with PT.
Proton Therapy Planning and Delivery
All patients were immobilized with a thermoplastic mask or vacuum-assisted bite-block system. Eight children (29%) were anesthetized using a conscious sedation protocol because of their young age, and patient positioning was verified before every fraction, as published previously [17]. Gross tumor volume was defined as a macroscopic tumor identified on brain magnetic resonance imaging (MRI) performed before initiation of PT, with residual tumor, if any, identified on pre-PT brain MRI, and the tumor bed identified on planning computed tomography (CT) during simulation. The clinical target volume included the gross tumor volume plus a 10-mm margin extension modified anatomically for microscopic involvement. The planning target volume encompassed the clinical target volume plus a 4- to 5-mm margin. The median planned target volume was 117.3 cm3 (range, 35.0–202.0 cm3).
Patients were treated with PBS PT on 1 of 2 scanning gantries. Patients treated before 2007 were treated 4 d/wk with energy-degraded beams from the 590-MeV cyclotron (ACCEL-Varian Medical Systems, Troisdorf, Germany). Patients treated during and after 2007 were treated 5 d/wk with the 250-MeV medical-dedicated cyclotron. A relative biologic effectiveness (RBE) factor of 1.1 (relative to that of cobalt 60) was used, and proton doses were expressed in terms of Gy (RBE), where Gy (RBE) = proton Gy × 1.1. A median dose of 54 Gy (RBE), and a mean dose of 52.8 ± 7.1 Gy (RBE) in 1.8 Gy (RBE) fractions was administered. When possible, treatments were designed to keep the mean doses to the cochleae at < 35 Gy (RBE), to the pituitary gland at < 30 Gy (RBE), to the temporal lobes or hippocampi at < 20 Gy (RBE), and maximum dose to the optic nerves and chiasm of < 54 Gy (RBE). Treatment characteristics are listed in Table 2.
Table 2.
Treatment characteristics.
|
Characteristics |
Variables |
| PT at first diagnosis, No. (%) | 6 (21) |
| PT at progression or recurrence, No. (%) | 22 (79) |
| Chemotherapy before PT, No. (%) | 11 (39) |
| All PT delivered with IMPT (MFO), No. (%) | 6 (21) |
| Some series of PT delivered with IMPT (MFO), No. (%) | 2 (7) |
| All PT delivered with SFUD (SFO), No. (%) | 20 (71) |
| Median dose, Gy (RBE) | 54 (range, 46-64) |
| Median follow-up, mo (mean ± SD) | 30.7 (44.9 ± 42.9) |
| Median bilateral hippocampi mean dose in pediatric patients, Gy (RBE) | 32.5 (range, 0.05-53.8) |
| Median pituitary mean dose in pediatric patients, Gy (RBE) | 53.2 (range, 3-55.1) |
| Median bilateral hippocampi mean dose in adult patients,a Gy (RBE) | 10.9 (range, 0.04-50.2) |
| Median pituitary mean dose in adult patients,a Gy (RBE) | 23.3 (range, 0.01-55.0) |
Abbreviations: PT, proton therapy; IMPT, intensity-modulated proton therapy; MFO, multifield optimization; SFUD, single-field uniform dose; SFO, single-field optimization.
Data not available for 1 patient
Quality of Life
Beginning in 2005, a prospective assessment of the effect of PBS PT on QoL was performed in all pediatric patients at PSI in collaboration with the University of Münster (Germany) [18]. The self-administered, multidimensional questionnaire PEDQoL was used for patients aged 5 to 18 years. It was available as a self-rating version and as a proxy-rating version for parents. After collecting informed consent, parents were requested to complete a questionnaire (PEDQoL Proxy) on the QoL of their child. In addition, pediatric patients aged 5 years and older were asked to complete a self-questionnaire (PEDQoL Self) on their status. Questionnaires were completed at the start of PT, at 2 months, and at 1, 2, and 3 years after completion of PT. Eight dimensions of physical and psychosocial well-being were examined in the PEDQoL Proxy questionnaire (Table 3). Of the 20 children who received PBS PT for low-grade glioma, 4 were excluded from the QoL analysis, including 1 treated before opening of the QoL study and 3 who were younger than 4 years at the time of PBS PT. Because of the few completed PEDQoL Self questionnaires, only PEDQoL Proxy forms were analyzed. There was no QoL study performed for the adult patients.
Table 3.
Mean scores of PEDQoL Proxy scales in low-grade glioma patients treated with proton therapy.
| Baseline evaluation (E1)a |
Second evaluation (E2)b |
Third evaluation (E3)c |
Fourth evaluation (E4)d |
|
| Independence [SD] (no. of children) | 70.37 [±16.12] (12) | 68.55 [±17.13] (10) | 66.16 [±17.13] (11) | 72.59 [±16.84] (9) |
| Emotional ability [SD] (no. of children) | 73.44 [±19.06] (16) | 75.00 [±20.93] (14) | 77.78 [±18.23] (12) | 73.15 [±22.35] (9) |
| Body image [SD] (no. of children) | 73.96 [±27.87] (16) | 82.02 [±17.35] (14) | 70.83 [±18.70] (12) | 72.59 [±19.56] (9) |
| Learning ability [SD] (no. of children) | 73.85 [±19.36] (16) | 68.81 [±22.39] (14) | 67.36 [±19.63] (12) | 75.55 [±20.28] (9) |
| Bodily function [SD] (no. of children) | 60.94 [±9.96] (16) | 59.72 [±18.83] (14) | 56.25 [±14.27] (12) | 57.41 [±13.47] (9) |
| Social skills w/ peers [SD] (no. of children) | 74.48 [±15.05] (16) | 68.57 [±16.06] (14) | 66.67 [±20.91] (12) | 68.15 [±20.82] (9) |
| Social Skills w/ family [±SD] (no. of children) | 68.92 [±21.95] (16) | 64.68 [±22.05] (14) | 70.37 [±24.28] (12) | 75.39 [±14.70] (9) |
| Overall health [SD] (no. of children) | 65.63 [±30.71] (16) | 66.67 [±28.50] (14) | 70.83 [±24.75] (12) | 64.81 [±21.15] (9) |
Abbreviations: PEDQoL, pediatric quality of life ; SD, standard deviation
Prior to proton therapy
Approximately 2 mo after completion of proton therapy
Approximately 1 y after completion of proton therapy
Approximately 2 y after completion of proton therapy
Radiographic Response
The radiographic response of tumors to PT was determined by consensus of 2 neuroradiologists (S.U., F.J.A) using the Response Evaluation Criteria in Solid Tumors (RECIST). Response was categorized as complete (complete absence of tumor), partial (≥ 30% decrease in maximum diameter), stable disease (tumor < 20% increase or < 30% decrease in maximum diameter), or progressive disease (≥ 20% increase in maximum diameter). Pre-PT and post-PT MRI and CT images were used to evaluate radiographic response.
Follow-up
Patients were followed clinically and with MRI ± CT of the brain every 3 to 6 months for the first 3 years and then annually by the PSI Study and Research Office, which maintained a prospective database. Tumor control was defined as lack of tumor enlargement on subsequent brain MRI. When Digital Imaging and Communications in Medicine (DICOM, National Electrical Manufacturers Association, Rosslyn, Virginia) images were not available, the radiologist's reports and clinical notes were used to assess tumor control. Late toxicities were defined as side effects observed > 90 days after completion of PT and were categorized according to the National Cancer Institute (Bethesda, Maryland) Common Terminology Criteria for Adverse Events (version 4.0) grading system.
Statistical Analysis
Freedom from local progression and OS were calculated by the Kaplan-Meier method and were compared between groups with log-rank statistics. Analyses were performed with the Statistical Package for Social Sciences (version 18.0, formerly SPSS Inc, now, IBM Corporation, Armonk, New York).
Results
Radiographic Response
All patients completed the prescribed course of PT without interruption. After treatment with PBS PT, the authors had access to adequate patient follow-up DICOM images, which met RECIST criteria, to assess radiographic response for 11 patients. One of those patients (9%) showed a complete response at 6 months after PBS PT, 1 (9%) developed a partial response at 4 months, and 9 (82%) had stable disease. No cases of pseudoprogression were observed.
Survival Outcomes
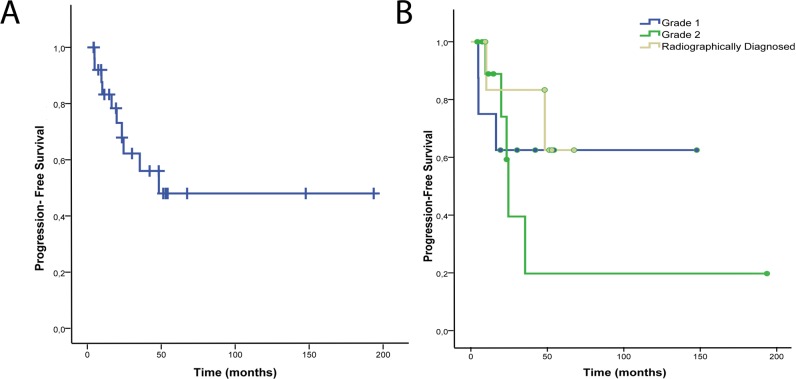
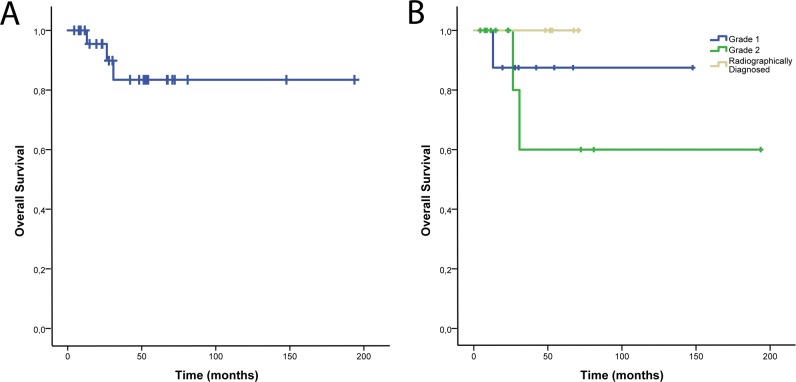
After a median follow-up of 30.5 months (mean ± SD, 44.9 ± 42.9 months) for all patients and 42.1 months (47.5 ± 44.6 months) for living patients, 10 developed local tumor progression. No cases of distant central nervous system progression were noted. Two- and 3-year PFS for all patients was 67.9% and 56.0%, respectively. Respective 2- and 3-year PFS for patients with grade I gliomas was 62.5% and 62.5%, for grade II gliomas was 59.3% and 19.8%, and for radiographically diagnosed patients was 83.3% and 83.3% (Figure 1). All deaths resulted from local tumor progression. Two- and 3-year OS for all patients was 95.5% and 83.4%, respectively. Respective 2- and 3-year OS for patients with grade I gliomas was 87.5% and 87.5% and for grade II gliomas was 100% and 60.0%. For radiographically diagnosed patients, the 3-year OS was 100% (Figure 2). One adult patient was diagnosed with a grade II oligodendroglioma of the frontal lobe and underwent a biopsy without resection followed by PT. That patient is without recurrence 16 years after treatment. Two other adults were diagnosed with fibrillary grade II astrocytomas and underwent subtotal resection or biopsy alone followed by PT. Neither received chemotherapy. One of the 2 patients had an 8% proliferative index, experienced local tumor recurrence 23 months after PT, and died 6 months later. The other had local tumor recurrence 22 months after PT and died 14 months later.
Figure 1.
Progression-free survival for all patients (A) and patients stratified by tumor grade or radiographic diagnosis (B).
Figure 2.
Overall survival for all patients (A) and patients stratified by tumor grade or radiographic diagnosis (B).
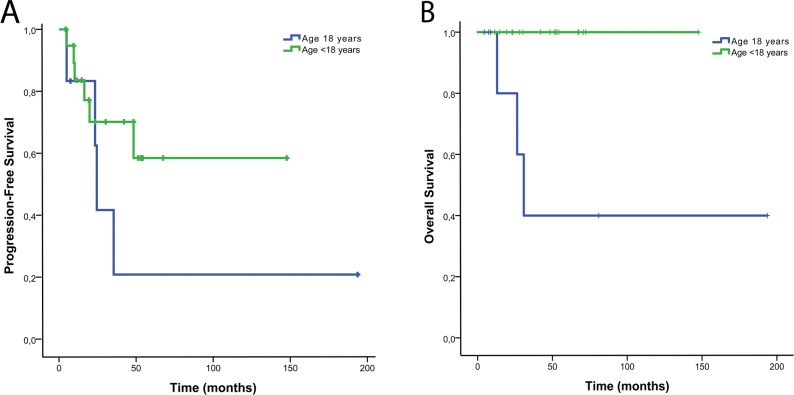
When stratified by age, patients younger than 18 years had a 2- and 3-year PFS of 70.1% and 70.1%, and 3-year OS was 100%. Patients 18 years and older had a 2- and 3-year PFS of 62.5% and 20.8%, respectively and a 2- and 3-year OS of 80% and 40%, respectively (Figure 3).
Figure 3.
Progression-free survival (A) and overall survival (B) stratified by patient age.
Healthy Tissue-Dose Distributions
At least 1 hippocampus was able to be spared, which was defined as a mean dose < 20 Gy (RBE) in 13 of 27 patients (48%), including both hippocampi in 8 patients (30%) (1 adult patient's dosimetric data was not retrievable). The mean pituitary gland dose to 11 of 27 patients (41%) was < 30 Gy (RBE) (Table 2).
Toxicity
No instances of ≥ grade III acute toxicity, and 1 case each (4%) of acute grade II alopecia, nausea, and headache was observed. Thirteen cases (46%) of acute grade I alopecia; 8 (29%) of acute, grade I radiation dermatitis; 2 each (7%) of nausea, headache, and fatigue; and 1 case each (4%) of insomnia and conjunctivitis were observed. No instances of ≥grade 4 late toxicity were observed. One patient (4%) developed late, grade III radiation necrosis; 2 (7%) developed late, grade I radiation necrosis. Nine patients (32%) developed late, grade II hypopituitarism, and 1 each (4%) developed late, grade II cognitive impairment and memory impairment, with one case of late, grade I alopecia. No radiation-induced tumors were observed.
PEDQoL Proxy Results
All parents completed all domains of the PEDQoL Proxy questionnaire at baseline (E1), except for the independence domain, which was completed by 12 of 16 sets of parents. A second evaluation (E2) was performed 2 months after completion of PT, with third (E3) and fourth (E4) evaluations after 1 and 2 years post-PT, respectively. The PEDQoL Proxy scores in all 8 domains at the E2, E3, and E4 time points showed no appreciable differences compared with baseline evaluation (E1) scores, although the sample size was too small for statistical analysis (Table 3).
Discussion
This analysis includes what is, to our knowledge, the first prospective QoL data on children with LGG treated with PBS PT. Children in our cohort had local control and OS rates similar to previously reported studies of children treated with photons [19] and passive-scattering PT [13]. Adults in our study experienced poorer local control outcomes than did patients treated with photon irradiation [6, 20] and passive-scattering PT [15], although the sample size was small.
Multiple studies have evaluated the potential benefits of PT over photon irradiation for treatment of brain tumors. Risk-modeling studies have estimated that PT results in a significantly lower risk of secondary tumor formation compared with various photon-irradiation techniques [21, 22]. In addition, radiation therapy and the volume of brain irradiated have been found to be highly correlated with neurocognitive sequelae [23, 24]. In addition, PT has the potential to further reduce cognitive sequelae by decreasing the volume of irradiated healthy brain tissue. Hearing loss and hypopituitarism have been shown to be directly correlated with mean radiation dose to the cochlea [25] and pituitary gland [26], respectively, and PT has the potential to reduce the dose those organs receive.
Previous publications on PT for children with LGG have reported excellent tumor control and long-term survival outcomes, but data are limited. Greenberger et al [13] reported tumor control and neurocognitive outcomes for 32 pediatric patients treated with passive-scattering PT for LGG and found 8-year PFS and OS rates of 82.8% and 100%, respectively, with some decline in neurocognitive function in children younger than 7 years and those with significant dose to the left temporal lobe. Hug et al [16] described 27 children treated with passive-scattering PT and, after a median follow-up of 3.3 years, reported a 78% local control rate and 85% OS. The only study, to our knowledge, reporting outcomes of children with LGG treated with PBS PT was that of Hauswald et al [14], whose long-term outcomes were limited by a short median follow-up of 5 months. Published data on outcomes after PT for adults with LGG is also limited. Shih et al [15] reported 5-year PFS and OS rates of 40% and 84%, respectively, in a cohort of adults treated with PT in a prospective clinical trial.
Because of high rates of long-term survival after LGG, long-term toxicities and patient QoL are of great importance when determining optimal timing of radiation treatment. Physicians frequently must weigh the potential benefits of immediate radiation therapy (such as tumor and seizure control) against potential toxicities (such as radiation necrosis, neurocognitive impairment, hearing loss, and hypopituitarism). Radiation necrosis is an infrequent consequence of radiation therapy for LGG. Olson et al [27] reported radiation necrosis in 9 of 106 patients at a median of 52 months after photon treatment. Two of 28 patients in our cohort developed grade I radiation necrosis and were observed, never requiring treatment. One patient developed grade III radiation necrosis and was treated with steroids, pentoxifylline, and vitamin E, with complete resolution of symptoms.
Hypopituitarism is a well-documented, dose-dependent, and long-term toxicity of radiotherapy for brain tumors [26]. Although the conformality of PT may decrease the incidence of hypopituitarism compared with conventional radiotherapy, the pituitary gland typically receives significant doses of radiation in patients with centrally located tumors near the hypothalamic-pituitary axis, regardless of the type of radiotherapy used. In our cohort, we reported 9 patients (32%) with hypopituitarism requiring hormone replacement, 7 of whom received a mean dose of ≥50 Gy (RBE) to the pituitary gland.
Cranial irradiation is an established risk factor for neurocognitive impairment for survivors of pediatric brain tumors, and neurocognitive impairment has been associated with increased dose and volume of brain irradiated [28–35]. However, PT has the potential to result in less cognitive dysfunction than conventional radiotherapy because of the decreased radiation dose to the surrounding brain. However, there are few data on neurocognitive outcomes and patient-reported QoL after PT for children with brain tumors. The rate of cognitive dysfunction in LGG patients ranges from 19% to 83% [36]. Numerous factors have a role in long-term cognitive and memory dysfunction, including age at treatment, disease progression, location of the tumor near critical structures, tumor-related epilepsy, and toxicities of surgery, chemotherapy, and radiation therapy [37]. Thus, quantification of cognitive dysfunction resulting from PT is challenging outside of a randomized clinical trial with formal neurocognitive testing. In our cohort, we reported 1 case of grade II cognitive dysfunction and 1 of grade II memory impairment, both in children who received a mean doses of ≥ 50 Gy (RBE) to the left hippocampus. Neurocognitive function was not assessed prospectively in our study, but efforts are made during treatment planning to spare the dominant hippocampus and to limit the dose to the hippocampi and temporal lobes when possible.
The QoL of patients with LGG can be affected by control or progression of their tumor but also by the toxicities of therapy [38]. Gustaffson et al [39] evaluated 39 adult patients with LGG. They found that 45% of patients reported overall low QoL and that treatment-related mental toxicities were more associated with a decreased QoL than were physical toxicities. Yavas et al [40] prospectively evaluated QoL in 43 adults with LGG. They found improvements in QoL after radiotherapy in a variety of domains, including global QoL, communication deficits, and short term memory recall. Our study is, to our knowledge, the first in the literature to present prospectively collected QoL data on children treated with PBS PT for LGG. Yock et al [41] reported on 6 children with LGG treated with passive-scattering PT, but the specific QoL outcome of those patients was not detailed. We found no appreciable decline in QoL as reported by parents in the 8 domains assessed, although our sample size was too small for statistical analyses. Our study's QoL findings in children were similar to those of Shih et al [15] in adults with LGG undergoing PT. Shih and colleagues [15] prospectively assessed patient-reported QoL at baseline and at multiple times after passive-scattering PT and found no significant decline over time. The future, more matured QoL data in our study and other studies that incorporate neurocognitive assessments will be invaluable in understanding the complex interconnection between partial-brain PT and the dose to critical structures of the brain, local tumor progression, chemotherapy, surgery and associated complications of tumor progression and treatment, and their effect on neurocognition and QoL in pediatric patients. If future data were to demonstrate that, in certain subgroups of pediatric patients with LGG, neurocognition and QoL are unaffected by PT, then PT could potentially be used at diagnosis, rather than as salvage therapy, as it is currently, frequently prescribed. Our study was limited by its small sample size. Although data were collected from the 16 pediatric patients enrolled in the QoL study prospectively, data collection from the remaining 4 children and all adults was retrospective. Thus, although patients were followed closely by our physicians and our research office, with office visits when possible and frequent questionnaires sent to patients, their families, and their referring physicians, it is possible that late toxicities and events were underreported. Although some patients in our study were treated starting in 1997, most of our patients (23 of 28; 82%) were treated beginning in 2008, which limited the duration of follow-up. Our study was also limited by LGG comprising a very heterogeneous group of tumors, with outcomes varying widely depending on the subtype of LGG [42]. In addition, age is a well-known prognostic factor in patients with LGG, and the age range of patients in our study was from 2 to 53 years. As expected, children in our cohort had significantly superior survival outcomes. No children in our cohort died, whereas 3 of 8 (38%) adults are deceased, all from tumor progression.
Although our institution began treating young children requiring anesthesia in 2004, PT for adults and adolescents with LGG was initiated in 1997. The treatment of LGG has evolved significantly since 1997, and this is reflected in our study. Pignatti et al [43] identified prognostic factors for patients with LGG enrolled in 2 European Organisation for Research and Treatment of Cancer trials, enabling physicians to appropriately select patients for more-intensive therapy. Of the 8 adults in our study, 4 (50%) were treated with PBS PT for recurrent or progressive disease, and none ever underwent gross total resection. In addition, the discovery of critical gene mutations that predict for a favorable response to chemotherapy, such as the 1p/19q codeletion and isocitrate dehydrogenase 1 (IDH1) mutation, has given us a better understanding of the heterogeneity of this disease and has helped physicians tailor therapy to individual patients [44]. For example, 2 adults treated early in our experience had large, centrally located, unresectable tumors and were treated with PT alone. Today, those patients would likely be treated with chemotherapy in addition to radiotherapy.
Another limitation of our study was that not all patients underwent histologic confirmation of their diagnoses. Because of the risk of biopsy for centrally located tumors and pathognomonic features of LGG on MRI, 25% of patients in our study did not undergo a biopsy. In determining the grade of a suspected glioma, conventional MRI examinations have been shown to have a specificity of only 65%, making it possible that some patients in our study, who did not undergo histologic confirmation, actually had higher-grade gliomas, although that is unlikely because of the superior outcomes in the cohort of patients diagnosed radiographically [45]. In addition, it is possible that patients who underwent stereotactic biopsy alone for diagnosis were undergraded [46]. Two of the adult patients had stereotactic biopsy alone for diagnosis, 1 of whom had a proliferative index of 8%. Both patients died within 27 months of PT. That, along with the other factors discussed, may explain why 4 of the 8 adult patients in our study developed local tumor progression. It is unlikely that the poor tumor control in those adult patients was due to PBS PT because the location of tumor progression in each of the patients does not appear to be at the margin of the PT dose distribution, as it might if rapid dose fall off resulted in underdosing of infiltrative tumor at the margins of the treatment field. In addition, if poor local control was due to the rapid dose fall off from PT, poor local-control outcomes would be expected in the pediatric patients, which was not seen.
Conclusion
In conclusion, PBS PT is a highly conformal and effective treatment for LGG. Acute toxicity is minimal, with no appreciable decline in QoL for children after PBS PT. More patients and longer follow-ups are needed to determine long-term efficacy and toxicity of PT for LGG.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Acknowledgments: This work was partially funded by a project grant from Onco Suisse, entitled “Gesundheitsbezogene Lebensqualität bei Kindern und Jugendlichen mit Hirntumoren und Protonenbestrahung” (grant 0169404-2005). Data collection since 2010 was continued by PSI resources. The PEDQoL project is supported in part by a grant from the Swiss Cancer League.
References
- 1.Freeman CR, Farmer J, Taylor RE. Central nervous system tumors in children. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez & Brady's Principles and Practice of Radiation Oncology. 6th ed: Lippincott Williams & Wilkins;; 2013. pp. 1632–1655. chapter 84. [Google Scholar]
- 2.Gondi V, Vogelbaum MA, Grimm S, Mehta MP. Primary intracranial neoplasm. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Perez and Brady's Principles and Practice of Radiation Oncology. 6th ed: Baltimore, MD: Williams & Wilkins;; 2013. pp. 649–676. chapter 35. [Google Scholar]
- 3.Buckner JC, Pugh SL, Shaw EG, Gilbert MR, Barger G, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K, Brachman D, Suh JH, Schultz CJ, Bahary JP, Fisher BJ, Kim H, Murtha AD, Curran WJ, Mehta MP. Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG [abstract 2000] J. Clin Oncol. 2014;32(suppl):5. [Google Scholar]
- 4.Eyre HJ, Crowley JJ, Townsend JJ, Eltringham JR, Morantz RA, Schulman SF, Quagliana JM, al-Sarraf M. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a Southwest Oncology Group study. J Neurosurg. 1993;78:909–14. doi: 10.3171/jns.1993.78.6.0909. [DOI] [PubMed] [Google Scholar]
- 5.Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, Guo D, Ullrich NJ, Robison NJ, Chi SN, Beroukhim R, Kieran MW, Manley PE. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61:1173–9. doi: 10.1002/pbc.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB, EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults. Lancet. 2006;366:985–90. doi: 10.1016/S0140-6736(05)67070-5. the EORTC 22845 randomised trial [published correction appears in. 367:1818]. Lancet 2005; [DOI] [PubMed] [Google Scholar]
- 7.Fisher BJ, Hu C, Macdonald DR, Lesser GJ, Coons SW, Brachman DG, Ryu S, Werner-Wasik M, Bahary JP, Liu J, Chakravarti A, Mehta M. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91:497–504. doi: 10.1016/j.ijrobp.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nageswara Rao AA, Packer RJ. Advances in the management of low-grade gliomas. Curr Oncol Rep. 2014;16:398. doi: 10.1007/s11912-014-0398-9. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald SM, Safai S, Trofimov A, Wolfgang J, Fullerton B, Yeap BY, Bortfeld T, Tarbell NJ, Yock T. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71:979–86. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 10.Lomax AJ, Böhringer T, Bolsi A, Coray D, Emert F, Goitein G, Jermann M, Lin S, Pedroni E, Rutz H, Stadelmann O, Timmermann B, Verwey J, Weber DC. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys. 2004;31:3150–7. doi: 10.1118/1.1779371. [DOI] [PubMed] [Google Scholar]
- 11.Lomax AJ. Physics of Treatment Planning Using Scanned Beams. Boca Raton, FL: CRC;; 2011. [Google Scholar]
- 12.Hälg RA, Besserer J, Boschung M, Mayer S, Lomax AJ, Schneider U. Measurements of the neutron dose equivalent for various radiation qualities, treatment machines and delivery techniques in radiation therapy. Phys Med Biol. 2014;59:2457–68. doi: 10.1088/0031-9155/59/10/2457. [DOI] [PubMed] [Google Scholar]
- 13.Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, Huang MS, Marcus KJ, Oberg JA, Tarbell NJ, Yock TI. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89:1060–8. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Hauswald H, Rieken S, Ecker S, Kessel KA, Herfarth K, Debus J, Combs SE. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol. 2012;7:189. doi: 10.1186/1748-717X-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih HA, Sherman JC, Nachtigall LB, Colvin MK, Fullerton BC, Daartz J, Winrich BK, Batchelor TT, Thornton LT, Mancuso SM, Saums MK, Oh KS, Curry WT, Loeffler JS, Yeap BY. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer. 2015;121:1712–9. doi: 10.1002/cncr.29237. [DOI] [PubMed] [Google Scholar]
- 16.Hug EB, Muenter MW, Archambeau JO, DeVries A, Liwnicz B, Loredo LN, Grove RI, Slater JD. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol. 2002;178:10–7. doi: 10.1007/s00066-002-0874-2. [DOI] [PubMed] [Google Scholar]
- 17.Weber DC, Ares C, Malyapa R, Albertini F, Calaminus G, Kliebsch U, Mikroutsikos L, Morach P, Bolsi A, Lomax T, Schneider R. Tumor control and QoL outcomes of very young children with atypical teratoid/rhabdoid tumor treated with focal only chemo-radiation therapy using pencil beam scanning proton therapy. J Neurooncol. 2015;121:389–97. doi: 10.1007/s11060-014-1648-2. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 19.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, Mascarenhas F, Horiot JC, Parvinen LM, van Reijn M, Jager JJ, Fabrini MG, van Alphen AM, Hamers HP, Gaspar L, Noordman E, Pierart M, van Glabbeke M. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–56. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 21.Dennis ER, Bussiere MR, Niemierko A, Lu MW, Fullerton BC, Loeffler JS, Shih HA. A comparison of critical structure dose and toxicity risks in patients with low grade gliomas treated with IMRT versus proton radiation therapy. Technol Cancer Res Treat. 2013;12:1–9. doi: 10.7785/tcrt.2012.500276. [DOI] [PubMed] [Google Scholar]
- 22.Winkfield KM, Niemierko A, Bussiere MR, Crowley EM, Napolitano BN, Beaudette KP, Loeffler JS, Shih HA. Modeling intracranial second tumor risk and estimates of clinical toxicity with various radiation therapy techniques for patients with pituitary adenoma. Technol Cancer Res Treat. 2011;10:243–51. doi: 10.7785/tcrt.2012.500199. [DOI] [PubMed] [Google Scholar]
- 23.Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702–13. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH, Beute GN, Sluimer JD, Slotman BJ, Reijneveld JC, Heimans JJ. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 25.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, Mendenhall WM. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76:S50–7. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez A, Brada M, Zabuliene L, Karavitaki N, Wass JA. Radiation-induced hypopituitarism. Endocr Relat Cancer. 2009;16:733–72. doi: 10.1677/ERC-08-0231. [DOI] [PubMed] [Google Scholar]
- 27.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54:1442–8. doi: 10.1212/wnl.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 28.Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A, Donaldson SS, Stovall M, Kadan-Lottick N, Armstrong G, Robison LL, Zeltzer LK. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–17. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–7. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, Xiong X, Mulhern RK. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–55. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 31.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–13. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 32.Brinkman TM, Krasin MJ, Liu W, Armstrong GT, Ojha RP, Sadighi ZS, Gupta P, Kimberg C, Srivastava D, Merchant TE, Gajjar A, Robison LL, Hudson MM, Krull KR. Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: results from the St Jude Lifetime Cohort Study. J Clin Oncol. 2016;34:1358–67. doi: 10.1200/JCO.2015.62.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merchant TE, Mulhern RK, Krasin MJ, Kun LE, Williams T, Li C, Xiong X, Khan RB, Lustig RH, Boop FA, Sanford RA. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–62. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 34.Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–54. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Merchant TE, Sharma S, Xiong X, Wu S, Conklin H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2014;90:547–53. doi: 10.1016/j.ijrobp.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Loon EM, Heijenbrok-Kal MH, van Loon WS, van den Bent MJ, Vincent AJ, de Koning I, Ribbers GM. Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47:481–8. doi: 10.2340/16501977-1975. [DOI] [PubMed] [Google Scholar]
- 37.Klein M, Heimans JJ, Aaronson NK, van der Ploeg HM, Grit J, Muller M, Postma TJ, Mooij JJ, Boerman RH, Beute GN, Ossenkoppele GJ, van Imhoff GW, Dekker AW, Jolles J, Slotman BJ, Struikmans H, Taphoorn MJ. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2011;360:1361–8. doi: 10.1016/s0140-6736(02)11398-5. [published correction appears in. 377:1654]. Lancet 2002; [DOI] [PubMed] [Google Scholar]
- 38.Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas: a review. J Neurosurg. 2011;115:240–4. doi: 10.3171/2011.3.JNS1153. [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14:1205–12. doi: 10.1007/s00520-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 40.Yavas C, Zorlu F, Ozyigit G, Gurkaynak M, Yavas G, Yuce D, Cengiz M, Yildiz F, Akyol F. Prospective assessment of health-related quality of life in patients with low-grade glioma: a single-center experience. Support Care Cancer. 2012;20:1859–68. doi: 10.1007/s00520-011-1288-4. [DOI] [PubMed] [Google Scholar]
- 41.Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, MacDonald SM, Pulsifer MB, Hill KS, DeLaney TF, Ebb D, Huang M, Tarbell NJ, Fisher PG, Kuhlthau KA. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol. 2014;113:89–94. doi: 10.1016/j.radonc.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumthekar P, Raizer J, Singh S. Low-grade glioma. Cancer Treat Res. 2015;163:75–87. doi: 10.1007/978-3-319-12048-5_5. [DOI] [PubMed] [Google Scholar]
- 43.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB. European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–84. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 44.Olar A, Sulman EP. Molecular markers in low-grade glioma-toward tumor reclassification. Semin Radiat Oncol. 2015;25:155–63. doi: 10.1016/j.semradonc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 46.Muragaki Y, Chernov M, Maruyama T, Ochiai T, Taira T, Kubo O, Nakamura R, Iseki H, Hori T, Takakura K. Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg. 2008;51:275–9. doi: 10.1055/s-0028-1082322. [DOI] [PubMed] [Google Scholar]