Abstract
We describe the first reported use of neutron radiation therapy for successful palliation of treatment refractory Merkel cell carcinoma. The patient was a 78-year-old man with Merkel cell carcinoma involving the scalp and bilateral cervical lymph nodes. The extensive coalescing scalp lesions were locally destructive, painful, and highly detrimental to his overall quality of life, and he had previously progressed through 3 courses of conventional x-ray–based radiation therapy and multiple immunotherapy regimens. We treated him with neutron radiation therapy, and he experienced a complete and durable local response with minimal toxicity. High linear energy transfer particle therapy approaches deserve consideration as a treatment option in cancers that are refractory to standard radiation therapy.
Keywords: neutron radiation therapy, high LET radiation, Merkel cell carcinoma, palliation, particle therapy
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous neuroendocrine malignancy associated with older age (>50 years), Caucasian race, ultraviolet radiation exposure, Merkel cell polyoma virus and immune suppression [1, 2]. The 5-year survival rate is 64% for localized disease, 39% with lymph node metastases and 18% with distant metastases [2].
In general, MCC is considered a radiosensitive malignancy [3], and conventional x-ray–based radiation therapy (XRT) plays an important role in both curative and palliative settings. For palliation in patients with stage IV disease , impressive response rates and durable control were observed with XRT using a single fraction of 8 Gy [4]. However, MCC can be refractory to XRT [5, 6], particularly in the recurrent setting. Uncontrolled treatment refractory tumors, especially in the head and neck region, cause considerable morbidity. They are frequently unsightly and cause symptoms such as pain, bleeding, and foul odor that can be detrimental to the patient's quality of life.
While systemic chemotherapy can provide transient palliation with objective responses in advanced MCC, responses are seldom durable, with a median progression-free survival of 3 months [7]. In addition, elderly patients often struggle with the significant adverse effects of chemotherapy [8]. Recently, programmed death 1 blockade with pembrolizumab was reported to lead to durable objective responses in ∼50% of patients with advanced MCC [9]. Avelumab has also shown a benefit in patients with chemorefractory metastatic MCC [10]. However, around half of patients with advanced MCC do not respond to programmed death 1 blockade. Furthermore, many patients are not eligible for immunotherapy due to presence of systemic immunosuppression. Additionally, those with a favorable response to systemic therapies may have an increasingly important need for local control, especially in isolated lesions that become refractory to treatment. Hence, there is an unmet need to find effective local salvage therapies in this patient population.
The response to reirradiation with XRT tends to be less successful, likely secondary to selection of radioresistant tumor and hypoxia [11]. Furthermore, delivering an adequate radiation dose may be limited by the tolerance of previously irradiated surrounding normal tissues.
Neutron radiation therapy (NRT) is a special high linear energy transfer (LET) type of radiation with a greater relative biological effectiveness (RBE) compared with XRT. While NRT has a higher tumoricidal effect than XRT, it was historically fraught with increased complication rates [12]. Currently, NRT is reserved for select radioresistant cancers such as salivary gland malignancies. Owing to our institution's extensive experience with this modality, we have used NRT for unresectable melanoma, soft tissue sarcomas, other treatment-refractory tumors, and effective palliation of metastases.
Here, after obtaining informed consent, we report on a patient with extensive, recurrent MCC of the head and neck that was refractory to multiple XRT courses and local and systemic immunotherapy. He was treated with NRT and had a rapid and durable response, minimal side effects, and improved quality of life. The tumor remains under local regional control now, nearly two years after treatment.
Case Report
A previously healthy 78-year-old Caucasian man with no history of immunosuppression was diagnosed with a <5 mm MCC of the left frontal scalp in February 2013, for which he underwent a surgical excision with clear margins. He developed a local recurrence in the vicinity of his initial MCC and underwent another wide local excision and sentinel lymph node biopsy in February 2014. Pathology results revealed multiple dermal deposits of residual MCC with lymph-vascular invasion. A left preauricular lymph node was positive for MCC. In June 2014, he completed XRT to 56 Gy in 2 Gy fractions to the scalp, and 52 Gy in 1.8 Gy fractions to the left neck and parotid region.
Three months later, the patient developed a biopsy proven MCC recurrence diffusely on the scalp both within and outside the previously irradiated fields. Staging scans confirmed that he was free of distant metastatic disease. He then received multiple local and systemic therapies as summarized in the Table.
Table.
Summary of the patient's local and systemic therapies.
|
Status |
Surgery |
XRT |
Drug |
Outcome |
|
| February 2013 | Diagnosis | WLE | Path: negative margins. | ||
| February 2014 | First recurrence | WLE, SLNB | Path: multiple dermal deposits, positive margin, positive LVSI, positive left preauricular LN | ||
| May–June 2014 | XRT: scalp, 56 Gy/28 fx IMRT: left neck, 52 Gy/28 fx |
NED | |||
| October 2014 | Diffuse scalp recurrence in/outside XRT field | October 23, electrons: scalp, 8 Gy/1 fx | October 23–24: intralesional Interferon to multiple scalp lesions ×2 weeks: topical Imiquimod to scalp | One month after treatment, complete resolution of lesions. Response persisted for ∼1 month | |
| January 2015 | Diffuse scalp recurrence, palpable left preauricular LN | - | - | Pembrolizumab × 5 (trial) | PD |
| May–June 2015 | PD | - | 30 Jun, electrons: frontal scalp lesions, 8 Gy/1 fx | Intratumoral GLA-SE injection ×8 (trial) | PD |
| Total RT dose before NRTa | Entire scalp: 73.6 Gy | PD | |||
| Frontal scalp lesion: 91.2 Gy | |||||
| Left neck: 52 Gy | |||||
Abbreviations: XRT, x-ray radiation therapy; WLE, wide local excision; SLNB, sentinel lymph node biopsy; LVSI, lymphovascular space invasion; LN, lymph node; IMRT, intensity modulated radiation therapy; NED, no evidence of disease; fx, fraction of radiation; PD, progressive disease .
Cumulative XRT doses are converted to conventional 2 Gy fractions using EQD2Gy (α/β=3). Assuming an alpha/beta ratio of 3 according to the linear quadratic model, the cumulative 2 Gy equivalent normal tissue doses with XRT from all XRT treatments were 73.6 Gy to the entire scalp, 91.2 Gy to the frontal scalp, and 52 Gy to the left neck and parotid region.
However, the patient continued to have progressive disease with bulky, symptomatic tumors on his scalp and neck. He was then treated with NRT to the scalp in July 2015 and received 18 neutron Gray (nGy) in 12 fractions. He had a rapid and robust response to treatment (Figure 1). Encouraged by this excellent response, the treatment field was expanded to include his bilateral parotid/cervical lymph nodal disease. The nodal disease was treated to 18 nGy in 10 fractions. Additional scalp fields were used to include 2 separate and smaller scalp lesions. These were treated to16 nGy in 8 fractions. His composite treatment plan can be seen in Figure 2. He had a complete clinical and radiologic response that was confirmed on computed tomography (Figure 3).
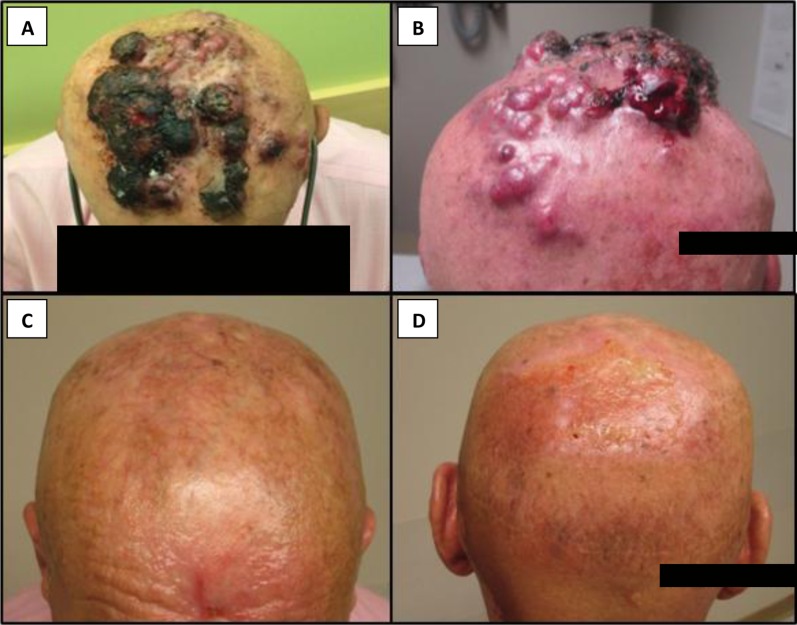
Figure 1.
(A, B) Progressive Merkel cell carcinoma involving the scalp prior to the start of neutron radiation. (C, D) Complete response of Merkel cell carcinoma to neutron radiation approximately 4 weeks after treatment.
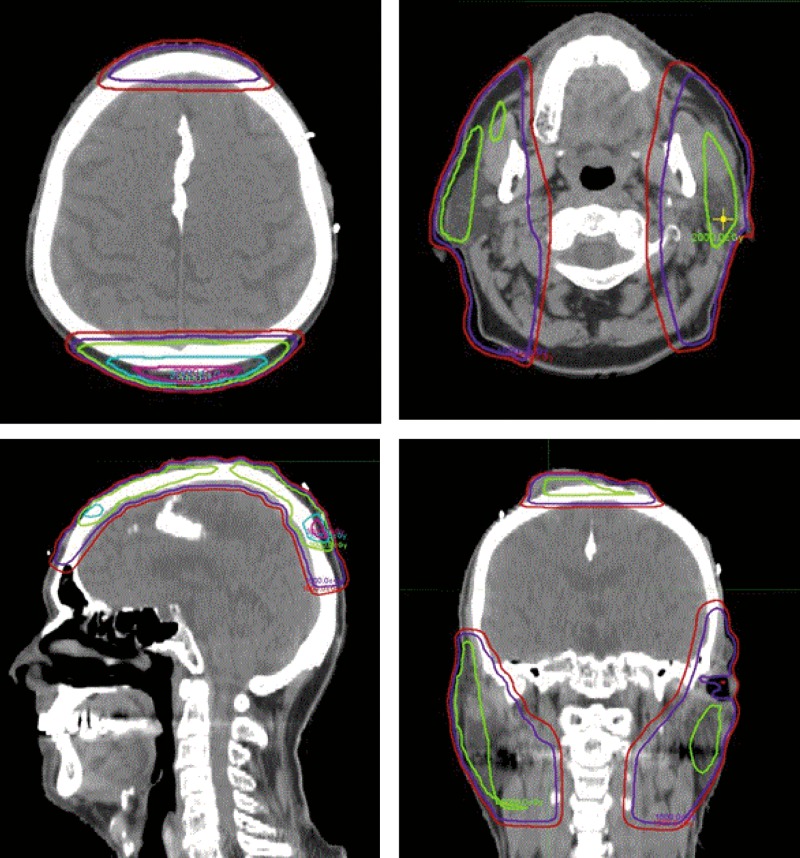
Figure 2.
Dose distribution for neutron therapy plans in axial, sagittal, and coronal planes. These are fusion images displaying the composite dose delivered with neutron therapy. Red, 10 neutron Gray (nGy); purple, 15 nGy; green, 20 nGy; blue, 30 nGy; pink, 35 nGy.
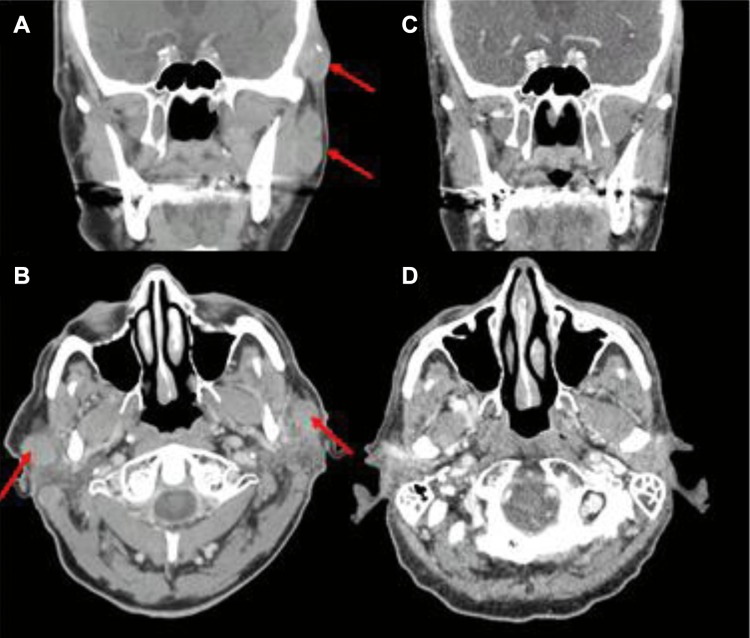
Figure 3.
(A, B) Coronal and axial images of bilateral parotid lymph nodes with Merkel cell carcinoma involvement prior to the start of neutron radiation. (C, D) Durable response of disease continues nearly two years after neutron radiation treatment.
Notably, the patient did not experience any serious complications after reirradiation with NRT. He developed mild skin telangiectasia in the treatment field, moderate xerostomia, and decreased hearing on the left side. Several months after NRT, he had small recurrences outside of the high-dose neutron treatment field. He was then able to enroll in another immunotherapy clinical trial and had a good response on the same. The NRT-treated extensive local regional disease, both on his scalp and in the lymph node regions, remains controlled as of June 2017, nearly two years after completion of NRT. The patient is currently enjoying an excellent quality of life and remains disease free.
Discussion
Local control of treatment-resistant tumors presents a profound challenge, particularly when prior radiation therapy has exhausted the tolerance of normal tissues. Poor blood perfusion and hypoxia play a major role in such treatment resistance. in addition to other molecular mechanisms [11, 13]. Neutron radiation is known for its lesser dependence on tissue oxygenation status compared with XRT [14] and can potentially overcome hypoxia-related treatment resistance to XRT, as demonstrated impressively in our patient with multiply recurrent MCC. His tumors responded completely and durably, and he experienced a significant palliative benefit from NRT after a poor response to 3 prior XRT courses and multiple immunotherapy regimens. Only moderate and acceptable toxicity from NRT was observed up to over a year after treatment, despite the reported propensity for normal tissue injury from NRT [12, 15]. Importantly, the durable local disease control enabled “bridging” to a systemic immunotherapy trial, which became available only several months later and has likely contributed to systemic disease control as well.
The biologic mechanism of cytotoxicity of NRT is distinct from XRT due to differences in physical and radiobiologic properties. The higher density of ionization in tissue by neutron radiation results in higher LET. The higher LET in turn damages large clusters of double strands of DNA, impairing DNA repair in tumors, and produces irreversible DNA disruption with less dependence on the cell cycle phase. Unlike XRT, the cellular interactions of NRT are relatively independent of the tumor microenvironment. In particular, the presence of oxygen is not required, and NRT is therefore more likely than XRT to be effective in hypoxic tumors. This results in a higher RBE that is estimated to be 3 to 3.5 times that of XRT in most normal tissues [16].
The therapeutic advantage of NRT over XRT is best established in unresectable salivary gland malignancies [17]. These tumors have a neutron RBE of 8, (versus 3–3.5 RBE for most normal tissues), resulting in a 2- to 2.5-fold therapeutic differential [18]. Notably, NRT has been tested on other tumor types, including tumors that would otherwise be considered resistant to XRT. In studies dating back to the 1980s, favorable response of bulky, symptomatic, soft tissue masses of radiation therapy–resistant tumors to neutron therapy was observed [19]. One study found an overall response rate of 78% in patients treated for recurrent disease with NRT after prior XRT. Toxicity was dose dependent, and approximately 25% of patients had adverse events [20]. among patients with unresectable adenoid cystic carcinomas, soft tissue sarcomas, rectal recurrences, and previously irradiated head and neck tumors, NRT appears to provide efficacious palliation [21].
Though NRT has a higher tumoricidal effect than XRT, it was historically fraught with increased complication rates [12]. However, some of the reported serious toxicity came from a time before the appropriate doses/RBE factors for neutrons had been defined. Greater toxicity was also seen when treating very large volumes to high doses, and many facilities using neutrons had more limited treatment platforms (eg, fixed beam rooms in research facilities, lack of multileaf collimator beam shaping). Subsequent randomized trials found the clinical utility of NRT limited to a few rare malignancies [15]. While the toxicity in those studies was not as concerning as the initial experience, it was still significantly worse than with XRT [17]. Currently, the University of Washington is the only clinically operational center for NRT in the United States, and it has maintained an active clinical NRT program. The technology includes a modern isocentric gantry and multileaf collimators, which permit 3-dimensional conformal techniques that allow better normal tissue sparing, thereby minimizing the toxicity of NRT [22]. Additionally, fast neutron therapy at our facility is delivered with a high-energy, hospital-based, Scanditronix MC 50 cyclotron (Seattle, Washington, USA) utilizing a 50.5 MeV proton –> beryllium reaction as previously described [23]. This is a higher energy than that of most facilities that were previously operational, and perhaps allowed for better depth-dose profiles and fewer complications.
In view of the past reports on major toxicity with NRT, it is notable that NRT was not associated with major treatment-related toxicity in our patient despite prior high-dose irradiation. The addition of our prescribed NRT dose to the previously received cumulative normal tissue dose of 73.6 Gy to the entire scalp, 91.2 Gy to the frontal scalp, and 52 Gy to the left neck (Table) is significant. The highly targeted delivery techniques employed in our NRT program may have contributed to minimizing complications nearly two years after completion of treatment. The patient continues under close surveillance.
Conclusion
Although the vast majority of radiation therapy in the world is currently delivered with XRT, alternative approaches of high LET radiation, such as neutrons or heavy charged particle therapy, employed with modern treatment delivery techniques, hold promise to salvage unresponsive focal tumor involvement in patients with recurrent MCC. The success of our case could apply to other treatment-resistant and/or hypoxic recurrent tumors. Such capabilities will likely become increasingly important as the advances in chemotherapies, targeted therapies, and immunotherapies result in improved systemic disease control and thereby a greater need to provide effective targeted palliation for unresponsive recurrent or metastatic tumor foci to improve patients' quality of life.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, Nghiem P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W, MacDonald M, You J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr Opin Virol. 2016;20:20–7. doi: 10.1016/j.coviro.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veness M, Foote M, Gebski V, Poulsen M. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78:703–9. doi: 10.1016/j.ijrobp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Iyer JG, Parvathaneni U, Gooley T, Miller NJ, Markowitz E, Blom A, Lewis CW, Doumani RF, Parvathaneni K, Anderson A, Bestick A, Liao J, Kane G, Bhatia S, Paulson K, Nghiem P. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4:1161–70. doi: 10.1002/cam4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunaratne DA, Howle JR, Veness MJ. Merkel cell carcinoma: a case of palliative upper limb amputation in a patient with refractory in-transit metastases. Australas J Dermatol. 2016;57:e53–6. doi: 10.1111/ajd.12291. [DOI] [PubMed] [Google Scholar]
- 6.Meeuwissen JA, Bourne RG, Kearsley JH. The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:325–31. doi: 10.1016/0360-3016(94)E0145-A. [DOI] [PubMed] [Google Scholar]
- 7.Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, Anderson A, Ma C, Bestick A, Parvathaneni U, Bhatia S, Nghiem P. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5:2294–301. doi: 10.1002/cam4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garneski KM, Nghiem P. Merkel cell carcinoma adjuvant therapy: current data support radiation but not chemotherapy. J Am Acad Dermatol. 2007;57:166–9. doi: 10.1016/j.jaad.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbe C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(suppl 1):i90–i8. doi: 10.1093/jrr/rrw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catterall M, Bewley DK, Sutherland I. Second report on results of a randomised clinical trial of fast neutrons compared with chi or gamma rays in treatment of advanced tumours of head and neck. Br Med J. 1977;1:1642. doi: 10.1136/bmj.1.6077.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oehler C, Dickinson DJ, Broggini-Tenzer A, Hofstetter B, Hollenstein A, Riesterer O, Vuong V, Pruschy M. Current concepts for the combined treatment modality of ionizing radiation with anticancer agents. Curr Pharm Des. 2007;13:519–35. doi: 10.2174/138161207780162935. [DOI] [PubMed] [Google Scholar]
- 14.Hall E. Radiobiology for the Radiologist 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins;; 2012. [Google Scholar]
- 15.Russell KJ, Laramore GE, Krieger JN, Wiens LW, Griffeth JT, Koh WJ, Griffin BR, Austin-Seymour MM, Griffin TW, Davis LW. Transient and chronic neurological complications of fast neutron radiation for adenocarcinoma of the prostate. Radiother Oncol. 1990;18:257–65. doi: 10.1016/0167-8140(90)90061-z. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson L, Tepper J. Clinical Radiation Oncology 4th ed. Philadelphia, PA: Elsevier Saunders;; 2012. [Google Scholar]
- 17.Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, Maor MH, Davis LW. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235–40. doi: 10.1016/0360-3016(93)90233-l. [DOI] [PubMed] [Google Scholar]
- 18.Battermann JJ, Breur K, Hart GA, van Peperzeel HA. Observations on pulmonary metastases in patients after single doses and multiple fractions of fast neutrons and cobalt–60 gamma rays. Eur J Cancer. 1981;17:539–48. doi: 10.1016/0014-2964(81)90056-6. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickson FR. The use of neutron beam therapy in the management of locally advanced nonresectable radioresistant tumors. CA Cancer J Clin. 1988;38:353–61. doi: 10.3322/canjclin.38.6.353. [DOI] [PubMed] [Google Scholar]
- 20.Saroja KR, Hendrickson FR, Cohen L, Mansell J, Lennox A. Re-irradiation of locally recurrent tumors with fast neutrons. Int J Radiat Oncol Biol Phys. 1988;15:115–21. doi: 10.1016/0360-3016(88)90354-9. [DOI] [PubMed] [Google Scholar]
- 21.Micke O, Schafer U, Prott FJ, Schuller P, Scheuber M, Willich N. Fast neutron irradiation in advanced pre-irradiated head and neck tumors. Tumori. 2000;86:393–8. doi: 10.1177/030089160008600505. [DOI] [PubMed] [Google Scholar]
- 22.Douglas JG, Lee S, Laramore GE, Austin-Seymour M, Koh W, Griffin TW. Neutron radiotherapy for the treatment of locally advanced major salivary gland tumors. Head Neck. 1999;21:255–63. doi: 10.1002/(sici)1097-0347(199905)21:3<255::aid-hed11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Risler R, Eenmaa Y, Jacky J, Kalet I, Wootton P. Installation of the cyclotron based clinical neutron therapy system in Seattle. In: Marti E, editor. Tenth International Conference on Cyclotrons and Their Applications. Piscataway, NJ: IEEE Service Center;; 1984. pp. 428–30. [Google Scholar]