Abstract
Purpose:
We dosimetrically compared pencil beam scanning (PBS) proton therapy and intensity-modulated radiation therapy (IMRT) for pelvic and para-aortic lymph node disease in endometrial carcinoma and present acute toxicities associated with extended-field PBS.
Patients and Methods:
Twenty-five patients with locally advanced endometrial malignancies were enrolled in an image-guided registry study. Seven of these patients were treated with PBS, and 18 patients were treated with IMRT. Organs at risk included pelvic bone marrow (PBM), small bowel (SB), large bowel (LB), rectum, bladder, and kidneys. The IMRT and PBS dosimetric parameters were compared using Wilcoxon rank-sum tests.
Results:
Compared with IMRT PBM dose-volume histograms, PBS resulted in significantly lower dose volumes from 0 to 26.0 Gy (P < .05) and higher dose volumes from 33.9 to 42.9 Gy (P < .05). Overall, PBS resulted in 22% lower median PBM volume irradiated to 10 Gy (RBE) (PBS 71.3% versus IMRT 93.4%, P < .001) and 14% lower median volume irradiated to 20 Gy (RBE) (PBS 65.1% versus IMRT 79.4%, P < .001). Compared with IMRT, PBS also significantly reduced SB dose volumes from 0 to 27.5 Gy, LB dose volumes from 0 to 31.6 Gy, bladder dose volumes from 0 to 27.3 Gy, and rectal dose volumes from 0 to 7.6 Gy (all P < .05). However, PBS resulted in higher rectal dose volumes compared with IMRT from 26.0 to 48.4 Gy. Grade 3+ hematologic toxicities were present in 2 (11%) IMRT-treated patients and no PBS-treated patients. No grade 3+ gastrointestinal or genitourinary toxicities were present in either treatment group.
Conclusion:
In endometrial carcinoma, extended-field PBS is clinically feasible, resulting in statistically significant dose reduction to PBM as well as SB, LB, and bladder in the lower dose regions.
Keywords: proton therapy, extended field pelvic radiotherapy, endometrial carcinoma, pelvic bone marrow
Introduction
Extended-field radiation therapy (RT) that includes the pelvis and para-aortic lymph nodes (PALN) is indicated in patients with gynecologic malignancies with suspected or confirmed PALN disease (gross disease, fluorodeoxyglucose (FDG) avid, or pathologically confirmed). However, extended-field RT is frequently given concurrently or sequentially with chemotherapy, and this treatment strategy is limited by acute and late pelvic bone marrow (PBM) and gastrointestinal (GI) toxicities. While 3-dimensional conformal RT has traditionally been used for extended-field RT, use of intensity-modulated RT (IMRT) has increased due to its improved dosimetry with respect to the PBM and favorable hematologic and GI toxicity profiles in retrospective and prospective trials [1–6].
Currently, there are no clinical reports of proton therapy for pelvic and PALN RT in women with gynecologic cancers. We have previously shown the clinical feasibility, toxicity, and dosimetric advantages of proton therapy for women requiring pelvic RT for gynecologic cancers. Our results demonstrated that pencil beam scanning (PBS) proton therapy is able to significantly reduce dose to normal tissues in the pelvis, particularly the PBM, bladder, and small bowel (SB) compared with IMRT while maintaining adequate target coverage [7]. In an early dosimetric study, we also compared IMRT and 2 different proton therapy techniques for PALN irradiation, demonstrating that both proton therapy techniques resulted in statistically significant dose reduction to SB, large bowel (LB), and kidney dose compared with IMRT [8].
In this first clinical report of proton beam therapy for women requiring pelvic and PALN RT for endometrial malignancies, we compare the dosimetry and acute toxicities associated with PBS and IMRT.
Patients and Methods
Patients
This study enrolled 7 women receiving PBS for locally advanced endometrial carcinoma from November 2013 to April 2015. All patients underwent hysterectomy, bilateral salphingo-oophorectomy, and pelvic-para-aortic lymph node dissection. Indications for extended-field RT include evidence of para-aortic lymph node positivity (gross, fluorodeoxyglucose avid, or pathologically confirmed) or positive pelvic lymph nodes without surgical sampling of PALN. Each subject consented to our institutional review board–approved prospective image-guided RT study. Baseline inclusion hematologic parameters included hemoglobin >10g/dL, platelets >150 000, and absolute neutrophil count >1500/μL within 30 days before initiating RT. We identified an additional 18 patients consecutively treated with pelvic and PALN IMRT for endometrial cancer from May 2008 to July 2013 for comparison. These patients were identified from an institutional review board–approved retrospective study with similar hematologic criteria for consideration for pelvic and PALN irradiation and who underwent complete surgical staging. All patients were treated at the University of Pennsylvania Department of Radiation Oncology.
Patient and treatment characteristics are summarized in Table 1. Median follow-up for the entire cohort was 33 months (range, 3 to 88 months). Median follow-up for IMRT patients was 44.5 months (range, 14 to 88 months), and median follow-up time for PBS patients was 19 months (range, 3 to 25 months). All patients received RT to the pelvis and PALN. Median dose of PBS was 50.4 Gy-equivalent (GyE, using relative biological effectiveness of 1.1 for proton therapy) (range, 45.0 to 50.4 GyE). Median dose of IMRT was 45 Gy (range, 45.0 to 50.4 Gy). Of the PBS patients, 2 received a boost to lymph nodes (total dose, 59.4 GyE), and 3 received brachytherapy boost (median, 12 Gy; range, 8 to 12 Gy, in 2 to 3 fractions). Of the IMRT patients, 3 received a boost to lymph nodes (total dose, 59.4 Gy) and 11 received brachytherapy (median, 12 Gy; range, 8 to 15 Gy, in 3 to 4 fractions).
Table 1.
Patient characteristics for pencil beam scanning and IMRT patients.
|
No. of proton patients (n=7) |
No. of IMRT patients (n=18) |
|
| Age | ||
| Median, years | 66 | 65 |
| Range, years | 59 to 74 | 60 to 69 |
| Race | ||
| Black | 3 (42%) | 7 (39%) |
| White | 4 (57%) | 11 (61%) |
| Chemotherapy | ||
| Sandwich | 3 (33%) | 12 (67%) |
| Sequential | 4 (44%) | 3 (17%) |
| Concurrent | 0 (0%) | 0 (0%) |
| Combined | 0 (0%) | 1 (6%) |
| None | 0 (0%) | 2 (11%) |
| Radiation dose | ||
| Median | 46.8 GyE | 45.0 Gy |
| Range | 45.0 to 50.4 GyE | 45.0 to 50.4 Gy |
Abbreviations: IMRT, intensity-modulated radiation therapy; combined, concurrent + sandwich chemotherapy; GyE, Gray equivalent using relative biological effectiveness of 1.1 for proton beam therapy.
All patients receiving proton therapy had carboplatin and paclitaxel chemotherapy; 3 patients received sandwich chemotherapy and 4 received chemotherapy followed by radiation. Of the IMRT patients, 11 received sandwich chemotherapy with carboplatin and/or paclitaxel, 1 received sandwich chemotherapy with gemcitabine and docetaxel, 2 received sequential carboplatin and paclitaxel after radiation, 1 received sequential radiation after chemotherapy with carboplatin and paclitaxel, 1 received concurrent cisplatin and sandwich carboplatin/paclitaxel, and 2 received no chemotherapy.
Computed Tomography Simulation
All patients underwent computed tomography (CT) and magnetic resonance simulation for treatment planning. Patients started simethicone with meals 1 week before simulation, maintained a clear liquid diet starting at noon of simulation, drank 16 ounces of fluid 30 minutes before simulation, and received at least 1 enema within 2 hours of simulation. Throughout treatment, patients were instructed to continue simethicone before meals and drink 16 ounces of fluid approximately 30 minutes before each RT session.
The CT simulation was conducted in the supine position with immobilization via an indexed knee-foot lock device. Initial scan was done with full bladder and rectal balloon (Radiadyne, Houston, Texas) inflated with 50 to 100 cm3 of water. The second CT scan (empty bladder scan) was done after patient voiding with identical rectal balloon. A rectal balloon was exclusively used in patients receiving PBS proton therapy.
Contouring
The vaginal internal target volume was created by fusing the full and empty bladder CT image sets and contouring the vaginal target to cover its maximum range between scans. Pelvic lymph nodes (internal and external iliac, common iliac, and obturator) and PALN were contoured according to the Radiation Therapy Oncology Group (RTOG) guidelines [9, 10]. Treatment clinical target volume (CTV) included the vaginal target and nodal volume. The planning target volume (PTV) included an asymmetric margin of 10 to 13 mm added to the vaginal target and a 7 to 8 mm margin added to the nodal volume.
The organs at risk (OARs) included the PBM, SB, LB, rectum, kidneys, and bladder. The PBM was composed of the ilium, lower pelvis (ischium, pubis, and proximal femora), and lumbosacral spine (LSS) [11]. The LSS was contoured from L1 to the sacrum. The rectum was contoured from the rectosigmoid junction to the anus and loops of bowel (small and large) were contoured within the field and up to 2 cm above the PTV.
Treatment Planning
Plans for photon and proton treatments were generated using the Eclipse planning software Version 11.0, (Varian Medical Systems, Palo Alto, California). The planned dose was 45.0 to 50.4 Gy in 1.8 Gy fractions (or GyE for proton plans). Proton treatment plans were developed using 2 PBS posterior oblique beams at 15° to 30° [7]. As described in Lin et al [7], a PBS treatment volume was created to correct for beam calibration and Hounsfield Unit (HU) conversion uncertainty upfront. The PBS treatment volume included the PTV with a 3.5% range margin in the beam direction to correct for HU to proton stopping power calibration uncertainties and an additional 1-mm margin to correct for beam calibration uncertainty. The IMRT plans were generated using coplanar 7-field plans using 6 MV and/or 15 MV photons.
Planning constraints for total PBM were volume irradiated to 10 Gy (RBE) (V10) <95% and volume irradiated to 20 Gy (RBE) (V20) <76%, LB and SB were volume irradiated to 40 Gy (RBE) (V40) <30% and V40 less than 300 cm3, and kidney was volume irradiated to 18 Gy (RBE) <66%. Bladder and rectal doses were as low as reasonably achievable.
Treatment plan target coverage robustness was evaluated with respect to patient setup and HU uncertainties by forward-calculating the dose distribution for the PBS plan with 5 mm isocenter shifts introduced in the anterior-posterior, cranio-caudal, and lateral directions combined with 3.5% range uncertainties (a total of 12 perturbations per patient) on the planning CT. The worst-case scenario for CTV coverage, D98 and D95 dose-volume histogram (DVH) indicators, was evaluated for each plan to assess the target coverage robustness relative to the setup variations. The plans were considered robust if the worst-case CTV coverage DVH indicators were within 3% of the nominal planned coverage.
Image guidance during treatment for patients undergoing proton therapy was achieved through daily orthogonal x-ray imaging and weekly or biweekly CT scans for treatment verification. For patients receiving IMRT, daily orthogonal kilovoltage imaging was used with alignment to bony anatomy followed by cone beam CT to ensure a full bladder and that the vaginal CTV was within the PTV.
Toxicity
Toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 4. Toxicity assessments were completed before initiation of RT, weekly during RT, and within 90 days of treatment initiation. Hematologic, GI, genitourinary, gynecologic, and neurologic acute toxicities were recorded.
Statistics
Statistical analysis was performed using STATA Version 13.1, (StataCorp, College Station, TX). The Wilcoxon rank sum test was used to compare IMRT and PBS extended-field RT dosimetric differences for OARs. Lymph node and brachytherapy boost doses were not included in the dose-volume comparisons to ensure dose and field homogeneity in comparison arms. Differences were considered statistically significant when P < .05. All P values were obtained using 2-sided tests. Average DVHs for OARs were generated to compare IMRT and PBS extended-field RT plans as previously described by Thompson [12], and statistically significant differences were identified. Logistic and linear regression models were used to test for association between dosimetric variables and presence of toxicities.
Results
Dosimetric Comparison between IMRT and PBS Organs at Risk
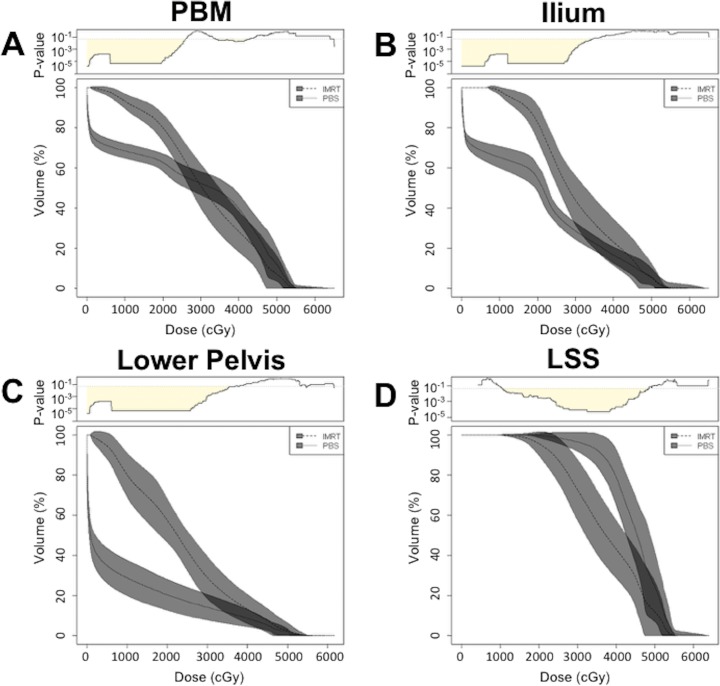
Use of PBS resulted in lower PBM V10 and V20 compared with IMRT (Table 2). The PBM median V10 was 71.3% for PBS (range, 54.0% to 76.3%) compared with IMRT 93.4% (range, 87.4% to 100%) (P < .001), representing a 22% volume reduction at V10. The median PBM V20 was 65.1% for PBS (range, 57.2% to 68.8%) compared with IMRT 79.4% (range, 66.4% to 94.7%) (P < .001), representing a 14% volume reduction at V20. Comparing DVH averages between the 2 RT techniques, PBS resulted in significantly lower PBM doses at all dose points from 0–26.0 Gy, and significantly higher doses compared with IMRT from 33.9 to 42.9 Gy (Figure 1A). Median PBM V40 was 41.6% for PBS (range, 26.1% to 47.6%) compared with IMRT 27.0% (range, 11.1% to 48.2%) (P = .03). Grade 3 or greater hematologic toxicity was not significantly associated with increased PBM V10, V20, or V40 (P = .27, P = .16, and P = .14 respectively).
Table 2.
Comparison between PBS and IMRT patient plans for median dose and range in PBM and component parts: ilium, lower pelvis, LSS. Percent change indicates percent change in median dose volumes from IMRT to PBS. P values were determined by Wilcoxon rank sum test.
|
IMRT median in % (range) |
PBS median in % (range) |
% change (IMRT to PBS) |
P
value |
|
| PBM | ||||
| V10 | 93.4 (87.4 to 100) | 71.3 (54.0 to 76.3) | –22% | <.001 |
| V20 | 79.4 (66.4 to 94.7) | 65.1 (57.2 to 68.8) | –14% | <.001 |
| Ilium | ||||
| V10 | 98.1 (92.4 to 100) | 67.9 (56.4 to 81.6) | –30% | <.001 |
| V20 | 81.8 (67.8 to 98.7) | 59.0 (45.1 to 66.8) | –23% | <.001 |
| Lower pelvis | ||||
| V10 | 80.7 (62.7 to 100) | 32.0 (20.3 to 42.9) | –49% | <.001 |
| V20 | 54.8 (40.6 to 84.6) | 23.1 (13.1 to 33.1) | –32% | <.001 |
| LSS | ||||
| V10 | 100 (99.5 to 100) | 100 (100 to 100) | 0% | .09 |
| V20 | 98.4 (78.1 to 100) | 100 (97.2 to 100) | +2% | .005 |
Abbreviations: PBS, pencil beam scanning; IMRT, intensity-modulated radiation therapy; PBM, pelvic bone marrow; LSS, lumbosacral spine; V10, volume irradiated to 10 Gy (RBE); V20, volume irradiated to 20 Gy (RBE).
Figure 1.
Dose-volume histogram comparison between pencil beam scanning (PBS) and intensity-modulated radiation therapy (IMRT) for (A) pelvic bone marrow, (B) ilium, (C) lower pelvis, and (D) lumbosacral spine. For PBS, cGy was calculated using relative biological effectiveness 1.1. Top: continuous corresponding P values calculated using Wilcoxon rank-sum test. Shading under P = .05 represents doses for which there is a statistically significant difference between PBS and IMRT. Gray bands represent standard deviation.
Compared with IMRT, PBS resulted in reduced dose to ilium and lower pelvis but higher dose to LSS (Table 2). Ilium median V10 for PBS was 67.9% (range, 56.4% to 81.6%) compared with IMRT 98.1% (range, 92.4% to 100%) (P < .001). The median V20 for PBS was 59.0% (range, 45.1% to 66.8%) compared with IMRT 81.8% (range, 67.8% to 98.7%) (P < .001). Compared with IMRT, PBS resulted in 30% and 23% volume reduction at the ilium V10 and V20, respectively. The DVH comparisons demonstrated that PBS resulted in statistically significant (P < .05) lower doses at the ilium compared with IMRT from 0 to 34.5 Gy (Figure 1B).
At the lower pelvis, median V10 for PBS was 32.0% (range, 20.3% to 42.9%) compared with 80.7% for IMRT (range, 62.7% to 100%) (P < .001). The median V20 for PBS was 23.1% (range, 13.1% to 33.1%) compared with IMRT 54.8% (range, 40.6% to 84.6%) (P < .001). Compared with IMRT, PBS resulted in 49% and 32% volume reduction at the lower pelvis V10 and V20, respectively. DVH averages for the lower pelvis demonstrated that PBS resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 37.0 Gy (Figure 1C)
The LSS median V10 for PBS was 100% (range, 100% to 100%) compared with IMRT 100% (range, 99.5% to 100%) (P = .09). The median V20 for PBS was 100% (range, 97.2% to 100%) compared with IMRT 98.4% (range, 78.1% to 100%) (P = .005), representing a 2% volume increase. Average DVH data for LSS demonstrated that PBS resulted in statistically significant (P < .05) higher doses than IMRT from 10.4 to 48.5 Gy (Figure 1D). Grade 3 or greater hematologic toxicity was not significantly associated with increased LSS V10 or V20 (P = .83, P = .58, respectively).
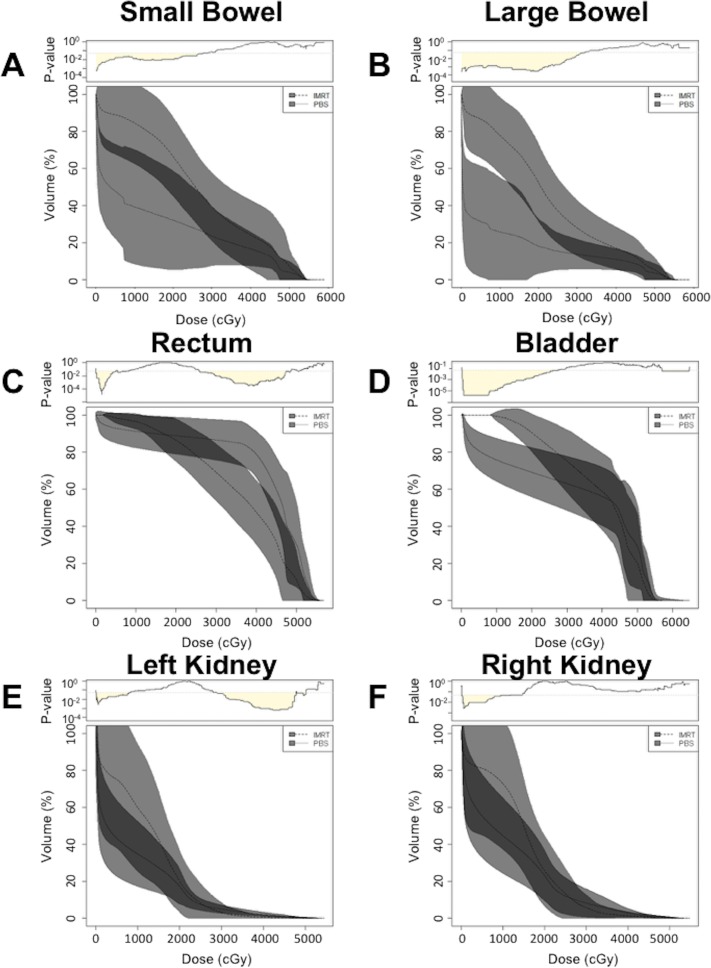
We next compared SB and LB dosimetric parameters between the 2 treatment modalities. In percent volume, PBS resulted in median SB V40 of 20.7% (range, 12.0% to 27.3%) compared with 19.1% (range, 3.3% to 88.8%) for IMRT (P = .81). In absolute volume, PBS resulted in median SB V40 of 155.0 cm3 (range, 46.2-331.0 cm3) compared with 139.8 cm3 (range, 17.4-336.2 cm3) for IMRT (P = .72). For PBS, one plan did not meet the V40 <300 cm3 dose constraint for SB, but all plans met the V40 <30% dose constraint. In contrast, for IMRT, one plan did not meet the V40 <300 cm3 dose constraint and 5 plans did not meet the V40 <30% dose constraint. Comparing average SB DVHs for PBS and IMRT, PBS resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 27.5 Gy (Figure 2A). In percent volume, PBS resulted in median LB V40 15.0% (range, 7.9% to 18.5%) compared with 16.5% (range, 2.9% to 52.5%) for IMRT (P = .51). In absolute volume, PBS resulted in median LB V40 of 87.4 cm3 (range, 60.0-184.9 cm3) compared with 139.0 cm3 (range, 17.3-752.1 cm3) for IMRT (P = .20). All PBS plans met absolute volume and percent dose constraints for LB V40, while one IMRT plan exceeded V40 <300 cm3 and 2 plans exceeded V40 <30% dose constraints. Average LB DVH comparisons between PBS and IMRT resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 31.6 Gy (Figure 2B).
Figure 2.
Dose-volume histogram comparison between pencil beam scanning (PBS) and intensity-modulated radiation therapy (IMRT) for (A) small bowel, (B) large bowel, (C) rectum, (D) bladder, (E) left kidney, and (F) right kidney. For PBS, cGy was calculated using relative biological effectiveness 1.1. Top: continuous corresponding P values were calculated using Wilcoxon rank-sum test. Shading under P = .05 represents doses for which there is a statistically significant difference between PBS and IMRT. Gray bands represent standard deviation.
We also compared DVHs for the rectum, bladder, and right and left kidneys. For the rectum, PBS resulted in statistically significant (P < .05) lower doses from 0 to 7.6 Gy and significantly (P < .05) higher doses from 26.0 to 48.4 Gy compared with IMRT (Figure 2C). For the bladder, PBS resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 27.3 Gy (Figure 2D). The DVH averages for the left kidney demonstrated that PBS resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 8.0 Gy and higher doses compared with IMRT from 28.9 to 47.8 Gy (Figure 2E). The DVH averages for the right kidney demonstrated that PBS resulted in statistically significant (P < .05) lower doses compared with IMRT from 0 to 11.8 Gy (Figure 2F).
The PBS plans were found to be robust with respect to 5-mm isocenter shifts combined with 3.5% range shifts. All but one patient met the coverage robustness criteria described previously. For the one patient whose worst case D98 for the CTV was >3% less than planned coverage, it was only the worst case DVH (1 of the 12 calculated perturbations) that differed by >3%, and the worst case D95 for the same patient was within 1% of the planned D95. The PBS plan target coverage robustness analysis showed that the worst case scenario of D98 for the CTV among the patients differed by 1.6% (range, 0.5% to 3.5%) from the nominal D98 and the D95 differed 1.0% (range, 0.5% to 2.0%) from the nominal D95.
Acute Toxicities
Acute hematologic and nonhematologic toxicities are described in Table 3. The only observed toxicity of grade 3 or higher was in 2 (11%) IMRT patients receiving either sandwich carboplatin/paclitaxel or sandwich carboplatin/paclitaxel with concurrent cisplatin. No patients had grade 3 or higher GI toxicities; grade 1 or 2 acute GI toxicities were nearly universal among both PBS and IMRT patients (94% to 100%) and included increased stool frequency, abdominal pain, nausea, diarrhea, constipation, or rectal mucositis. No patients had grade 3 or higher GU toxicities. Grade 1 or 2 GU toxicities were common and occurred in 10 (56%) PBS and 4 (57%) IMRT patients; toxicities included dysuria, microscopic hematuria, urinary frequency or urgency, or urinary retention. No patients had grade 3 or higher gynecologic toxicities; grade 1 or 2 gynecologic toxicities occurred in 4 (22%) IMRT patients and 3 (43%) PBS patients with vaginal dryness, discharge, bleeding, or hot flashes. Grade 1 or 2 neurologic toxicities occurred in 22% of IMRT and 43% of PBS patients and were related to peripheral sensory neuropathy.
Table 3.
Acute hematologic and nonhematologic toxicities tabulated by number of patients per grade.
|
No. (%) of proton patients |
No. (%) of IMRT patients |
|||||
|
Grade 1 to 2 |
Grade 3 |
Grade 4 |
Grade 1 to 2 |
Grade 3 |
Grade 4 |
|
| Hematologic | 7 (100%) | 0 (0%) | 0 (0%) | 11 (61%) | 2 (11%) | 0 (0%) |
| Gastrointestinal | 7 (100%) | 0 (0%) | 0 (0%) | 17 (94%) | 0 (0%) | 0 (0%) |
| Genitourinary | 4 (57%) | 0 (0%) | 0 (0%) | 10 (56%) | 0 (0%) | 0 (0%) |
| Gynecologic | 3 (43%) | 0 (0%) | 0 (0%) | 4 (22%) | 0 (0%) | 0 (0%) |
| Neurologic | 3 (43%) | 0 (0%) | 0 (0%) | 4 (22%) | 0 (0%) | 0 (0%) |
Abbreviation: IMRT, intensity-modulated radiation therapy.
Discussion
The purpose of this study was to report our initial clinical experience with proton therapy for pelvic and PALN RT for locally advanced endometrial malignancies and to dosimetrically compare IMRT and PBS. Prior dosimetric data suggested that proton therapy may reduce OAR dosing, particularly the PBM, SB, and LB, which may correlate with decreased hematologic toxicity and enable increased tolerance of chemoradiotherapy and dose escalation, particularly in the setting of gross disease. The results from this study suggest that PBS reduces dose relative to IMRT for the PBM, SB, LB, bladder, and kidneys.
Compared with IMRT, PBS resulted in lower PBM DVHs at low dose regions (0-26.0 Gy) (Figure 1A). These findings are consistent with those of prior dosimetric studies for proton therapy, which demonstrated lower PBM dose compared with IMRT in this dose region [7, 8]. The PBM, which accounts for up to 50% of total body bone marrow by weight [13], is significantly impacted by pelvic irradiation due to its anatomical proximity to the RT target. Prior studies support V10 and V20 as significant dosimetric parameters for hematologic toxicity in patients receiving chemotherapy and pelvic RT for locally advanced cervical and anal cancers [11, 14, 15]. Rose et al [14] recommend dose constraints of PBM V10 <95% and V20 <76% to reduce grade 3 or higher hematologic toxicities. In this study, the PBS median PBM V10 was 71.3%—and V20 was 65.1%—well below the recommended dose constraints. In contrast, IMRT plans marginally met the V10 and were unable to meet the V20 dose constraints (median V10 93.4% and V20 79.4%). Compared with IMRT, PBS resulted in 14% to 22% volume reduction at V10 and V20.
At higher dose regions between 33.9 and 42.9 Gy, PBS resulted in statistically significant higher doses to the PBM compared with IMRT, with PBS V40 of 41.6% compared with IMRT V40 of 27.0% (P = .03). We did not observe increased grade 3 or higher hematologic toxicities despite RTOG 0418 findings suggesting that V40 >37% is associated with greater hematologic toxicities. Unlike the patients in RTOG 0418, however, our patients did not have cervical cancer nor did they receive chemoradiotherapy [16]. Thus, our results demonstrate that PBS was superior to IMRT at meeting existing V10 and V20 PBM parameters. Despite increased volume of high dose deposition in PBS, we did not observe increased hematologic toxicities, although only 7 PBS patients were evaluated and studies with more patients are needed to validate these findings.
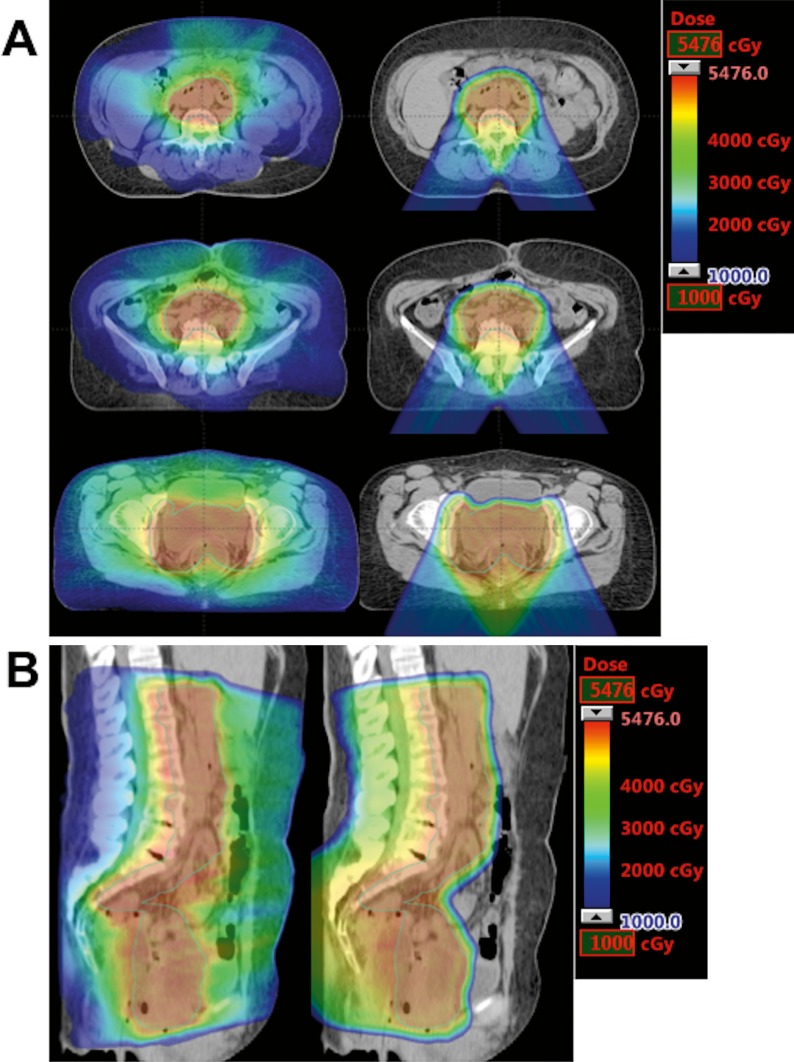
Breaking down PBM into its component categories, PBS outperformed IMRT in the ilium and lower pelvis but was similar to IMRT at the LSS. In the ilium and lower pelvis, PBS resulted in 23% to 49% volume reduction relative to IMRT plans at V10 and V20. This was consistent with our previous study comparing PBS and IMRT for PALN irradiation, which found statistically significant dose reduction of proton therapy at the ilium and ischium and no statistically significant changes at the sacrum (lumbar spine analysis was not performed) [8]. Increased LSS dose is expected in PBS given that PALN stations are in close proximity to the lumbar spine and the posterior oblique beam approach is more likely to distribute dose across the vertebrae compared with a multi-beam IMRT approach (Figure 3). The hematologic significance of increased LSS dose in PBS, however, is uncertain because previously described PBM dose constraints do not distinguish between component parts [11, 14, 15]. Nevertheless, increased lower pelvis and LSS dose has been associated with increased hematologic toxicity, and efforts should be made to reduce dose to the PBM whenever possible [15]. Additional studies to evaluate LSS and lower pelvis contribution to hematologic toxicity in both photon and proton approaches, as well as the use of intensity-modulated proton therapy to further increase dose conformality, may be needed to determine the best approach to PALN coverage in patients with locally advanced gynecologic malignancies.
Figure 3.
Comparison of dose distribution between intensity-modulated radiation therapy plans (left) and pencil beam scanning plans (right) in axial (A) and sagittal (B) planes.
Anterior structures, such as the SB and LB, also benefited from significantly lower doses in PBS plans. Although no significant differences were observed between SB and LB V40 parameters and the majority of plans were able to meet predefined dose constraints, comparisons of overall DVHs demonstrated decreased volumes receiving low doses (0-27.5 Gy in SB and 0-31.6 Gy in LB) in favor of proton therapy. While the patients in this analysis were mainly treated to doses of 45 to 50 Gy, and therefore below the dose tolerance of the SB and LB, dose escalation in the setting of gross nodal disease is frequently performed and has been previously reported to result in severe GI toxicities in the IMRT era [17, 18]. As a result, reducing dose to the SB and LB, especially the duodenum, continues to be an important clinical priority.
Posterior and inferior OAR structures, such as the rectum, did not benefit as significantly, likely due to issues with beam orientation. Use of PBS resulted in lower rectum dose volumes from 0 to 7.6 Gy and was found to deliver higher dose volumes from 26.0 to 48.4 Gy. Despite the increased dose relative to IMRT, both techniques were able to meet the dose constraint guidelines of V50 <50%, and no grade 3 or higher GI toxicities were noted in the PBS group [19].
Both IMRT and PBS to the pelvis and PALN were generally well tolerated. No patients experienced grade 3 or higher nonhematologic acute toxicities, and no PBS patients experienced grade 3 or higher hematologic toxicities. Although 2 IMRT patients (11%) were found to have grade 3 hematologic toxicities, this is improved compared with previously reported rates of hematologic toxicity for extended-field RT with concurrent chemotherapy, which range from 15% to 46% [3, 5]. Overall, toxicity comparisons between the PBS and IMRT cohort are limited due to differences in chemotherapy regimens. The PBS patients received carboplatin and paclitaxel, either as sandwich chemotherapy or sequentially, whereas IMRT patients tended to have more heterogeneous chemotherapy combinations and sequencing, with 2 patients receiving no chemotherapy. Therefore, more studies with larger patient populations are needed to better characterize the toxicities in extended-field proton therapy treatment.
Although our study investigated a homogeneous population of patients treated for endometrial carcinoma, this study is limited by small sample size and heterogeneous chemotherapy treatment regimens. More patients with homogeneous chemotherapy regimens and treatment schedules are needed to better assess toxicity data and ascertain the relative contribution to hematologic toxicity of RT doses to the ilium, lower pelvis, and LSS versus total PBM. This dosimetric analysis also only applies to a subset of proton therapy techniques; the use of intensity-modulated proton therapy may enable further OAR dose reduction that is not reflected in this analysis. Despite these limitations, however, our study builds on our previous dosimetric analysis that compared IMRT versus passive scattering proton therapy versus intensity-modulated proton therapy for treatment of the para-aortic region matched to a pelvic IMRT plan [8]. This study is the first study that we are aware of to report on the clinical feasibility and toxicities associated with pelvis and para-aortic radiotherapy for endometrial carcinoma.
Conclusion
Our early clinical experience demonstrates the feasibility of PBS proton therapy for women requiring pelvic and PALN RT for endometrial carcinoma. We demonstrate statistically significant dose reduction to the PBM, its component parts (ilium and lower pelvis), SB, and LB using PBS with favorable GI and hematologic toxicity profiles compared with IMRT.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Barillot I, Tavernier E, Peignaux K, Williaume D, Nickers P, Leblanc-Onfroy M, Lerouge D. Impact of post operative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: results of the phase II RTCMIENDOMETRE French multicentre trial. Radiother Oncol. 2014;111:138–43. doi: 10.1016/j.radonc.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Poorvu PD, Sadow CA, Townamchai K, Damato AL, Viswanathan AN. Duodenal and other gastrointestinal toxicity in cervical and endometrial cancer treated with extended-field intensity modulated radiation therapy to paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2013;85:1262–8. doi: 10.1016/j.ijrobp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Salama JK, Mundt AJ, Roeske J, Mehta N. Preliminary outcome and toxicity report of extended-field, intensity-modulated radiation therapy for gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2006;65:1170–6. doi: 10.1016/j.ijrobp.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Chen CC, Wang L, Lu CH, Lin JC, Jan JS. Comparison of clinical outcomes and toxicity in endometrial cancer patients treated with adjuvant intensity-modulated radiation therapy or conventional radiotherapy. J Formos Med Assoc. 2014;113:949–55. doi: 10.1016/j.jfma.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Sood BM, Timmins PF, Gorla GR, Garg M, Anderson PS, Vikram B, Goldberg GL. Concomitant cisplatin and extended field radiation therapy in patients with cervical and endometrial cancer. Int J Gynecol Cancer. 2002;12:459–64. doi: 10.1046/j.1525-1438.2002.01172.x. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich A, Bernard L, Ramanakumar AV, Stroian G, Gotlieb WH, Lau S, Bahoric B. Para-aortic and pelvic extended-field radiotherapy for advanced-stage uterine cancer: dosimetric and toxicity comparison between the four-field box and intensity-modulated techniques. Curr Oncol. 2015;22:405–11. doi: 10.3747/co.22.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin LL, Kirk M, Scholey J, Taku N, Kiely JB, White B, Both S. Initial report of pencil beam scanning proton therapy for posthysterectomy patients with gynecologic cancer. Int J Radiat Oncol Biol Phys. 2016;95:181–9. doi: 10.1016/j.ijrobp.2015.07.2205. [DOI] [PubMed] [Google Scholar]
- 8.Milby AB, Both S, Ingram M, Lin LL. Dosimetric comparison of combined intensity-modulated radiotherapy (IMRT) and proton therapy versus IMRT alone for pelvic and para-aortic radiotherapy in gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2012;82:e477–84. doi: 10.1016/j.ijrobp.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Kabolizadeh P, Fulay S, Beriwal S. Are radiation therapy oncology group para-aortic contouring guidelines for pancreatic neoplasm applicable to other malignancies—assessment of nodal distribution in gynecological malignancies. Int J Radiat Oncol Biol Phys. 2013;87:106–10. doi: 10.1016/j.ijrobp.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Small W, Jr, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, Jhingran A, Portelance L, Schefter T, Iyer R, Varia M, Winter K, Mundt AJ. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428–34. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mell LK, Tiryaki H, Ahn KH, Mundt AJ, Roeske JC, Aydogan B. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1504–10. doi: 10.1016/j.ijrobp.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RF. RadOnc: an R package for analysis of dose-volume histogram and three-dimensional structural data. J Radiat Oncol Inform. 2014;6:98–110. [Google Scholar]
- 13.Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–8. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 14.Rose BS, Aydogan B, Liang Y, Yeginer M, Hasselle MD, Dandekar V, Bafana R, Yashar CM, Mundt AJ, Roeske JC, Mell LK. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:800–7. doi: 10.1016/j.ijrobp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, Hurteau JA, Collins YC, Lengyel E, Mundt AJ. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–65. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Klopp AH, Moughan J, Portelance L, Miller BE, Salehpour MR, Hildebrandt E, Nuanjing J, D'Souza D, Souhami L, Small W, Jr, Gaur R, Jhingran A. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90. doi: 10.1016/j.ijrobp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirvani SM, Klopp AH, Likhacheva A, Jhingran A, Soliman PT, Lu KH, Eifel PJ. Intensity modulated radiation therapy for definitive treatment of paraortic relapse in patients with endometrial cancer. Pract Radiat Oncol. 2013;3:e21–8. doi: 10.1016/j.prro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanic S, Mayadev JS. Tolerance of the small bowel to therapeutic irradiation: a focus on late toxicity in patients receiving para-aortic nodal irradiation for gynecologic malignancies. Int J Gynecol Cancer. 2013;23:592–7. doi: 10.1097/IGC.0b013e318286aa68. [DOI] [PubMed] [Google Scholar]
- 19.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]