Abstract
Purpose:
To report prostate cancer outcomes, toxicity, and quality of life (QOL) in men treated with proton beam therapy (PBT).
Patients and Methods:
Patients were enrolled in a prospective trial. All participants received 75.6 to 78 Gy (RBE). Up to 6 months of luteinizing hormone-releasing hormone agonist therapy was allowed. The Phoenix definition defined biochemical failure. Modified Radiation Therapy Oncology Group criteria defined toxicity. Expanded Prostate Cancer Index Composite questionnaires objectified QOL. Clinically significant QOL decrement was defined as ≥0.5 × baseline standard deviation.
Results:
In total, 423 men were analyzed. The National Comprehensive Cancer Network risk classification was used (low 43%; intermediate 56%; high 1%). At the 5.2-year median follow-up, overall and disease-specific survival rates were 99.8% and 100%, respectively. Cumulative biochemical failure rate was 5.2% (95% confidence interval [CI] = 3.0%-8.3%); acute grade 2 genitourinary (GU) toxicity was 46.3%; acute grade 2 gastrointestinal (GI) toxicity was 5.0% (95% CI = 3.1%-7.3%). There was no acute grade ≥3 GI or GU toxicity. Cumulative late grade 2 GU and GI toxicity was 15.9% (95% CI = 13%-20%) and 9.7% (95% CI = 6.5%-12%), respectively. There were 2 grade 3 late GI toxicities (rectal bleeding) and no late grade ≥3 GU toxicity. The 4-year mean Expanded Prostate Cancer Index Composite urinary, bowel, sexual, and hormonal summary scores (range; standard deviation) were 89.7 (43.8-100; 11), 91.3 (41.1-94.6; 10), 57.8 (0.0-96.2; 27.1), and 92.2 (25-95.5; 10.5), respectively. Compared with baseline, there was no clinically significant decrement in urinary, sexual, or hormonal QOL after treatment completion. A modest (<10 points), yet clinically significant, decrement in bowel QOL was appreciated throughout follow-up.
Conclusion:
Contemporary PBT resulted in excellent biochemical control, minimal risk of higher-grade toxicity, and modest QOL decrement. Further investigation comparing PBT with alternative prostate cancer treatment strategies are warranted.
Keywords: proton therapy; particle therapy; intensity-modulated proton therapy, prostate cancer, Expanded Prostate Cancer Index Composite
Introduction
Although proton beam therapy (PBT) has been used as curative intent prostate cancer treatment for more than 40 years [1], the application of this technology has been relatively limited due to the paucity of proton therapy centers. Distinct physical properties afford PBT radiation dose deposition advantages to nontarget anatomy while facilitating dose escalation to intended treatment targets compared with alternative forms of external beam radiation [2, 3]. Recent advancements in PBT delivery systems, such as intensity-modulated proton therapy (IMPT) and image-guided treatment systems, represent further evolution of this technology and a resultant burgeoning of interest among patients and practitioners. Despite excellent outcomes with extended follow-up in men treated with PBT alone [4–6] or in combination with alternative forms of external radiation [7–10], the routine application of PBT for prostate cancer has remained controversial [11–14].
In 2006, our institution opened the third clinically active proton center in North America with the goal of defining the role of PBT for the treatment of neoplastic diseases. Institutional protocol 2005-0956 is a single-arm prospective registry trial designed to estimate survival, cancer control, toxicity, and patient-reported quality of life (QOL) in men receiving contemporary dose-escalated PBT for localized prostate cancer. Results from this trial are presented here.
Patients and Methods
Patients
Patients were enrolled on an institutional review board approved, prospective QOL trial at a single tertiary cancer center from 2006 to 2012. All patients provided written informed consent for participation. Men with previously untreated, nonmetastatic prostate cancer were eligible. The study cohort for this analysis consists of the subset of registered patients with at least 4 years of follow-up.
Data Collection and Follow-up
The combined SF-12-AUA-IPSS-Expanded Prostate Cancer Index Composite questionnaire (EPIC-50) was administered before any treatment, at the conclusion of PBT, and at each follow-up evaluation. Follow-up evaluations were conducted every 3 to 6 months for the first year and every 6 to 12 months thereafter. Gastrointestinal (GI) and genitourinary (GU) toxicity was scored using modified Radiation Therapy Oncology Group (RTOG) toxicity criteria (see Supplemental Material (6.7MB, zip) ). Toxicity was assessed/graded by the treating physician, reviewed by the clinically trained research nursing core, and verified for accuracy/attribution by the principal investigator. Events occurring within 6 months of PBT initiation were recorded as acute, and any event persisting or occurring thereafter was recorded as late. Reported toxicity between defined follow-up visits was also captured and recorded. As the role of short-course androgen-deprivation therapy combined with high-dose radiation therapy was controversial at the time of trial activation, inclusion of a luteinizing hormone-releasing hormone agonist up to a total of 6 months was allowed at the discretion of the treating physician. When utilized, androgen deprivation was scheduled neoadjuvantly (2-3 months), concurrently, and adjuvantly with PBT.
Treatment Planning Technique
All patients underwent computed tomography simulation including ultrasound bladder volume quantification and conventional leg and thigh immobilization for pelvic radiotherapy. An endo-rectal balloon for rectal volume standardization and prostate immobilization during treatment was applied in the vast majority. Kilovoltage x-ray verification facilitated image-guided radiation therapy before each treatment. Although there was a transition toward utilization of intra-prostatic fiducial markers during the treatment interval for this cohort, image-guided radiation therapy verification primarily utilized pelvic bone anatomy. Choice of PBT delivery system, either passively scattered proton therapy (PSPT) or IMPT, was at the discretion of the treating physician.
Both PSPT and IMPT consisted of opposed right and left lateral beam arrangements with incident proton beam energies typically from 150 to 225 MeV. Both fields were treated daily. The clinical target volume (CTV) was generally customized according to National Comprehensive Cancer Network risk stratification as follows: low risk (prostate only), intermediate-risk (prostate + proximal seminal vesicle), and high risk (prostate + full seminal vesicle). For PSPT, an evaluation target volume was applied as a 6-mm radial expansion of the CTV except posteriorly; where the margin was limited to 4 to 5 mm. Proximal and distal margins were typically 9 to 12 mm based on the formula derived by Moyers et al [15]. For IMPT, a scanning target volume margin was applied as follows: 12 mm laterally and 6 mm in all other dimensions, except 4 to 5 mm posteriorly to the CTV. The IMPT treatment-planning technique and robustness have been described previously [16–20]. The total prescribed dose was 75.6 to 78 Gy (RBE), delivered in equivalent 1.8 to 2 Gy (RBE) fractions. The relative biological effectiveness correction factor for physical to biological dose was 1.1. Treatment was designed to cover 100% of the CTV and >95% of the scanning target volume/evaluation target volume. The intended total dose was prescribed to an isodose line above 95% for optimal homogeneity.
Statistical Analysis
The methods of Gooley et al [21] were used to estimate the cumulative incidence of acute and late lower GI and GU toxicity, considering death as a competing event. We similarly analyzed cumulative incidence of argon plasma coagulation (APC) and time to prostate-specific antigen (PSA) failure as defined by the Phoenix definition of biochemical failure following radiation therapy (PSA nadir + 2 ng/mL). Ninety-five percent confidence intervals (95% CIs) were calculated for relevant event and patient-reported QOL outcomes.
The methods of Fine and Gray [22] were used to model potential risk factors for cumulative incidence of toxicity, considering death as a competing event in a univariate fashion. We then included all factors with a P value < .25 in a saturated model and used backward elimination to remove factors until all remaining factors were significant at the .05 level. Wilcoxon rank sum test was used to compare groups over continuous variables. Fisher's exact test was used to compare groups over categorical variables.
The QOL was evaluated primarily as the change over time from baseline stratified within each treatment group and across EPIC-50 domains of sexual function/bother, urinary incontinence, urinary obstruction/irritation, urinary function/bother, and bowel function/bother. Scores for patient-reported outcomes, as measured by EPIC-50, were calculated according to the instrument instructions [23]. Resulting domain scores range from 0 to 100, with higher values representing more favorable QOL. To assess which QOL domains were affected, we evaluated the time profiles of EPIC-50 scores to assess whether the mean score changes from baseline at each follow-up time point were different using a one-sample t test. Statistical significance was defined as a P value < .05. A change from baseline equal to or greater than half the baseline standard deviation for a domain or subdomain was considered clinically significant [24]. Analyses were performed with the use of SAS 9.3 for Windows (SAS Institute Inc, Cary, North Carolina).
Results
Patients
Table 1 shows baseline demographic and clinical characteristics of the 423 patients included in this analysis.
Table 1.
Demographics and clinical characteristics (N = 423).a, b
|
Characteristic |
No. of Patients (%) |
| Race | |
| White | 389 (92) |
| Black | 14 (3) |
| Hispanic | 10 (2) |
| Asian | 7 (2) |
| Other | 2 (<1) |
| NCCN risk group | |
| Low | 182 (43) |
| Intermediate | 238 (56) |
| High | 3 (1) |
| T-stage | |
| T1b | 1 (<1) |
| T1c | 322 (76) |
| T2a | 66 (16) |
| T2b | 32 (8) |
| T2c | 2 (<1) |
| Gleason score | |
| 6 | 189 (45) |
| 7 | 233 (55) |
| 8 | 1 (<1) |
| % total biopsy cores with cancer | |
| <35 | 305 (72) |
| 35-50 | 65 (15) |
| >50 | 46 (11) |
| Unknown | 7 (2) |
| Hormone therapy | |
| No | 265 (63) |
| Yes | 158 (37) |
| Anticoagulant medications | |
| No | 237 (56) |
| Yes | 186 (44) |
| History of hemorrhoids | |
| No | 265 (63) |
| Yes | 158 (37) |
| Diabetes mellitus | |
| No | 377 (89) |
| Yes | 46 (11) |
| History of vascular disease | |
| No | 374 (88) |
| Yes | 49 (12) |
| History of rectal surgery | |
| No | 393 (93) |
| Yes | 30 (7) |
| Charlson comorbidity score | |
| 0 | 350 (83) |
| 1 | 56 (13) |
| ≥2 | 17 (4) |
| Proton therapy technique | |
| PSPT | 344 (81) |
| IMPT | 79 (19) |
Abbreviations: NCCN, National Comprehensive Cancer Network; PSPT, passively scattered proton therapy; IMPT, intensity modulated proton therapy; PSA, prostate-specific antigen.
Median age at diagnosis was 65 years (range = 45 to 82 years).
Mean pretreatment PSA was 4.9 ng/ml (standard deviation = 2.8).
Survival, Biochemical Control, and Patterns of Failures
At a median follow-up of 5.2 years, there was 1 patient death unrelated to prostate cancer for crude overall and disease-specific survival rates of 99.8% and 100% respectively. The cumulative incidence of biochemical failure was 5.2% (95% CI = 3.0%-8.3%) Biochemical failure over time is represented in Figure 1. At the time of biochemical recurrence, advanced imaging and transrectal ultrasound-guided prostate biopsy were routinely used. Of the 17 observed biochemical failures, 2 local recurrences, 2 regional nodal recurrences, and 2 distant metastases were confirmed. The remaining 11 recurrences were PSA only failures. Twelve patients received salvage therapy. At the time of last contact, 96.9% (crude rate) of patients were alive without evidence of disease.
Figure 1.
Cumulative incidence of prostate-specific antigen (PSA) failure (nadir + 2 ng/mL) in men treated with proton beam therapy for localized prostate cancer.
Toxicity
Observed toxicity by grade is represented in Table 2. Grade 2 GU toxicity was relatively common as events were observed in 196 patients, resulting in a cumulative incidence of 46.3% (95% CI = 42%-51%). Twenty-one men experienced acute grade 2 GI toxicity, resulting in a cumulative incidence of 5.0% (95% CI = 3.1%-7.3%). There was no acute grade ≥3 GI or GU toxicity.
Table 2.
Modified Radiation Therapy Oncology Group toxicity by grade in men treated with proton beam therapy for localized prostate cancer.
|
No. of patients (%) |
|||
|
Grade 2 |
Grade 3 |
Grade 4 |
|
| Acute | |||
| Genitourinary | 196 (46)a | 0 (0) | 0 (0) |
| Lower gastrointestinal | 21 (5) | 0 (0) | 0 (0) |
| Late | |||
| Genitourinary | 68 (16) | 0 (0) | 0 (0) |
| Lower gastrointestinal | 41 (10) | 2 (<1) | 0 (0) |
Initiation or dose escalation of alpha-blocker therapy was categorized as grade 2 genitourinary toxicity.
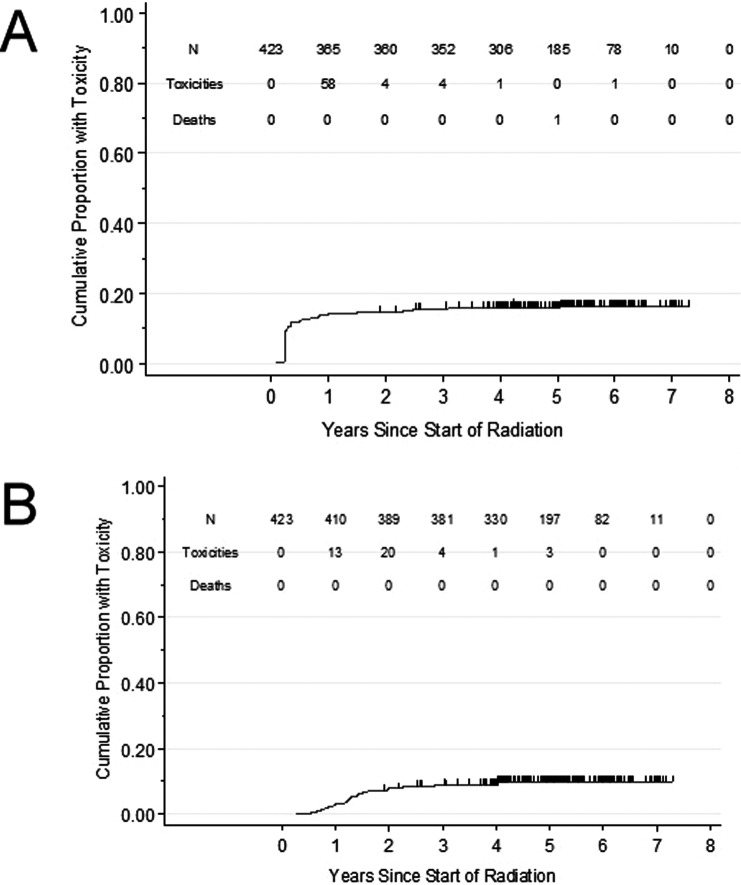
The cumulative incidence of late grade 2 toxicity is graphically represented in Figure 2A and 2B. Sixty-eight men experienced late grade 2 GU toxicity resulting in a cumulative incidence of 15.9% (95% CI = 13-20%). There was no late grade ≥ 3 GU toxicity. The last grade 2 GU toxicity was observed at 5-years. Forty-one men experienced late grade 2 GI toxicity, resulting in a cumulative incidence of 9.7% (6.5%-12%). One man experienced grade 3 late GI toxicity (rectal bleeding; cumulative incidence <1%). There was no late grade 4 toxicity. The last grade ≥ 2 GI toxicity was observed at 4 years. Rectal bleeding was the predominant late GI toxicity with subsequent management qualifying as grade escalation (initiation of medications and/or APC application). Twenty-three men received APC for rectal bleeding for a cumulative incidence of 5.6% (95% CI = 3.7%-8.2%). Both late grade 3 GI toxicities were for multiple APC applications. Management of late effects was noted to be effective as 422 patients (99.5%) had resolution of GI toxicity at last follow-up.
Figure 2.
Cumulative incidence of modified Radiation Therapy Oncology Group (RTOG) grade 2 (A) genitourinary and (B) lower gastrointestinal toxicity in men treated with proton beam therapy for localized prostate cancer.
Quality of Life
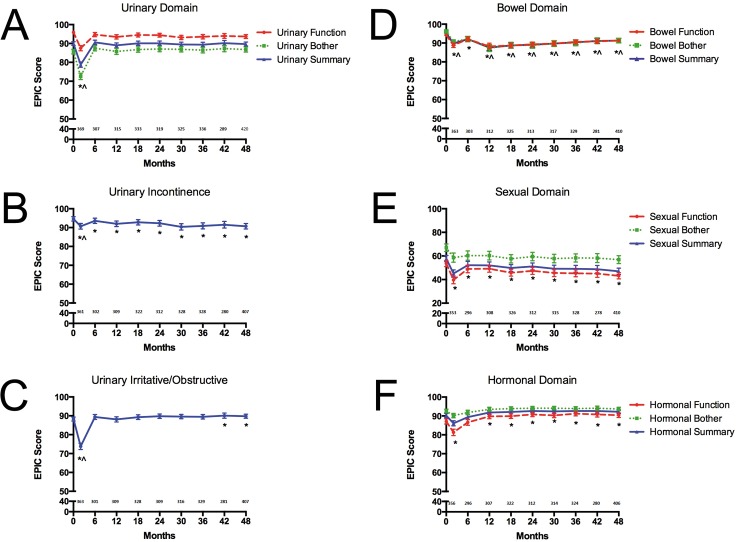
Mean EPIC-50 scores over time across bowel, sexual, hormonal, and urinary domains are illustrated in Figure 3A through 3F. Specific levels of symptom-related distress are characterized in Table 3.
Figure 3.
Patient reported quality of life as measured by the Expanded Prostate Index Composite (A-C) urinary, (D) bowel, (E) sexual, and (F) hormonal domain and subdomain scores in men treated with proton beam therapy for localized prostate cancer (*statistically significant and ^clinically significant decline from baseline).
Table 3.
Percentage of patients reporting various domain-specific levels of distress 4 years after proton beam therapy for localized prostate cancer, as queried by the SF-12-AUA-IPSS-Expanded Prostate Cancer Index Composite survey.
|
No problem |
Very small problem |
Small problem |
Moderate problem |
Big Problem |
Missing |
|
| Urine leaking/dribbling | 68 | 22 | 6 | <1 | <1 | 3 |
| Urine function | 56 | 32 | 7 | 3 | <1 | 1 |
| Bloody stools | 74 | 15 | 6 | 1 | <1 | 3 |
| Bowel function | 65 | 24 | 6 | 2 | 1 | <1 |
| Functional erections | 26 | 19 | 10 | 16 | 27 | 2 |
| Overall sexual function | 28 | 21 | 14 | 16 | 21 | <1 |
Bowel Domain
Mean EPIC baseline bowel summary, function, and bother scores were 95.2 (range = 57.1-96.4; SD = 5.5), 94.2 (57.1-96.4; 6.6), and 96.1 (57.1-100; 6.3), respectively. The 4-year mean EPIC bowel summary, function, and bother scores were 91.3 (41.1-94.6; 10), 91.3 (46.4-92.9; 9.1), and 91.3 (25-96.4; 12.4), respectively. There were statistically significant changes from baseline in bowel QOL which persisted through 4 years of follow-up. The 4-year bowel bother decrement was both statistically and clinically significant from baseline; while the decrement in bowel function was statistically but not clinically significant. Mean bowel domain QOL scores stabilized without subsequent decline after 12 months. Although, the mean bowel summary, function, or bother decrement never exceeded 10 points from baseline at any assessment time point, the bowel summary decrement from baseline met criteria for clinical significance at all time points, with the exception of 6 months. Distress associated with bowel QOL was low, with only 1% and 3% of respondents reporting a “moderate or big” problem from bloody stools or bowel function, respectively, at the 4-year follow-up.
Sexual Domain
There was sexual QOL impairment at baseline with substantial variability within the analyzed cohort. The mean EPIC baseline sexual summary, function, and bother scores were 57.8 (0.0-96.2; 27.1), 53.6 (0.0-94.4; 27.0), and 67.0 (0.0-100; 32.6), respectively. The 4-year mean EPIC sexual summary, function, and bother scores were 47.0 (0.0-98.1; 27.3), 43.2 (0.0-97.2; 27.2), and 56.9 (0.0-100; 34.2) , respectively. There were statistically significant changes from baseline in sexual domain QOL which persisted through 4-year follow-up; however, no sexual function or bother decrement was considered clinically significant at any assessment period. Although an initial decline in sexual function was observed, both sexual function and bother scores remained stable after treatment. Distress from erection function and overall sexual function was considerable at 4 years, with 43% and 37%, respectively, reporting a “moderate or big” problem.
Urinary Domain
Mean EPIC baseline urinary summary, function, and bother scores were 90 (range = 31-100; SD = 9.9), 95.9 (38.2-100; 8.4), and 85.7 (20.8-100; 13.1), respectively. The 4-year mean EPIC urinary summary, function, and bother scores were 89.7 (43.8-100; 11), 93.7 (5-100; 10.6), and 86.8 (14.3-100; 14), respectively. There were statistically significant and clinically significant changes from baseline in mean urinary function and bother at the end of treatment, with return to baseline by 6 months posttreatment and without significant QOL decrement thereafter. On subdomain assessment, a modest decrement in mean urinary incontinence scores was observed, though this was not considered clinically significant. There was no distress from urinary incontinence. Statistically significant and clinically significant urinary irritative/obstructive QOL decrement was observed at treatment conclusion; with subsequent resolution by 6 months. The 4-year mean urinary irritative/obstructive scores were statistically superior to baseline levels; likely reflecting liberal use of selective alpha-blocker therapy. Distress associated with urinary QOL was low, with <1% and 3% of respondents reporting a “moderate or big” problem from urinary dribbling/leaking or urinary function, respectively, at the 4-year follow-up.
Hormonal Domain
The mean EPIC-50 baseline hormonal summary, function, and bother scores were 90.2 (range = 45.5-95.5; SD = 11.4), 87.1 (35-95; 14.2), and 92.6 (45.8-95.8; 10), respectively. Of those patients receiving androgen-deprivation therapy, the median duration was 6 months. The 4-year mean EPIC-50 hormonal summary, function, and bother scores were 92.2 (25-95.5; 10.5), 90.4 (15-95; 12.8), and 93.7 (33.3-100; 9.5), respectively. There was a statistically significant change from baseline in mean hormonal summary QOL at treatment conclusion. No hormonal function or bother decrement was considered clinically significant at any assessment interval.
Discussion
In this analysis of a prospectively assessed cohort of men with localized prostate cancer, PBT resulted in excellent biochemical control, low cumulative incidence of higher grade toxicity, and a clinically modest effect on hormonal, sexual, bowel, and urinary QOL. These results represent one of the most mature prospective analyses of men treated with high-dose, image-guided PBT for localized prostate cancer inclusive of patient-reported outcome measures.
Our findings are both consistent with and additive to previous reports using PBT for localized prostate cancer. Most recently, the University of Florida reported the intermediate-term results of 3 clinical trials using high-dose, image-guided PBT for men at low, intermediate, and high risk of prostate cancer recurrence [4]. Treatments were prescribed as follows: 78 Gy (RBE) (low risk), 78-82 Gy (RBE) (intermediate risk), and 78 Gy (RBE) with concomitant docetaxel followed by 6 months androgen deprivation (high risk). With a median follow-up of 5.2 years in 211 patients, the 5-year rate of biochemical and clinical freedom from progression were 99%, 99%, and 76% in the low, intermediate, and high risk cohorts, respectively. Similar to the current series, higher-grade GI or GU toxicity was rare, with grade 3 or greater events observed in 0.5% and 1%, respectively. The 5.2% cumulative incidence of PSA failure, 100% disease-specific survival, and <1% cumulative incidence of grade ≥3 GI or GU toxicity in our series add further support toward the safety and efficacy of PBT for localized prostate cancer.
In our analysis, patient-reported QOL outcome assessment revealed clinically significant urinary obstructive/irritative decrements at the conclusion of PBT and a modest, yet clinically significant, decrement in bowel QOL which persisted through the 4-year follow-up. These results are also consistent with and additive to previously published patient-reported QOL outcome studies after dose-escalated, image-guided PBT for prostate cancer. The University of Florida reported QOL outcomes using the EPIC questionnaire in 262 men ≤60 years old treated with definitive PSPT with median 2-year follow-up [25]. The change in median urinary, bowel, and sexual summary scores from baseline at 24 months were −3.1, −4.8, and −12.6, respectively. Using the conventional definition of 0.5 multiplied by the baseline standard definition, only bowel QOL decrement was clinically significant. In the 3 University of Florida clinical trials mentioned earlier, there was no change in median urinary irritative/obstructive or urinary incontinence from baseline compared with 4+ years of follow-up. However, there was a significant decrement in bowel and sexual summary score. Although we detected clinically significant decrements in bowel QOL which persisted throughout 4-year follow-up, the magnitude of the decrement was modest and calls into question the definition of clinical significance in patient-reported outcomes. Overall, our results support a modest QOL impact following PBT and compare favorably to the University of Florida experience.
The current analysis has some other limitations, the most obvious being that it does not facilitate scientifically valid comparative effectiveness between the multiple management modalities currently presented to men with localized prostate cancer. Although the results presented in this analysis might compare favorably to any intermediate/long-term outcomes following prostate cancer therapy, many have argued that unfavorable comparative cost-effectiveness assessments prohibit routine PBT application [12, 26, 27]. Furthermore, the enrolled cohort possessed relatively favorable prognostic features, had minimal comorbidity, and was predominantly white. This homogeneity limits generalizability of these results. Although the reported toxicity in this cohort was highly favorable compared with historical precedents, physician-reported toxicity is prone to reporting bias and confers an element of subjectivity; particularly for low-grade events. Furthermore, despite multiple years of follow-up for this cohort, late occurring toxicity following high dose radiotherapy remains possible; long-term follow-up is desirable. Despite these limitations, it can be concluded that dose-escalated, image-guided PBT for localized prostate cancer is highly efficacious, with modest QOL impact and minimal potential for high-grade toxicity. Future efforts should focus on defining the long-term value proposition of PBT for localized prostate cancer. Fortunately, randomized data comparing PBT to intensity-modulated radiation therapy is forthcoming, as a multicenter randomized trial comparing intensity-modulated radiation therapy to PBT for men with localized prostate cancer, is in active accrual (NCI registration NCT01617161).
Conclusions
Use of PBT for localized prostate cancer provided excellent intermediate-term biochemical control with minimal risk of grade ≥3 toxicity and without clinically significant decrement in urinary, sexual, or hormonal QOL after treatment completion. A modest, but clinically significant, decrement in bowel QOL was appreciated throughout the 4-year follow-up. Thus, PBT is a safe and highly efficacious treatment for localized prostate cancer. Further investigation comparing PBT to alternative prostate cancer treatment strategies are warranted.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose. This work was presented as an abstract at the 2014 Annual Meeting of the American Society of Radiation Oncology.
Acknowledgments: This research is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant (CA016672).
References
- 1.Shipley WU, Tepper JE, Prout GR, Jr, Verhey LJ, Mendiondo OA, Goitein M, Koehler AM, Suit HD. Proton radiation as boost therapy for localized prostatic carcinoma. JAMA. 1979;241:1912–5. [PubMed] [Google Scholar]
- 2.Trofimov A, Nguyen PL, Coen JJ, Doppke KP, Schneider RJ, Adams JA, Bortfeld TR, Zietman AL, Delaney TF, Shipley WU. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–53. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas C, Fryer A, Mahajan C, Indelicato D, Horne D, Chellini A, McKenzie C, Lawlor P, Henderson R, Li Z, Lin L, Olivier K, Keole S. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–51. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall NP, Hoppe BS, Nichols RC, Mendenhall WM, Morris CG, Li Z, Su Z, Williams CR, Costa J, Henderson RH. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602. doi: 10.1016/j.ijrobp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Slater JD, Rossi CJ, Jr, Yonemoto LT, Bush DA, Jabola BR, Levy RP, Grove RI, Preston W, Slater JM. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Coen JJ, Paly JJ, Niemierko A, Weyman E, Rodrigues A, Shipley WU, Zietman AL, Talcott JA. Long-term quality of life outcome after proton beam monotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:e201–9. doi: 10.1016/j.ijrobp.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, Bush DA, Lunt M, Spiegel DY, Skowronski R, Jabola BR, Rossi CJ. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nihei K, Ogino T, Ishikura S, Kawashima M, Nishimura H, Arahira S, Onozawa M. Phase II feasibility study of high-dose radiotherapy for prostate cancer using proton boost therapy: first clinical trial of proton beam therapy for prostate cancer in Japan. Jpn J Clin Oncol. 2005;35:745–52. doi: 10.1093/jjco/hyi193. [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr, Miller DW, Adams JA, Shipley WU. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 10.Yonemoto LT, Slater JD, Rossi CJ, Jr, Antoine JE, Loredo L, Archambeau JO, Schulte RW, Miller DW, Teichman SL, Slater JM. Combined proton and photon conformal radiation therapy for locally advanced carcinoma of the prostate: preliminary results of a phase I/II study. Int J Radiat Oncol Biol Phys. 1997;37:21–9. doi: 10.1016/s0360-3016(96)00311-2. [DOI] [PubMed] [Google Scholar]
- 11.Hahn C, Kavanagh B, Bhatnagar A, Jacobson G, Lutz S, Patton C, Potters L, Steinberg M. Choosing wisely: the American Society for Radiation Oncology's top 5 list. Pract Radiat Oncol. 2014;4:349–55. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–8. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 13.Suit H, Kooy H, Trofimov A, Farr J, Munzenrider J, DeLaney T, Loeffler J, Clasie B, Safai S, Paganetti H. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother Oncol. 2008;86:148–53. doi: 10.1016/j.radonc.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Terasawa T, Dvorak T, Ip S, Raman G, Lau J, Trikalinos TA. Systematic review: charged-particle radiation therapy for cancer. Ann Intern Med. 2009;151:556–65. doi: 10.7326/0003-4819-151-8-200910200-00145. [DOI] [PubMed] [Google Scholar]
- 15.Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–38. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 16.Meyer J, Bluett J, Amos R, Levy L, Choi S, Nguyen QN, Zhu XR, Gillin M, Lee A. Spot scanning proton beam therapy for prostate cancer: treatment planning technique and analysis of consequences of rotational and translational alignment errors. Int J Radiat Oncol Biol Phys. 2010;78:428–34. doi: 10.1016/j.ijrobp.2009.07.1696. [DOI] [PubMed] [Google Scholar]
- 17.Pugh TJ, Amos RA. John Baptiste S, Choi S, Nhu Nguyen Q, Ronald Zhu X, Palmer MB, Lee AK. Multifield optimization intensity-modulated proton therapy (MFO-IMPT) for prostate cancer: robustness analysis through simulation of rotational and translational alignment errors. Med Dosim. 2013;38:344–50. doi: 10.1016/j.meddos.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu XR, Sahoo N, Zhang X, Robertson D, Li H, Choi S, Lee AK, Gillin MT. Intensity modulated proton therapy treatment planning using single-field optimization: the impact of monitor unit constraints on plan quality. Med Phys. 2010;37:1210–9. doi: 10.1118/1.3314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu XR, Li Y, Mackin D, Li H, Poenisch F, Lee AK, Mahajan A, Frank SJ, Gillin MT, Sahoo N, Zhang X. Towards effective and efficient patient-specific quality assurance for spot scanning proton therapy. Cancers (Basel) 2015;7:631–47. doi: 10.3390/cancers7020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XR, Poenisch F, Song X, Johnson JL, Ciangaru G, Taylor MB, Lii M, Martin C, Arjomandy B, Lee AK, Choi S, Nguyen QN, Gillin MT, Sahoo N. Patient-specific quality assurance for prostate cancer patients receiving spot scanning proton therapy using single-field uniform dose. Int J Radiat Oncol Biol Phys. 2011;81:552–9. doi: 10.1016/j.ijrobp.2010.11.071. [DOI] [PubMed] [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 24.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wei JT. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe BS, Nichols RC, Henderson RH, Morris CG, Williams CR, Costa J, Marcus RB, Jr, Mendenhall WM, Li Z, Mendenhall NP. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer. 2012;118:4619–26. doi: 10.1002/cncr.27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin NP, Sher DJ, Konski AA. Systematic review of the cost effectiveness of radiation therapy for prostate cancer from 2003 to 2013. Appl Health Econ Health Policy. 2014;12:391–408. doi: 10.1007/s40258-014-0106-9. [DOI] [PubMed] [Google Scholar]
- 27.Peeters A, Grutters JP, Pijls-Johannesma M, Reimoser S, De Ruysscher D, Severens JL, Joore MA, Lambin P. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95:45–53. doi: 10.1016/j.radonc.2009.12.002. [DOI] [PubMed] [Google Scholar]