Abstract
After >60 years since the first treatment, particle radiation therapy (RT) is now used to treat various types of tumors worldwide. Particle RT results in favorable outcomes, especially in local control, because of its biological properties and excellent dose distribution. However, similar to other types of cancer treatment, metastasis control is a crucial issue. Notably, immunotherapy is used for cancer treatment with high risk for recurrence and/or metastasis. These 2 cancer therapies could be ideal, complementary partners for noninvasive cancer treatment. In this review, we will focus on preclinical studies combining particle RT, especially carbon ion RT, and immunotherapy.
Keywords: immunotherapy, mouse, radiotherapy, dendritic cell; carbon-ion
Introduction
When compared with conventional photon radiation therapy (RT), carbon ion (C-ion) RT has a higher antitumor effect and is less damaging to normal tissues surrounding the tumor [1–3]. For some difficult cancers, such skull base, sarcoma, and pancreatic cancer, C-ion RT alone or combined with chemotherapy significantly prevented or delayed the development of distant metastasis, with improved survival and local control [1]. However, metastasis and local recurrence are crucial issues for the improvement of the C-ion RT outcomes.
In this review, we discuss the progress of animal models treated with C-ion RT combined with immunotherapy (IT) and future developments of this combination therapy.
Immunotherapy as a Partner of Radiation Therapy
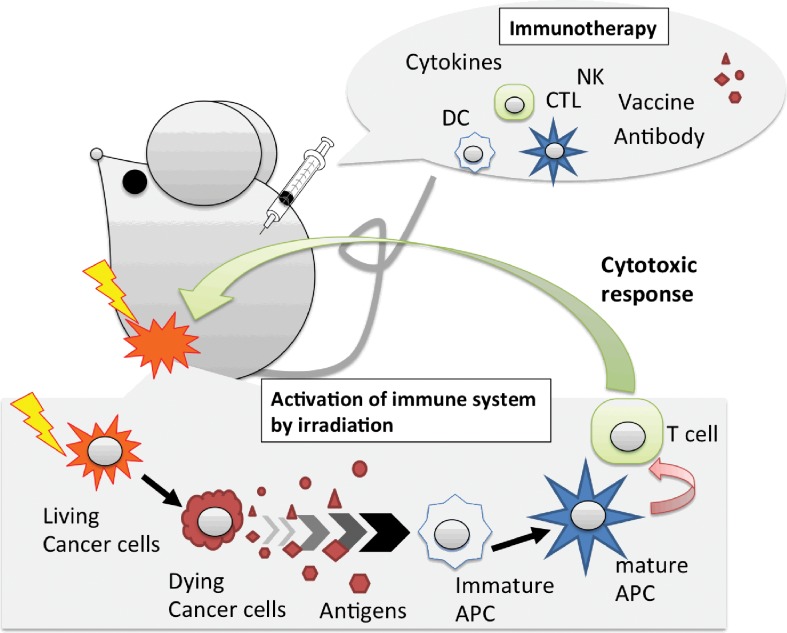
The human immune system has potential to eliminate cancer, as shown by spontaneous regression in some cases. However, the immune system is not able to block tumor growth for several reasons, such as immunosuppression caused by myeloid-derived suppressor cells, regulatory T cells, and cytokines [4–6] during the escape phase of cancer immunoediting [7, 8]. Therefore, IT was designed to boost the natural immune system and eliminate cancer by administering cancer-specific antibodies, cytokines, cancer vaccines, and immune-checkpoint inhibitors, as well as other therapies (Figure 1). In addition, IT is able to attack small tumors that cannot be detected by conventional methods and can retain the ability to kill cancer cells long after treatment [9].
Figure 1.
Radiation-induced immune activation and combination with immunotherapy. Radiation therapy is able to induce cancer cell death, including immunogenic cell death. The dying cells act as a source of tumor antigen to antigen-presenting cells, such as dendritic cells and macrophages. Mature antigen-presenting cells display tumor antigens combined with major histocompatibility complexes and stimulate T cells to become cytotoxic T cells. The activated cytotoxic T cells are expected to attack cancer, including micrometastases and circulating tumor cells. If we find ways to control the mechanisms in any patients, radiation therapy is able to use an inducer of in situ vaccine. For immunotherapy, these key factors, such as immune cells and cytokines, are used for the treatment. Therefore, combination immunotherapy–radiation therapy is expected to enhance the antitumor effects.
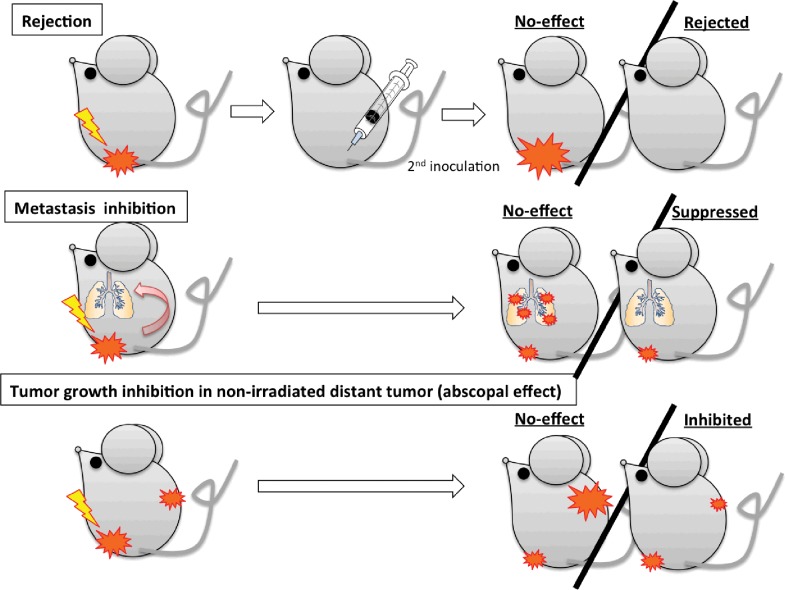
It is thought that most cancer treatments, including RT, involve immune-mediated systems. In particular, RT is believed to possess strong potential in combination with IT. Unlike surgical therapy, irradiated cancer cells die in the body after irradiation. These dead cancer cells may act as tumor antigens and be taken up by phagocytes (Figure 1). In previous reports [10–13], RT- and chemoRT-induced immunogenic cell death is preceded or accompanied by the emission of a series of immunostimulatory, damage-associated molecular patterns in a precise spatiotemporal configuration [14, 15]. The radiation-induced immunogenic cell death might act as an in situ tumor vaccine and is a crucial process to initiate anticancer immune responses [16]. In addition, clinical case reports demonstrated that RT sometimes induced tumor regression of nonirradiated tumors [17–21]. These phenomena, known as the abscopal effect, may be involved in the immune response, secondary to irradiation-induced cancer cell death. If it becomes possible to regulate and/or enhance the RT-induced abscopal effect, we may move a step closer to cancer control. Therefore, an increasing body of basic research combining RT and IT has been carried out in immune-competent animal models [13, 22–40]. These studies can be categorized based on the index of evaluation, as shown Figure 2. There may be a tendency showing that the abscopal effect was enhanced by, or was only functional by, combination treatment with IT. However, even though it does not show a clear abscopal effect, RT combined with IT treatment prevented tumor growth or distant metastasis [41]. In addition, several clinical trials have already been started for combined photon RT (or chemoRT) and IT [42–46].
Figure 2.
Types of evaluation for effectiveness of radiation therapy–induced immune response. If irradiation induces antitumor immune response in the tumor-bearing mouse, evaluation of activation is possible by the following methods: rejection—evaluate rejection rate of secondary tumor inoculation after treatment of the first tumor; metastasis suppression—evaluate number of metastases after treatment; and abscopal effect—evaluate nonirradiated tumor growth after irradiation of another tumor.
Basic Studies on the Combination of Particle Beam and Immunotherapy
Even though particle beams might have several biological advantages for use in combination with IT, basic science studies on the combination are still limited (Table 1).
Table 1.
Immune-competent mouse model experiments combining particle radiation therapy with immunotherapy.
|
Source, y |
Cell line |
Mouse strain |
Radiation therapy |
Immunotherapy |
Immunotherapy administration |
Effect |
| Matsunaga et al, 2010 [47] | SCC VII; FM3A, mammary carcinoma | C3H/He; BALB/c–nude | 290 MeV/n C-ion; 77 keV/μm; <10 Gy/min | BM-derived; DCs (SCC VII lysate treated, then rSeV/dF infected) | Immunotherapy, d 2, 9, 17 after IR | Second tumor rejection |
| Ohkubo et al, 2010 [48] | NR-S1, SCC | C3H/He | 290 MeV/n C-ion; 6 cm SOBP; 6 Gy | BM-derived; α-GalCer–pulsed DCs | Immunotherapy, d 1.5 after IR | Lung metastasis |
Abbreviations: BM, bone marrow; C-ion, carbon ion; DC, dendritic cell; SCC, squamous cell carcinoma; SOBP, spread-out Bragg peak; α-GalCer, α-galactosylceramide.
Mouse studies on the combination of IT with C-ion were published in 2010 by 2 independent groups [47, 48]. Both articles used bone marrow (BM)-derived dendritic cells (DCs) as IT. The DCs were administered by intratumoral injection after irradiation. They are the most potent population of the antigen-presenting cells and the key mediator in generating therapeutic immunity against cancer [49, 50]. Matsunaga et al [47] used 2 experimental models: (1) syngeneic C3H/He mice inoculated with poorly immunogenic squamous cell carcinoma (SCC VII), and (2) mammary carcinoma FM3A cells. Before administration, DCs were activated by infection with recombinant Sendai virus after a coculture with SCC VII lysate as the tumor antigen. These were administered multiple times following irradiation. Researchers showed that C-ion irradiation resulted in tumor elimination, rejection of secondary tumor inoculation, and T-cell activation. These antitumor effects were enhanced by the combination of DC IT.
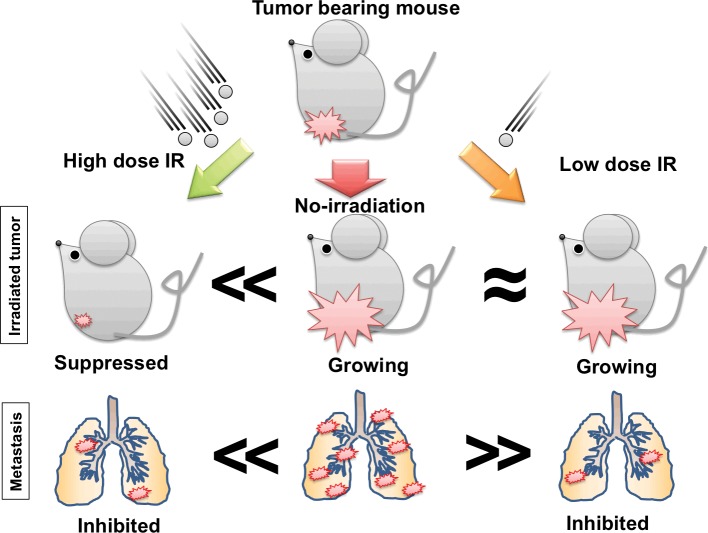
On the other hand, we reported a study [48] that used a syngeneic model of highly metastatic SCC NR-S1-implanted C3H/He mouse. In the article [48], we evaluated the effect of the combination treatment based on metastasis suppression. For IT, we administrated α-galactosylceramide (α-GalCer)–treated, BM-derived DCs on day 1.5 after irradiation by intratumoral injection. We chose lower radiation doses, which could not significantly repress the growth of the irradiated tumor. Because high-dose irradiation significantly inhibits tumor growth, it is difficult to evaluate the nature of C-ion–induced metastasis inhibition. Specifically, it is unclear whether such irradiation has a direct effect on the metastatic process, such as the invasive potential or if it is just a consequence of growth inhibition (Figure 3). In this setting, treatments with either α-GalCer DCs or C-ion irradiation decreased the numbers of metastatic nodules. When the combination of immature DCs IT and C-ion irradiation was used, the number of lung metastatic nodules was drastically reduced. Interestingly, DC treatment had no effect on tumor growth whether with C-ion treatment or without. We further showed that the lung tissue of the NR-S1–implanted mice exhibited increased expression of S100A8, which is a marker of premetastatic change. The S100A8 protein and messenger RNA expression were not affected by either C-ion irradiation or DC treatment. However, the lung tissues of the combination therapy group showed repressed S100A8 expression at 7 days after C-ion irradiation.
Figure 3.
The possible mechanisms of metastasis reduction. High-dose irradiation is able to suppress the growth of the irradiated tumor, and distant lung metastasis may also be inhibited. However, it is difficult to evaluate whether the inhibition of metastasis results from immune response or is a consequence of primary tumor regression. In contrast, low-dose irradiation has less effect on tumor growth. The irradiated tumor is able to sustain growth. If lung metastasis is inhibited significantly in this condition, it might indicate a direct effect of carbon-ion radiation therapy in metastases. Moreover, the radiation-induced immune response may be evaluated.
In many reports, BM-derived DCs were used as IT in tandem with RT. However, the DCs were treated with different activators or modifiers, such as α-GalCer [48] or tumor lysate [47], even though the combination treatment resulted in significant effects in all cases. In addition, radiation treatment combined with the administration of Flt3 ligand, which stimulates the proliferation and differentiation of DCs, also expanded radiation-induced antitumor effects [34, 38]. This suggests that BM-derived DCs themselves or the various treatments for immune activation were the essential factors. For example, DCs with α-GalCer treatment are thought to activate natural killer T cells. Therefore, we evaluated whether these modifications are essential or not for the combination treatment. When we used BM-derived immature DCs or α-GalCer-treated DCs in combination with C-ion RT, lung metastasis was suppressed in both cases [51]. This result highlights that C-ion irradiation has enough potential to activate immature DCs without pre-treatment. Furthermore, we also compared different methods for the administration of DCs. Because particle therapy has an advantage in treating tumors that are located deep in the body, intratumoral injection is not a suitable way for combination treatment. Among the methods compared, intravenous injection was shown to be highly effective in preventing lung metastasis. Further investigation is necessary to elucidate the precise mechanisms involved, such as tracking the injected DCs. In addition, we confirmed the efficacy with other mouse models using different cancer cell lines and mouse strains to expand the application of combination C-ion RT and DC IT. As a result, C-ion RT combined with DC IT significantly suppressed lung metastasis. Furthermore, we have shown that, even when exposed to the equivalent, relative biological effectiveness dose of C-ion and photon, the combination with photon could not be induced to the same level [51]. This result highlights that C-ions may be more effective in activating the immune system.
Advantage of Particle Radiotherapy for Use in Combination with Immunotherapy
As a partner to immune combination therapy, are particle beams as effective as, or more advantageous than, photon beams? Even when exposed to the same relative biological effectiveness dose, particle and photon beams are known to induce different bioresponses.
There are several reports demonstrating the effect of particle beams on metastatic potential. In some cases, x-ray treatment–enhanced metastatic potential [52], but the C-ion beam effectively suppressed it [53], with examples like migration [54–58] and invasion [55–57, 59] of cancer cell lines with in vitro assays. In addition, C-ion beam treatment inhibited in vitro angiogenesis at sublethal doses [60] as well as the expression of angiogenesis mediators [59, 61]. In immune-competent mouse models, C-ion significantly suppressed lung metastasis [55, 62].
In recent years, increasing amounts of research assert that substantial heterogeneity derived from clonal evolution exists within tumors, leading to varying radioresistance within a target [63–66]. Coupled with cancer stem cell (CSC)-like cells and also quiescent cells are known for their photon irradiation resistance and are correlated with repopulation of local recurrence after treatments, and the treatment of any individual tumor grows increasingly complex. In vitro experiments showed that CSC-like cells and quiescent cells have more resistance to irradiation with x-rays than C-ion compared with non-CSC-like cells or total cells [67–69]. In addition, Zhang et al [58] reported that CSC-like cells were more sensitive to proton irradiation than photons were. These findings indicated that particle RT is suitable for suppressing the dependency on the heterogeneity within tumors.
Some articles showed that particle beams induce the opposite results or that they induce similar bioresponses as photon irradiation [70–73]. The presence of contradicting reports may indicate the need to clarify the differences in outcome between the RT types used and between different cancers. Basic biological research focused on particle beams is still limited. In particular, to compare the effects on immunological response between different types of RT and/or methods of therapy (hypofractionation, hyperfractionation, among others) are essential issues for further development of particle RT combined with IT.
Future Developments
Despite the superior local control achieved with particle therapy, improvement in overall survival is limited because of distant metastasis [74]. Therefore, it is essential to find the optimal “combination partner” for particle RT to improve clinical outcomes.
Basic research on particle beams combined with IT is still limited to experimental models and the types of IT. However, these results show that, even with a lower dose, C-ion RT has substantial potential to activate the immune system. Such lower irradiated dose might be able to induce cell death in some tumor cells. However, it is enough to activate the immune system in both in vitro assays and mouse models [41]. Excellent dose distribution of particle therapy reduced damaged volume of normal tissues, including the BM and skin. In addition, C-ion RT showed good local control. These features allow us to select different types of IT as a partner for C-ion RT, such as immune system modulators, immune checkpoint inhibitors, myeloid-derived suppressor cells inhibitors, and cytokines, which act via the immune system of the patient.
Preclinical experiments for combining photon RT with various types of IT were previously reported. Combination with an immune-checkpoint inhibitor was first reported in 2005 [37], and many reports were published in the past few years [25, 28, 31, 35, 36, 40, 75, 76]. In addition, clinical trials for photon RT combined with immune-checkpoint inhibitors have already been started. Because particle RT could have an advantage for cancer treatment, it is expected that immune-checkpoint inhibitors may also be efficient in combination with particle RT. Moreover, because of the difference in mechanisms involved among conventional ITs and immune-checkpoint inhibitors, it may be of value for evaluating triple-combination antitumor effects, as already described in some reports [28, 35]. However, there are many variations in immune response, including the differing effects of immune cell lines. Combination therapy approaches considering the tumor microenvironment could serve as starting points [77].
The reports regarding RT combined with IT demonstrate drastic antitumor effects, but there have not been any reports, to our knowledge, on mouse models in which the combination effect could not be observed (except for immunodeficient or CD8-depleted mice). Because the clinical results showed that spontaneous regression (or abscopal effects) was not observed in all cases [78], it is important to investigate inefficient models for combination RT as well as the efficient cases. In addition, very aggressive or treatment-resistance models, such as the 4T1 breast cancer model [4, 39], are required to develop C-ion combined IT because these difficult-to-cure cancers are the targets for C-ion RT. It is expected that comparison among these mouse models might lead us to further understand the underlying mechanisms of combination RT, which influence the outcomes of individual patients.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol. 2012;42:670–685. doi: 10.1093/jjco/hys104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen AD, Munter MW, Debus J. Review of clinical experience with ion beam radiotherapy. Br J Radiol. 2011;84:S35–S47. doi: 10.1259/bjr/71511359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res. 2011;711:150–157. doi: 10.1016/j.mrfmmm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19:565–582. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011. 3:95ra73. [DOI] [PMC free article] [PubMed]
- 10.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis. 2013;4:e688. doi: 10.1038/cddis.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 13.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 14.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, Buque A, Castro MG, Cirone M, Colombo MI, Cremer I, Demaria S, Dini L, Eliopoulos AG, Faggioni A, Formenti SC, Fucikova J, Gabriele L, Gaipl US, Galon J, Garg A, Ghiringhelli F, Giese NA, Guo ZS, Hemminki A, Herrmann M, Hodge JW, Holdenrieder S, Honeychurch J, Hu HM, Huang X, Illidge TM, Kono K, Korbelik M, Krysko DV, Loi S, Lowenstein PR, Lugli E, Ma Y, Madeo F, Manfredi AA, Martins I, Mavilio D, Menger L, Merendino N, Michaud M, Mignot G, Mossman KL, Multhoff G, Oehler R, Palombo F, Panaretakis T, Pol J, Proietti E, Ricci JE, Riganti C, Rovere-Querini P, Rubartelli A, Sistigu A, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Sukkurwala AQ, Tartour E, Thorburn A, Thorne SH, Vandenabeele P, Velotti F, Workenhe ST, Yang H, Zong WX, Zitvogel L, Kroemer G, Galluzzi L. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adkins I, Fucikova J, Garg AD, Agostinis P, Spisek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology. 2014;3:e968434. doi: 10.4161/21624011.2014.968434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Rubner Y, Wunderlich R, Ruhle PF, Kulzer L, Werthmoller N, Frey B, Weiss EM, Keilholz L, Fietkau R, Gaipl US. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2012;2:75. doi: 10.3389/fonc.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Hatzi VI, Laskaratou DA, Mavragani IV, Nikitaki Z, Mangelis A, Panayiotidis MI, Pantelias GE, Terzoudi GI, Georgakilas AG. Non-targeted radiation effects in vivo: a critical glance of the future in radiobiology. Cancer Lett. 2015;356:34–42. doi: 10.1016/j.canlet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 21.de la Cruz-Merino L, Illescas-Vacas A, Grueso-Lopez A, Barco-Sanchez A, Miguez-Sanchez C, Cancer Immunotherapies Spanish Group Radiation for awakening the dormant immune system, a promising challenge to be explored. Front Immunol. 2014;5:102. doi: 10.3389/fimmu.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zegers CM, Rekers NH, Quaden DH, Lieuwes NG, Yaromina A, Germeraad WT, Wieten L, Biessen EA, Boon L, Neri D, Troost EG, Dubois LJ, Lambin P. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21:1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 23.Son CH, Bae JH, Shin DY, Lee HR, Jo WS, Yang K, Park YS. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys. 2015;92:390–398. doi: 10.1016/j.ijrobp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Ma SY, Song H, Park JH, Choi JH, Kim JH, Kim KH, Park S, Park DH, Kang MS, Kwak M, Fu YX, Choi I, Cho H, Park S. Addition of anti-neu antibody to local irradiation can improve tumor-bearing BALB/c mouse survival through immune-mediated mechanisms. Radiat Res. 2015;183:271–278. doi: 10.1667/RR13800.1. [DOI] [PubMed] [Google Scholar]
- 25.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14:1159–66. doi: 10.1158/1078-0432.CCR-07-4485. [DOI] [PubMed] [Google Scholar]
- 27.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimoto Y, Suzuki Y, Mimura K, Ando K, Oike T, Sato H, Okonogi N, Maruyama T, Izawa S, Noda SE, Fujii H, Kono K, Nakano T. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One. 2014;9:e92572. doi: 10.1371/journal.pone.0092572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Ramakrishnan R, Lavilla-Alonso S, Chinnaiyan P, Rao N, Fowler E, Heine J, Gabrilovich DI. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother. 2014;63:1009–1021. doi: 10.1007/s00262-014-1573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholch S, Rauber C, Tietz A, Rahbari NN, Bork U, Schmidt T, Kahlert C, Haberkorn U, Tomai MA, Lipson KE, Carretero R, Weitz J, Koch M, Huber PE. Radiotherapy combined with TLR7/8 activation induces strong immune responses against gastrointestinal tumors. Oncotarget. 2015;6:4663–4676. doi: 10.18632/oncotarget.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, Haynes NM. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 37.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 38.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 39.Filatenkov A, Baker J, Muller AM, Ahn GO, Kohrt H, Dutt S, Jensen K, Dejbakhsh-Jones S, Negrin RS, Shizuru JA, Engleman EG, Strober S. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat Res. 2014;182:163–169. doi: 10.1667/RR13471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jing W, Gershan JA, Weber J, Tlomak D, McOlash L, Sabatos-Peyton C, Johnson BD. Combined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myeloma. J Immunother Cancer. 2015;3:2. doi: 10.1186/s40425-014-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L, Ando K, Irie D, Sato K, Imai T, Shimokawa T. Poster 4-PS8D-14 presented at: 15th International Congress of Radiation Research; May 25–29, Kyoto, Japan.: 2015. Analysis of underlying mechanisms for combination therapy of carbon-ion irradiation and dendritic cell immunotherapy. [Google Scholar]
- 42.Persa E, Balogh A, Safrany G, Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015;368:252–261. doi: 10.1016/j.canlet.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–2763. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, Demaria S, Formenti S. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol. 2015;25:54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasumi K, Aoki Y, Wantanabe R, Mann DL. Clinical response of advanced cancer patients to cellular immunotherapy and intensity-modulated radiation therapy. Oncoimmunology. 2013;2:e26381. doi: 10.4161/onci.26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsunaga A, Ueda Y, Yamada S, Harada Y, Shimada H, Hasegawa M, Tsujii H, Ochiai T, Yonemitsu Y. Carbon-ion beam treatment induces systemic antitumor immunity against murine squamous cell carcinoma. Cancer. 2010;116:3740–3748. doi: 10.1002/cncr.25134. [DOI] [PubMed] [Google Scholar]
- 48.Ohkubo Y, Iwakawa M, Seino KI, Nakawatari M, Wada H, Kamijuku H, Nakamura E, Nakano T, Imai T. Combining carbon ion radiotherapy and local injection of α-galactosylceramide–pulsed dendritic cells inhibits lung metastases in an in vivo murine model. Int J Radiat Oncol Biol Phys. 2010;78:1524–1531. doi: 10.1016/j.ijrobp.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 49.Bhardwaj N. Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol Med. 2001;7:388–394. doi: 10.1016/s1471-4914(01)02101-3. [DOI] [PubMed] [Google Scholar]
- 50.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 51.Ando K, Fujita H, Hosoi A, Nakawatari M, Nakamura E, Kakimi K, Nakano T, Imai T, Shimokawa T. Effective suppression of pulmonary metastasis in combined carbon ion radiation therapy with dendritic-cell immunotherapy in murine tumor models. Int J Radiat Oncol Biol Phys. 2013;87:S642. [Google Scholar]
- 52.Kargiotis O, Geka A, Rao JS, Kyritsis AP. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J Neurooncol. 2010;100:323–338. doi: 10.1007/s11060-010-0199-4. [DOI] [PubMed] [Google Scholar]
- 53.Lee KS, Lee DH, Chun SY, Nam KS. Metastatic potential in MDA-MB-231 human breast cancer cells is inhibited by proton beam irradiation via the Akt/nuclear factor-κB signaling pathway. Mol Med Rep. 2014;10:1007–1012. doi: 10.3892/mmr.2014.2259. [DOI] [PubMed] [Google Scholar]
- 54.Goetze K, Scholz M, Taucher-Scholz G, Mueller-Klieser W. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int J Radiat Biol. 2007;83:889–896. doi: 10.1080/09553000701753826. [DOI] [PubMed] [Google Scholar]
- 55.Ogata T, Teshima T, Kagawa K, Hishikawa Y, Takahashi Y, Kawaguchi A, Suzumoto Y, Nojima K, Furusawa Y, Matsuura N. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005;65:113–120. [PubMed] [Google Scholar]
- 56.Ogata T, Teshima T, Inaoka M, Minami K, Tsuchiya T, Isono M, Furusawa Y, Matsuura N. Carbon ion irradiation suppresses metastatic potential of human non-small cell lung cancer A549 cells through the phosphatidylinositol-3-kinase/Akt signaling pathway. J Radiat Res. 2011;52:374–379. doi: 10.1269/jrr.10102. [DOI] [PubMed] [Google Scholar]
- 57.Akino Y, Teshima T, Kihara A, Kodera-Suzumoto Y, Inaoka M, Higashiyama S, Furusawa Y, Matsuura N. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2009;75:475–481. doi: 10.1016/j.ijrobp.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Lin SH, Fang B, Gillin M, Mohan R, Chang JY. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. J Thorac Oncol. 2013;8:1484–1491. doi: 10.1097/JTO.0b013e3182a5fdcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girdhani S, Lamont C, Hahnfeldt P, Abdollahi A, Hlatky L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat Res. 2012;178:33–45. doi: 10.1667/rr2724.1. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi Y, Teshima T, Kawaguchi N, Hamada Y, Mori S, Madachi A, Ikeda S, Mizuno H, Ogata T, Nojima K, Furusawa Y, Matsuura N. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003;63:4253–4257. [PubMed] [Google Scholar]
- 61.Kamlah F, Hanze J, Arenz A, Seay U, Hasan D, Juricko J, Bischoff B, Gottschald OR, Fournier C, Taucher-Scholz G, Scholz M, Seeger W, Engenhart-Cabillic R, Rose F. Comparison of the effects of carbon ion and photon irradiation on the angiogenic response in human lung adenocarcinoma cells. Int J Radiat Oncol Biol Phys. 2011;80:1541–1549. doi: 10.1016/j.ijrobp.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 62.Tamaki T, Iwakawa M, Ohno T, Imadome K, Nakawatari M, Sakai M, Tsujii H, Nakano T, Imai T. Application of carbon-ion beams or gamma-rays on primary tumors does not change the expression profiles of metastatic tumors in an in vivo murine model. Int J Radiat Oncol Biol Phys. 2009;74:210–218. doi: 10.1016/j.ijrobp.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 63.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foo J, Michor F. Evolution of acquired resistance to anti-cancer therapy. J Theor Biol. 2014;355:10–20. doi: 10.1016/j.jtbi.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerdes MJ, Sood A, Sevinsky C, Pris AD, Zavodszky MI, Ginty F. Emerging understanding of multiscale tumor heterogeneity. Front Oncol. 2014;4:366. doi: 10.3389/fonc.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui X, Oonishi K, Tsujii H, Yasuda T, Matsumoto Y, Furusawa Y, Akashi M, Kamada T, Okayasu R. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 2011;71:3676–3687. doi: 10.1158/0008-5472.CAN-10-2926. [DOI] [PubMed] [Google Scholar]
- 68.Oonishi K, Cui X, Hirakawa H, Fujimori A, Kamijo T, Yamada S, Yokosuka O, Kamada T. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother Oncol. 2012;105:258–265. doi: 10.1016/j.radonc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Masunaga S, Ando K, Uzawa A, Hirayama R, Furusawa Y, Koike S, Ono K. The radiosensitivity of total and quiescent cell populations in solid tumors to 290 MeV/u carbon ion beam irradiation in vivo. Acta Oncol. 2008;47:1087–1093. doi: 10.1080/02841860701821999. [DOI] [PubMed] [Google Scholar]
- 70.Fujita M, Imadome K, Endo S, Shoji Y, Yamada S, Imai T. Nitric oxide increases the invasion of pancreatic cancer cells via activation of the PI3K-AKT and rhoA pathways after carbon ion irradiation. FEBS Lett. 2014;588:3240–3250. doi: 10.1016/j.febslet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Fujita M, Otsuka Y, Imadome K, Endo S, Yamada S, Imai T. Carbon-ion radiation enhances migration ability and invasiveness of the pancreatic cancer cell, PANC-1, in vitro. Cancer Science. 2012;103:677–683. doi: 10.1111/j.1349-7006.2011.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ando S, Nojima K, Ishihara H, Suzuki M, Ando M, Majima H, Ando K, Kuriyama T. Induction by carbon-ion irradiation of the expression of vascular endothelial growth factor in lung carcinoma cells. Int J Radiat Biol. 2000;76:1121–1127. doi: 10.1080/09553000050111596. [DOI] [PubMed] [Google Scholar]
- 73.Yoshimoto Y, Oike T, Okonogi N, Suzuki Y, Ando K, Sato H, Noda SE, Isono M, Mimura K, Kono K, Nakano T. Carbon-ion beams induce production of an immune mediator protein, high mobility group box 1, at levels comparable with X-ray irradiation. J Radiat Res. 2015;56:509–514. doi: 10.1093/jrr/rrv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 75.Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin Oncol. 2015;42:363–377. doi: 10.1053/j.seminoncol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 78.Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]