Abstract
Many manned missions to the Moon and Mars are scheduled in the near future. However, space radiation presents a major hazard to humans, and astronauts are constantly exposed to radiation, including high linear energy transfer (LET) radiation, which differs from radiation on Earth. Thus, there is thus an urgent need to clarify the biological effects of space radiation and reduce the associated risks. In this review, we consider the role of high-LET radiobiology in relation to space-radiation exposure.
Keywords: radiobiology, space radiation, linear energy transfer, risk assessment
Introduction
Many manned space missions are scheduled in the near future, during which astronauts will be constantly exposed to space radiation [1]. The radiation environment in deep space and low Earth orbit is very different from that at the Earth's surface (Figure 1), and space radiation, including high linear energy transfer (LET) radiation, is a serious hazard to people outside the protection of Earth's magnetic field and atmosphere, even at low doses and low dose rates. A lack of knowledge regarding the biological effects of space radiation is considered to be the most important factor limiting the prediction of radiation risk associated with long-term space travel. Accumulating evidence has refuted the classic target theory of radiation biology, and studies have found inverse low-dose-rate effects, an adaptive response, and bystander effects after exposure to low doses of high-LET radiation at a low dose rate [2]. The existence of space-radiation-induced nontargeted effects thus influences our understanding of the health risks associated with exposure to low fluences of high-charge and high-energy particles (HZE).
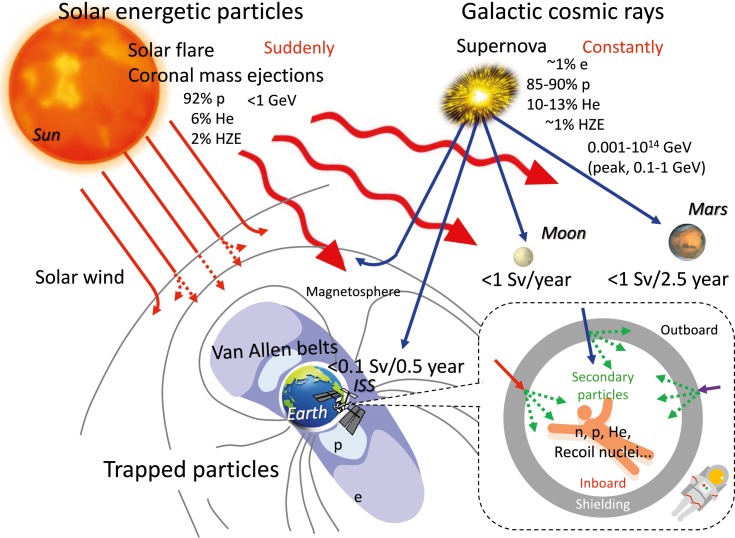
Figure 1.
Environment of space radiation with galactic cosmic rays, solar energetic particles, and trapped particles. Abbreviations: e, electron; p, proton; He, helium ion; HZE, high-charge and high-energy particle; n, neutron.
The present review summarizes the characteristics and environment of space radiation and the biological effects of exposure to high-LET radiation at a low dose rate and considers the status of biological research into space radiation.
Characteristics of Space Radiation
Galactic Cosmic Rays
Galactic cosmic rays (GCRs) come from outside the solar system but generally from within the Milky Way galaxy. The GCR particles consist of about 1% electrons, 85% to 90% protons, 10% to 13% helium ions, and about 1% HZEs [3]. Because the proton flux is generally high compared with the HZE flux, it is assumed that cells are likely to be hit by a proton before being hit by an HZE ion. GCRs include fully stripped ions to uranium; even-numbered elements, such as carbon, oxygen and iron, are more abundant than odd-numbered elements, although the flux of ions above nickel is low. GCR energy has a wide range from 0.001 to 1014 GeV/n, with a peak at 0.1 to 1 GeV/n. Cosmic particles with energies >10 GeV/n do not contribute to the radiation dose because they have a very low flux and are emitted isotropically [4, 5].
Though GCRs are considered to originate from high-energy explosions of supernovae [6], the mechanism of GCR acceleration is unknown. However, GCRs with less than about 106 GeV energy are generally accepted to be accelerated by shock waves from supernova explosions within the Milky Way galaxy [7, 8]. A supernova remnant (SNR), which is the structure resulting from a supernova explosion, is bounded by an expanding shock wave and consists of ejected material expanding from the explosion. The main protons of GCRs were recently reported to be accelerated in SNRs [9]. In contrast, GCRs with >109 GeV energy are believed to originate outside our galaxy [10], having been accelerated to nearly the speed of light within the past few million years and traveled across the galaxy.
The heliosphere shields the solar system from GCRs. The GCRs are pushed back out by the solar wind, such that they are ejected out from the Sun with the magnetic field because the charged GCRs are affected by Lorentz forces. Thus, GCRs are subjected to heliospheric (solar) modulation associated with the 11-year solar activity cycle and show an inverse correlation with solar activity [11].
Solar Energetic Particles
The Sun emits radiation across most of the electromagnetic spectrum, including high-energy γ-rays and x-rays, ultraviolet, visible, and infrared light, microwaves, and ultra-long-wavelength radio waves. The frequencies, but not the sizes, of solar flares (SFs) and coronal mass ejections (CMEs) are related to the cycle of solar activity. A solar radiation storm (solar energy particle [SEP] event) comprises high-energy particles from the Sun originating in SFs and/or CMEs. The exposure durations of the SEPs from SFs are short (several hours) while those from CMEs are longer (a few days). These SEP events are sporadic and difficult to predict. They consist of 92% protons, 6% helium ions, and 6% HZE ions [4], with the proton energy ranging from 0.01 to 10 GeV/n. The peak proton flux of SEP events varies as a function of both SF and CME properties [12].
Trapped Radiation
The rotation of the Earth's molten iron core creates a magnetic field around the Earth that resembles a dipole field. This field traps high-energy protons (10-100 MeV/n) and electrons (0.1-10 MeV) in 2 annular doughnut-shaped zones known as the inner (1,000-5,000 km) and outer (15 000-25 000 km) Van Allen radiation belts [5]. Although protons and electrons may be produced by solar ultraviolet-induced and x-ray-induced dissociation of hydrogen atoms, the inner-belt protons and electrons are mainly derived from cosmic-ray albedo neutron decay [13]. Because Earth's rotational and magnetic axes are different, the South Atlantic Anomaly is formed by the Van Allen radiation belt dropping to low altitude (about several hundred kilometers) over the south Atlantic Ocean.
Environment of Space Radiation
Low-Earth Orbits and the International Space Station
The International Space Station (ISS) flies at an average altitude of about 400 km above Earth, and the astronauts are exposed to high-energy radiation originating from GCRs and SEPs, largely due to trapped radiation in the South Atlantic Anomaly [14]. The dose rate outside the ISS is greater than that inside the ISS, and the dose inside the ISS depends on the location and overall shielding at the location, which can vary significantly [4]. The radiation field varies spatially and temporally depending on the Earth's magnetic field and the solar cycle. High-energy charged particles of space radiation produce secondary particles, mostly neutrons, through nuclear reactions, when they strike a spacecraft or an astronaut. Exposure doses in the ISS have been estimated to be about 0.5 mSv/day [15, 16], which is about 100 times the dose on the ground, with the dose-equivalent rate remaining below 100 mSv during a 6-month stay on the ISS. However, exposure to 100 mSv is associated with a 0.5% increase in the risk of fatal cancer. The dramatically higher dose rates at solar minimum than at solar maximum are attributed to higher GCR fluxes around several hundred MeV/n, which are affected by solar modulation, particularly in the absence of magnetic shielding [17].
Deep Space, the Moon, and Mars
Deep space refers to space outside Earth's protective magnetic field, and the flow of high-energy charged particles of SEPs and GCRs presents a major problem relating to long-duration manned missions in deep space. Astronauts will be exposed to trapped radiation as they travel through the Van Allen radiation belts before reaching deep space. The GCR dose rate also depends on the solar cycle, and the total dose from SEP particles during an event can be 19.5 mSv/event or higher in a spacecraft traveling through interplanetary space. [18].
The lunar surface is exposed to much higher levels of radiation than the Martian surface because there is little gas in the lunar atmosphere, though the atmosphere of Mars is also much thinner (0.75%) than that of Earth. Nevertheless, the dose rates at the surfaces of the Moon and Mars are still lower than those inside a spacecraft traveling through interplanetary space [17].
It has been recognized that the albedo, or backscattered, radiation environment near the surfaces of the Moon and Mars may pose a biological risk to astronauts. The Mars Science Laboratory's Curiosity Rover measured dose-equivalent rates of GCRs and SEPs of 0.64 mSv/day and 0.025 mSv/event, respectively, on the Martian surface during a period of solar maximum [19]. A Mars mission comprising 360 days return space flight and 500 days on the Martian surface would be associated with a total exposure of around 1 Sv [19], while the exposure rate on the lunar surface has been calculated at 1 Sv/year [20]. Although the total dose exposure during a deep-space mission may vary depending on the solar cycle, an exposure to 1 Sv is anticipated to be associated with a 5% increase in the risk of fatal cancer. Consideration of the radiation environment on the Martian and lunar surfaces also needs to take account of albedo neutrons, but not other secondary reaction products (eg, photons, electrons, positrons, pions.). In general, the contribution of albedo neutrons to the effective radiation dose ranges from 1% to 32%, depending on the environmental model, shielding material, and shielding thickness [21]. The most intense region of the albedo neutron spectrum occurs at low thermal energies (E <1 eV), where the biological risk is smaller [20, 21], while the flux of neutrons is smaller at higher thermal energies (up to hundreds of MeV), but the biological risk is greater due to recoil nuclei and target fragmentation [22].
Detection of DNA Damage Induced by Space Radiation
Space radiation–induced damage to DNA samples during space flight has been observed using 2 methods: postlabeling and immunocytochemistry. Postlabeling detects DNA strand breaks as grains on fixed silver emulsion resulting from β-rays emitted from 3H-atoms in the nuclei of the cells. Fixed cultured human cells spent 9 days onboard the Space Shuttle and 40 days onboard the Mir Space Station in 1997 [23], and space radiation–induced DNA strand breaks were then labeled by enzymatic incorporation of [3H]-dATP with terminal deoxyribonucleotidyl transferase. The number of cells with many grains was higher in the Mir Space Station samples compared with the Space Shuttle samples, while virtually no DNA damage was detected in the ground control sample. These results suggest that space radiation causes DNA damage, with the amount of damage depending on the duration of the space flight [23].
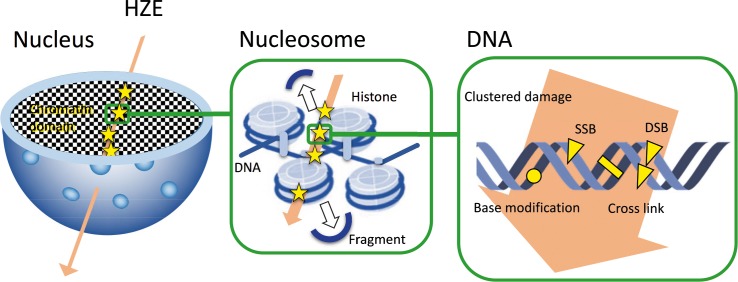
Immunocytochemistry detects DNA damage by recognizing phospho-histone H2AX (γH2AX) foci, which has become the gold standard for detecting DNA double-strand breaks. High-LET radiation damages DNA along the track of particle irradiation, leaving fragments of DNA and clusters of DNA damage, including double-strand breaks [24–26] (Figure 2). This differs from the damage normally produced by sparse low-LET radiation, such as x-rays or γ-rays, and the differential spatial distribution of the energy deposited along the core and penumbra of the track creates DNA lesions that are complex and difficult to repair [27]. In 2008–2009, human frozen cells spent 133 days in an ISS freezer [28], after which they were cultured for 30 minutes and then fixed. γH2AX was detected in the nuclei in space samples but not in the ground control samples, providing the first report of high-LET space radiation–induced γH2AX tracks in cell nuclei. These results confirmed that space flight damaged nuclear DNA along tracks reflecting the tracks of space-radiation exposure [28]. Other recent studies have reported γH2AX signals in cells flown in space [29], as well as other forms of DNA damage, including chromosome aberrations in astronauts' lymphocytes [30]. High-LET radiation can thus induce permanent genetic changes in somatic and germ cells and is usually more effective per unit of absorbed dose than low-LET radiation.
Figure 2.
DNA damage due to high-energy particles. High linear energy transfer radiation induces clustered DNA damage, involving 2 or more closely spaced damages (strand breaks, base modifications, or cross links) within 1 or 2 DNA helix turns, as well as small fragments of DNA (<40 base pairs) along the track of particle irradiation in the cell nucleus.
Biological Effect of a Low Dose Rate of High-LET Radiation
Inverse Dose-Rate Effect
Low-LET radiation received at a low dose rate usually reduces the effectiveness of a given dose [31], but decreasing the dose rate also increases the risk of carcinogenesis and other biological effects (eg, gene mutation, chromosome aberration, and oncogenic transformation). This inverse dose-rate effect [32, 33] has been supported by many in vitro and in vivo studies.
One possible mechanism for this inverse dose-rate effect is that there may be a narrow window during the cell cycle when the cells are particularly sensitive to oncogenic transformation [34], and high-LET radiation, even at a low dose rate, is thought to be able to deposit enough energy to initiate the required effect in cycling cells. Bystander effects, which involve intercellular signaling, are another possible mechanism for the inverse dose-rate effect of high-LET radiation [35, 36].
If the suggested model is realistic, high-LET radiation, such as HZE cosmic rays, would be expected to have relatively little effect, though trapped protons might have an effect on astronauts in Earth orbit [34]. However, the inverse dose-rate effects were reported around 20 years ago and were observed in such end points as cell transformation. Although cell transformation is relevant to cancer risks, the impact of inverse dose-rate effects on risk projections needs to be confirmed in animal studies.
Adaptive Response
The ability of living organisms to resist stress damage through prior exposure to reduced stress levels is referred to as an adaptive response. This adaptive response manifests through decreased levels of cell death, gene mutation, micronuclei formation, chromosome aberration, and malignant transformation [37, 38]. The dose window (ie, the specific range of doses and/or dose rates) and the interval between conditioning and challenging irradiation are important factors affecting the adaptive response [39].
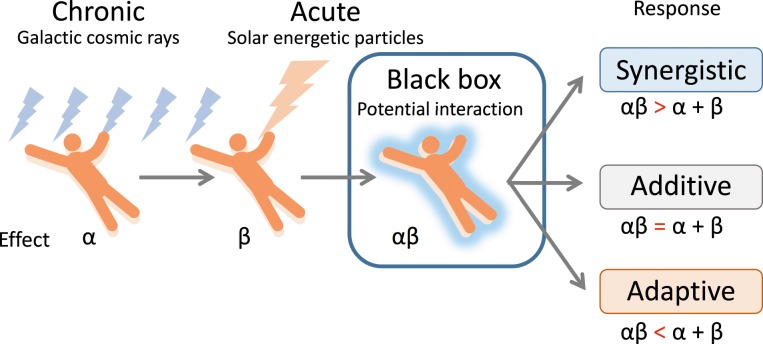
The possible mechanisms of the adaptive response include the transcription and translation of DNA repair-regulated and cell cycle–regulated genes [40, 41], the activation of poly (ADP-ribose) polymerase [42], protein kinase C [43, 44], p53 [45, 46], and signal transducer and activator of transcription1 [47], and the secretion of nuclear clusterin [48]. Although radioadaptation was assumed to occur only when cells were exposed to a priming dose of low-LET, but not high-LET, irradiation [49], both low-LET and high-LET irradiation have been reported to act as challenging doses for radioadaptation. Although chronic exposure to GCRs was reported to reduce the radiation susceptibility and help to protect astronauts against unpredictable exposure to a sudden and dramatic increase in flux due to SEPs during a mission [50], this finding remains highly speculative (Figure 3). This may be particularly important to understanding the radiation risks involved in space travel, because the GCR environment comprises predominantly protons, and it is realistic to expect that cells will be exposed to multiple hits from protons before being traversed by an HZE [51]. Notably unirradiated cells co-cultured with proton-irradiated cells were also significantly protected from the DNA-damaging effects of the challenge dose with iron ions [52], indicating that protective adaptive responses can spread from cells targeted by low-LET space radiation to unirradiated cells in their vicinity.
Figure 3.
Possibility of adaptation to sudden high doses of radiation due to solar energetic particles after exposure to chronic galactic cosmic rays (based on [50]).
Human Effect of Space Radiation
Chronic exposure to space radiation on long-duration and exploration spaceflights increases the risk of cancer [53, 54], can lead to tissue degeneration and cataracts [55, 56], and can affect the central nervous system [57–59] and immune function [60]. Furthermore, it was recently shown that the risk of cardiovascular disease may also be increased by traveling into deep space [61], although the study sample was small and the results were not statistically valid [62]. However, this finding is supported by the results of the Radiation Effects Research Foundation studies on a cohort of Japanese atomic-bomb survivors, who showed that low-dose total-body irradiation could be responsible for increased cardiovascular mortality rates [63].
Several factors contribute to the large uncertainties in risk projection and hinder evaluations of the effectiveness of possible countermeasures, including radiation-quality effects at a low dose rate (ie, the inverse dose-rate effect, adaptive response, bystander effect, and cell competition) and microgravity [64]. To allow the assessment and management of human health risks in space, it is necessary to obtain more basic data on the combined effects of radiation under microgravity [30, 65]. To address these serious problems, we developed 3-dimensional clinostat-synchronized heavy-ion and x-ray irradiation systems [66, 67], which are expected to provide significant contributions to space radiation research, as a valuable platform for studies on the relative biological effectiveness and the combined effects of radiation under microgravity.
The basic mechanisms underlying the molecular, cellular, and tissue responses to radiation with different ion species and LET remain under investigation [68, 69]. Although the risks of acute exposure to space radiation with a complicated mixed beam can be estimated, the effects of chronic and fractionated exposures have not yet been resolved. The risks of late tissue effects, including cancer, are important, and further analysis of the effects of mixed radiation beams is required [70]. A GCR simulator was recently designed in the NASA Space Radiation Laboratory to deliver a mixed radiation field comprising different ions, from protons to iron ions, at energies ranging from about 100 to 1,500 MeV/u for all ions and up to 2,500 MeV for protons [71]. This simulator will become a powerful tool in basic data acquisition for space radiobiology.
Conclusion
The present review clarifies the characteristics and biological effects of space radiation, including charged particles at a low dose and low dose rate. While some effects, such as cataracts, have been observed, others, such as cancer, are still being investigated. Furthermore, some health consequences of spaceflight, such as cardiovascular disease and immune dysfunction, are primarily caused by microgravity, and the contribution of radiation to these effects remains unclear. Further research is necessary to identify these risks of space radiation.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Acknowledgments: This work was supported by a MEXT Grant-in-Aid for Scientific Research on Innovative Areas, Grant Number JP15H05935 “Living in Space,” Research Projects with Heavy Ions at the Gunma University Heavy Ion Medical Center (GHMC). We thank the Edanz Editing Group for editing a draft of this manuscript.
References
- 1.Blakely EA. Biological effects of cosmic radiation: deterministic and stochastic. Health Phys. 2000;79:495–506. doi: 10.1097/00004032-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Waldren CA. Classical radiation biology dogma, bystander effects and paradigm shifts. Hum Exp Toxicol. 2004;23:95–100. doi: 10.1191/0960327104ht425oa. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JA. Elemental and isotopic composition of the galactic cosmic rays. Ann Rev Nuclear Part Sci. 1983;33:323–82. [Google Scholar]
- 4.Maalouf M, Durante M, Foray N. Biological effects of space radiation on human cells: history, advances and outcomes. J Radiat Res. 2011;52:126–46. doi: 10.1269/jrr.10128. [DOI] [PubMed] [Google Scholar]
- 5.Nelson GA. Space radiation and human exposures, a primer. Radiat Res. 2016;185:349–58. doi: 10.1667/RR14311.1. [DOI] [PubMed] [Google Scholar]
- 6.Baade W, Zwicky F. Cosmic rays from super-novae. Proc Natl Acad Sci U S A. 1934;20:259–63. doi: 10.1073/pnas.20.5.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell AR. The acceleration of cosmic rays in shock fronts – I. Mon Not R Astron Soc. 1978;182:147–56. [Google Scholar]
- 8.Blandford RD, Ostriker JP. Particle acceleration by astrophysical shocks. Astrophys J. 1978;221:L29–32. [Google Scholar]
- 9.Malkov MA, Diamond PH, Sagdeev RZ. Mechanism for spectral break in cosmic ray proton spectrum of supernova remnant W44. Nat Commun. 2011;2:194. doi: 10.1038/ncomms1195. [DOI] [PubMed] [Google Scholar]
- 10.Nagano M, Watson AA. Observations and implications of the ultrahigh-energy cosmic rays. Rev Mod Phys. 2000;72:689–732. [Google Scholar]
- 11.Forbush SE. Cosmic-ray intensity variations during two solar cycles. J Geophys Res. 1958;63:651–69. [Google Scholar]
- 12.Papaioannou A, Sandberg I, Anastasiadis A, Kouloumvakos A, Georgoulis MK, Tziotziou K, Tsiropoula G, Jiggens P, Hilgers A. Solar flares, coronal mass ejections and solar energetic particle event characteristics. J Space Weather Space Clim. 2016;6:A42. [Google Scholar]
- 13.Li X, Selesnick R, Schiller Q, Zhang K, Zhao H, Baker DN, Temerin MA. Measurement of electrons from albedo neutron decay and neutron density in near-Earth space. Nature. 2017;552:382–5. doi: 10.1038/nature24642. [DOI] [PubMed] [Google Scholar]
- 14.Badavi F, Walker S, Santos Koos L. Evaluation of the new radiation belt AE9/AP9/SPM model for a cis lunar mission. Acta Astronautica. 2014;102:156–68. [Google Scholar]
- 15.Cucinotta FA, Kim MH, Willingham V, George KA. Physical and biological organ dosimetry analysis for international space station astronauts. Radiat Res. 2008;170:127–38. doi: 10.1667/RR1330.1. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi T, Takahashi A, Nagamatsu A, Omori K, Suzuki H, Shimazu T, Ishioka N. Detection of space radiation-induced double strand breaks as a track in cell nucleus. Biochem Biophys Res Commun. 2009;390:485–8. doi: 10.1016/j.bbrc.2009.09.114. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Nagamatsu A, Ueno H, Kataoka R, Miyake S, Takeda K, Niita K. Comparison of cosmic-ray environments on Earth, Moon, Mars and in spacecarft using PHITS. Radiat Prot Dosimetry. 2017;29:1–4. doi: 10.1093/rpd/ncx192. [DOI] [PubMed] [Google Scholar]
- 18.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, Kang S, Weigle G, Böttcher S, Böhm E, Burmeister S, Guo J, Köhler J, Martin C, Posner A, Rafkin S, Reitz G. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science. 2013;340:1080–4. doi: 10.1126/science.1235989. [DOI] [PubMed] [Google Scholar]
- 19.Hassler DM, Zeitlin C, Wimmer-Schweingruber RF, Ehresmann B, Rafkin S, Eigenbrode JL, Brinza DE, Weigle G, Böttcher S, Böhm E, Burmeister S, Guo J, Köhler J, Martin C, Reitz G, Cucinotta FA, Kim MH, Grinspoon D, Bullock MA, Posner A, Gómez-Elvira J, Vasavada A, Grotzinger JP. MSL Science Team. Mars' surface radiation environment measured with the Mars Science Laboratory's Curiosity rover. Science. 2014;343:1244797. doi: 10.1126/science.1244797. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y, Lin ZW. The radiation environment on the Moon from galactic cosmic rays in a lunar habitat. Radiat Res. 2010;173:238–44. doi: 10.1667/RR1846.1. [DOI] [PubMed] [Google Scholar]
- 21.Slaba TC, Blattnig SR, Clowdsley MS. Variation in lunar neutron dose estimates. Radiat Res. 2011;176:827–41. doi: 10.1667/rr2616.1. [DOI] [PubMed] [Google Scholar]
- 22.Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 2006;7:431–5. doi: 10.1016/S1470-2045(06)70695-7. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi T, Ohnishi K, Takahashi A, Taniguchi Y, Sato M, Nakano T, Nagaoka S. Detection of DNA damage induced by space radiation in Mir and space shuttle. J Radiat Res. 2002;43:S133–6. doi: 10.1269/jrr.43.s133. [DOI] [PubMed] [Google Scholar]
- 24.Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 25.Rydberg B. Clusters of DNA damage induced by ionizing radiation: formation of short DNA fragments. II. Experimental detection. Radiat Res. 1996;145:200–9. [PubMed] [Google Scholar]
- 26.Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res. 2008;49:203–10. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- 27.Durante M. New challenges in high-energy particle radiobiology. Br J Radiol. 2014;87:20130626. doi: 10.1259/bjr.20130626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi T, Takahashi A, Nagamatsu A, Omori K, Suzuki H, Shimazu T, Ishioka N. Detection of space radiation-induced double strand breaks as a track in cell nucleus. Biochem Biophys Res Commun. 2009;390:485–8. doi: 10.1016/j.bbrc.2009.09.114. [DOI] [PubMed] [Google Scholar]
- 29.Lu T, Zhang Y, Wong M, Feiveson A, Gaza R, Stoffle N, Wang H, Wilson B, Rohde L, Stodieck L, Karouia F, Wu H. Detection of DNA damage by space radiation in human fibroblasts flown on the International Space Station. Life Sci Space Res. 2017;12:24–31. doi: 10.1016/j.lssr.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Villanueva M, Wong M, Lu T, Zhang Y, Wu H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity. 2017;3:14. doi: 10.1038/s41526-017-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells RL, Bedford JS. Dose-rate effects in mammalian cells. IV. Repairable and nonrepairable damage in noncycling C3H 10T 1/2 cells. Radiat Res. 1983;94:105–34. [PubMed] [Google Scholar]
- 32.Hill CK, Han A, Elkind MM. Possible error-prone repair of neoplastic transformation induced by fission-spectrum neutrons. Br J Cancer. 1984;6:97–101. [PMC free article] [PubMed] [Google Scholar]
- 33.Charles M, Cox R, Goodhead D, Wilson A. CEIR forum on the effects of high-LET radiation at low-dose and dose-rates. Int J Radiat Biol. 1990;58:859–85. [Google Scholar]
- 34.Brenner DJ, Hall EJ. The inverse dose-rate effect for oncogenic transformation by neutrons and charged particles: a plausible interpretation consistent with published data. Int J Radiat Biol. 1990;58:745–58. doi: 10.1080/09553009014552131. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DJ, Sachs RK. Do low dose-rate bystander effects influence domestic radon risks? Int J Radiat Biol. 2002;78:593–604. doi: 10.1080/09553000210121740. [DOI] [PubMed] [Google Scholar]
- 36.Shuryak I, Brenner DJ, Ullrich RL. Radiation-induced carcinogenesis: mechanistically based differences between gamma-rays and neutrons, and interactions with DMBA. PLoS One. 2011;6:e28559. doi: 10.1371/journal.pone.0028559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–7. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 38.Shadley JD, Afzal V, Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat Res. 1987;111:510–7. [PubMed] [Google Scholar]
- 39.Yonezawa M, Takeda A, Misonoh J. Acquired radio-resistance after low dose X- irradiation in mice. J Radiat Res. 1990;31:256–62. doi: 10.1269/jrr.31.256. [DOI] [PubMed] [Google Scholar]
- 40.Wolff S. Is radiation all bad? The search for adaptation. Radiat Res. 1992;131:117–23. [PubMed] [Google Scholar]
- 41.Boothman DA, Meyers M, Odegaard E, Wang M. Altered G1 checkpoint control determines adaptive survival responses to ionizing radiation. Mutat Res. 1996;358:143–53. doi: 10.1016/s0027-5107(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 42.Wiencke JK, Afzal V, Olivieri G, Wolff S. Evidence that the [3H]thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1986;1:375–80. doi: 10.1093/mutage/1.5.375. [DOI] [PubMed] [Google Scholar]
- 43.Woloschak GE, Chang-Liu CM, Jones PS, Jones CA. Modulation of gene expression in Syrian hamster embryo cells following ionizing radiation. Cancer Res. 1990;50:339–44. [PubMed] [Google Scholar]
- 44.Sasaki MS. On the reaction kinetics of the radioadaptive response in cultured mouse cells. Int J Radiat Biol. 1995;68:281–91. doi: 10.1080/09553009514551211. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi A. Different inducibility of radiation- or heat-induced p53-dependent apoptosis after acute or chronic irradiation in human cultured squamous cell carcinoma cells. Int J Radiat Biol. 2001;77:215–24. doi: 10.1080/09553000010009495. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi A, Su X, Suzuki H, Omori K, Seki M, Hashizume T, Shimazu T, Ishioka N, Iwasaki T, Ohnishi T. p53-dependent adaptive responses in human cells exposed to space radiations. Int J Radiat Oncol Biol Phys. 2010;78:1171–6. doi: 10.1016/j.ijrobp.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 47.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101:1714–9. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278:11590–600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto H, Hamada N, Takahashi A, Kobayashi Y, Ohnishi T. Vanguards of paradigm shift in radiation biology: radiation-induced adaptive and bystander responses. J Radiat Res. 2007;48:97–106. doi: 10.1269/jrr.06090. [DOI] [PubMed] [Google Scholar]
- 50.Mortazavi SM, Cameron JR, Niroomand-rad A. Adaptive response studies may help choose astronauts for long-term space travel. Adv Space Res. 2003;31:1543–51. doi: 10.1016/s0273-1177(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 51.Huff J, Carnell L, Blattnig S, Chappell L, Kerry G, Lumpkins S, Simonsen L, Slaba T, Werneth C. Evidence Report: Risk of Radiation Carcinogenesis. Houston, TX: NASA; 2016. pp. 1–70. [Google Scholar]
- 52.Buonanno M, De Toledo SM, Howell RW, Azzam EI. Low-dose energetic protons induce adaptive and bystander effects that protect human cells against DNA damage caused by a subsequent exposure to energetic iron ions. J Radiat Res. 2015;56:502–8. doi: 10.1093/jrr/rrv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cucinotta FA. A new approach to reduce uncertainties in space radiation cancer risk predictions. PLoS One. 2015;10:e0120717. doi: 10.1371/journal.pone.0120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcellos-Hoff MH, Blakely EA, Burma S, Fornace AJ, Jr, Gerson S, Hlatky L, Kirsch DG, Luderer U, Shay J, Wang Y, Weil MM. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci Space Res. 2015;6:92–103. doi: 10.1016/j.lssr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B, Wear M. Space radiation and cataracts in astronauts. Radiat Res. 2001;156:460–6. doi: 10.1667/0033-7587(2001)156[0460:sracia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 56.Jones JA, McCarten M, Manuel K, Djojonegoro B, Murray J, Feiversen A, Wear M. Cataract formation mechanisms and risk in aviation and space crews. Aviat Space Environ Med. 2007;78:A56–66. [PubMed] [Google Scholar]
- 57.Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, Olschowka JA, O'Banion MK. Galactic cosmic radiation leads to cognitive impairment and increased Aβ plaque accumulation in a mouse model of Alzheimer's disease. PLoS One. 2012;7:e53275. doi: 10.1371/journal.pone.0053275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cucinotta FA, Alp M, Sulzman FM, Wang M. Space radiation risks to the central nervous system. Life Sci Space Res. 2014;2:54–69. [Google Scholar]
- 59.Parihar VK, Allen B, Tran KK, Macaraeg TG, Chu EM, Kwok SF, Chmielewski NN, Craver BM, Baulch JE, Acharya MM, Cucinotta FA, Limoli CL. What happens to your brain on the way to Mars. Sci Adv. 2015;1:e1400256. doi: 10.1126/sciadv.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Gonzalo R, Baatout S, Moreels M. Impact of particle irradiation on the immune system: from the clinic to Mars. Front Immunol. 2017;8:177. doi: 10.3389/fimmu.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delp MD, Charvat JM, Limoli CL, Globus RK, Ghosh P. Apollo lunar astronauts show higher cardiovascular disease mortality: possible deep space radiation effects on the vascular endothelium. Sci Rep. 2016;6:29901. doi: 10.1038/srep29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cucinotta FA, Hamada N, Little MP. No evidence for an increase in circulatory disease mortality in astronauts following space radiation exposures. Life Sci Space Res. 2016;10:53–6. doi: 10.1016/j.lssr.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Takahashi I, Shimizu Y, Grant EJ, Cologne J, Ozasa K, Kodama K. Heart disease mortality in the life span study, 1950-2008. Radiat Res. 2017;187:319–32. doi: 10.1667/RR14347.1. [DOI] [PubMed] [Google Scholar]
- 64.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer. 2008;8:465–72. doi: 10.1038/nrc2391. [DOI] [PubMed] [Google Scholar]
- 65.Yatagai F, Ishioka N. Are biological effects of space radiation really altered under the microgravity environment? Life Sci Space Res. 2014;3:76–89. [Google Scholar]
- 66.Ikeda H, Souda H, Puspitasari A, Held KD, Hidema J, Nikawa T, Yoshida Y, Kanai T, Takahashi A. Development and performance evaluation of a three-dimensional clinostat synchronized heavy-ion irradiation system. Life Sci Space Res. 2017;12:51–60. doi: 10.1016/j.lssr.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Ikeda H, Souda H, Puspitasari A, Held KD, Hidema J, Nikawa T, Yoshida Y, Kanai T, Takahashi A. A new system for three-dimensional clinostat synchronized X-irradiation with a high-speed shutter for space radiation research. Biol Sci Space. 2016;30:8–16. doi: 10.1016/j.lssr.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Tsuruoka C, Suzuki M, Hande MP, Furusawa Y, Anzai K, Okayasu R. The difference in LET and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiat Res. 2008;170:163–71. doi: 10.1667/RR1279.1. [DOI] [PubMed] [Google Scholar]
- 69.Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol. 2009;85:715–28. doi: 10.1080/09553000903072470. [DOI] [PubMed] [Google Scholar]
- 70.Blakely EA. New measurements for hadrontherapy and space radiation: biology. Phys Med. 2001;17:50–8. [PubMed] [Google Scholar]
- 71.La Tessa C, Sivertz M, Chiang IH, Lowenstein D, Rusek A. Overview of the NASA space radiation laboratory. Life Sci Space Res. 2016;11:18–23. doi: 10.1016/j.lssr.2016.10.002. [DOI] [PubMed] [Google Scholar]