Abstract
Purpose:
In order to take full advantage of proton radiotherapy, the biological effect of protons in normal and tumor tissue should be investigated and understood in detail. The ongoing discussion on variable relative biological effectiveness along the proton depth dose distribution (eg, Paganetti 2015), and also the administration of concomitant treatments, demands dedicated in vitro trials that prepare the translation into the clinics. Therefore, a setup for radiobiological studies and the corresponding dosimetry should be established that enables in vitro experiments at a horizontal proton beam and a clinical 6 MV photon linear accelerator (Linac) as reference.
Methods:
The experimental proton beam at the University Proton Therapy Dresden is characterized by high beam availability and reliability throughout the day in parallel to patient treatment. For cell irradiation, a homogeneous 10 × 10 cm2 proton field with an optional spread-out Bragg-peak can be formed. A water-filled phantom was installed that allows for precise positioning of different sample geometries along the proton path.
Results:
Depth-dose profiles within the phantom and dose homogeneity over different cell samples were characterized for the proton beam and the photon reference source. A daily quality assurance protocol was implemented that provides absolute dose information required for significant and reproducible in vitro experiments. Cell survival test experiments were performed to demonstrate the feasibility of such experiments.
Conclusion:
In the experimental room of the University Proton Therapy Dresden, clinically relevant conditions for proton in vitro experiments have been realized. The established cell phantom and dosimetry facilitate irradiation in an aqueous environment and are transferable to other proton, photon and ion beam facilities. Precise positioning and easy exchange of cell samples, monitor unit-based dose delivery, and high beam availability allow for systematic in vitro experiments. The close vicinity to the radiotherapy and radiobiology departments provides access to clinical linacs and the interdisciplinary basis for further translational steps.
Keywords: proton radiobiology, proton RBE, University Proton Therapy Dresden, proton in vitro setup
Introduction
Motivated by its potential to better spare normal tissue than photon-based radiation therapy (RT), proton therapy is increasingly used to treat tumors of various entities [1]. This is reflected by a steep increase in proton therapy centers in operation, and more than 40 centers are under construction worldwide [2].
Beside the physical characterization of the proton beam, there is also a need for a more profound understanding of the biological action associated with proton radiation to fully utilize the advantages of proton RT. Compared with clinical photons, proton irradiation has a greater biological effectiveness per unit dose than a photon dose yielding the same biological effect, which can be quantified by the relative biological effectiveness (RBE). The RBE is defined as the ratio between photon and particle doses resulting in a biological isoeffect. Although the biological effectiveness of proton irradiation is known to be variable with beam energy and other additional factors that are still subject to debate [eg, 3–7], in clinical routine a generic RBE of 1.1 is used to calculate proton doses. In particular, the influence of physical and biological parameters, such as linear energy transfer (LET) variations along the proton depth-dose distribution, fraction size, and tissue type on RBE [5–11], are under discussion. Moreover, the clinical application of proton RT prompts questions, for example, on normal tissue side effects [12–14], the influence of virus infections [15] and chemotherapy [16], as well as the potential for personalized treatment by means of appropriate biomarkers [1] that have been primarily considered for photon RT.
During the last decade numerous in vitro experiments, in vivo preclinical studies, and clinical trials were performed to resolve some of the questions regarding the biological effect of proton therapy in a translational manner. Exemplarily, variable RBE between ∼1.1 in the mid–spread-out Bragg peak (SOBP) and a maximum of 1.7 in the distal fall-off region were revealed by means of in vitro studies addressing the clonogenic survival after proton versus photon treatment [5]. In vivo experiments so far could not confirm much higher RBE values than 1.1 [6, 17], and clinical evidence for an altered RBE is still sparse [eg, 7, 14].
Despite important limitations for direct translation of cellular findings to in vivo experiments and the clinical setting, a number of important questions related to proton interactions still require elucidation by in vitro investigations: (1) determining RBE values along the depth-dose profile, (2) exploring the combined effect of chemotherapy and proton RT [16], and (3) determining the differential processing of DNA damage after proton and photon irradiation, which might influence treatment outcome and long-term effects [18, 19]. To do so, systematic in vitro experiments are required with human normal tissue and tumor cell lines using the same experimental setup and identical exposure conditions for both the proton beam and the photon reference [8, 20]. Moreover, the translational strategy demands in vitro experiments as close as possible to clinical settings, that is, clinically relevant treatment fields, dosimetry, and irradiation regimens. This includes a clinical linear accelerator (Linac) as photon reference since commonly used orthovoltage x-rays introduce an additional LET effect on the RBE measurement [20].
To meet these requirements, an experimental room has been set up at the University Proton Therapy Dresden (UPTD) providing a fixed horizontal beam line with high proton beam availability for research as well as a clinical Linac in close proximity. Here, the establishment of an irradiation setup is reported that enables in vitro experiments in a patient-like aqueous environment and can be applied at the proton beam and Linac reference. Similar to the clinical routine, daily quality assurance (QA) including absolute dosimetry was introduced, and the experimental workflow was optimized to facilitate a high throughput of cell samples.
Methods and Results
In the following, the features of a flexible setup for clinically relevant irradiation of biological samples with protons and Linac photons at UPTD are described. The basic parameters of the proton beam and the experimental area are briefly summarized [21] followed by a description of the setup for precise positioning, dosimetric characterization, and irradiation of different sample geometries. Finally, first in vitro experiments are described aiming at the clonogenic survival of LN-229 glioblastoma cells after irradiation with a proton SOBP relative to 6 MV Linac photons and the combined effect of temozolomide (TMZ) and proton radiation.
UPTD Proton Beam and Experimental Beamline
The UPTD is clinical therapy facility with a single treatment room and an additional room for experimental purposes allowing for radiation experiments in parallel to medical treatment, that is, on a regular basis and during daytime. Experiments can be performed between patient treatments when the beam is not delivered to the treatment room, for example, while a patient is positioned. A dedicated in-house developed interface and beam control software set the beam parameters for the experimental beamline and ensures the priority of beam requests from the treatment beamline [22]. These settings result in high beam availability for experiments with about 3 time slots of approximately 15 minutes per hour through about 10 hours per day (Figure 1).
Figure 1.
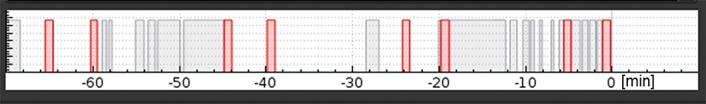
Characteristic beam use of University Proton Therapy Dresden proton beam either by the therapy (red) or by the experimental room (gray).
A fixed horizontal beam line is installed in the experimental room delivering a continuous monoenergetic pencil-like proton beam with energies in the range of 70 to 230 MeV and energy-dependent maximal currents up to 100 nA. A double-scattering device with a ridge-filter for 150 MeV protons is used to provide a laterally extended proton field and an SOBP of variable width for sample irradiation [21]. The extended proton field shows a high degree of dose uniformity in the lateral direction with a relative dose variation of ±2% within the 10 × 10 cm2 field comparable to that in patient treatment at UPTD. In beam direction, homogeneous depth-dose distributions with SOBP widths between 20 and 32 mm in water can be formed by inserting a ridge filter in different angular orientations. Dose rates up to 10 Gy/min in the SOBP are reliably delivered. The double-scattering device is mounted on an optical table and can be quickly installed or removed if necessary.
Cell Phantom for Exact Positioning and Irradiation of Cell Samples
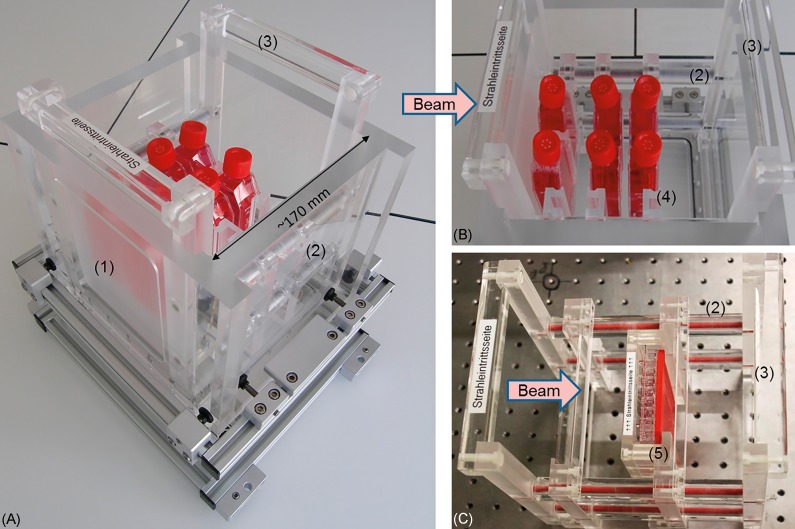
A dedicated water phantom (Figure 2A, hereafter called cell phantom) was designed and built in-house for cell irradiation at well-defined depth-dose positions. It allows for a precise and reproducible positioning of varying cell sample geometries at horizontal beams and, furthermore, provides the option to perform the necessary absolute dosimetry. The phantom consists of an outer polymethylmethacrylate (PMMA) box (PLEXIGLAS GS Röhm AG Switzerland) and vessel holders that can be customized for individual cell sample geometries. A fixed, stable, and adjustable aluminum underframe allows for the fast positioning of the whole phantom relative to the beam axis. All elements of the phantom within the irradiation field are made of PMMA. The outer 18-mm-thick PMMA box with an inner volume of 180 × 180 × 171.9 mm3 (width × height × length, that is, horizontal × vertical lateral extension × depth along the beam axis) can be filled with water to generate a patient-like radiation field. The beam is entering the phantom through a milled 120 × 140 mm2 (width × height) window (Figure 2A) with a reduced thickness of 12.00 ± 0.05 mm (13.96 mm water equivalent path length). The bottom of the phantom has an outlet for fast water exchange and can be supplemented with a heatable aluminum plate to control the temperature of the cell samples during irradiation. The phantom inserts (Figure 2B and 2C) are equipped with four polyvinylchloride bars parallel to the beam axis, whereas PMMA cylinders of varying size on these bars are deployed as spacers to fix individual sample positions in a reproducible way. The inserts, and the respective sample holders mounted thereon, can be completely exchanged in less than 2 minutes.
Figure 2.
(A) Cell phantom including 1 insert with holders for 25 cm2 cell culture flasks at 3 depth positions. (B) The insert with holders for the 25 cm2 cell culture flasks and (C) with holders for the 96-well plates is easily exchangeable and could be equipped with a varying number of holders (see Figure 4 for details). The numbering denotes (1) the beam entrance window, (2) polyvinylchloride bars with polymethylmethacrylate spacers, (3) the exchangeable cell phantom insert, (4) holders for 25 cm2 cell culture flasks, and (5) holders for 96-well plates.
The vessel holders for 25 cm2 cell culture flasks (Figure 2B) allow for fast and reproducible positioning of 2 cell culture flasks within the irradiation field. A cell monolayer can be positioned with an accuracy of ±0.2 mm over the beam axis of ∼170 mm inside the cell phantom by using spacers of appropriate length, whereby production tolerances of the 25 cm2 flasks are considered. Up to 4r vessel holders for 25 cm2 cell culture flasks can be irradiated at the same time in different depths (Figure 2B).
Similar to the holder for cell culture flasks, a holder for 96-well plates was built (Figure 2C). The plates can be positioned upright with a positioning accuracy of ±0.2 mm along the beam axis in the cell phantom. To avoid leakage of culture medium and to limit the volume of the wells, the wells of the 96-well plate can either be closed with a sealing foil or with a 3-dimensional (3D) printed watertight acrylonitrile butadiene styrene (ABS) plastic stamp, which matches the reverse profile of the inner wells (Figures 2C and 3C). The outer rows of the 96-well plates that exceed the 10 × 10 cm2 irradiation field are not used for experiments.
Figure 3.
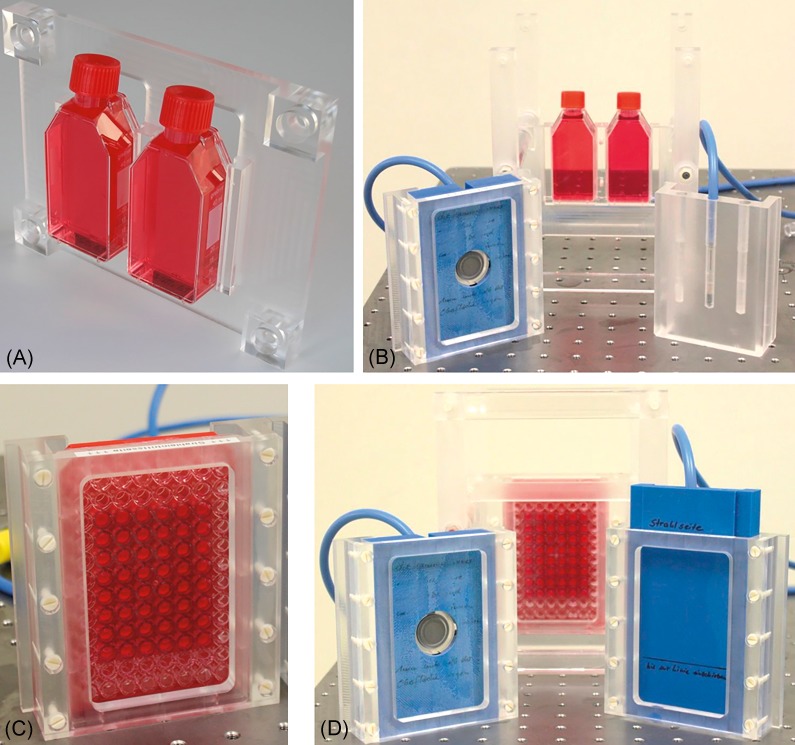
The holders for two 25 cm2 cell culture flasks (A) and one 96-well plate (C) are shown together with the corresponding holders for Markus and semiflex ionization chambers (B, D) that can be used within the cell phantom. Both holders for the semiflex ionization chamber allow measuring at 3 lateral positions, whereas the Markus chamber is positioned on the beam axis.
Proton Depth-Dose Distribution Within the Cell Phantom
The relative depth-dose distribution generated from the 150 MeV proton beam (Figure 4, gray line) by double-scattering and ridge-filter was measured with a capped Markus ionization chamber (IC; model 23343, PTW, Freiburg, Germany) inside a clinical water phantom (BluePhantom, IBA Dosimetry, Schwarzenbruck, Germany). Due to the different design of BluePhantom and cell phantom, the depth-dose position of the cell sample with respect to the relative depth-dose distribution measured within the BluePhantom had to be verified. For this purpose, 2 ICs were subsequently applied— a parallel plate Markus IC and a cylindrical semiflex IC (type: 31010, PTW), both read out online by a Unidos electrometer (PTW). A 3D printed holder (Figure 3B and 3D, material: ABS) was designed to center the Markus IC on the central beam axis and to place the measurement point at the same depth as the cell monolayer in a culture vessel. Similarly, the holders for the semiflex IC ensures that the center of the IC tis aligned with the cell monolayer in a 25 cm2 culture flask (Figure 3B, material: PMMA) and in a 96-well plate (Figure 3d, material: ABS), respectively. It allows for placing the IC at three different lateral positions (left, right, and on beam axis) to check for field homogeneity.
Figure 4.
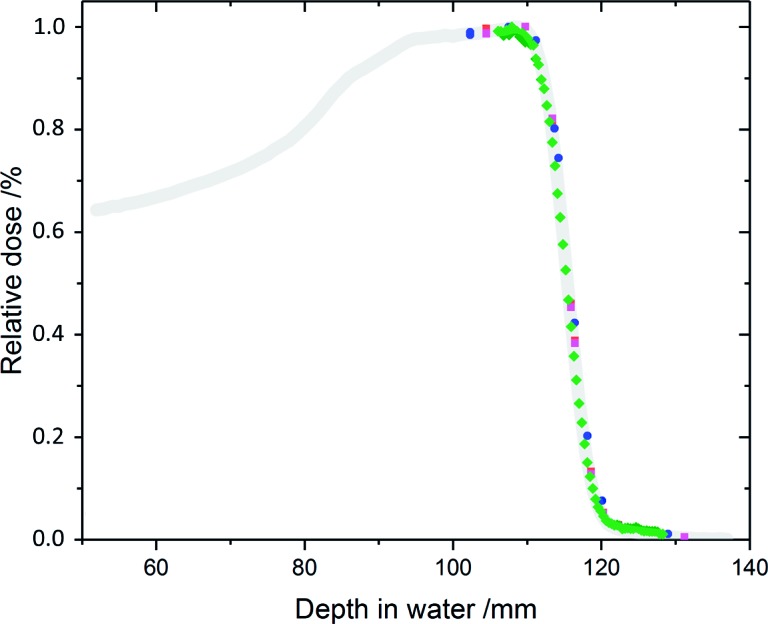
Relative depth-dose distributions measured in the cell phantom applying the semiflex IC (blue) on beam axis, the Markus ionization chamber in flasks right (black) and left (red) from beam axis and a stack of 62 EBT3 films (green) at left flask position. For comparison, the relative depth-dose distribution measured with the Blue Phantom (grey, [20]) is shown.
Both IC (more specifically the effective point of measurement of the Markus IC and the center of the semiflex IC, respectively) were placed at a distance of 89.5 mm from the inner beam entrance window, corresponding to a cell monolayer position in the middle of the SOBP (mid-SOBP). Additionally, relative depth-dose distributions were determined by placing a Markus IC in a cell culture flask with minimum distance to the flask bottom. This setup enables positioning of the Markus IC left and right from the beam axis (see Figure 4 for depth-dose distributions) and considers the influence of the cell culture flask bottom (∼1 mm polystyrene with a water equivalent path lengths (WEPL) of 1.023 [23]). By inserting polycarbonate (PC) and PMMA plates of different thickness in front of the cell phantom, the depth-dose distribution (Figure 4, semiflex on axis) was measured considering a water equivalent thickness of 1.163 for PMMA and 1.150 for PC penetrated by 150 MeV protons. For the cylindrical semiflex IC, a shift of the effective point of measurement of –1.4 ± 0.3 mm (ie, to lower depth) from the cylinder axis was determined and considered for the depth-dose distribution.
Supplementary to the IC measurements, GafChromic EBT3 dosimetry films (ISP Corp, New York, New York) were applied. Stacks of 62 EBT3 films were placed inside a halved cell culture flask with the first film attached to the flask bottom facing the beam in a distance of 89.5 mm from the inner beam entrance window. Additionally, a 2.1-mm PMMA plate was inserted in front of the cell phantom to shift the depth position of the first film of the stack to the last third of the SOBP width. Directly after irradiation, the films were air dried; at least 2 days later they were scanned with an Epson Perfection Flatbed Scanner (Epson, Meerbusch, Germany) and analyzed by IDL (Interactive Data Language, Harris Geospatial Solutions, Exelis Visual Information Solutions GmbH, Gilching, Germany) based software [24]. Relative dose values of the EBT3 films were correlated to the water equivalent depth of the films (Figure 4) using a measured relative WEPL (Ep = 150 MeV) of 1.30 and assuming a film thickness of 280 μm with the sensitive layer at 140 μm. The WEPL was derived from depth-dose measurements (R90 and R50 values) with both the Markus IC in the BluePhantom and the Giraffe detector (IBA Dosimetry) each with and without a stack of 107 EBT3 films (total thickness of 30.0 mm) inserted in the proton beam path.
The comparison of the relative depth-dose distributions (Figure 4) measured inside the cell phantom with Markus and semiflex IC and by EBT3 film stacks revealed consistency between the methods and relative to the measurements with the BluePhantom. Inside the cell phantom, R90 and R50 values (depth where the dose is reduced to 90% and 50% of the dose maximum) of 111.9 ± 0.3 mm and 115.6 ± 0.2 mm, respectively, were derived from the different methods (Semiflex IC, Markus IC, EBT3 films).
For dedicated and systematic experiments, the stability of the proton range is very crucial, since the advantageous depth-dose profile, compared with photons, might be impaired by uncertainties in the precise stopping location of the Bragg peak in stopping materials. Also, range uncertainty is a major source of uncertainty in the dose and radiation quality characterized by LET. Therefore, 2 aspects are important: the stability of the beam energy and, correspondingly, the beam range and precision of the setup. Regular range measurements with the Zebra detector (IBA Dosimetry) revealed a very stable range for the 150 MeV proton beam with 1 standard deviation of ±0.1 mm governed by the measurement uncertainty of the Zebra detector itself. Regarding the second aspect, a precision of ±0.2 mm could be achieved for the positioning of a cell monolayer along the depth dose profile by keeping the cell sample holders on fixed positions defined by certain PMMA spacers. Thereby, production tolerances of the thicknesses of the plastic bottoms of the 25 cm2 are already considered.
Proton Field Dose Homogeneity
The irradiation of extended or multiple samples in parallel requires a laterally homogeneous proton dose at different positions in depth. During setup of the experiment, the lateral dose uniformity of the unaffected radiation field, that is, without cell phantom, was monitored by the 2-dimensional (2D) dose measurements with a Lynx scintillation detector (IBA Dosimetry). Measurements at different depth positions were realized by inserting PC and PMMA plates in the beam path. Lateral dose homogeneity inside the cell phantom could be monitored pointwise by IC measurements left, right, or center on beam axis (as described earlier). For example, the Markus IC readings left and right from the beam axis did not differ significantly in different depth (Figure 4). Likewise, measurements with the semiflex IC in the 3 lateral holder positions revealed dose differences of <0.5%.
Using EBT3 films, the dose homogeneity was investigated at the position of the cell monolayer to take the combined influence of the cell phantom and the cell culture vessel geometry into account. A maximal dose inhomogeneity of ±3.0% was observed for the cell-covered area of 25 cm2 culture flasks positioned either left or right from the beam axis and irradiated in mid-SOBP (see earlier). Taking the inhomogeneity of the radiochromic films and the scanner field into account, the homogeneity measured was compatible with the ±2% dose deviation obtained for the irradiation field without cell phantom. For other cell vessel geometries, such as 96-well plates, EBT3 films or the Lynx detector were positioned behind an empty culture vessel. The resulting dose distributions revealed a homogeneous exposure of the inner 8 × 10 wells of a 96-well plate.
Absolute Dose Delivery and Experiment Routine, Including QA
Similar to RT, the dose delivery in the experimental room of UPTD is controlled by a segmented beam transmission chamber (model 34058, PTW) at beam exit that switches the beam off after reaching a requested number of monitor units (MUs). For all cell culture vessels and sample positions, the correlation between irradiated MUs and absolute dose at cell position was established.
Daily QA measurements ensure sample positioning relative to the depth-dose curve and the lateral dose homogeneity. Unintended shifts in the position of the 2 scatterers or the ripple filter are determined by their influence on lateral dose homogeneity using Lynx measurements and corrected before sample irradiation. After positioning the cell phantom, the depth-dose distribution is verified by semiflex or Markus IC at the desired positions. Moreover, Markus IC measurements at the beginning of each experiment day were applied to correlate the requested MU to absolute dose at cell position for all irradiated sample geometries and beam intensities. For absolute dose determination, a maximum uncertainty of 4.7% was estimated considering the uncertainties of temperature, air pressure, IC calibration, and beam quality correction factor. These measurements were verified at the end of the day and during sample irradiation pauses.
Dosimetric Characterization of the Clinical 6 MV Photon Reference
Reference irradiations with 6 MV photons were performed at a clinical Linac type Artiste (Siemens AG, Erlangen, Germany) with rotating gantry for horizontal beam delivery comparable to the protons.
Radiobiological experiments at the Linac also require knowledge of the photon depth-dose curve, lateral field homogeneity, and absolute dose at cell or sample position but can be related to clinical dosimetry. The lateral homogeneity of the standardized 10 × 10 cm2 field of the 6 MV photon beam is measured in different depths within a BluePhantom equipped with an IC10 (Wellhöfer/IBA Dosimetry; DIN6800-2) calibrated for MV photons.
In addition to the standard clinical output factors, absolute dose, and required MU at cell position in the cell phantom were verified by semiflex IC measurement. The maximum absolute dose uncertainty was 3.7%, including the uncertainty of clinical standard dosimetry and that arising from uncertainties in temperature, air pressure, and depth position. Using EBT3 films, the lateral dose homogeneity inside the cell culture vessels was determined revealing, for example, a dose inhomogeneity <2% for a cell monolayer inside a 25 cm2 cell culture flask.
Proof of Principle Cell Irradiation Experiment
First cell irradiation experiments were performed comparing the clonogenic survival of LN-229 glioblastoma (GBM) cells after irradiation with the proton SOBP and the 6 MV photon reference to demonstrate the applicability of the setup and experimental routines (experiment 1). Moreover, the possibility of experiments with critical timing was proven by investigating the combined effects of temozolomide (TMZ, Sigma Aldrich, Taufkirchen, Germany) and proton irradiation on the cellular survival (experiment 2). Cell line LN-229 was purchased from ATCC (LGC Standards, Wesel, Germany) and maintained as exponential cultures in MEM supplemented with 10% FCS and 1% penicillin/streptomycin (all from Pan-Biotech, Aidenbach, Germany) at 37°C and 5% CO2. For cell survival experiments, 2.5 × 105 cells were seeded in 25 cm2 culture flasks 48 hours before treatment. Following the clinical treatment sequence, one group was pretreated with 50 μM TMZ for 1 hour before irradiation [25]. Directly before treatment at the horizontal beam lines, cell culture flasks were completely filled with cell culture medium, capped with nonventilated caps, and sealed with Parafilm (Pechiney Plastic Packaging, USA via VWR, Dresden, Germany). Control samples were prepared and treated in the same way, except for irradiation; transport and storage of all samples outside the cell laboratory took place in insulated Styropor (Styrofoam) boxes.
Two cell culture flasks were positioned side by side in the cell phantom with the cell monolayer positioned 89.5 mm away from the inner beam entrance window, that is, in mid- SOBP position (see first position of the Markus IC in Figure 4). The proton treatment was performed at a dose rate of 3 Gy/min, comparable to the clinical reference beams, whereas a dose rate of 1.96 Gy/min was achieved at this position for Linac irradiation.
After irradiation, the exposed flasks were stored at 37°C in a shielded part of the experimental room until their activation was below 5 μSv/h local dose rate, which lasts about 5 to 10 minutes depending on the dose delivered to the cell samples. In total, the cell samples have been kept for a maximum of 60 minutes outside the cell laboratory, including transport, positioning, sample exchange, and activity decay. Back in the cell laboratory, the cells were immediately trypsinized, counted, and seeded for colony formation. After 12 days, surviving colonies were stained with Coomassie brilliant blue (Carl Roth, Karlsruhe, Germany). Colonies with more than 50 cells were counted, and the data were normalized to the unexposed controls. The linear quadratic model was used to fit the surviving fraction, SFD = exp(–αD–βD2), as a function of dose D, whereby mean SF values were determined on the basis of 4 and 8 experiment replications for the comparison of photons and protons and the influence of radiochemotherapy, respectively. It has to be noted, that the normalization has been done for each individual replication, that is, before averaging SF values.
The obtained fit parameters for the linear quadratic model for all experiments are summarized in the Table.
Table.
Fit parameters (± uncertainty) returned from a linear quadratic fit of the dose-dependent survival data obtained for photon and proton irradiation alone (experiment 1) and for proton irradiation with or without combined temozolomide (TMZ) treatment (experiment 2).
|
Treatment regimen |
α [Gy−1] |
Δα [Gy−1] |
β [Gy−2] |
Δβ [Gy−2] |
| Experiment 1: Determination of relative biological effectiveness (Figure 5A) | ||||
| Proton | 0.191 | 0.065 | 0.074 | 0.009 |
| Photon | 0.274 | 0.041 | 0.046 | 0.006 |
| Experiment 2: Influence of TMZ on cellular survival (Figure 5B) | ||||
| Single proton treatment (normalized to k1) | 0.129 | 0.050 | 0.100 | 0.011 |
| Combined effect of proton treatment with TMZ (normalized to k2) | 0.387 | 0.095 | 0.085 | 0.024 |
The RBE values were analyzed by comparing the dose values at 10% survival level for mid-SOBP protons relative to 6 MV photons (Figure 5A). No significant difference in clonogenic survival was observed for this exemplary test experiment. Further, the influence of TMZ on the proton dose response of LN-229 glioblastoma was investigated. The obtained survival data were normalized to the respective unirradiated controls, that is, k1 for proton treatment without and k2 for proton treatment with TMZ. The corresponding dose response curves for proton treatment alone (open squares) and for the combined treatment with protons and TMZ (filled squares) are shown in Figure 5B. The effect of the combination treatment on cell survival was larger with a significantly increased α parameter (P = .016) than radiation alone, whereas no significant change was observed for the β value. Hence, the treatment with TMZ increased the radiation sensitivity of the cells. In order to illustrate the combined effect of TMZ for proton irradiation, the dose response curve for combined treatment is additionally shown (red dots) after multiplying it with a factor of 0.15, that is, normalization to the ratio of the averaged k1 and averaged k2 (<k1>/<k2>).
Figure 5.
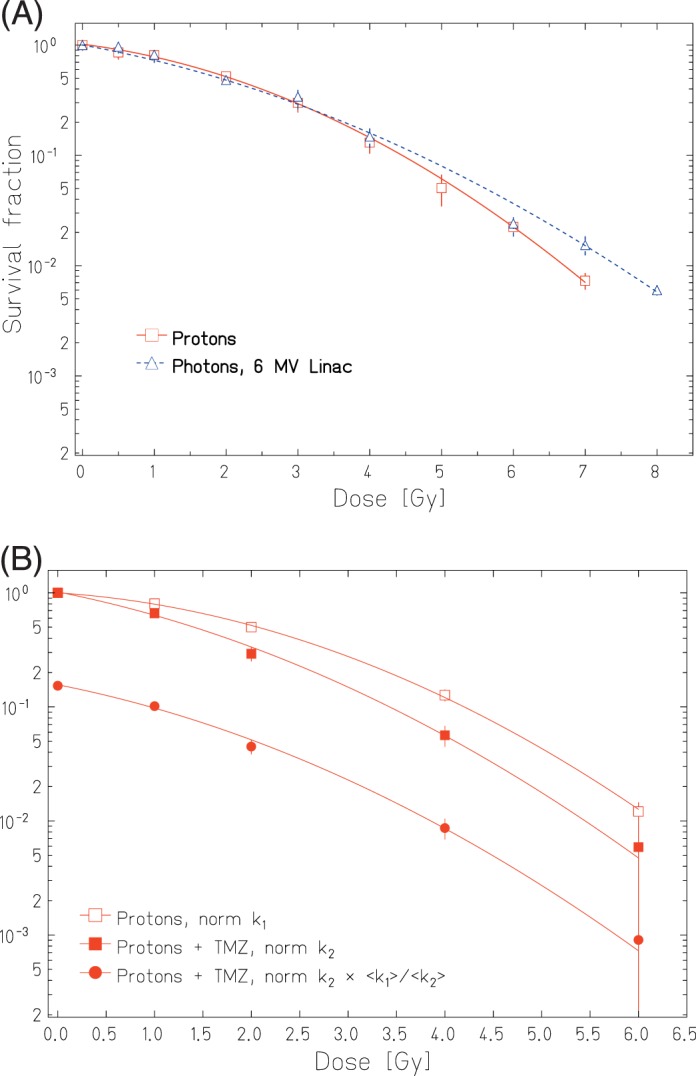
(A) Experiment 1: Cellular survival curves measured for LN229 cells treated with 150 MeV protons in mid–spread-out Bragg peak and with 6 MV Linac photons. (B) Experiment 2: Dose-dependent clonogenic survival obtained after treatment with 150 MeV mid–spread-out Bragg peak protons alone (open squares) and in combination with (B) Experiment 2: Dose-dependent clonogenic survival obtained after treatment with 150 MeV mid-SOBP protons alone (open squares) and in combination with TMZ (filled symbols). The dose response curves for combined treatment are shown twice: normalized to the unirradiated control k2 (filled squares) and additionally multiplied by the ratio of the averaged k1 and k2 (filled dots). The plotted survival fraction values are shown with their corresponding standard error of the mean.
Discussion
In the experimental room at UPTD, a cell phantom and corresponding absolute and relative dosimetry were established in combination with a beam shaping setup that provides clinical relevant SOBPs and a 10 × 10 cm2 homogenous proton field for 150 MeV protons [21]. The cell phantom facilitates in vitro experiments by providing fast, reproducible, and precise positioning of various cell culture vessels at dedicated depth-dose positions for both the proton beam and the 6 MV clinical photon reference. Additionally, different types of ICs can be integrated providing the dose at cell position for different cell culture vessels from rather small 25 cm2 culture flasks to extended 96-well plates (Figure 3). The established daily QA validates homogeneity of the irradiation field, depth-dose and actual MU-to-absolute-dose correlation at cell position. The application of the cell phantom together with the high proton beam availability enable high throughput and systematic in vitro experiments, making UPTD an attractive research site for internal and potential external users [26]. Moreover, the cell phantom and the dosimetric protocols for protons and the MV photon reference are also easily transferable to other proton or ion facilities.
Here, the dosimetry inside the cell phantom was only demonstrated for 1 standard proton field, while the established methodology enables acquisition of all necessary parameters for other proton energies and field geometries as well. In doing so, the application of the cell phantom is not limited to 2D cell monolayers; the irradiation of 3D cell spheroids of about half a millimeter in size to millimeter-thick patient-derived organoids and zebra fish embryos is also feasible. Moreover, following the clinical trend of using pencil beam scanning (PBS) instead of passive scattering, a new beamline is currently installed in the experimental room of the UPTD providing PBS for radiobiological and physical experiments. Again, the established dosimetry protocols and cell phantom will enable rapid implementation of radiobiological experiments at the new PBS beamline.
To summarize, all necessary requirements for systematic radiobiological in vitro studies are established at UPTD, and, after the first successful experiments, a variety of such studies are currently ongoing. The high accuracy of cell culture vessel positioning along the depth-dose distribution allows for the investigation of the proton RBE dependence on particle energy and LET. A survey of the existing literature reveals that RBE values higher than the clinical applied RBE of 1.1 were found at the end of the SOBP by in vitro [3–5] and in vivo [6, 27] experiments. However, owing to the uncertainties associated with a direct translation of in vitro results to the patient world and a lack of clinical evidence for an enhanced RBE, the value of 1.1 is still applied in the clinics [28]. To reduce the RBE uncertainty in proton RT treatment planning [5, 7, 10, 29, 30], in vitro data need to be backed up by in vivo studies. Accordingly, the described setup was currently extended to allow for small animal irradiation studies [25, 26, 31].
However, before entering the phase of animal or even clinical trials, cell studies are needed to identify underlying mechanisms of action. Exemplarily, potential influence of proton RT on the action of chemotherapeutic substances, an issue that is thoroughly investigated for photon RT, could be investigated on different normal and malignant cell lines [1, 32]. Moreover, the influence of virus infections [15], the usage of different DNA repair ways after proton compared with photon RT [18,19] or the detection and validation of biomarkers that might help to stratify patients in responders and nonresponders to proton RT [1] could be studied using a dedicated panel of relevant cell lines. Additionally, systematic in vitro data are required to feed Monte-Carlo simulations that model the RBE of proton beams and serve as basis for treatment planning software [26, 29, 30].
As a next step, the translation of in vitro findings and research questions, such as the quantification of normal tissue toxicity and potential differences of the immune response after proton and photon irradiation, demand hypothesis-driven in vivo trials, which are presently prepared at UPTD [26, 27, 31]. The ongoing installation of the PBS beam line will further increase the experimental possibilities, for example, direct side-by-side comparison of the impact of passive and active beam shaping on the biological dose response, and allow researchers to answer questions prompted by the increasing application of PBS for patient treatment at UPTD.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts to disclose.
Acknowledgements: The authors are grateful to Dr Cordelia Hoinkis, Dr Stefan Menkel, and Dr Daniela Kunath for information and discussions on clinical dosimetry of the linear accelerator and the proton beam. We also thank Dr Benjamin Lutz for experimental assistance and the local IBA team at UPTD for technical support and stable beam delivery. The authors wish to thank Dorothee Pfitzmann and Nadine von Auw for excellent technique assistance with cell experiments.
References
- 1.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, Richter C, Zips D, Bortfeld T. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–49. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Particle Therapy Co-Operative Group. Proton centers and carbon ion centers worldwide. http://www.ptcog.ch/index.php/facilities-in-operation Updated May 2017. Accessed May 18, 2018.
- 3.Britten RA, Nazaryan V, Davis LK, Klein SB, Nichiporov D, Mendonca MS, Wolanski M, Nie X, George J, Keppel C. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res. 2013;179:21–8. doi: 10.1667/RR2737.1. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary P, Marshall TI, Perozziello FM, Manti L, Currell FJ, Hanton F, McMahon SJ, Kavanagh JN, Cirrone GA, Romano F, Prise KM, Schettino G. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys. 2014;90:27–35. doi: 10.1016/j.ijrobp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–72. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 6.Slabbert J, Martinez J, De Coster BM, Gueulette J. Increased proton relative biological effectiveness at the very end of a spread-out Bragg peak for jejunum irradiated ex vivo. Int J Particle Ther. 2015;2:37–43. [Google Scholar]
- 7.Lühr A, von Neubeck C, Krause M, Troost EGC. Relative biological effectiveness in proton beam therapy—current knowledge and future challenges. Clin Transl Radiat Oncol. 2018;9:35–41. doi: 10.1016/j.ctro.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzscheiter MH, Bassler N, Dosanjh M, Sørensen BS, Overgaard J. A community call for a dedicated radiobiological research facility to support particle beam cancer therapy [editorial] Radiother Oncol. 2012;105:1–3. doi: 10.1016/j.radonc.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones B. Why RBE must be a variable and not a constant in proton therapy. Br J Radiol. 2016;89:20160116. doi: 10.1259/bjr.20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underwood T, Paganetti H. Variable proton relative biological effectiveness: how do we move forward? Int J Radiat Oncol Biol Phys. 2016;95:56–8. doi: 10.1016/j.ijrobp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Giantsoudi D, Sethi RV, Yeap BY, Eaton BR, Ebb DH, Caruso PA, Rapalino O, Chen YL, Adams JA, Yock TI, Tarbell NJ, Paganetti H, MacDonald SM. Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: LET and RBE associations for areas of injury. Int J Radiat Oncol Biol Phys. 2016;95:287–96. doi: 10.1016/j.ijrobp.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, Debus J, Adeberg S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192:759–69. doi: 10.1007/s00066-016-1005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, Mohan R, Grosshans DR. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401. doi: 10.1016/j.radonc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohaus F, Linge A, Tinhofer I, Budach V, Gkika E, Stuschke M, Balermpas P, Rödel C, Avlar M, Grosu AL, Abdollahi A, Debus J, Bayer C, Belka C, Pigorsch S, Combs SE, Mönnich D, Zips D, von Neubeck C, Baretton GB, Löck S, Thames HD, Krause M, Baumann M. DKTK-ROG. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Radiother Oncol. 2014;113:317–23. doi: 10.1016/j.radonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Grassberger C, Paganetti H. Methodologies in the modeling of combined chemo-radiation treatments. Phys Med Biol. 2016;61:R344–69. doi: 10.1088/0031-9155/61/21/R344. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen BS, Bassler N, Nielsen S, Horsman MR, Grzanka L, Spejlborg H, Swakoń J, Olko P, Overgaard J. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol. 2017;56:1387–91. doi: 10.1080/0284186X.2017.1351621. [DOI] [PubMed] [Google Scholar]
- 18.Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA, Pruschy MN. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol. 2015;116:374–80. doi: 10.1016/j.radonc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary P, Marshall TI, Currell FJ, Kacperek A, Schettino G, Prise KM. Variations in the processing of DNA double-strand breaks along 60-MeV therapeutic proton beams. Int J Radiat Oncol Biol Phys. 2016;95:86–94. doi: 10.1016/j.ijrobp.2015.07.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid TE, Greubel C, Dollinger G, Schmid E. The influence of reference radiation photon energy on high-LET RBE: comparison of human peripheral lymphocytes and human-hamster hybrid AL cells. Radiat Environ Biophys. 2017;56:79–87. doi: 10.1007/s00411-016-0680-3. [DOI] [PubMed] [Google Scholar]
- 21.Helmbrecht S, Baumann M, Enghardt W, Fiedler F, Krause M, Lühr A. Design and implementation of a robust and cost-effective double-scattering system at a horizontal proton beamline. J Instrum. 2016;11:T11001. [Google Scholar]
- 22.Kormoll T, Duplicy A, Enghardt W, Helmbrecht S, Gonzalez FH. A beam control system for an experimental beam line operated parallel to a therapeutic beam line. Radiother Oncol. 2014;110:S52–3. [Google Scholar]
- 23.Zhang R, Taddei PJ, Fitzek MM, Newhauser WD. Water equivalent thickness values of materials used in beams of protons, helium, carbon and iron ions. Phys Med Biol. 2010;55:2481–93. doi: 10.1088/0031-9155/55/9/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeil K, Beyreuther E, Lessmann E, Wagner W, Pawelke J. Cell irradiation setup and dosimetry for radiobiological studies at ELBE. Nucl Instrum Methods Phys Res B. 2009;267:2403–10. [Google Scholar]
- 25.Kil WJ, Cerna D, Burgan WE, Beam K, Carter D, Steeg PS, Tofilon PJ, Camphausen K. In vitro and In vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–8. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 26.Dosanjh M, Jones B, Pawelke J, Pruschy M, Sørensen BS. Overview of research and therapy facilities for radiobiological experimental work in particle therapy. Report from the European Particle Therapy Network radiobiology group. Radiother Oncol. doi: 10.1016/j.radonc.2018.03.008. [DOI] [PubMed]
- 27.Lühr A, von Neubeck C, Helmbrecht S, Baumann M, Enghardt W, Krause M. Modeling in vivo relative biological effectiveness in particle therapy for clinically relevant endpoints. Acta Oncol. 2017;56:1392–8. doi: 10.1080/0284186X.2017.1356468. [DOI] [PubMed] [Google Scholar]
- 28.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50:135–42. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 29.Grün R, Friedrich T, Krämer M, Scholz M. Systematics of relative biological effectiveness measurements for proton radiation along the spread out Bragg peak: experimental validation of the local effect model. Phys Med Biol. 2017;62:890–908. doi: 10.1088/1361-6560/62/3/890. [DOI] [PubMed] [Google Scholar]
- 30.Patel D, Bronk L, Guan F, Peeler CR, Brons S, Dokic I, Abdollahi A, Rittmüller C, Jäkel O, Grosshans D, Mohan R, Titt U. Optimization of Monte Carlo particle transport parameters and validation of a novel high throughput experimental setup to measure the biological effects of particle beams. Med Phys. 2017;44:6061–73. doi: 10.1002/mp.12568. [DOI] [PubMed] [Google Scholar]
- 31.Müller J, Neubert C, von Neubeck C, Baumann M, Krause M, Enghardt W, Bütof R, Dietrich A, Lühr A. Proton radiography for inline treatment planning and positioning verification of small animals. Acta Oncol. 2017;56:1399–1405. doi: 10.1080/0284186X.2017.1352102. [DOI] [PubMed] [Google Scholar]
- 32.Coleman CN, Higgins GS, Brown JM, Baumann M, Kirsch DG, Willers H, Prasanna PG, Dewhirst MW, Bernhard EJ, Ahmed MM. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res. 2016;22:3138–47. doi: 10.1158/1078-0432.CCR-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]