Abstract
Purpose:
Clinical trials (CTs) in proton beam therapy (PBT) are important for determining its benefits relative to other treatments. An analysis of PBT trials is, thus, warranted to understand the current state of PBT CTs and the factors affecting current and future trials.
Materials and Methods:
We queried the clinicaltrials.gov Website using the search terms: proton beam therapy, proton radiation, and protons. A total of 152 PBT CTs were identified. We used χ2 analysis and logistic regression to evaluate trial characteristics.
Results:
Most CTs were recruiting (n = 79; 52.0%), phase II (n = 95; 62.5%), open label (n = 134; 88.2%), single-group assignment (n = 84; 55.3%), and with primary treatment endpoints of safety and efficacy (n = 94; 61.8%). The primary treatment sites included gastrointestinal (n = 32; 21.1%), central nervous system (n = 31; 20.4%), lung (n = 21; 13.8%), prostate (n = 19; 12.5%), sarcoma (n = 15; 9.9%), and others (n = 24; 15.8%). Comparison studies between radiation modalities involved PBT and intensity-modulated photon therapy (n = 11; 7.2%), PBT and general photon therapy (n = 8; 5.3%), and PBT and carbon-ion therapy (n = 7; 4.6%). The PBT CTs underwent substantial growth after 2008 but now appear to be in decline. Nongovernmental institutions, comprising university centers, hospital systems, and research groups, have funded the greatest number of CTs (n= 106; 69.7%). The National Institutes of Health (NIH) were more likely to fund CTs involving the central nervous system (P = 0.02). Trials involving NIH funding were more likely to result in successful trial completion (P = 0.02).
Conclusion:
Among PBT CTs, most were phase II trials, with a very few being phase III CTs. Funding of PBT CTs originating from industry or the NIH is limited. Recently, there has been a declining trajectory of newly initiated PBT trials. It is not yet clear whether this represents a true trend or just a pause in CT implementation. Despite multiple impediments to PBT CTs, the particle therapy community continues to work toward evidence generation.
Keywords: proton beam therapy, clinical trials, clinicaltrials.gov
Introduction
There is increasing interest in proton beam therapy (PBT) [1]. Because the physical properties of charged particle therapy result in superior dose distribution, PBT offers potential opportunities to reduce toxicity and increase tumor control [1, 2]. However, the high cost of PBT has led to scrutiny regarding the quality of evidence required to accept the superiority of PBT over standard photon therapy [3]. Current evidence most strongly supports PBT in the treatment of pediatric malignancies [4–7] and sarcomas of the skull base and spine [3, 8–10]. Nevertheless, a recent systematic review highlighted the lack of high-quality, prospective, and long-term clinical data, even in the field of PBT for pediatric malignancies [11]. With more operating proton centers in the United States and worldwide, opportunities for prospective clinical trials have never been greater [12, 13].
As part of the US Food and Drug Administration (FDA) Modernization Act of 1997, the clinicaltrials.gov registry was established to provide accessible data to patients about ongoing clinical trials (CTs). To ensure compliance with trial registration on clinicaltrials.gov, a Food and Drug Administration Modernization Act amendment was passed to increase the scope of trials meeting the threshold of mandatory registration [14]. Additionally, the International Committee of Medical Journal Editors began requiring CT registration as a prerequisite for trial publication [14, 15]. Consequently, clinicaltrials.gov provides a large repository of CT data for analysis. Trials registered on clinicaltrials.gov are those meeting the criteria of the FDA Amendment Act Section 801, and its definition of applicable clinical trials [16]. Briefly, applicable CTs include those using drugs and products subject to regulation by the FDA. Additionally, applicable CTs must have one or more sites in the United States, have a drug or device registered for FDA investigational drug approval, or involve a drug or device manufactured for research in the United States or its territories. Using the clinicaltrials.gov database, we sought to evaluate the landscape of CTs involving PBT during the past 20 years to understand the focus and trends in PBT CTs, the factors predictive of trial funding and trial completion, and the present direction of PBT CTs.
Materials and Methods
On February 16, 2016, we performed an advanced search on clinicaltrials.gov using the search terms proton beam therapy OR proton radiation OR protons. Additionally, we used the following exclusion terms to narrow our search to only radiation therapy–related trials: NOT proton pump inhibitors, NOT proton spectroscopy. Trials were then limited to interventional CTs only, and observational trials were excluded. The resulting CTs were downloaded and individually analyzed to identify trials incorporating PBT. This yielded 152 CTs.
From this portfolio of trials, we extracted relevant trial characteristics and categorized them as follows: sex of enrollees (males, females, or both), trial recruitment status (active, completed, withdrawn, suspended, or terminated), age categorization of enrollees (pediatric, adults, or seniors), trial location (North America, Europe, Asia), randomization (randomized or nonrandomized), masking (open label, single blind, or double blind), primary treatment site (prostate, gastrointestinal tract, central nervous system [CNS], lungs, and other), endpoint classification (safety, efficacy, safety and efficacy, or bioequivalence), intervention model (single group or parallel group), and trial phase (0, I, II, III, or IV). Funding sources were classified as National Institutes of Health (NIH), industry, or “other.” Trials classified as other encompassed university centers, hospital systems, and research groups. For the purposes of this study, university centers, hospital systems, and research groups will be termed nongovernmental institutions (NGIs).
We used χ2 analysis to determine the role of trial characteristics in successful trial completion. In addition, χ2 analysis and univariate logistic regression were employed to compare trial characteristics on funding sources. Multivariate analysis was not used because of limitations arising from the small cohort. Statistical significance was established as P < 0.05. STATA, version14.0 was used for all statistical computations.
Results
Clinical Trial Demographics, Study Design, and Treatments
We analyzed 152 CTs involving PBT (Table 1). We found most CTs to be active and recruiting (n = 79; 52.0%), involving adults only (n = 124; 81.6%), and open to both sexes (n = 121; 79.6%). Trials were predominately located in North America (all in the United States; n = 131; 86.2%). European CTs constituted 6.5% (n = 10) of all trials, with 70% (7 of 10) of those originating from Germany. Trials conducted in Asia accounted for 7.2% (n = 11) of all CTs; among which, 82% (9 of 11) originated from South Korea. The median and mean target enrollments were 55.8 and 112.0 patients, respectively. Final results were available for only 7.2% (n = 11) of the trials at the time of this study.
Table 1.
Characteristics of proton beam therapy clinical trials.
|
Characteristics |
No. of trials (N = 152) |
Trials, % |
| Primary site | ||
| Gastrointestinal system | 32 | 21.1 |
| Central nervous system | 31 | 20.4 |
| Lung | 21 | 13.8 |
| Prostate | 19 | 12.5 |
| Breast | 10 | 6.6 |
| Sarcoma | 15 | 9.9 |
| Eye | 8 | 5.3 |
| Other | 16 | 10.5 |
| Sex | ||
| Female | 10 | 6.6 |
| Male | 21 | 13.8 |
| Both | 121 | 79.6 |
| Age | ||
| Children included | 28 | 18.4 |
| Adult Only | 124 | 81.6 |
| Location | ||
| North America | 131 | 86.2 |
| Europe | 10 | 6.6 |
| Asia | 11 | 7.2 |
| Randomization | ||
| Randomized | 35 | 23.0 |
| Nonrandomized | 37 | 24.3 |
| Unspecified | 80 | 52.6 |
| Treatment endpoint | ||
| Safety and efficacy | 94 | 61.8 |
| Efficacy | 28 | 18.4 |
| Safety | 7 | 4.6 |
| Bioequivalence | 1 | 0.7 |
| Unspecified | 22 | 14.5 |
| Intervention model | ||
| Single group | 84 | 55.3 |
| Parallel group | 55 | 36.2 |
| Unspecified | 11 | 7.2 |
| Masking | ||
| Open label | 134 | 88.2 |
| Single blind | 4 | 2.6 |
| Double blind | 2 | 1.3 |
| Unspecified | 12 | 7.9 |
| Recruiting status | ||
| Active, recruiting | 79 | 52.0 |
| Active, not recruiting | 37 | 24.3 |
| Complete | 13 | 8.6 |
| Terminated | 12 | 7.9 |
| Not yet recruiting | 6 | 3.9 |
| Withdrawn | 5 | 3.3 |
An analysis of the CT study design showed distinct characteristics. Trial masking involved open-label (n = 134; 88.2%), single-blind (n = 4; 2.6%), double-blind (n = 2; 1.3%), or unspecified (n = 12; 7.9%) parameters. Trial intervention models were single-group assignment (n = 84; 55.3%), parallel-group assignment (n = 55; 36.2%), or unspecified (n = 11; 7.2%). Primary endpoint classifications of CTs were safety and efficacy (n = 94; 61.8%), efficacy only (n = 28; 18.4%), safety only (n = 7; 4.6%), or unspecified (n = 22; 14.5%) (Table 1).
The primary treatment sites were the gastrointestinal tract (n = 32; 21.1%), CNS (n = 31; 20.4%), lung (n = 29; 13.8%), prostate (n = 19; 12.5%), sarcoma (n = 15; 9.9%), breast (n = 10; 6.5%), eye (n = 8; 5.3%), and others (n = 16; 10.5%) (Table 1). Trials categorized as others referred to gynecologic, lymphoma, bladder, testicular, and head and neck sites. There were 134 CTs (88.2%) in which the primary treatment modality was PBT. The remaining CTs (n = 18; 11.8%) involved chemotherapy, surgery, hormones, or monoclonal antibodies with PBT serving as only one radiation therapy treatment option among other alternatives, such as intensity-modulated photon radiation therapy and 3-dimensional conformal radiation therapy.
Radiation Modality Comparisons and Trial Randomizations
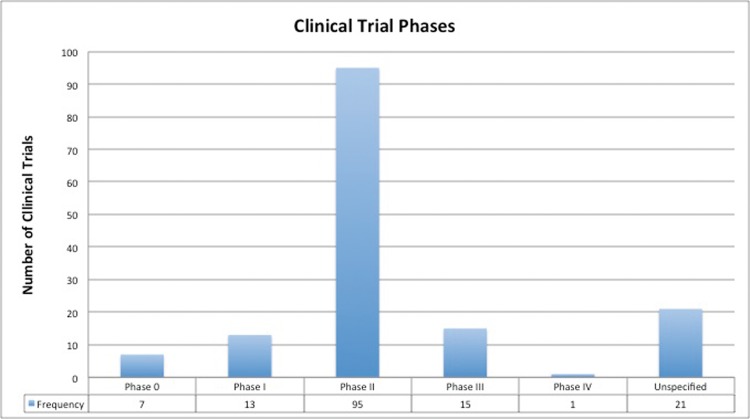
There were 28 (18.4%) CTs that involved direct comparison of PBT with other radiation modalities. Those comparisons centered on efficacy, safety, or both. A total of 11 CTs involved comparisons specifically between PBT and intensity-modulated photon radiation therapy (7.2%), 8 CTs involved comparisons between PBT and photon therapy (without specifying photon technique; 5.3%), 7 CTs involved comparisons between PBT and carbon ion (4.6%), and 2 CTs compared PBT with radiofrequency ablation (1.3%). Only 72 (47.4%) CTs were labeled as either randomized (n = 35; 23.0%) or nonrandomized (n = 37; 24.3%), with the remainder being unspecified (n = 80; 52.6%). Phase II CTs (n = 95; 62.5%) were the most predominant trial design, followed by phase III, phase I, and phase 0 CTs (Figure 1). Of the trials that were classified as randomized, 12 were phase III randomized CTs (RCTs). These RCTs included prostate cancer (n = 6), chondrosarcoma (n = 1), chordoma (n = 1), hepatocellular carcinoma (n = 1), non–small cell lung cancer (n = 1), esophageal cancer (n = 1), and head and neck cancer (n = 1). They were conducted in the United States (n = 9), Germany (n = 2), and Taiwan (n = 1). Only 1 RCT was conducted before the year 2000, another RCT occurred between 2000 and 2009, whereas the remainder occurred subsequent to 2009.
Figure 1.
Phases of clinical trials in proton beam therapy.
Frequency of Proton Beam Therapy Trials
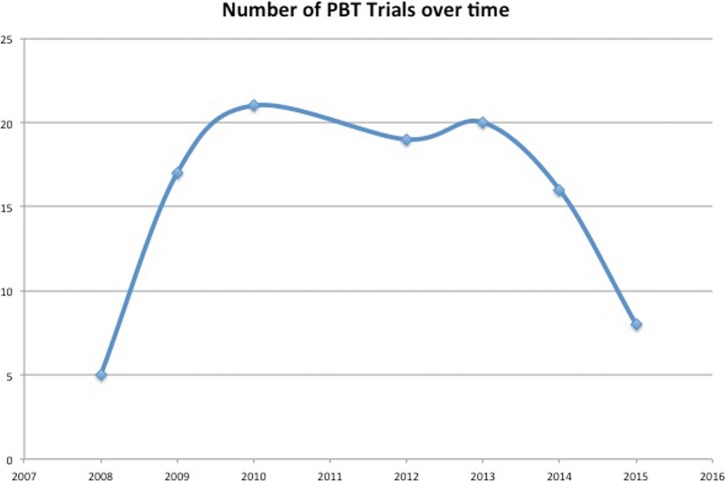
Although, in this study, we report on CTs that were initiated after 1996, we limited our assessment of the rate of change of new CTs to those beginning in 2008 because compliance with trial registration increased after the federal mandate in 2007 requiring registration of trials on clinicltrials.gov. A total of 5 CTs were initiated in 2008 and reached a peak of 21 CTs initiated in 2010. After that, there was a gradual decrease in new trial initiation, with the most recent results from 2015 showing only 8 new PBT CTs (Figure 2). This decline, however, was not found to be statistical significant (P > 0.05) during the past 5 years, probably because of the small cohort.
Figure 2.
Number of proton beam therapy clinical trials over time.
Factors Influencing Funding of Proton Beam Therapy Clinical Trials
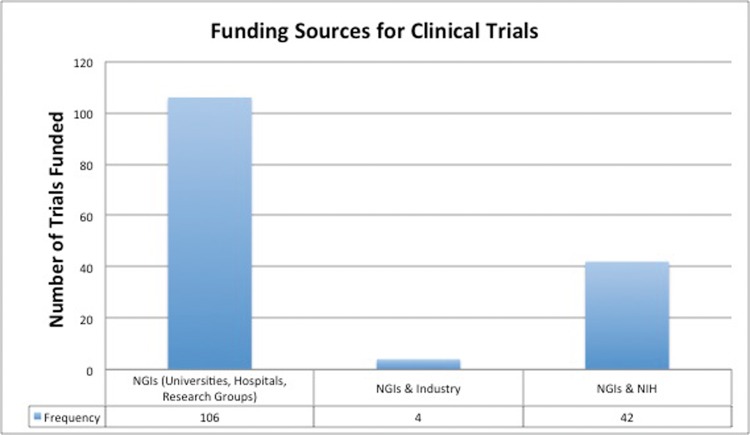
University centers, hospital systems, and research groups, labeled as NGIs in this study, funded the most CTs (n = 106; 69.7%), followed by a collaborative work between the NIH and NGIs (n = 42; 27.6%), and industry and NGIs (n = 4; 2.6%). There were no PBT CTs funded exclusively by industry or the NIH (Figure 3). When NIH funded trials were compared with non–NIH-funded trials, primary disease site and the location of trial were significant (P < 0.05; Table 2). Specifically, the NIH was more likely to fund CTs involving CNS and lung sites (P < 0.01) and CTs located in North America (P < 0.01). On univariate analysis, CTs involving CNS treatment was predictive of NIH funding (P = 0.02), whereas CTs involving lung treatments trended toward significance (P = 0.07; Table 2).
Figure 3.
Funding sources for proton beam therapy clinical trials.
Table 2.
Funding source by trial characteristics.
|
Characteristics |
NIH |
Non-NIH institutions |
Univariate regression |
|||||
|
No. of trials |
Trials, % |
No. of trials |
Trials, % |
P
value |
OR |
95% CI |
P
value |
|
| Primary site | ||||||||
| Prostate | 3 | 7 | 16 | 15 | 0.01 | 1.00 | 1.00–1.00 | - |
| Gastrointestinal tract | 6 | 14 | 26 | 24 | 1.23 | 0.27–5.62 | 0.79 | |
| Central nervous system | 16 | 38 | 15 | 14 | 5.69 | 1.37–23.54 | 0.02 | |
| Lung | 9 | 21 | 12 | 11 | 4.00 | 0.89–18.03 | 0.07 | |
| Other | 8 | 19 | 41 | 37 | 1.04 | 0.24–4.42 | 0.96 | |
| Sex | ||||||||
| Female | 2 | 5 | 8 | 7 | 0.26 | 1.00 | 1.00–1.00 | - |
| Male | 3 | 7 | 18 | 16 | 0.67 | 0.09–4.80 | 0.69 | |
| Both | 37 | 88 | 84 | 76 | 1.76 | 0.36–8.70 | 0.49 | |
| Location | ||||||||
| North America | 42 | 100 | 89 | 81 | 0.01 | NA | NA | NA |
| Europe | 0 | 0 | 10 | 9 | NA | NA | NA | |
| Asia | 0 | 0 | 11 | 10 | NA | NA | NA | |
| Trial phase | ||||||||
| Phase 1 | 2 | 6 | 11 | 12 | 0.65 | 1.00 | 1.00–1.00 | NA |
| Phase 2 | 27 | 79 | 68 | 76 | 2.18 | 0.45–10.51 | 0.33 | |
| Phase ¾ | 5 | 15 | 11 | 12 | 2.50 | 0.40–15.75 | 0.33 | |
| Randomization | ||||||||
| Nonrandomized | 10 | 50 | 27 | 52 | 0.88 | 1.00 | 1.00–1.00 | NA |
| Randomized | 10 | 50 | 25 | 48 | 1.08 | 0.38–3.03 | 0.88 | |
| Age group | ||||||||
| Adults only | 32 | 76 | 92 | 84 | 0.29 | 1.00 | 1.00–1.00 | NA |
| Pediatric allowed | 10 | 24 | 18 | 16 | 1.60 | 0.67–3.82 | 0.29 | |
| Trial status | ||||||||
| Active/completed | 39 | 93 | 90 | 82 | 0.37 | 1.00 | 1.00–1.00 | NA |
| Terminated or withdrawn | 2 | 5 | 15 | 14 | 0.32 | 0.07–1.45 | 0.14 | |
Abbreviations: CI, confidence interval; NA, not applicable; NIH, National Institutes of Health; OR, odds ratio.
Trial Success and Failure
Trials were classified as active and recruiting (n = 79; 52.0%), active and not recruiting (n = 37; 24.3%), completed (n = 13; 8.6%), terminated (n = 12; 7.9%), not yet recruiting (n = 6; 3.9%), or withdrawn (n = 5; 3.3%). For the purposes of our study, we classified a CT as successful if it was active or completed. On χ2 analysis, trials involving collaboration between the NIH and NGIs were more likely to result in successful trial outcomes in comparison to trials involving NGIs alone or an NGIs-industry partnerships (P = 0.02; Table 3).
Table 3.
Trial failure by trials characteristics.
|
Characteristics |
Success |
Failure |
|||
|
No. of trials |
Trials, % |
No. of trials |
Trials, % |
P
value |
|
| Primary Site | |||||
| Prostate | 18 | 13 | 1 | 6 | 0.07 |
| Gastrointestinal tract | 27 | 20 | 5 | 29 | |
| Central nervous system | 31 | 23 | 0 | 0 | |
| Lung | 16 | 12 | 5 | 29 | |
| Other | 43 | 32 | 6 | 35 | |
| Sex | |||||
| Female | 8 | 6 | 2 | 12 | 0.65 |
| Male | 19 | 14 | 2 | 12 | |
| Both | 108 | 80 | 13 | 76 | |
| Location | |||||
| North America | 115 | 85 | 16 | 94 | 0.49 |
| Europe | 10 | 7 | 0 | 0 | |
| Asia | 10 | 7 | 1 | 6 | |
| Trial phase | |||||
| Phase I | 9 | 8 | 4 | 9 | 0.09 |
| Phase II | 87 | 78 | 8 | 87 | |
| Phase III | 14 | 13 | 1 | 14 | |
| Phase IV | 1 | 1 | 0 | 1 | |
| Randomization | |||||
| Nonrandomized | 30 | 48 | 7 | 78 | 0.09 |
| Randomized | 33 | 52 | 2 | 22 | |
| Age group | |||||
| Adults only | 110 | 81 | 14 | 82 | 0.93 |
| Pediatric allowed | 25 | 19 | 3 | 18 | |
| Funding source | |||||
| Industry | 2 | 1 | 2 | 12 | 0.02 |
| National Institutes of Health | 40 | 30 | 2 | 12 | |
| Other | 93 | 69 | 13 | 76 | |
Discussion
In this study, we report on the status of 152 PBT CTs that were initiated between 1996 and 2016 and were registered on clinicaltrials.gov. We observed marked variability in the frequency of new proton trials over time, with recent years showing a declining trajectory. We found most trials to be phase II trials, with few randomized phase III CTs. Universities, hospitals, and research groups were the largest source of trial funding, with little contribution from industry sources. Treatment site was predictive of the source of funding. Lastly, the likelihood of the successful trial completion was associated with the source of the trial funding.
The number of proton centers in the United States has increased in recent years. Within the past 6 years (June 2010 to June 2016), 16 of the 23 proton treatment centers (70%) in the United States began operations [13, 17]. This increase, however, has been accompanied by increasing calls for more evidence-based CTs to corroborate the theoretic benefits of PBT [3, 18]. In the present study, we observed that in the past 5 years, there actually has been a trend toward a decrease in the number of new PBT trials, although that trend was not statistically significant.
Phase III CTs accounted for only 9% of all PBT trials. Some have challenged the need for randomized prospective CTs in PBT, largely because equipoise cannot be maintained in the setting of the improved dose distribution of PBT [19, 20]. Moreover, cost effectiveness alone may be insufficient grounds to launch a phase III trial [21]. Cox [22] detailed challenges inherent in conducting PBT-specific CTs. Among many concerns, he noted the difficulty associated with conducting an unbiased CT when study participants do not have equal access to both treatment arms because of current restrictions associated with PBT insurance coverage. In response to those concerns, other models for acquiring high-quality evidence have been suggested, such as registries, case control, and cohort studies [18, 23, 24].
Funding for CTs evaluating advanced technologies such as PBT is a major impediment to reversing the downward trend that we observed [22]. In the present study, we sought to investigate the state of funding of PBT CTs. We observed funding of PBT CTs from industry sources to be significantly limited, accounting for only 3% of all PBT trials. In contrast to those findings, results from another study investigating the proportion of funding from industry sources among all oncologic CTs, showed that industry funding constituted 42% of funding efforts [25]. This variance is significant, but not unexpected, given the limitations both in insurance coverage, even for patients on CT [26], and in the involvement of the pharmaceutical industry. Consequently, the funding to establish the value of PBT has been placed uniquely on the shoulders of universities, hospitals, and research groups, who at present, are independently funding 70% of all PBT CTs. Expanding coverage with evidence development would provide both a needed financial foundation and an incentive to expand clinical trial participation [27].
Although initiating clinical trials is important to the future of PBT, successful completion of clinical trials is equally critical. Poor accrual and other barriers of completion are not necessarily unique to PBT, but randomization between standard and advanced technologies must be carefully considered in trial design. We noted 11% of CTs experiencing termination, withdrawal, or suspension. A similar incidence rate of 12% was reported by another study looking at CTs involving all types of radiation therapy [28]. In the present study, we found that, among CTs involving funding from the NIH, there was a greater likelihood of successful trial completion in comparison to non–NIH-funded CTs. This underscores the potential importance of collaborations between the NIH and other institutions in PBT CTs.
We note several limitations in our study. The quality of our study was dependent on the degree of compliance with which administrators registered CTs on the clinicaltrials.gov Website. Although regulations have been implemented to ensure timely trial registration, the degree of improved compliance is unclear. Additionally, a few of the CT characteristics were not reported by CT administrators, resulting in incomplete details about trial characteristics, design, and outcomes. Finally, in light of the specific FDA stipulations required for qualification to register CTs on clinicaltrials.gov [16], it is likely that PBT CTs conducted outside the United States may not meet the criteria for mandatory reporting. This could result in limited capture of international PBT CTs.
Conclusion
We note that PBT CTs focused on a diverse range of malignancies. In spite of the appeal to increase PBT CTs, there has not been an associated increase in recent years. Phase II trials represent the largest type of PBT CTs, and only a few trials employed a phase III design. Phase III RCTs may be appropriate for some, but certainly not all, these questions. There are challenges to PBT trial funding, with minimal support originating from industry, and only modest support from the NIH. Prospective clinical trial data may best answer many critical questions raised by the increasing use of PBT. The goal should be to employ the most appropriate clinical trial design to ensure successful trial completion and generation of the highest-quality evidence.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Zietman AL. Particle therapy at the “tipping point”: an introduction to the Red Journal's special edition. Int J Radiat Oncol Biol Phys. 2016;95:1–3. doi: 10.1016/j.ijrobp.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 2.Foote RL, Stafford SL, Petersen IA, Pulido JS, Clarke MJ, Schild SE, Garces YI, Olivier KR, Miller RC, Haddock MG, Yan E, Laack NN, Arndt CA, Buskirk SJ, Miller VL, Brent CR, Kruse JJ, Ezzell GA, Herman MG, Gunderson LL, Erlichman C, Diasio RB. The clinical case for proton beam therapy. Radiat Oncol. 2012;7:174. doi: 10.1186/1748-717X-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brada M, Pijls-Johannesma M, De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15:319–24. doi: 10.1097/PPO.0b013e3181b6127c. [DOI] [PubMed] [Google Scholar]
- 4.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, Jones RM, MacDonald SM, Pulsifer MB, Lavally B, Abrams AN, Huang MS, Marcus KJ, Tarbell NJ. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17:287–98. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald SM, Trofimov A, Safai S, Adams J, Fullerton B, Ebb D, Tarbell NJ, Yock TI. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121–9. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 6.De Amorim Bernstein K, Sethi R, Trofimov A, Zeng C, Fullerton B, Yeap BY, Ebb D, Tarbell NJ, Yock TI, MacDonald SM. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. Int J Radiat Oncol Biol Phys. 2013;86:114–20. doi: 10.1016/j.ijrobp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93:400–7. doi: 10.1016/j.ijrobp.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, Schuller JC, Pedroni E, Goitein G. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–8. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 9.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, Chen YL, Schwab JH, Hornicek FJ. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–22. doi: 10.1002/jso.23617. [DOI] [PubMed] [Google Scholar]
- 10.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, Plastaras J, Bucci MK, Yock TI, Bonilla L, Price R, Harris EE, Konski AA. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol. 2012;103:8–11. doi: 10.1016/j.radonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Leroy R, Benahmed N, Hulstaert F, Van Damme N, De Ruysscher D. Proton therapy in children: a systematic review of clinical effectiveness in 15 pediatric cancers. Int J Radiat Oncol Biol Phys. 2016;95:267–78. doi: 10.1016/j.ijrobp.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Jarosek S, Elliott S, Virnig BA. Data Points Publication Series. Rockville, MD: Agency for Healthcare Research and Quality; 2012; Proton beam radiotherapy in the U.S. Medicare population: growth in use between 2006 and 2009—data point 10. [PubMed] [Google Scholar]
- 13.National Association for Proton Therapy. Proton therapy centers. 2016 http://www.proton-therapy.org/map.htm Accessed May 30,
- 14.US National Institutes of Health. History of Clinicaltrials.gov. 2016 https://clinicaltrials.gov/ct2/about-site/history Accessed May 30,
- 15.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–47. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 16.US National Institutes of Health. Which trials must be registered and have results submitted to clinicaltrials.gov? 2016 https://clinicaltrials.gov/ct2/manage-recs/fdaaa#WhichTrialsMustBeRegistered Accessed May 30,
- 17.Particle Therapy Co-Operative Group. Proton centers and carbon ion centers worldwide. 2016 http://www.ptcog.ch/index.php/facilities-in-operation Accessed May 30,
- 18.Macbeth FR, Williams MV. Proton therapy should be tested in randomized trials. J Clin Oncol. 2008;26:2590–1. doi: 10.1200/JCO.2008.16.5514. author reply 3–6. [DOI] [PubMed] [Google Scholar]
- 19.Suit H, Kooy H, Trofimov A, Farr J, Munzenrider J, DeLaney T, Loeffler J, Clasie B, Safai S, Paganetti H. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted?: no. Radiother Oncol. 2008;86:148–53. doi: 10.1016/j.radonc.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26:175–6. doi: 10.1200/JCO.2007.14.4329. [DOI] [PubMed] [Google Scholar]
- 21.Glimelius B, Montelius A. Proton beam therapy—do we need the randomised trials and can we do them? Radiother Oncol. 2007;83:105–9. doi: 10.1016/j.radonc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Cox JD. Impediments to comparative clinical trials with proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:4–8. doi: 10.1016/j.ijrobp.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Miller RC, Lodge M, Murad MH, Jones B. Controversies in clinical trials in proton radiotherapy: the present and the future. Semin Radiat Oncol. 2013;23:127–33. doi: 10.1016/j.semradonc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Widder J, van der Schaaf A, Lambin P, Marijnen CA, Pignol JP, Rasch CR, Slotman BJ, Verheij M, Langendijk JA. The quest for evidence for proton therapy: model-based approach and precision medicine. Int J Radiat Oncol Biol Phys. 2016;95:30–6. doi: 10.1016/j.ijrobp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K, Schulman KA, Dilts DM, Abernethy AP. Characteristics of oncology clinical trials: insights from a systematic analysis of clinicaltrials.gov. JAMA Intern Med. 2013;173:972–9. doi: 10.1001/jamainternmed.2013.627. [DOI] [PubMed] [Google Scholar]
- 26.Kerstiens J, Johnstone PA. Proton therapy expansion under current United States reimbursement models. Int J Radiat Oncol Biol Phys. 2014;89:235–40. doi: 10.1016/j.ijrobp.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Bekelman JE, Hahn SM. Reference pricing with evidence development: a way forward for proton therapy. J Clin Oncol. 2014;32:1540–2. doi: 10.1200/JCO.2014.55.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cihoric N, Tsikkinis A, Aebersold DM, Lössl K, Zaugg K. Analysis of clinical trials in radiation oncology: a systematic characterization of the clinicaltrials.gov database. Int J Radiat Oncol Biol Phys. 2014;90:S581–2. [Google Scholar]