Abstract
Purpose:
Radiation therapy (RT) improves local tumor control in axial chondrosarcomas (CS). It is, however, often difficult to safely deliver the high doses (range, 70.2-77.4 Gy) required for achieving a high likelihood of local control, especially in the spine, using photons. This, however, can be achieved with proton beam therapy (PBT) due to its unique physical characteristics. The main goal of our study is to evaluate the outcomes of CS patients treated with passive scattered PBT.
Materials and Methods:
Forty-four patients (N = 44) were identified who received PBT as part of their treatment from 1990 to 2012. A retrospective review of their medical and RT treatment records was conducted. Multivariate analyses were performed to identify patient- and tumor-related factors predicting for improved local control and overall survival.
Results:
Median age was 45.5 years and 55% were female. Median tumor size was 13 cm. Most common anatomical location was the spine (80%). Median follow-up was 29.1 months. Median external beam RT dose was 70.2 Gy relative biological effectiveness (RBE) at 1.8 Gy (RBE) per fraction typically administered using a combination of photon RT + PBT (77%) or PBT alone (23%). Local control was 76% and 57%, and overall survival was 90% and 68% at 2 and 5 years, respectively. Toxicity was acceptable, with the most frequent being wound complications (16%). On multivariate analyses, grade III tumors were significantly associated with decreased local control (P = 0.019), while female sex (P = 0.037) and grade III tumors (P = 0.005) were associated with a poorer overall survival.
Conclusions:
High-dose proton-based RT in combination with surgery resulted in local tumor control in most of these high-risk CS patients. Female sex was predictive for decreased survival, while higher tumor grade (grade III) was predictive of decreased local control and survival. Proton beam therapy is an attractive treatment modality for these challenging tumors.
Keywords: chondrosarcoma, extracranial, proton beam therapy, particle therapy, radiation therapy
Introduction
Chondrosarcomas (CS) are a rare heterogeneous group of bone tumors characterized by cartilaginous matrix deposition with an incidence of 1 in 200 000 per year in the United States [1]. These tumors can occur in both the appendicular and axial skeleton. Although surgical resection is the mainstay of local management, tumors in the spine and some pelvic sites are often difficult to resect with negative margins, given their proximity to and often involvement of adjacent critical organs [2–4]. Depending on their grade, they can be indolent or aggressive [4, 5]. Surgical margins correlate with prognosis with higher local control (∼80%) reported for en bloc resections with negative margins [6, 7]. Significant factors associated with worse prognosis include increasing patient age, primary surgery outside of a tumor center, and an incisional biopsy versus a noninvasive diagnostic procedure [4].
Adjuvant radiation therapy (RT) has been added to surgery to improve local control in CS [3, 8, 9]. Radiation techniques have included conformal radiation, stereotactic body RT, image-guided RT, and intensity-modulated RT (IMRT) to stereotactic radiosurgery [8, 10, 11]. Radiation therapy has also been used definitively in select patients who cannot undergo a surgery or elect not to [9, 12]. Chondrosarcomas are relatively radioresistant, and high RT doses are required to achieve local control rates in the absence of surgery, using doses like those employed for most soft tissue sarcomas [9, 13]. It is dosimetrically challenging to deliver doses >70 Gy safely using photons without exceeding the tolerance of adjacent or surrounding normal tissues because of the immediate proximity of a critical normal structure (eg, spinal cord) or a large volume of normal tissue exposed to a high integral dose (eg, bowel). Protons (and other heavy charged particles) deposit most of their energy in a relatively narrow “Bragg peak” at the end of their range, in tissue that allows for a sharp dose falloff with no exit dose beyond the tumor target. This, along with a sharp lateral dose falloff, allows for dose escalation to the tumor, while better sparing the surrounding normal healthy tissues [14]. A previous study evaluated the role of high-dose RT incorporating proton beam therapy (PBT) in spine sarcomas but included only 14 (28%) patients with CS [9]. The role of PBT in management of skull-base CS has been well established, with excellent local control rates as high as 98% at 10 years [15–17]. We conducted the current study with the goal to evaluate the outcomes in patients with extracranial non–skull-base CS treated with PBT.
Materials and Methods
Under an institutional review board–approved protocol, 44 patients (N = 44) who received PBT as part of their treatment for CS from 1990 to 2012 were identified from institutional proton and sarcoma databases. A retrospective review of their medical and RT treatment records was then conducted. External beam RT was delivered once daily using PBT alone (n = 10) or a combination of PBT and photons (n = 34). Photon technique was 3-dimensional (3-D) conformal initially and IMRT after 2003. Proton beam therapy was delivered using 3-D, passively scattered protons in all patients. Radiation Therapy was delivered with a component of preoperative RT in 50% of patients, as exclusively postoperative RT in 41% and as definitive nonsurgical therapy in 9%.
Our approach to treatment field design, outlining volumes, and radiation techniques has been previously described in detail [3, 9, 18].
Preoperative RT was given with 2 types of fractionation schemes. One consisted of a short course of preoperative RT 19.8 Gy (RBE) in 11 fractions to an initial clinical target volume (CTV1) that generally included approximately 1- to 2-cm radial margins on gross disease; and for spine tumors, this included the entire involved vertebra(e) plus 1 vertebra above and below the involved segment. This short course preoperative RT was primarily used to prevent tumor-cell seeding in cases where tumor cut-through or marginal resection was anticipated; use of this low dose obviated the need to include operative field (all incision, hardware, drain sites) in the radiation target volume. Surgery, performed within 2 weeks of completion of radiation, was done with the availability of intraoperative dural plaque brachytherapy for spinal tumors touching the dural surface, followed by additional postoperative radiation of another 30.6 Gy (RBE) in 17 fractions to a CTV similar to the initial CTV1 to bring the total dose to CTV1 to 50.4 Gy (RBE). Additional boost radiation of 19.8 Gy (RBE) in 11 fractions was delivered to the tumor bed with 0.5 to 1.0 cm margin (CTV2; ie, high-risk margin) to bring the total dose to 70.2 Gy (RBE).
The other preoperative RT scheme employed 50.4 Gy (RBE) in 28 fractions with surgery (with availability of intraoperative dural plaque) performed 4 to 6 weeks after RT. Postoperatively, an additional 19.8 Gy (RBE) was delivered to CTV2.
For patients undergoing exclusively postoperative radiation, 50.4 Gy (RBE) was delivered to CTV1 as described above, enlarged at the discretion of the treating physician to include the operative field (incision, hardware, drain sites at risk for tumor implants) if felt to be technically feasible and physiologically tolerable. The high-risk tumor bed CTV2 was boosted to 70.2 Gy (RBE) for microscopic margins. With all 3 types of adjuvant RT described above, any gross residual disease would receive an additional 7.2 Gy (RBE) for a cumulative total of 77.4 Gy (RBE).
For patients who were inoperable or declined surgery, definitive RT consisted of 50.4 Gy (RBE) delivered to CTV1 as described above followed by a boost of 27 Gy (RBE) to the gross tumor for a cumulative dose of 77.4 Gy (RBE).
Univariate and multivariate analyses were performed to identify any patient- or tumor-related factors predictive for improved local control and overall survival. Kaplan-Meier method was used to estimate local control and overall survival.
Results
The median age was 45.5 years (range, 4-83 years). Median follow-up was 29.1 months (range, 3-96.8 months). Most patients were female (55%). The median tumor size was 13 cm (range, 1-27 cm). The most common anatomic location was the spine (80%). Proton beam therapy was used in the primary setting in the majority (73%) of patients and less frequently for recurrent CS (27%). Tumor grades were I (27%), II (59%), and III (14%). Most common histology was mixed myxoid and hyaline type (43%) followed by hyaline type (25%). Proton beam therapy was used preoperatively in 50%, postoperatively alone in 41%, and as definitive, nonsurgical therapy in 9%. At surgery, an R0 resection was achieved in 25%, an R1 in 14%, an R2 in 50%, and it was not reported (NR) in 11%. Patient and tumor characteristics are listed in Table 1.
Table 1.
Patient and tumor characteristics.
|
Characteristics |
No. of patients |
Percentage (%) |
| Sex | ||
| Male | 20 | 45 |
| Female | 24 | 55 |
| Anatomic site | ||
| Spine | 35 | 80 |
| Pelvis | 6 | 14 |
| Head and neck | 2 | 4 |
| Thorax | 1 | 2 |
| Pathology | ||
| Not specified | 6 | 14 |
| Myxoid | 2 | 5 |
| Mesenchymal | 4 | 8 |
| Hyaline | 11 | 25 |
| Dedifferentiated | 2 | 5 |
| Mixed | 19 | 43 |
| American Joint Committee on Cancer Stage | ||
| IA | 20 | 45 |
| IB | 8 | 18 |
| IIA | 2 | 5 |
| IIB | 2 | 5 |
| Recurrent | 12 | 27 |
| Primary versus recurrence | ||
| Primary | 32 | 73 |
| Recurrence | 12 | 27 |
| Intraspinal versus extraspinal | ||
| Intraspinal | 30 | 68 |
| Extraspinal | 14 | 32 |
| Tumor grade | ||
| I | 12 | 27 |
| II | 26 | 59 |
| III | 6 | 14 |
| Surgery, extent | ||
| Gross total resection | 22 | 50 |
| Subtotal resection | 18 | 41 |
| Biopsy | 4 | 9 |
| Margin status | ||
| R0 | 11 | 25 |
| R1 | 6 | 14 |
| R2 | 19 | 50 |
| Not reported | 8 | 11 |
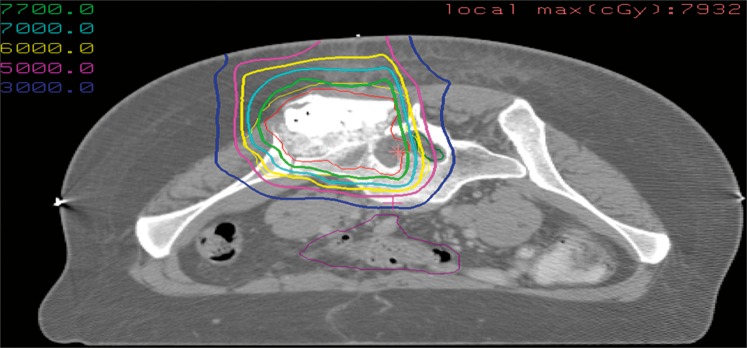
Median external beam RT dose was 70.2 Gy (RBE) (range, 19.8-77.4 Gy) at 1.8 Gy per fraction, typically administered using a combination of photon RT + PBT (77%) or PBT alone (23%). One patient only received 19.8 Gy preoperatively and was noted to have negative margins. Owing to wound-healing concerns, this patient was subsequently not given a postoperative boost. Intraoperative RT was used in 5 patients. This delivered a boost dose of 10 to 12 Gy using electrons (2 patients), or dural isotope–based plaque brachytherapy with yttrium (Y90, 2 patients), or phosphorus (P32, 1 patient). A representative PBT plan with treatment schema is illustrated in Figure 1. Lumbar instillation of contrast material was used to help better delineate the spinal cord and aid planning.
Figure 1.
A representative proton beam therapy plan with treatment schema.
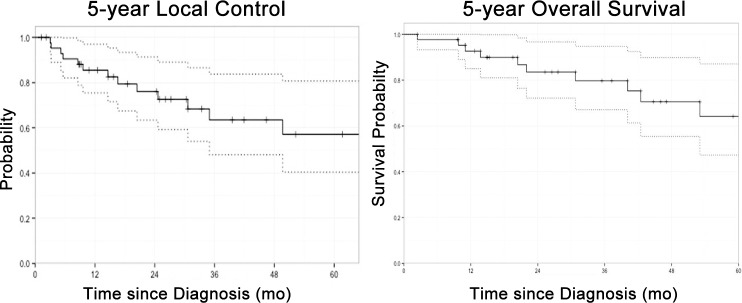
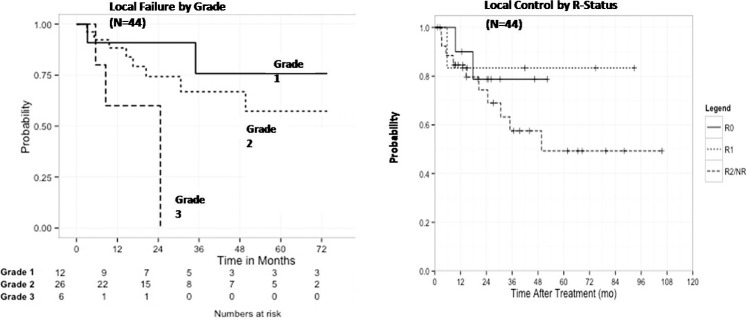
On multivariate analyses, grade III tumors were significantly associated with decreased local control (P = .019) (Table 2; Figures 2 and 3), while female sex (P = .037) and grade III tumors (P = .005) were associated with a poorer overall survival (Table 3; Figures 2 and 3). Local control at 3 years as a factor of extent of resection was 78.8% (R0), 83.3% (R1), and 57.5% (R2/NR) (P = .547) (Figure 3). Eleven patients failed locally at median time of 30.6 months (range, 13-88 months). Overall local control was 76% at 3 years and 57% at 5 years. Three patients had progressive disease locally immediately after completion of treatment. Distant metastases developed in 10 patients. Sites of distant failure were in the lungs (n = 7), bones (n = 1), and both bone and lungs (n = 2). Salvage surgery and chemotherapy were attempted when possible. The impact of chemotherapy was very limited. Overall survival was 90% at 2 years and 68% at 5 years.
Table 2.
Multivariate analysis, local control.
|
Variable |
Hazard ratio |
95% Confidence interval |
P
value |
| Sex | |||
| Female | 1.00 | - | - |
| Male | 0.34 | 0.06 to 2.01 | .233 |
| Tumor grade | |||
| I | 1.00 | - | - |
| II | 2.29 | 0.23 to 22.48 | .476 |
| III | 26.46 | 1.73 to 405.27 | .019 |
| Primary or recurrence | |||
| Primary | 1.00 | - | - |
| Recurrence | 1.92 | 0.43 to 8.46 | .391 |
| Site of tumor | |||
| Non-spine | 1.00 | - | - |
| Spine | 1.45 | 0.11 to 18.48 | .777 |
| Resection margin | |||
| R0 | 1.00 | - | - |
| R1 | 0.88 | 0.05 to 16.07 | .928 |
| R2 | 1.69 | 0.22 to 12.91 | .611 |
| Not reported | 0.25 | 0.01 to 6.47 | .405 |
Figure 2.
The 5-year local control (left) and the 5-year overall survival (right).
Figure 3.
Local failure by grade (left) and local control by R status (right).
Table 3.
Multivariate analysis, overall survival.
|
Variable |
Hazard ratio |
95% Confidence interval |
P
value |
| Sex | |||
| Male | 1.00 | - | - |
| Female | 13.33 | 1.16 to 152.63 | .037 |
| Tumor grade | |||
| I | 1.00 | - | - |
| II | 1.37 | 0.14 to 13.08 | .782 |
| III | 62.91 | 3.46 to 1144.52 | .005 |
| Primary or recurrence | |||
| Primary | 1.00 | - | - |
| Recurrence | 4.16 | 0.67 to 25.87 | .127 |
| Resection margin | |||
| R0 | 1.00 | - | - |
| R1 | 0.16 | 0.01 to 2.95 | .220 |
| R2 | 0.79 | 0.11 to 5.80 | .813 |
| Not reported | 0.0 | 0.00 to inf | .999 |
Toxicities were acceptable, with the most frequent being wound complications seen in 5 of 22 patients (23%) treated preoperatively and 2 of 18 (11%), treated postoperatively. This was most commonly treated with debridement and packing. Only 1 of these 7 patients had a major reconstruction failure requiring surgical correction with instrumentation. There was one grade 2 and one grade 3 radiation neuropathy observed but no myelopathies. Of these 2, one patient developed new-onset paresthesia approximately 27 months after receiving 74 Gy to the pelvis with definitive intent, while another developed bowel and bladder dysfunction 12 months after receiving 77.4 Gy to the sacrum for a subtotally resected tumor. Other toxicities included chronic pain (5%), sacral fracture (5%), and hypothyroidism (2%).
Discussion
Surgical resection remains the mainstay of therapy in CS. This procedure is unfortunately often not possible because of the risk of surgical morbidity resulting in high rates of local failure of 24% to 75% [2, 7, 13]. Although CS is the most common primary bone tumor in adults, fewer than 12% occur in the spine [7, 13]. Boriani et al [7] showed that en bloc resection with negative margins can reduce the local failure rate to as low as 8%, even in the spine location. Radiation therapy is a useful adjuvant treatment and appears to offer excellent and durable local control where wide surgical resection is difficult to accomplish [8]. Chemotherapy has unfortunately not proven to be effective in improving outcomes [19], and distant metastases remain a significant problem in high-grade CS, with rates as high as 43% [13].
At our center, high-dose proton-based RT (>70 Gy) and has been our preferred approach resection for patients with axial CS [9, 13]. Radiation therapy dose limitations of bowel and spinal cord are considered to be approximately 50 Gy [20, 21]. Conventional photon techniques were not able to achieve high tumor doses without exceeding these constraints, whereas PBT can achieve doses >70 Gy with significantly better sparing of these critical organs at risk [12, 22]. Dosimetric studies have shown a significant advantage of PBT over photon techniques, including IMRT, in normal tissue sparing [14]. Long-term results with median follow-up of 7.3 years (from a prospective phase II study of 50 patients with spine sarcomas, of which 14 [28%] were CS), showed a local control rate of 85% at 8 years for primary tumors; however, local control for patients with primary tumors recurrent after prior surgery was only 50%, suggesting that high-risk patients are best treated with adjuvant radiation at the time of initial presentation [23]. Late morbidity was acceptable with no late neurologic toxicities noted at doses ≤72.0 Gy, while 3 sacral neuropathies appeared after doses of 75.6 to 77.4 Gy among patients with definitively treated tumors or R1/R2 resection.
There are few series evaluating the role of RT in CS in the extracranial setting. In a study from Princess Margaret Hospital, Goda et al [8] reported on 60 patients who underwent surgery and photons for extracranial high-risk CS. Preoperative RT (median dose 50 Gy) and postoperative RT (median dose 60 Gy) were used in 40% and 60% of the patients, respectively. Sites included pelvis/lower extremity (48%), chest wall (22%), spine/paraspinal (17%), and head and neck (13%). Median follow-up was 75 months. The crude local control rate was 90%. Patients with R0, R1, and R2 resections had local control of 100%, 94%, and 42%, respectively. Several important distinctions exist between our study and that by Goda et al [8], which could explain the comparatively lower local control rates seen in our experience. Our patients had tumors that were approximately twice as large (median size, 13 versus 7 cm), and we had more spine/paraspinal cases (80% versus 17%); thus, as expected, we had fewer R0 resections (25% versus 50%) and more R2 resections (50% versus 13%). Of note, in the study by Goda et al [8], the local control in patients with R2 resection was only 42% as compared with 57.5% in our study when resection was R2/NR. Several series have documented poor local control rates in CS of the spine with failure occurring in 24% to 75% of cases [2, 7, 13]. Despite these high-risk features, our reported local control rates of 76% at 2 years and 57% at 5 years compare favorably.
Tumor grade is a well-established prognostic factor, with higher grades having worse outcomes [1, 4–6, 15]. In the study by Goda et al [8], grade III tumors had a significantly poorer progression-free survival (PFS) (P = .0003). Our study also found this to be true with grade III tumors significantly associated with both decreased local control (P = .019) and a poorer overall survival (P = .005). Sex differences in outcomes of CS, unlike chordomas, are not well established or understood, with decreased survival seen in our study but not in others [24, 25].
Techniques in PBT delivery have ranged from a passive delivery system [9, 16], which incorporates beam modifiers to produce dose distribution via passive scattering, to recently a more refined active spot scanning, which utilizes narrow pencil-beam scanning protons [24, 25]. Charged particles heavier than protons, such as carbon ions, are also being evaluated. Carbon may have a therapeutic advantage over protons because of its excellent dose profile, higher RBE, and lower oxygen dependence. Randomized studies comparing carbon ion and protons for skull-base CS as well as skull-base and sacral chordomas are currently in progress at the Heidelberg Ion-Beam Therapy Center, University of Heidelberg, Germany [26]. Kamada et al [27] reported on 57 patients with 64 sites of bone and soft tissue sarcomas, not suited for resection, who received carbon ion radiotherapy; the overall local control rates were 88% and 73% at 1 year and 3 years, respectively.
A large systematic review of 63 articles [28] concluded that when wide or marginal margins (en bloc) are achieved in surgical resection of CS (and chordomas) of the spine, there is a decrease in local recurrence and mortality (Strong Recommendation, Moderate Quality Evidence). Radiation therapy of at least 60 to 65 Gy is indicated as an adjuvant treatment for CS (and chordomas) of the spine when there has been incomplete resection or an intralesional margin (Weak Recommendation, Low Quality Evidence). We thus believe that our study contributes to existing literature on the use of RT for CS. We acknowledge that because of the rarity of spine CS and the limited (although increasing) number of PBT centers in the United States, our study has some limitations as a retrospective study with a relatively small sample size. Nevertheless, our study elucidates the role and clinical feasibility of PBT in high-risk, critically located tumors, where a sufficiently high dose may not be achievable with conventional photons and may be less satisfactory with IMRT. A unique feature of our study is the use of PBT in all patients. Another unique feature of our study is the use of intraoperative RT in 5 patients. The technique used for dural plaque brachytherapy has been previously described [3, 18], and this approach helps deliver a boost dose safely to the involved dura, while sparing the spinal cord. To the best of our knowledge, our series represents the largest report on the use of PBT in the management of non–skull-base extracranial CS.
Conclusion
High-dose PBT in combination with surgery resulted in good local control rates despite the presence of multiple high-risk factors (eg, large tumors, 50% R2, spine location). Notably, the use of PBT allowed for safe dose escalation (>70 Gy) even in the challenging spinal location. Female sex was predictive for decreased survival, while grade III tumors predicted for decreased local control and overall survival. Proton beam therapy should be considered an important treatment modality in the management of these challenging tumors.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Acknowledgments: The project was supported in part by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
References
- 1.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 2.York JE, Berk RH, Fuller GN, Rao JS, Abi-Said D, Wildrick DM, Gokaslan ZL. Chondrosarcoma of the spine: 1954 to 1997. J Neurosurg. 1999;90:73–8. doi: 10.3171/spi.1999.90.1.0073. [DOI] [PubMed] [Google Scholar]
- 3.Wagner TD, Kobayashi W, Dean S, Goldberg SI, Kirsch DG, Suit HD, Hornicek FJ, Pedlow FX, Raskin KA, Springfield DS, Yoon SS, Gebhardt MC, Mankin HJ, Delaney TF. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys. 2009;73:259–66. doi: 10.1016/j.ijrobp.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 4.Bergh P, Gunterberg B, Meis-Kindblom JM, Kindblom LG. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–12. doi: 10.1002/1097-0142(20010401)91:7<1201::aid-cncr1120>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–19. [PubMed] [Google Scholar]
- 6.Talac R, Yaszemski MJ, Currier BL, Fuchs B, Dekutoski MB, Kim CW, Sim FH. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res. 2002;397:127–32. doi: 10.1097/00003086-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Boriani S, De Iure F, Bandiera S, Campanacci L, Biagini R, Di Fiore M, Bandello L, Picci P, Bacchini P. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25:804–12. doi: 10.1097/00007632-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Goda JS, Ferguson PC, O'Sullivan B, Catton CN, Griffin AM, Wunder JS, Bell RS, Kandel RA, Chung PW. High-risk extracranial chondrosarcoma: long-term results of surgery and radiation therapy. Cancer. 2011;117:2513–9. doi: 10.1002/cncr.25806. [DOI] [PubMed] [Google Scholar]
- 9.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, McManus P, Rosenberg AE, Nielsen GP, Harmon DC, Spiro IJ, Raskin KA, Suit HD, Yoon SS, Hornicek FJ. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–9. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthukumar N, Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for chordoma and chondrosarcoma: further experiences. Int J Radiat Oncol Biol Phys. 1998;41:387–92. doi: 10.1016/s0360-3016(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Lovelock DM, Yenice KM, Bilsky MH, Hunt MA, Zatcky J, Leibel SA. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53–61. doi: 10.1016/j.ijrobp.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Hug EB, Fitzek MM, Liebsch NJ, Munzenrider JE. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1995;31:467–76. doi: 10.1016/0360-3016(94)00390-7. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld AJ, Hornicek FJ, Pedlow FX, Kobayashi W, Raskin KA, Springfield D, DeLaney TF, Nielsen GP, Mankin HJ, Schwab JH. Chondrosarcoma of the mobile spine: a review of 21 cases treated at a single center. Spine (Phila Pa 1976) 2012;37:119–26. doi: 10.1097/BRS.0b013e31823d2143. [DOI] [PubMed] [Google Scholar]
- 14.Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596–606. doi: 10.1016/j.ijrobp.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, Rosenberg AE, Slater JM. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–9. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 16.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(suppl 2):57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg AE, Nielsen GP, Keel SB, Renard LG, Fitzek MM, Munzenrider JE, Liebsch NJ. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23:1370–8. doi: 10.1097/00000478-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 18.DeLaney TF, Chen GT, Mauceri TC, Munro JJ, Hornicek FJ, Pedlow FX, Suit HD. Intraoperative dural irradiation by customized 192iridium and 90yttrium brachytherapy plaques. Int J Radiat Oncol Biol Phys. 2003;57:239–45. doi: 10.1016/s0360-3016(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–38. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 21.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093–112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 22.Austin-Seymour M, Munzenrider J, Goitein M, Verhey L, Urie M, Gentry R, Birnbaum S, Ruotolo D, McManus P, Skates S, Ojemann RG, Rosenberg A, Schiller A, Koehler A, Suit HD. Fractionated proton radiation therapy of chordoma and low-grade chondrosarcoma of the base of the skull. J Neurosurg. 1989;70:13–7. doi: 10.3171/jns.1989.70.1.0013. [DOI] [PubMed] [Google Scholar]
- 23.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, Chen YL, Schwab JH, Hornicek FJ. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–22. doi: 10.1002/jso.23617. [DOI] [PubMed] [Google Scholar]
- 24.Rutz HP, Weber DC, Goitein G, Ares C, Bolsi A, Lomax AJ, Pedroni E, Coray A, Hug EB, Timmermann B. Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: initial experience at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2008;71:220–5. doi: 10.1016/j.ijrobp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Weber DC, Rutz HP, Pedroni ES, Bolsi A, Timmermann B, Verwey J, Lomax AJ, Goitein G. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institute experience. Int J Radiat Oncol Biol Phys. 2005;63:401–9. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Nikoghosyan AV, Rauch G, Münter MW, Jensen AD, Combs SE, Kieser M, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study HIT-1-study. BMC Cancer. 2010 doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed]
- 27.Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Tateishi A. Working Group for the Bone and Soft Tissue Sarcomas. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–71. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine (Phila Pa 1976) 2009;34(suppl):S48–57. doi: 10.1097/BRS.0b013e3181b969ac. [DOI] [PubMed] [Google Scholar]